Abstract
Adolescence is associated with potentially stressful challenges, and adolescents may differ from adults in their stress responsivity. To investigate possible age-related differences in stress responsiveness, the consequences of repeated restraint stress (90 min/day for 5 days) on anxiety, as indexed using the elevated plus-maze (EPM) and modified social interaction (SI) tests, were assessed in adolescent and adult Sprague-Dawley male and female rats. Control groups at each age included non-stressed and socially deprived animals, with plasma corticosterone (CORT) levels also measured in another group of rats on days 1 and 5 of stress (sampled 0, 30, 60, 90, and 120 min following restraint onset). While repeatedly restrained animals exhibited similar anxiety levels compared to non-stressed controls in the EPM, restraint stress increased anxiety at both ages in the SI test (as indexed by reduced social investigation and social preference). Daily weight gain measurements, however, revealed more marked stress-related suppression of body weight in adolescents versus adults. Analysis of stress-induced increases in CORT likewise showed that adolescents demonstrated less habituation than adults, embedded within typical sex differences in CORT magnitude (females greater than males) and age differences in CORT recovery (adolescents slower than adults). Despite no observable age-related differences in the behavioral response to restraint, adolescents were more sensitive to the repeated stressor in terms of physiological indices of attenuated weight gain and habituation of stress-induced CORT.
Keywords: rat, adolescence, elevated plus-maze, social interaction, anxiety, restraint stress, corticosterone, body weight gain
Introduction
Adolescence is a developmental period in which an individual transitions from immaturity and dependence on the family unit into maturity and independence. This transformation is accompanied by a number of rapid hormonal, neural, and behavioral changes, with similar alterations identified across a variety of mammalian species (see [1] for review and references). In humans, adolescence is usually defined as the second decade of life [2], with females showing more rapid maturation than males [3]. In rats, postnatal days (P) 28–42 provide a conservative age range during which rats of both sexes and most breeding stock exhibit adolescent-typical neurobehavioral features [1]. Sex differences in the timing of adolescent-related changes may occur in rats as well, with females demonstrating certain maturational changes indicative of impending adolescence as early as P20 and male rats displaying some adolescent-associated characteristics as late as approximately P55 [4].
Adolescence is also a time of considerable behavioral and cognitive change, with adolescents sometimes differing dramatically from those younger or older in the ways they respond to and interact with stimuli in their environment [1]. It has been suggested that human adolescents experience more stressors and negative life events than children or adults [3,5]. Indeed, the developmental transition towards maturity is often stressful, especially given that the magnitude and speed of adolescent-associated changes can overburden the capacity of some adolescents to cope with different environmental and social challenges [6–8]. Furthermore, this adolescent-related stress is strongly associated with the emergence of anxiety disorders either during adolescence or later in life [9], with the median age of onset of anxiety disorders approximately 15 years [10].
It is still not clear, however, whether human adolescents differ from their adult counterparts in terms of how they behaviorally and hormonally respond to stressors and whether the adverse consequences of stress exposure would more profoundly impact the development of anxiety disorders during adolescence than during adulthood. Since human research is limited in its ability to address age-related differences in stress responsiveness, animal models provide an important tool for examining these issues. Similarities found between human adolescents and adolescents of various mammalian species in terms of developmental history, behavioral changes, as well as neural and hormonal responses provide sufficient face and construct validity to support the use of animal models to explore certain basic features of adolescence [1,11].
Research conducted in laboratory animals, albeit limited, has revealed evidence that the responsiveness of adolescents to stressors might differ from that of adults. For instance, adolescent male mice were found to be more sensitive to stress-induced reductions in food intake and showed greater anxiety-like responses than their more mature counterparts following repeated stressor exposure [12]. Additionally, several studies have demonstrated that both male and female adolescent rats show a more prolonged hormonal stress response than do adults when exposed to stressors such as footshock [13] and restraint [14,15].
Importantly, not only have age differences in stress responsiveness been demonstrated in animal models, but also pronounced sex-related differences in both the behavioral and hormonal response to stressors have been reported in studies using adult rats. For instance, repeated restraint stress impairs performance of cognitive tasks in male rats, whereas females show stress-enhanced performance [16–19]. Other experiments using adult rats have shown sex differences in the hormonal response to stressors, with stress-induced elevations in corticosterone (CORT) greater in mature females than males (e.g., [20–22]). Sex differences in stress responsiveness within the period of adolescence have been less well studied, however, with experiments comparing the hormonal and behavioral response to stressors during adolescence and adulthood in both sexes scarce.
Repeated exposure to stressors has been found to increase anxiety-like behaviors in rodents using a number of behavioral assays, including the elevated plus-maze (EPM) and social interaction (SI) tests [23–25]. In the EPM, greater levels of anxiety are indexed by avoidance of the open arms of the maze and greater proportions of time spent in protected areas [26], with this test validated pharmacologically using anxiolytic and anxiogenic compounds (for review and references see [27]). The SI test in rats exploits the sensitivity of social behavior to environmental factors, with social interactions notably suppressed by unfamiliar relative to familiar testing situations [27,28]. Low basal levels of social interaction in unfamiliar testing situations are typically restored by anxiolytic compounds and manipulations [27], whereas high levels of social interactions under familiar circumstances are often sensitive to suppression by anxiogenic drugs/circumstances [28, 29].
The present study was designed to assess possible age- and sex-related differences in responsiveness to repeated stress in Sprague-Dawley rats. Stress-induced anxiety was examined in both the EPM and modified SI (familiar test condition) tests, whereas physiological responsiveness to the stressors was indexed via body weight gain and CORT levels. Use of two behavioral tests of anxiety was included to address the important issue of whether similar age differences in anxiogenic effects of repeated stress would be evident both when animals were tested alone (EPM) as well as when examined in a social context (SI).
Experiment 1
The purpose of Exp. 1 was to investigate possible age- and sex-related differences in the anxiogenic response to repeated restraint stress in the EPM test of anxiety. For this experiment, repeated restraint was chosen as the stressor, since it is primarily psychological in nature and does not involve physical pain or harm to the animal [30,31]. Rats assigned to the repeated restraint condition were exposed to this stressor once per day for 5 days. Since the restraint procedure required separation of animals from their cage mates and the home cage during the 90-min stressor period, two control groups were included in the design of this study. One group of animals was a non-stress control, with these animals receiving no manipulation other than daily recording of body weights. Animals in the other control group were socially isolated in novel holding cages with clean pine shavings for 90 min a day over the 5-day period. This group was included in order to account for the 90-min period of exposure to social isolation and novelty experienced by the stressed animals while in the restraint tubes. Since the EPM test, and also the SI test used in Exp. 2, have both been shown to be affected by experimental manipulations such as novelty exposure or social deprivation (e.g., [32,33]) this group provided the opportunity to determine whether any alterations in behavioral measures in either Experiment 1 or 2 were due to a combination of these variables or the restraint stress exposure itself.
Methods
Rats used in these experiments were maintained and treated in accordance with the guidelines for animal care established by the National Institutes of Health [34], and using protocols approved by the Binghamton University Institutional Animal Care and Use Committee (IACUC). In each experiment, no more than one male and one female animal from a given litter was placed into a particular experimental group [35].
Subjects and Experimental Design
Adolescent and adult Sprague-Dawley rats used in this study were obtained from established breeding pairs in a temperature-controlled vivarium on a 14:10 hr light:dark cycle (lights on at 0700 hr), with ad libitum access to water and food (Purina rat chow, Lowell, MA). On the day after birth, postnatal day (P) 1, litters were culled to 8 – 10 pups, with 6 animals of one sex and 4 animals of the other being retained whenever possible. Offspring then remained with their mother and father until the time of weaning on P21.
A total of 96 adolescent and adult rats were used in the 2 (age: adolescent vs. adult) × 2 (sex: male vs. female) × 3 (stress condition: non-manipulated vs. repeated restraint stress vs. repeated social deprivation) factorial design of this experiment, with 8 animals tested per group. Adolescents entered the experiment at P30-32, while adults began experimental procedures at P65-67, with final behavioral testing occurring on P34-36 (adolescents) or P69-71 (adults). These ages were selected since the majority of past data examining age-related differences in anxiety [32,36,37] and social behavior [33,38,39] from our laboratory has been obtained using mid-adolescent (approximately P35) and young adult rats (approximately P70).
Apparatus
The adult elevated plus-maze (EPM) consisted of two open arms, 48.3 × 12.7 cm, and two closed arms, 48.26 × 12.7 × 29.2 cm. The adolescent EPM was proportionately sized based on crown-rump length and confirmed by gait width analysis, and consisted of 30 × 8.9 cm open arms and 30 × 8.9 × 20.3 cm closed arms. Because our laboratory sometimes conducts EPM assessments following pharmacological manipulations that may disrupt balance/motor coordination, small plastic edges (0.6 cm in height for adolescents and 1.3 cm for adults) were located along each side and end of the open arms to minimize the possibility of falling during testing [40]. These edges ended 4.0 cm (adolescent) and 4.5 cm (adult) before the junctions of the open and closed arms to provide easy access below the plane of the maze, thereby allowing for protected head-dipping behavior. Both mazes were elevated to a height of 50 cm. During EPM sessions, the testing room was maintained under dim light (3 lux), with a fan running to mask extraneous sounds. Sessions were videotaped by a camera mounted at a height of 147 cm above the apparatus to allow testing of the animals without an experimenter present in the room. After testing each animal, the apparatus was cleaned with a 3% hydrogen peroxide solution and dried before the next animal was placed on the apparatus.
Testing Procedure
At the start of the EPM session, each subject was placed on the center platform facing a closed arm and its behavior on the maze videotaped for 5 min. Behavioral measures were later scored from the videotapes by an experimenter blind to the experimental condition of each animal. Measures assessed included time spent on the open (OAT) and closed (CAT) arms and number of entries into the open (OAE) and closed (CAE) arms. An arm entry was defined by all four paws being placed in the arm, whereas an exit was considered to have taken place when at least the two front paws were placed outside of the arm. Number of protected (PHD) and unprotected (UHD) head dips were also assessed; the former was defined as dipping the head over the sides of the maze with the body located in the center platform or a closed arm, whereas head dips were defined as unprotected when the same behavior occurred with the body positioned on an open arm. Similarly, stretched attend postures were recorded and separated into protected (PSAP) and unprotected (USAP) versions, with the protected form exhibited when the animal’s two hind feet remained in a closed arm or on the center platform while the animal elongated its head and shoulders, followed by subsequent retraction, whereas unprotected stretched attends were the same behavior but when emitted while the animal was on an open arm. Frequency of rearing behavior was also recorded.
Percentage of time spent on the open arms and percentage of open arm entries have repeatedly been shown to be reliable measures of anxiety on the EPM [41,42]. Additionally, percent protected head dips and percent protected stretched attend postures have been suggested to be even more sensitive measures of anxiety, based on ethological analysis and pharmacological manipulations [26,43,44]. Closed arm entries and number of rears have generally been considered indices of activity [44,45]. More recently, factor analyses have validated the EPM for use in both adolescent and adult rats, with similar primary and secondary behavioral components (i.e. anxiety and activity, respectively) emerging at both ages [36].
Stressor Procedures
On day 1 of the experiment (P30-32 for adolescents; P65-67 for adults), the experimental procedures began. Daily for 5 days, each animal was removed from its cage between 1000 – 1200 hr and its body weight recorded. Animals in the control condition were otherwise non-manipulated and immediately returned to their home cage with their social partner. Animals in the other control group (social deprivation with novelty) were individually placed into a clean novel holding cage immediately following weighing, and left undisturbed for 90 min before being returned to the home cage. For rats in the repeated stressor group, subjects were restrained in an age/sex size-adjusted restraint tube for 90 min in a novel holding cage. Restraint tubes (Braintree Scientific, Braintree, MA) were round slotted Plexiglas cylinders with sliding plugs to allow adjustment of the tube length for each animal’s size. Cylinders measured 18 × 4.7 cm for adolescent males and females, 20.5 × 7.0 cm for adult females, and 23 × 8.0 cm for adult males (length × diameter). Restraint is a mild stressor commonly used in studies of anxiety-related behavior in laboratory rodents. The duration of exposure to this stressor varied across studies from 30 min [24] to 6 hr [46]. The 90-min restraint stress period, which can be considered as relatively short, was chosen in the present study in order to better assess age- and sex-related differences in the dynamics of CORT response within the period of exposure to the stressor, as well as following the offset of the stressor.
Animals in the non-manipulated group were housed in standard cages (45.5 × 24.5 × 20 cm) with a same-sex littermate partner that was also non-manipulated (but not used as an experimental animal). Animals in the other two conditions were similarly pair-housed with a same-sex littermate, with one animal from a pair placed into the repeated restraint condition, and the other assigned to the repeated social deprivation group.
Testing Procedure
Immediately after the 90-min period of social isolation or restraint on day 5, rats were then isolated in a novel holding cage for 30 min prior to an EPM test. Animals in the non-manipulated group were also exposed to 30-min of pretest isolation at this time. Data from our lab [32] and others [42] has indicated that brief exposure to a novel setting prior to the 5-min EPM test increases subsequent open arm activity, thereby increasing the likelihood of detecting stress-induced anxiogenesis in this behavioral test.
Data Analysis
Data were checked for outliers before analysis with mixed-factor analyses of variance (ANOVA) at each age, with a score > 2.0 standard deviations from the mean of a particular experimental group being considered an outlier. Main effects of age emerged in the ANOVA for body weight gain and almost all of the EPM behavioral measures. Therefore, separate analyses were conducted at each age to explore the effects of stressor exposure on the weight gain and EPM behavior, with Fischer’s Least Significant Difference (LSD) tests used as post hoc comparisons to determine the locus of significant main effects and interactions.
Results
Three animals fell from the maze during testing: one adult male from the non-manipulated group and two adult males from the chronic restraint stress group. These animals were not replaced, and these data were not included in the analyses.
Percent body weight gain
Body weight gain (see Table 1) was analyzed as a percentage increase from day 1 to day 5 [e.g. (day 5 weight − day 1 weight)/day 1 weight * 100]. In this data set, three animals met the criterion for outliers and were removed from the analysis: two adolescent males (one repeatedly restrained and one from the social deprivation condition) and one adult male (from the non-manipulated group). Both repeated restraint stress and repeated social deprivation significantly suppressed weight gain of the adolescents when compared to their non-manipulated counterparts [stress main effect: F(2,40) = 4.23, p ≤ .05], whereas adults only showed a stress-induced suppression of weight gain following repeated restraint [stress main effect: F(2,37) = 5.09, p ≤ .05]. In both age groups, males gained significantly more weight than their female counterparts [sex main effect for adolescents: F(1,40) = 9.44, p ≤ .01; for adults: F(1,37) = 6.64, p ≤ .05].
Table 1.
Percent body weight gain from day 1 to day 5 in adolescent and adult male and female rats
| Adolescent | Adult | |||
|---|---|---|---|---|
| Condition | Male | Female | Male | female |
| Exp. 1 | ||||
| Non-manipulated | 26.9 ± 1.1 | 24.2 ± 1.7 | 4.7 ± .5 | 2.8 ± 1.5 |
| Repeated restraint stress | 22.2 ± .6* | 19.2 ± 3.1* | 1.8 ± .6* | −0.6 ± 1.1* |
| Repeated social deprivation | 25.8 ± 1.3* | 18.4 ± 1.1* | 3.3 ± .6 | 1.5 ± .8 |
|
| ||||
| Exp. 2 | ||||
| Non-manipulated | 25.0 ± .7 | 22.6 ± .7 | 4.0 ± 1.0 | 4.1 ± 1.4 |
| Repeated restraint stress | 19.2 ± 1.7* | 18.6 ± 1.0* | 2.4 ± .7 | 3.3 ± .9 |
| Repeated social deprivation | 25.3 ± .9 | 21.2 ± 1.0 | 3.2 ± .3 | 2.9 ± .6 |
Asterisks (*) denote a significant difference from the non-manipulated group, collapsed across sex (main effects of stressor).
Elevated plus-maze results
For the analyses of the EPM data, several animals were eliminated as outliers: one adolescent and one adult male, both from the social deprivation condition; and one adolescent female from the restraint stress condition.
Analysis of the classic anxiety-like behaviors, %OAE and %OAT (Figure 1a and b), showed that repeated restraint stress did not significantly impact anxiety levels in either adolescents or adults when compared to their non-manipulated controls. Repeated social deprivation, however, was found to significantly reduce anxiety levels (i.e. to increase %OAE and %OAT) with both measures in adults, regardless of sex [stress main effect for adults for %OAE: F(2,38) = 5.67, p ≤ .01; for %OAT: F(2,38) = 4.19, p ≤ .05]; these effects were not evident among adolescents.
Figure 1.
The effects of repeated (5 days) restraint stress (RS) or social deprivation (SD) on (a) percent open arm entries and (b) percent open arm time in both adolescent and adult male and female rats tested for 5 min in the elevated plus-maze. Asterisks (*) indicate a significant difference from non-manipulated (NM) control animals, with p ≤ .05. In this figure (and in all subsequent figures) bars depict the mean for a particular group, while vertical lines represent the standard error of the mean.
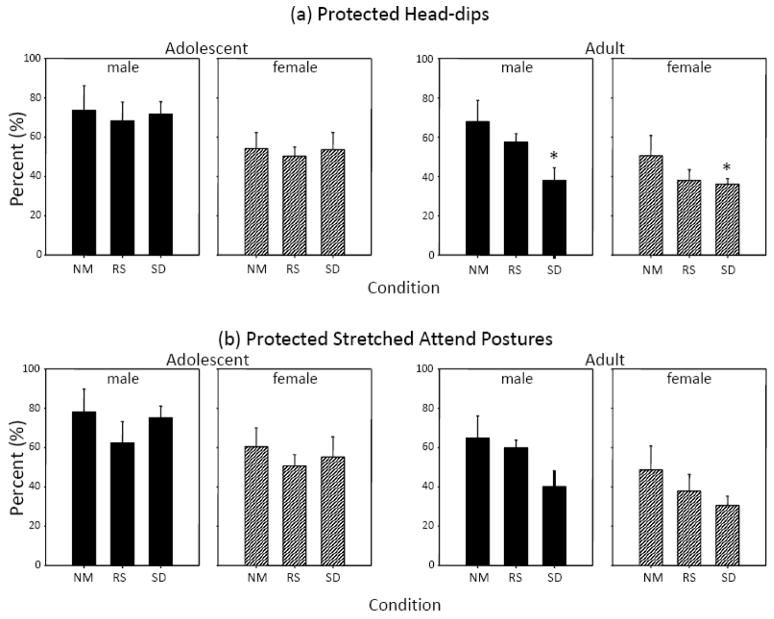
Examination of the more ethologically relevant anxiety measures, %PHD and %PSAP (Figure 2a and b), also revealed no effects of repeated restraint stress in animals of either age. Repeated social deprivation, however, significantly suppressed anxiety among adults but not adolescents when assessed via %PHD [stress main effect for adults: F(2,38) = 4.50, p ≤ .05], with a similar trend that did not quite reach significance for %PSAP (p = .07). Overall, with both the %PHD and %PSAP measures, adult females were found to be significantly less anxious than adult males [sex main effect for %PHD: F(1,38) = 4.39, p ≤ .05; for %PSAP: F(1,38) = 4.63, p ≤ .05], whereas no significant sex differences emerged in adolescents.
Figure 2.
The effects of repeated restraint stress (RS) or social deprivation (SD) on (a) percent protected head-dips and (b) percent protected stretched attend postures in both adolescent and adult male and female rats tested for 5 min in the elevated plus-maze. Asterisks (*) indicate a significant difference from non-manipulated (NM) control animals with p ≤ .05.
Activity in the EPM, as indexed via CAE, was not affected by either repeated restraint or social deprivation in animals of either age or sex. In general, adolescent (12.7 ± .6) and adult females (11.1 ± .5) were more active than their male counterparts (adolescent males: 9.3 ± .9; adult males: 9.3 ± .6) [sex main effect for adolescents: F(1,40) = 8.32, p ≤ .01; for adults: F(1,38) = 6.21, p ≤ .05].
Experiment 2
The results of Exp. 1 showed no significant effects of repeated restraint stress on anxiety-like behaviors in the EPM test in either adolescents or adults. The EPM assay of anxiety, however, has been shown to be quite vulnerable to methodological variations. In particular, data using adult subjects have shown that manipulations of many pretest variables including, but not limited to, pretest handling [47], pretest transportation via cart [48], pretest injection [49], exposure to varying periods of isolation [50,51], and exposure to a novel arena [42,52], are capable of altering expression of anxiety-like behaviors during the test. Such alterations in EPM behavior due to pretest variations may not be expressed similarly in adolescents and adults, however [32,37]. Given these issues, it is important to investigate anxiety-related behavior using other validated behavioral anxiety assays. The purpose of Exp. 2, therefore, was to assess potential age differences in the effects of repeated restraint stress and social deprivation in both adolescent and adult rats using another model of anxiety, the social interaction test. Use of the social interaction test as an anxiety assay allows for the assessment of anxiety behaviors in a social setting, with the expression of stress-induced anxiety potentially different in a social (SI) vs. non-social (EPM) testing situation.
Methods
Subjects and Experimental Design
All animals were housed in a temperature-controlled (22°) vivarium maintained on a 14/10-hr light/dark cycle (lights on at 0700) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. On the day of birth (P0), litters were culled to 10 pups (5 males and 5 females) within 24 hr and housed until weaning with their mothers in standard plastic opaque maternity cages (30 × 20 × 20 cm) with pine shavings as bedding material. Rats were weaned on P21 and placed into cages (48.5 × 39.0 × 20 cm) with same-sex littermates (5 animals per cage) until the start of the experiment at adolescence (P30-32) or adulthood (P65-67).
The design of this experiment was a 2 (age: adolescent vs. adult) × 2 (sex: male vs. female) × 3 (stress condition: non-manipulated vs. repeated social deprivation vs. repeated restraint stress) factorial, with 6 experimental animals tested per group. As in Exp. 1, no more than one animal of each sex from a given litter was placed into an experimental group, with remaining animals from a litter used in other projects.
Apparatus
Testing was conducted in Plexiglas (Binghamton Plate Glass, Binghamton, NY) chambers (30 × 20 × 20 cm for adolescents; 45 × 30 × 30 cm for adults) that contained clean pine shavings. Each test apparatus was divided into two compartments by a clear Plexiglas partition containing an aperture (7 × 5 cm for adolescents; 9 × 7 for adults) to allow movement of animals between compartments [38,39].
Each social interaction test session was 10 min in duration, with all testing procedures conducted under dim light (15–20 lux) between 1200 and 1400 hr, and a white noise generator used to attenuate extraneous sounds during testing. Social behavior emitted by the test subject during the session was recorded by a video camera mounted above the apparatus, with videotapes later scored by a trained experimenter who was blind to the experimental condition of each animal.
Frequencies of a number of social activities were analyzed [53–55]. Social investigation was defined as sniffing of any part of the body of the partner. Play fighting was scored as the sum of the frequencies of the following behaviors: pouncing or playful nape attack (experimental subject lunges at the partner with its forepaws extended outward); following and chasing (experimental animal rapidly pursues the partner); and pinning (the experimental subject stands over the exposed ventral area of the partner, pressing it against the floor). Play fighting differs from serious fighting in the laboratory rat according to the target of the attack—during play fighting, snout or oral contact is directed towards the partner’s nape, whereas during serious fighting the partner’s rump is the object of the attack [56]. Aggressive behavior (serious fighting) was not scored in these experiments, since subjects did not exhibit serious attacks or threats. The total number of crossovers (movements between compartments through the aperture) exhibited by each experimental subject was used as an index of general locomotor activity [57].
Social preference/avoidance was analyzed by separately measuring the number of crossovers demonstrated by the experimental subject towards as well as away from the social partner. Social motivation was assessed by means of a coefficient of preference/avoidance [coefficient (%) = (crossovers to the partner − crossovers away from the partner)/(total number of crosses both to and away from the partner) × 100]. Social preference was defined as positive values of the coefficient, while social avoidance was associated with negative values [38,39].
Procedure
Experimental animals were individually exposed to the social interaction test chamber one day prior to the test session (i.e. the fourth day of the repeated stressor period) for 30 min. For animals in the repeated stress or social deprivation conditions, this pre-test familiarization was conducted to increase baseline levels of social interaction on the test day, hence making potential anxiogenic effects of the chronic stressors easier to observe [27,58].
The stressor conditions and procedures in the current experiment were identical to those detailed in Exp. 1, with animals either non-manipulated, socially isolated or restrained repeatedly for 5 days. Social interaction testing was conducted after the final stressor exposure on day 5 of the experiment. Immediately following the stressor period on that day, subjects from all experimental conditions (including non-manipulated animals) were isolated in novel cages for 30 min prior to placement in the testing chamber. Social interactions between the experimental subject and a social partner were then recorded for 10 min. Each social partner was always a non-manipulated, same-sex, unfamiliar peer who was unfamiliar with the test situation and not socially deprived prior to testing. Weight differences between test subjects and their social partners were kept as small as possible.
Data Analysis
As in Exp. 1, data were checked for outliers before analysis. For these data, no outliers were present. Also similar to Exp. 1, a main effect of age emerged in the overall ANOVAs for almost all measures, and therefore separate analyses were conducted at each age to explore the effect of stressor exposure on anxiety levels, with stress-induced changes in social behavior and social motivation assessed by post hoc comparisons (Fisher’s LSD) to determine to locus of significant main effects and interactions. In addition, given the sex-related differences in play behavior observed in both adolescents and adults, separate 1-way ANOVAs were performed for each age and sex group to assess the effects of stress on play behavior.
Results
Percent Body Weight Gain
Percent Body weight gain from day 1 to day 5 (see Table 1) was calculated as in Exp. 1 (see Exp. 1 results). Assessment of adolescent body weight data revealed that, overall, adolescent males gained significantly more weight than their adolescent female counterparts [sex main effect: F(1,30) = 7.42, p ≤ .05], with both male and female adolescents showing significant suppression of weight gain when exposed to repeated restraint stress in comparison to non-manipulated adolescents [stress main effect: F(2,30) = 12.30, p ≤ .001]. In contrast to the adolescents, neither the repeated restraint stress nor the social deprivation influenced weight gain in adult animals of either sex when compared to their non-manipulated controls, with both male and female adults exhibiting similar weight gain during the experiment.
Social Interaction Results
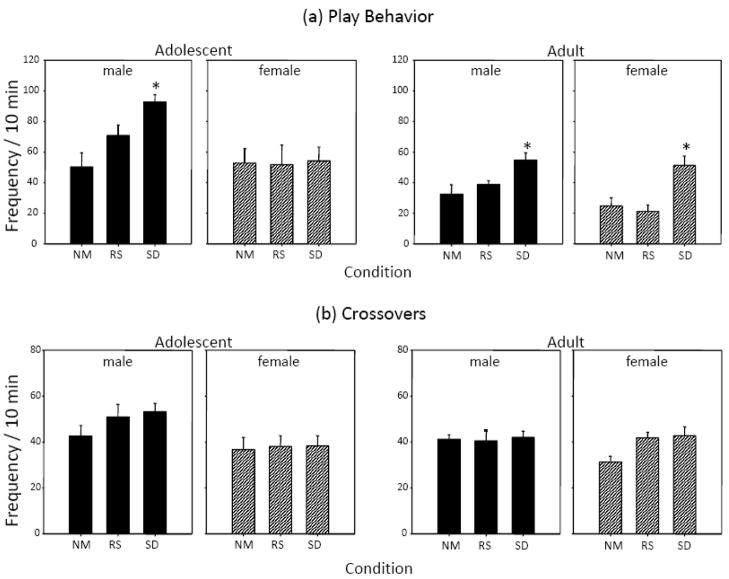
When frequency of social investigation was examined (Figure 3a), both adolescents and adults exhibited significant reductions in this measure following exposure to the repeated restraint stressor when compared to non-manipulated animals [stress main effect for adolescent: F(2,30) = 4.91, p ≤ .05; for adults F(2,30) = 6.53, p ≤.01]. However, a similar duration of repeated social deprivation was without effect on levels of social investigation in either age group.
Figure 3.
The effects or repeated restraint stress (RS) or social deprivation (SD) on (a) social investigation and (b) social preference/avoidance in both adolescent and adult male and female rats during a 10-min social interaction test. Asterisks (*) represent a significant difference from non-manipulated controls with p ≤ .05.
Results for the coefficient of social preference/avoidance (Figure 3b) demonstrated an overall preference for the social partner in all experimental groups. Analysis of the adolescent animals revealed that, regardless of sex, repeated restraint stress but not repeated social deprivation significantly decreased the level of social preference when compared to non-manipulated adolescents [stress main effect: F(2,30) = 9.03, p ≤ .001]. Whereas adult females demonstrated this same pattern in response to the repeated stressors, adult male animals exhibited equivalent levels of social preference across all test conditions [sex x stress interaction: F(2,30) = 4.72, p ≤ .05].
Males demonstrated more play behavior than females at each age group [sex main effect for adolescents: F(1,30) = 6.26, p ≤ .05; for adults: F(1,30) = 5.29, p ≤ .05], with separate analyses therefore conducted at each sex and age group. Overall, frequency of play behavior (Figure 4a) revealed few effects of the repeated restraint stressor, with adolescent and adult males and females demonstrating no significant alterations in play following this stress procedure. Interestingly, exposure to repeated social deprivation significantly elevated levels of play in the analysis of both male and female adult data [stress main effect for males: F(5.67, p ≤ .05; for females: F(2,15) = 9.49, p ≤ .01]. Similar to adults, adolescent males also exhibited social deprivation-induced increases in play behavior [stress main effect for adolescent males: F(2,15) = 8.81, p ≤ .01], with their female counterparts being the only group that did not show this alteration in play behavior.
Figure 4.
The effects of repeated restraint stress (RS) or social deprivation (SD) on (a) play behavior and (b) number of crossovers in both adolescent and adult male and female rats during a 10-min social interaction test. Asterisks (*) depict a significant differences from non-manipulated (NM) control animals with p ≤ .05.
Assessment of activity (as indexed via total number of crossovers) showed that neither stressor condition significantly altered activity levels (Figure 4b). Overall, adolescent males exhibited significantly more crossovers than adolescent females [sex main effect: F(1,30) = 8.31, p ≤ .01], a sex difference that appeared to be driven mostly by the adolescents in the restraint stress and social deprivation conditions, although the interaction of sex × condition did not reach significance. No sex differences emerged in the analysis of the adult data.
Experiment 3
Exposure to a repeated restraint stressor generally resulted in significant anxiogenesis for adolescent and adult rats of both sexes in the social interaction test (Exp. 2) but not in the EPM test (Exp. 1). Examination of body weight gain, however, revealed some age-related differences in stressor impact, suggesting that the physiological response to the repeated stressor many have differed from the behavioral expression of stress-induced anxiety behavior. Experiment 3 was therefore conducted to investigate possible age-related differences in the hormonal response (another physiological assessment of stressor impact) to the same repeated restraint stressor via measurement of CORT levels. In contrast to Experiments 1 and 2, animals exposed to repeated social deprivation were not included in this experiment. This decision was based on behavioral data showing a lack of any anxiogenic effect of this manipulation in either of the two previous experiments.
Methods
Subjects and Experimental Design
A total of 120 Sprague-Dawley rats bred and reared as in Exp. 2 were used in the current study. CORT levels were assessed in a 2 (age: adolescent vs. adult) × 2 (sex: male vs. female) × 5 (time: baseline, 15, 30, 60 and 120 min after stressor onset) × 2 (day: day 1 vs. day 5) factorial design (n = 6 animals per group).
Procedure
Stress-induced elevations in CORT were examined in animals subjected to five consecutive days of repeated restraint stress, using the same stressor procedure as in Exps. 1 and 2. On the first day of restraint, animals were randomly assigned for sampling at either 15, 30, or 60 min into stressor exposure or at 30 min after stressor offset (120 min time point; rats were removed from restraint tubes and left in the holding cage for this 30-min period). Although samples were not collected on days 2 – 4, the restraint procedure was repeated each day. On the final day of restraint (day 5), the same group of animals was sampled at the same time-point after onset of the stressor as on day 1. Baseline CORT levels were assessed in a separate group of non-stressed animals taken directly from the home cage, with the same animals sampled on day 1 and day 5.
Blood samples were collected via tail vein using heparinized tubes and were then centrifuged at 2°C for 20 min at 3,000 rpm. Plasma samples were frozen in a − 80° freezer until the time of assay. Plasma CORT levels were analyzed by radioimmunoassay using RIA kits obtained from ICN Biomedicals, Inc. (Orangeburg, NY). Since animals were restrained in the slotted Plexiglas tubes described in Exp. 1, CORT samples collected during the restraint period could be collected while animals remained confined in the tube.
Data Analysis
No outliers were present in the analyses of these CORT data. Data were analyzed with mixed factor ANOVA. Significant main effects and interactions were assessed with post hoc comparisons using Fisher’s LSD.
Results
When data from both age and sex groups were considered together, a significant main effect of sex emerged [F(1,100) = 51.23, p ≤ .0001] tempered by significant interactions of age and time with this variable [F(1,100) = 23.66, p ≤ .0001 and F(4,100) = 3.35, p ≤ .05, respectively]. As is usually observed in analyses of the CORT response (e.g. [21,59]), females exhibited significantly greater stress-induced increases in CORT and slower recovery than males. Given the large sex differences apparent in the magnitude of the CORT response, separate ANOVAs were conducted on data within each sex to further explore the CORT response over the course of the stressor exposure within and across days.
In the analysis of the CORT data in males (Figure 5a), a significant age × time × day interaction was observed [F(4,50) = 4.39, p ≤ .01]. On the first day of restraint stress, both adolescent and adult males exhibited a comparable increase in CORT levels, with only adult males showing a significant post-stressor decline. That is, adult males sampled at the post-stress sampling time point (120 min) had significantly lower CORT levels than adult males sampled during the stressor exposure (15, 30 and 60 min) and also than adolescent males examined at the 120 min sampling point. Dramatic ontogenetic differences in the CORT response emerged, however, by day 5. On the fifth day of stressor exposure, adult males exhibited a robust attenuation in stress-induced elevations of CORT. Specifically, at all time points examined following stressor onset, adult CORT levels were significantly lower on day 5 than on day 1 and were lower on day 5 than those of adolescents also sampled at the same post-onset time intervals on day 5. When habituation of CORT levels was examined in adolescent males, a more modest reduction in their CORT response to repeated restraint stress was observed, with CORT levels showing significant reductions from day 1 to day 5 only at the post-stress sampling point (120 min).
Figure 5.
The corticosterone response to acute (day 1) and repeated (day 5) restraint stress for both male and female adolescent and adult rats at 15, 30, 60, or 120 min after onset of the 90-min stressor. Baseline measurements (0 min assessments) were collected in a separate group of animals taken directly from the home cage. Dark (filled) symbols represent stress-induced elevations in corticosterone on the first day of stressor exposures, while light (open) symbols show the corticosterone response on the last (5th) day or stressor exposure. The same animals were sampled at a given time point on day 1 and day 5, with different animals sampled at each post-onset interval. Since the baseline data were uniformly low and did not differ across groups, data were not adjusted for these baselines.
Adult females exhibited a greater stress-induced elevation in CORT than their adolescent counterparts [main effect of age: F(1,50) = 4.95, p ≤ .05] (Figure 5b), an effect that did not interact significantly with the other variables. Regardless of age, on the first day of restraint stress a modest, but non-significant, recovery in the CORT response was observed by the post-stress time point (120 min). By the fifth day of restraint exposure, however, both adolescent and adult females showed significant recovery of CORT levels post-stress, with CORT significantly lower at the 60 and 120 min time points on day 5 versus day 1 and significantly lower at the 60 and 120 min time points than at 30 min following stressor onset [day x time interaction: F(4,50) = 5.51, p ≤ .001].
General Discussion
Overall, adolescent and adult males and females did not show increases in anxiety-like responses to repeated restraint stress when tested in the EPM, whereas reliable increases in anxiety were observed in the social interaction test, regardless of age. In contrast to the behavioral effects, the physiological effects of restraint stress differed as a function of age, with adolescent males and females showing greater restraint stress-induced reductions in body weight gain and adolescent males demonstrating less habituation to stress-induced elevations in CORT levels across days when compared to their adult counterparts.
Given the high sensitivity of the EPM to anxiogenic compounds [29], it was surprising that repeated restraint did not impact anxiety behavior in this test. Yet, findings from previous studies examining effects of repeated restraint on anxiety in the EPM are mixed, with increased [25,60] or unchanged anxiety levels [59,61,62] sometimes observed. Inconsistent results in the effects of repeated restraint stress on EPM anxiety levels may be related to methodological differences across laboratories, since the EPM has been shown to be sensitive to procedural differences, including different housing conditions [50,51] and handling procedures [47]. It is possible that the lack of restraint-induced anxiogenesis in the present study may reflect habituation to the anxiogenic effects of restraint as indexed within the non-social context of the EPM. Indeed, anxiogenesis following restraint stress is more consistently observed after acute stress, with reliable enhancement of anxiety observed following a single exposure to this stressor [61–63].
In contrast to the EPM experiment, repeated restraint resulted in a reliable increase in anxiety that was generally consistent across age and sex, with both male and female adolescents and adults demonstrating significant decreases in social investigation in response to repeated restraint stress, and play behavior not affected by this stressor. When social preference was examined, however, age-related differences in stress responsivity were evident in male subjects and sex-associated differences became apparent in adults, with adult males not showing the restraint stress-induced reduction in social preference observed in adolescent males or in females of either age. Therefore, two measures of social behavior, namely social investigation and social preference, were selectively sensitive to repeated restraint stress, results reminiscent of previous findings where only these measures of social behavior were found to be sensitive to the anxiolytic effects of ethanol in adolescents and adults [57,64]. Taken together, these results suggest that social investigation and the coefficient of social preference may be particularly sensitive measures for assessment of anxiolytic and anxiogenic manipulations in the SI test in both adolescents and adults.
While some SI studies have reported that repeated restraint increases anxiety (indexed by attenuated social interactions (present study, [23,24]), others have found no changes in social behavior after acute [63] or repeated restraint [46]. Analysis of the literature, however, would suggest that restraint-induced alterations in social behavior likely differ as a function of test situation familiarity. Specifically, animals tested in a familiar (and hence low anxiety-provoking) environment generally exhibit decreased social interactions following restraint stress (present study, [23,24]), whereas rats whose social behavior is already attenuated by testing in unfamiliar (anxiogenic) circumstances often show no further social suppression following exposure to restraint [46,63]. Together, these findings and the results of the present study suggest that the level of anxiety produced by familiarity/unfamiliarity of the test situation [28,65] is an extremely important variable when assessing anxiogenic effects of stressors.
When the behavioral effects of repeated social deprivation were considered, the pattern of results differed considerably from those induced by repeated restraint. In the EPM for instance, repeated social separation of adults produced anxiolytic effects, as indexed by significant increases in percent open arm entries and time, and decreases in protected head dips relative to non-manipulated adult control animals—an effect not observed among adolescents. While one might expect the opposite (i.e., that the stress of social isolation immediately prior to testing would increase anxiety), other studies have also found anxiolytic effects following various acute pretest perturbations. For example, recent results from our laboratory reported decreased EPM anxiety levels in both adult and adolescent rats following exposure to several pretest perturbations, with these anxiolytic effects sometimes differentially expressed across age and adults seemingly more sensitive than adolescents to these pretest perturbations [32]. Similarly, other researchers have reported anxiolysis in the EPM after acute social defeat [66], pretest handling [47], or a combination of mild social stress with brief isolation [67]. Thus, there is an increasing consensus that certain pretest perturbations serve to attenuate expression of anxiety in the EPM.
In the present study, age differences in the anxiolytic effects of pretest social deprivation were not related to age-associated variations in baseline anxiety levels, given that basal anxiety responses in the EPM were comparable across age for NM controls. Observed age differences in the effects of repeated social deprivation also were seemingly unrelated to age-associated alterations in response to the EPM itself, since principle components analyses have revealed the primary underlying components of EPM behavior to be similar in both adolescent and adult Sprague-Dawley rats [36]. The mechanisms underlying anxiolytic effects of repeated social deprivation prior to the EPM test, as well as age-related differences in these effects, however, remain to be investigated.
In the SI test, exposure to repeated social deprivation increased play behavior in adolescent males and adult animals of both sexes, whereas adolescent females did not show significant changes in play. Interestingly, social deprivation increased levels of play in adults to levels similar to those of their NM adolescent counterparts. Such findings suggest that periods of social deprivation can reinstate notable amounts of play behavior among adults and perhaps even increase the rewarding value of those interactions, as suggested by previous results using the conditioned place preference paradigm [68]. The sex difference in response to repeated social deprivation among adolescents was rather unexpected, given that previously both adolescents and adults were found to exhibit enhanced play behavior after 5 days of isolate-housing, with no sex differences being observed at any age [33]. While sex differences in play among adolescent rats have been reported previously (with males generally engaging in more play [55,69–71], these sex differences are not robust and appear to be partially strain-dependent [69,72].
Repeated restraint stress produced marked physiological effects in both adolescents and adults. In terms of body weight, restraint generally decreased weight gain, particularly in adolescent animals. Age differences in sensitivity to restraint-induced reductions in body weight have been previously reported in mice, with 5 days of repeated restraint decreasing body weight in 4-wk-old but not 8-wk-old male mice [12]. While similar age differences were observed in Exp. 2 of the present study, adults from Exp. 1 exhibited significant restraint-related reductions in weight gain, therefore attenuating age differences in this effect. It is possible that differences in housing conditions between the two experiments could have contributed to these inconsistent effects of restraint on weight gain among adults. Perhaps the larger social housing groups of Exp. 2 provided a buffering effect for adults against stress-induced attenuations in weight gain, and hence increased the likelihood of observing ontogenetic differences in the effects of stress on this measure.
Yet another physiological consequence of the repeated restraint procedure was an increase in CORT levels. In Experiment 3, pronounced age- and sex-associated differences were seen in terms of the CORT response to both acute and repeated stress. Adolescent and adult males demonstrated similar peak levels of CORT response to acute restraint stress (day 1), with adolescents maintaining higher CORT levels than adults after termination of the stressor. These findings are in agreement with the previous reports of delayed post-stressor recovery following acute stress among adolescents relative to adults [14,21,73–76]. According to some investigators, the prolonged release of CORT in young animals relative to their more mature counterparts is associated with an immature negative feedback system [21,77]. However, the inability to terminate the glucocorticoid response during adolescence is not related to glucocorticoid and mineralocorticoid receptor binding capacity or affinity [76], since the levels of these receptors do not differ between adolescent and adult rats [77]. The CORT response after repeated restraint stress exposure (day 5) differed dramatically between adolescent and adult males, with adults showing pronounced reduction in CORT levels relative to day 1 and also in comparison to adolescents on day 5 at the same sampling points. Among adolescents, a significant attenuation in the CORT response to restraint from day 1 to day 5 was only seen at the last sampling point (i.e. 120 min after stressor onset). While an adolescent-associated slower recovery to basal CORT levels following repeated restraint stress has not always been reported [14], it is likely that discrepancies between studies could reflect differences in experimental procedures such as the specific age at stress exposure/testing and potential differences in stress responsivity between shipped and non-shipped animals. The precise influence of such variables on ontogenetic differences in stress responsiveness will certainly require further study.
Overall, pronounced sex differences were seen in Exp. 3, with adult females demonstrating higher stress-induced CORT levels than adult males. Many studies conducted in laboratory rodents have demonstrated that the ACTH and CORT response to stressors is greater in adult females than males, with these sex differences in HPA function being related largely to the control of HPA axis by sex hormones (for references see [21]). This elevation in the CORT response to stress was age dependent, with adolescent females demonstrating lower stress-induced CORT levels than their adult counterparts, although both ages showed pronounced habituation of the CORT response following repeated restraint. These findings are in agreement with the previously reported enhancement of acute restraint stress-induced CORT response in adult females relative to their younger counterparts [78], although such age differences among females are not ubiquitous [15].
In summary, age-related differences in stress responsivity emerged when indexed via the physiological measures of body weight changes and CORT responses, whereas both ages responded similarly when anxiety-like behavior was assessed via the EPM and SI tests. These results demonstrate that conclusions regarding enhanced stress responsiveness during adolescence should be made cautiously, given that the stress-induced response under investigation, the test context, as well as sex of the experimental animals may impact observed age-associated differences or similarities in stress responsivity. Furthermore, the lack of age- and sex-related differences in stress-induced anxiety when using the SI test suggests that this paradigm may prove to be a particularly valuable measure for ontogenetic studies of responsiveness to anxiogenic influences and anxiolytic compounds in both males and females.
Acknowledgments
Supported by grants R01 DA019071, R37 AA12525 and R01 AA01735501 to Linda Spear and R01 AA12453 to Elena Varlinskaya.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 2.Petersen AC, Silbereisen RK, Sorensen S. Adolescent development: A global perspective. In: Hurrelmann K, Hamilton SF, editors. Social Problems and Social Contexts in Adolescence. New York: Aldine de Gruyter; 1996. pp. 3–37. [Google Scholar]
- 3.Buchanan CM, Eccles JS, Becker JB. Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. PsyB. 1992;111(1):62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Odell WD. Sexual maturation in the rat. In: Grumbach MM, Sizonenko PC, Aubert ML, editors. Control of the onset of puberty. Baltimore: Williams & Wilkins; 1990. pp. 183–210. [Google Scholar]
- 5.Larson R, Asmussen L. Anger, worry, and hurt in early adolescence: an enlarging world of negative emotions. In: Colten ME, Gore S, editors. Adolescent Stress: Causes and Consequences. New York: Aldine de Gruyter; 1991. pp. 21–41. [Google Scholar]
- 6.Collins ME. Transition to adulthood for vulnerable youths: A review of research and implications for policy. Soc Serv Rev. 2001;75:271–291. [Google Scholar]
- 7.Davis M. Addressing the needs of youth in transition to adulthood. Adm Policy Ment Health. 2003;30(6):495–509. doi: 10.1023/a:1025027117827. [DOI] [PubMed] [Google Scholar]
- 8.Jessor R. Successful adolescent development among youth in high-risk settings. Am Psychol. 1993;48(2):117–126. doi: 10.1037//0003-066x.48.2.117. [DOI] [PubMed] [Google Scholar]
- 9.Turner RJ, Lloyd DA. Stress burden and the lifetime incidence of psychiatric disorder in young adults: racial and ethnic contrasts. Arch Gen Psychiatry. 2004;61(5):481–488. doi: 10.1001/archpsyc.61.5.481. [DOI] [PubMed] [Google Scholar]
- 10.Kessler RC. The global burden of anxiety and mood disorders: putting the European Study of the Epidemiology of Mental Disorders (ESEMeD) findings into perspective. The J Clin Psychiatry. 2007;68 (Suppl 2):10–19. [PMC free article] [PubMed] [Google Scholar]
- 11.Spear LP. Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicol Teratol. 2007;29(1):1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone EA, Quartermain D. Greater behavioral effects of stress in immature as compared to mature male mice. Physiol Behav. 1998;63:143–145. doi: 10.1016/s0031-9384(97)00366-1. [DOI] [PubMed] [Google Scholar]
- 13.Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29(9):1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- 14.Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147(4):1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- 15.Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004;80(6):387–393. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- 16.Bowman RE. Stress-induced changes in spatial memory are sexually differentiated and vary across the lifespan. J Neuroendocrinol. 2005;17(8):526–535. doi: 10.1111/j.1365-2826.2005.01335.x. [DOI] [PubMed] [Google Scholar]
- 17.Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43(1):48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 18.Luine V. Sex differences in chronic stress effects on memory in rats. Stress (Amsterdam, Netherlands) 2002;5(3):205–216. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- 19.Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol. 2007;19(10):743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 20.Chisari A, Carino M, Perone M, Gaillard RC, Spinedi E. Sex and strain variability in the rat hypothalamo-pituitary-adrenal (HPA) axis function. J Endocrinol Invest. 1995;18(1):25–33. doi: 10.1007/BF03349692. [DOI] [PubMed] [Google Scholar]
- 21.McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86(2):220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Seale JV, Wood SA, Atkinson HC, Bate E, Lightman SL, Ingram CD, Jessop DS, Harbuz MS. Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamic-pituitary-adrenal axis activity in male and female rats. J Neuroendocrinol. 2004;16(6):516–524. doi: 10.1111/j.1365-2826.2004.01195.x. [DOI] [PubMed] [Google Scholar]
- 23.Gehlert DR, Shekhar A, Morin SM, Hipskind PA, Zink C, Gackenheimer SL, Shaw J, Fitz SD, Sajdyk TJ. Stress and central Urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur J Pharmacol. 2005;509(2–3):145–153. doi: 10.1016/j.ejphar.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 24.Sajdyk TJ, Fitz SD, Shekhar A. The role of neuropeptide Y in the amygdala on corticotropin-releasing factor receptor-mediated behavioral stress responses in the rat. Stress. 2006;9(1):21–28. doi: 10.1080/10253890600557315. [DOI] [PubMed] [Google Scholar]
- 25.Sevgi S, Ozek M, Eroglu L. L-NAME prevents anxiety-like and depression-like behavior in rats exposed to restraint stress. Methods Find Exp Clin Pharmacol. 2006;28(2):95–99. doi: 10.1358/mf.2006.28.2.977840. [DOI] [PubMed] [Google Scholar]
- 26.Rodgers RJ, Cole JC. The elevated plus-maze: pharmacology, methodology, and ethology. In: Cooper SJ, Hendrie CA, editors. Ethology and psychopharmacology. Chichester: John Wiley & Sons, Ltd; 1994. pp. 9–44. [Google Scholar]
- 27.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463(1–3):35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 28.File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62(1):19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24(3):525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 30.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg MS, Girotti M, Spencer RL. Restraint-induced fra-2 and c-fos expression in the rat forebrain: relationship to stress duration. Neuroscience. 2007;150(2):478–486. doi: 10.1016/j.neuroscience.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Pretest manipulations impact elevated plus-maze behavior differentially across age in adolescent and adult rats. Pharmacol Biochem Behav. 2009 doi: 10.1016/j.pbb.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188(2):398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Institute of Laboratory Animal Research, C. o. L. S., National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 35.Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14(3):221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- 36.Doremus TL, Varlinskaya EI, Spear LP. Factor analysis of elevated plus-maze behavior in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83(4):570–577. doi: 10.1016/j.pbb.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Doremus-Fitzwater TL, Spear LP. Developmental differences in acute ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2007;31(9):1516–1527. doi: 10.1111/j.1530-0277.2007.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner’s activity. Physiol Behav. 1999;67(4):475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- 39.Varlinskaya EI, Spear LP, Spear NE. Acute effects of ethanol on behavior of adolescent rats: role of social context. Alcohol Clin Exp Res. 2001;25(3):377–385. [PubMed] [Google Scholar]
- 40.Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol Biochem Behav. 1996;54(1):31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- 41.Lal H, Prather PL, Rezazadeh SM. Anxiogenic behavior in rats during acute and protracted ethanol withdrawal: reversal by buspirone. Alcohol. 1991;8(6):467–471. doi: 10.1016/s0741-8329(91)90153-n. [DOI] [PubMed] [Google Scholar]
- 42.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 43.Espejo EF. Structure of mouse behavior on the elevated plus-maze test of anxiety. Behav Brain Res. 1997;86:105–112. doi: 10.1016/s0166-4328(96)02245-0. [DOI] [PubMed] [Google Scholar]
- 44.Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21(6):801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 45.Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49(1):171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 46.Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res. 2005;156(1):105–114. doi: 10.1016/j.bbr.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Schmitt U, Hiemke C. Strain differences in open-field and elevated plus-maze behavior of rats without and with pretest handling. Pharmacol Biochem Behav. 1998;59(4):807–811. doi: 10.1016/s0091-3057(97)00502-9. [DOI] [PubMed] [Google Scholar]
- 48.Morato S, Brandao ML. Transporting rats to the test situation on a cart can modify rat exploratory behavior in the elevated plus-maze. Psychobiology. 1996;24:247–252. [Google Scholar]
- 49.Adamec RE, Sayin U, Brown A. The effects of corticotropin releasing factor (CRF) and handling stress on behavior in the elevated plus-maze test of anxiety. J Psychopharm. 1991;5:175–186. doi: 10.1177/026988119100500301. [DOI] [PubMed] [Google Scholar]
- 50.Haller J, Halasz J. Mild social stress abolishes the effects of isolation on anxiety and chlordiazepoxide reactivity. Psychopharmacology (Berl) 1999;144(4):311–315. doi: 10.1007/s002130051012. [DOI] [PubMed] [Google Scholar]
- 51.Karim A, Arslan MI. Isolation modifies the behavioural response in rats. Bangladesh Med Res Counc Bull. 2000;26(1):27–32. [PubMed] [Google Scholar]
- 52.Soderpalm B. The SHR exhibits less “anxiety” but increased sensitivity to the anticonflict effect of clonidine compared to normotensive controls. Pharmacol Toxicol. 1989;65(5):381–386. doi: 10.1111/j.1600-0773.1989.tb01193.x. [DOI] [PubMed] [Google Scholar]
- 53.Meaney MJ, Stewart J. Neonatal-androgens influence the social play of prepubescent rats. Horm Behav. 1981;15(2):197–213. doi: 10.1016/0018-506x(81)90028-3. [DOI] [PubMed] [Google Scholar]
- 54.Thor DH, Holloway WR., Jr Social play in juvenile rats: a decade of methodological and experimental research. Neurosci Biobehav Rev. 1984;8(4):455–464. doi: 10.1016/0149-7634(84)90004-6. [DOI] [PubMed] [Google Scholar]
- 55.Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21(3):309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 56.Pellis SM, Pellis VC. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Aggressive Behav. 1987;13:227–242. [Google Scholar]
- 57.Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26(10):1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- 58.File SE. The social interaction test of anxiety. Neuroscience Protocols. 1993;10(1):1–7. [Google Scholar]
- 59.Chadda R, Devaud LL. Differential effects of mild repeated restraint stress on behaviors and GABA(A) receptors in male and female rats. Pharmacol Biochem Behav. 2005;81(4):854–863. doi: 10.1016/j.pbb.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 60.Gameiro GH, Gameiro PH, Andrade Ada S, Pereira LF, Arthuri MT, Marcondes FK, Veiga MC. Nociception- and anxiety-like behavior in rats submitted to different periods of restraint stress. Physiol Behav. 2006;87(4):643–649. doi: 10.1016/j.physbeh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Albonetti ME, Farabollini F. Behavioral responses to single and repeated restraint in male and female rats. Behav Processes. 1992;28:97–110. doi: 10.1016/0376-6357(92)90052-F. [DOI] [PubMed] [Google Scholar]
- 62.Netto SM, Silveira R, Coimbra NC, Joca SR, Guimaraes FS. Anxiogenic effect of median raphe nucleus lesion in stressed rats. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(6):1135–1141. doi: 10.1016/s0278-5846(02)00248-8. [DOI] [PubMed] [Google Scholar]
- 63.Chaouloff F, Baudrie V, Coupry I. Effects of chlorisondamine and restraint on cortical [3H]ketanserin binding, 5-HT2A receptor-mediated head shakes, and behaviours in models of anxiety. Neuropharmacology. 1994;33(3–4):449–456. doi: 10.1016/0028-3908(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 64.Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48(2):146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- 65.File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2(3):219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- 66.Haller J, Halasz J, Makara GB, Kruk MR. Acute effects of glucocorticoids: behavioral and pharmacological perspectives. Neurosci Biobehav Rev. 1998;23(2):337–344. doi: 10.1016/s0149-7634(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 67.Morato S, Brandao ML. Paradoxical increase of exploratory behavior in the elevated plus-maze by rats exposed to two kinds of aversive stimuli. Braz J Med Biol Res. 1997;30(9):1113–1120. doi: 10.1590/s0100-879x1997000900010. [DOI] [PubMed] [Google Scholar]
- 68.Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45(3):153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- 69.Pellis SM, Field EF, Smith LK, Pellis VC. Multiple differences in the play fighting of male and female rats. Implications for the causes and functions of play. Neurosci Biobehav Rev. 1997;21(1):105–120. doi: 10.1016/0149-7634(95)00060-7. [DOI] [PubMed] [Google Scholar]
- 70.Thor DH, Holloway WR. Developmental analyses of social play behavior in juvenile rats. BPS. 1984;22(6):587–590. [Google Scholar]
- 71.Thor DH, Holloway WR., Jr Social play soliciting by male and female juvenile rats: effects of neonatal androgenization and sex of cagemates. Behav Neurosci. 1986;100(2):275–279. doi: 10.1037//0735-7044.100.2.275. [DOI] [PubMed] [Google Scholar]
- 72.Holloway KS, Suter RB. Play deprivation without social isolation: housing controls. Dev Psychobiol. 2004;44(1):58–67. doi: 10.1002/dev.10151. [DOI] [PubMed] [Google Scholar]
- 73.Goldman L, Winget C, Hollingshead GW, Levine S. Postweaning development of negative feedback in the pituitary-adrenal system of the rat. Neuroendocrinology. 1973;12(3):199–211. doi: 10.1159/000122169. [DOI] [PubMed] [Google Scholar]
- 74.Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004;79(3):125–132. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- 75.Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- 76.Vazquez DM, Akil H. Pituitary-adrenal response to ether vapor in the weanling animal: characterization of the inhibitory effect of glucocorticoids on adrenocorticotropin secretion. Pediatr Res. 1993;34(5):646–653. doi: 10.1203/00006450-199311000-00017. [DOI] [PubMed] [Google Scholar]
- 77.Vazquez DM. Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 1998;23(7):663–700. doi: 10.1016/s0306-4530(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 78.Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146(1):137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]