Abstract
Adipogenesis and ectopic lipid accumulation during aging have a great impact on the aging process and the pathogenesis of chronic diseases with age. However, at present, information on the age-related molecular changes in lipid redistribution patterns and their potential nutritional interventions is sparse. We investigated the mechanism underlying age-related lipid redistribution and its modulation using 5-, 17-, and 24-month-old male Fischer 344 rats fed ad libitum (AL) or a 3-week-long CR (40% less than AL) diet. Results revealed that the activities of adipogenic transcription factors were decreased in the white adipose tissue (WAT) of aged AL rats. In contrast, the skeletal muscle of aged AL rats showed increased fat accumulation through decreased carnitine palmitoyltransferase-1 activity, which was blunted by short-term CR. This study suggests an age-related shift in lipid distribution by reducing the adipogenesis of WAT while increasing intramyocellular lipid accumulation, and that CR can modulate age-related adipogenesis and ectopic lipid accumulation.
Keywords: Aging, Calorie restriction, Lipid accumulation, Peroxisome proliferators-activated receptors, Sterol regulatory element-binding protein-1, Skeletal muscle
Introduction
Adipogenesis is closely correlated with obesity and several obesity-related diseases, including type 2 diabetes mellitus (T2D), cardiovascular disease, hypertension, hypercholesterolemia, asthma, and certain forms of cancer (Weisberg et al. 2003; Shoelson et al. 2007). Visceral adiposity is shown to be particularly more closely associated with obesity-related pathology than central fat deposits. Although the underlying mechanisms are not known, peripheral subcutaneous fat tends to decrease with age, whereas visceral fat does not seem to be affected (Hughes et al. 2004; Cartwright et al. 2007).
Recently, many studies have shown that muscle triglyceride (TG) accumulation is an important causative factor for the progression of metabolic disorders (Tucker and Turcotte 2003; Kelley 2005; Corcoran et al. 2007; van Herpen and Schrauwen-Hinderling 2008), and that age-related intramyocellular TG accumulation is linked to increased insulin-stimulated fatty acid uptake by muscle tissue (Tucker and Turcotte 2003). Slawik and Vidal-Puig (2006) view increased intramyocellular lipid content as indicative of an age-related over-saturation of the lipid storage capacity in white adipose tissue (WAT), thereby creating a spillover phenomenon.
The notion of lipid storage over-capacity is supported by several studies in which cultured preadipocytes from old animals consistently showed reduced lipid accumulation, reduced lipogenic enzyme activities, and changes in differentiation-dependent gene expression (Djian et al. 1985; Hauner et al. 1989; Gregerman 1994; Karagiannides et al. 2001; Cartwright et al. 2007). Since adipogenesis is a result of transcriptional activities, a better way to understand the above-mentioned shift is to examine the molecular events involved in adipogenesis that are modulated by the activation of a large number of adipose-related genes (Fève 2005). Two key regulatory mediators of the adipogenesis process are the peroxisome proliferators-activated receptor (PPAR)γ and sterol regulatory element-binding protein-1 (SREBP-1) (MacDougald and Lane 1995; Fève 2005). Studies of differentiating preadipocytes isolated from old rats have shown that when the capacity of preadipocytes becomes fully lipid-loaded, adipocytes decreases with age (Karagiannides et al. 2001; Cartwright et al. 2007). In addition, the expression of PPARγ is substantially lowered (Karagiannides et al. 2006) and lower levels of SREBP-1 mRNA were observed in the WAT of old rats compared to that of young rats (Swierczynski 2006).
Thus, the decline of adipocyte differentiation induces a reduction in the size of fat depots, and the redistribution of fat among depots causes the “spillover” phenomenon by depositing fat outside fat depots (Slawik and Vidal-Puig 2006; Cartwright et al. 2007). Moreover, recent studies showing the characteristics of increased infiltration of macrophages into WAT documented that macrophages invading adipose tissue plays a constitutive role in eliciting increased inflammatory activity with age (Weisberg et al. 2003; Xu et al. 2003). Therefore, from a physiological and pathological perspective, it is important to prevent accumulation of visceral fat as well as accumulation of ectopic lipid during aging.
Calorie restriction (CR) exhibits an exceptionally broad protective effect against age-related changes in lipid metabolism, including adiposity, TG levels and lipolysis (Liepa et al. 1980; Bertrand et al. 1987). Although CR clearly reduces the age-related increase in adiposity and maintains lean body mass (Yu et al. 1982), the precise mechanisms of CR in relationship to adipogenesis and ectopic lipid accumulation are not known.
The purpose of the current study was to obtain evidence on (1) altered adipogenesis during aging as shown by decreased lipogenic transcription factors, namely SREBP-1 and PPARγ; (2) ectopic lipid accumulation in aged skeletal muscle as the result of decreased fatty acid oxidation as revealed by morphometric analysis; (3) the modulation of age-related alterations in intramyocellular lipid accumulation by short-term use of the anti-aging and anti-lipogenic actions of CR; and finally, (4) the consequential effect of lipid accumulation as expressed by increased age-related inflammation in skeletal muscle. We found that short-term CR increases expression and activity of adipogenic factors in aged WAT. In addition, our view is that modulation of lipid accumulation by CR through increased carnitine palmitolytransferase-1 (CPT-1) activity is physiologically as well as pathologically important to the anti-aging action of CR and to its ability to attenuate age-related chronic diseases.
Materials and methods
Animals
Specific pathogen-free male Fischer 344 rats were obtained from Samtako (Osan, Korea) and were fed a diet of the following composition: 21% soybean protein, 15% sucrose, 43.65% dextrin, 10% corn oil, 0.15% α-methionine, 0.2% choline chloride, 5% salt mix, 2% vitamin mix and 3% Solka-Floc. The ad libitum (AL)-fed group had free access to both food and water. The animals designated as CR were fed 60% of the food intake of their AL-fed littermates for 3 weeks. Rats were maintained in a temperature-controlled (25°C) facility with a strict 12 h light/dark cycle (6:00 a.m.–6:00 p.m.) in a separate vivarium at the Pusan National University. Body weight was measured in nonfasting state, and blood was collected. Rats at 5, 17, and 24 months of age were used in this study. Each group consisted of six animals, which were weighed daily. Animals with gross tumor pathologies were excluded from the study.
Rats at 5, 17, and 24 months of age were sacrificed by decapitation (10:00 a.m.–2:00 p.m.). After sacrifice, the abdominal WAT and gastrocneminus parts of the skeletal muscle were quickly removed and rinsed in iced-cold buffer [100 mM Tris, 1 mM ethylenediaminetetraacetate (EDTA), 0.2 mM phenylmethyl-sulfonylfluoride (PMSF), 1 μM pepstatin, 2 μM leupeptin, 80 mg/l trypsin inhibitor, 20 mM β-glycerophosphate, 20 mM sodium fluoride (NaF), 2 mM sodium orthovanadate (pH 7.4)]. The tissues were immediately frozen in liquid nitrogen and stored at −80°C.
Materials
All chemical reagents were obtained from Sigma (St. Louis, MO), except where noted. Assay kits for TG, cholesterol, and non-esterified fatty acid (NEFA) were obtained from Shinyang Chemical (Busan, Korea) and Daichi Pure Chemicals (Tokyo, Japan). The radionucleotide [γ-32P]-ATP was obtained from Amersham Life Sciences (Buckinghamshire, UK). West-zol Plus was from iNtRON Biotechnology (Seongsam, Korea). Trizol was obtained from Invitrogen (Carlsbad, CA). Primers for reverse transcriptase-polymerase chain reaction (RT-PCR) were synthesized by Bioneer (Daejeon, Korea). Anti-SREBP-1 antibody (rabbit polyclonal, sc-366), anti-PPARγ antibody (rabbit polyclonal, sc-7196), anti-PPARγ2 antibody (goat polyclonal, sc-220020), anti-PGC-1 antibody (rabbit polyclonal, sc-13067), anti-phospho-p65 antibody (rabbit polyclonal, sc-33020), anti-p65 antibody (rabbit polyclonal, sc-7151), and anti-Histone H1 antibody (mouse monoclonal, sc-8030) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Polyvinylidene difluoride (PVDF) membranes were obtained from Millipore (Bedford, MA). All other materials were obtained in the highest available grade.
Nuclear extract preparation
All solutions, tubes, and centrifuges were maintained at 0–4°C. For each nuclear extract preparation, WAT and skeletal muscle of six male Fischer rats were used. The tissues were placed in 1 ml hypotonic buffer A [10 mM HEPES (pH 7.8), 10 mM KCl, 2 mM MgCl2, 1 mM dithiothreitol (DTT), 0.1 mM EDTA, and 0.1 mM PMSF] and homogenized in an ice bath using a tissue homogenizer. To the homogenates was added 125 μl 10% Nonidet P-40 (NP-40) solution and the mixture was then centrifuged at 14,800 g for 5 min. The pelleted nuclei were washed once with 200 μl buffer A plus 25 μl 10% NP-40, centrifuged, resuspended in 30 μl of a solution consisting of 50 mM HEPES (pH 7.8), 50 mM KCl, 0.3 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 0.1 mM PMSF, and 10% glycerol, mixed for 30 min, and centrifuged at 14,800 g for 15 min. The supernatant containing nuclear proteins was collected and stored in aliquots at −80°C.
Western blotting
Western blotting was carried out as described previously (Kim et al. 2006). Homogenized samples were boiled for 5 min with a gel-loading buffer (0.125 M Tris-HCl, 4% SDS, 10% 2-mercaptoethanol, pH 6.8, 0.2% bromophenol blue) at a ratio of 1:1. Total protein-equivalents for each sample were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 10% acrylamide gels as described by Laemmli (1970), and transferred to PVDF membrane at 15 V for 1 h in a semi-dry transfer system. The membrane was immediately placed into blocking buffer (1% non-fat milk) in 10 mM Tris, pH 7.5, 100 mM NaCl, and 0.1% Tween 20. The blot was allowed to block at room temperature for 1 h. The membrane was incubated with specific primary antibody at 25°C for 3 h, followed by a horseradish peroxidase-conjugated anti-mouse antibody (Santa Cruz, 1:25,000), anti-rabbit antibody (Santa Cruz, 1:5,000), or anti-goat antibody (Santa Cruz, 1:5,000) at 25°C for 1 h. Antibody labeling was detected using West-zol Plus and chemiluminescence FluorchemTMSP (Alpha Innotech Corporation, San Leandro, CA). Pre-stained protein markers were used for molecular weight determinations.
Electrophoretic mobility shift assay
The electrophoretic mobility shift assay (EMSA) method was used to characterize the binding activities of NF-κB in nuclear extracts (Kerr 1995). The sequence of the SREBP-specific oligonucleotide was 5′-TGAAAATCACCCCACTGCAA-3′ and that of the PPAR oligonucleotide 5′-TGACCTTTGACCTAGTTTTG-3′ (Yang et al. 2004). Protein-DNA binding mixtures containing 25 μg nuclear protein extract were incubated for 40 min at room temperature in binding medium containing 10% glycerol, 1 mM MgCl2, 10 mM KCl, 0.2 mM EDTA, 1 mM DTT, 2 mM HEPES (pH 7.8), and 0.4 μg poly (dI-dC)•poly (dI-dC). Radiolabeled transcription factor consensus oligonucleotide [γ-32P]-ATP (Amersham, Pittsburgh, PA) was added, and the complex mixture was incubated at 25°C for an additional 20 min. DNA-binding complexes were resolved by 7% native PAGE with 0.5× TBE (0.045 M Tris-borate/0.001 M EDTA) with 5 mM Tris/38 mM glycine running buffer for 70 min at 200 V. The gel was dried, and complexes were detected by autoradiography. The identity of the complexes was established with excess unlabeled oligonucleotide.
RT-PCR
Isolation of RNA from tissue
Total RNA was isolated by the method of Puissant and Houdebine (1990). Briefly, tissue samples were homogenized in the presence of Trizol (2 ml per 100 mg tissue) with a few strokes in a tissue homogenizer. Aliquots of 0.2 ml chloroform per 2 ml homogenate was added, the samples were shaken vigorously for 15 s and kept in ice for 5 min. The suspension was centrifuged at 12,000 g at 4°C for 15 min. The aqueous phase was transferred to a fresh tube, to which an equal volume of isopropanol was added, and the samples were kept at 4°C for 15 min. Samples were again centrifuged at 12,000 g at 4°C for 15 min.
The supernatant was removed and the RNA pellet was washed once with 75% ethanol by vortexing and subsequent centrifugation at 7,500 g at 4°C for 8 min. The pellet was dried for 10–15 min. The RNA pellet was dissolved in diethylpyrocarbonate (DEPC)-treated water.
Reverse transcription
First strand cDNA was synthesized from 2 g total RNA. DEPC-treated water and 250 ng random primer were added, incubated at 75°C for 5 min, and then incubated on ice for 5 min. Aliquots of 2 μl 0.1 M DTT, 4 μl 5× buffer, 4 μl 2.5 mM deoxyribonucleotides (dNTP), 100 U reverse transcriptase, and 16.5 U RNase inhibitor were added and the mixture incubated at 37°C for 2 h. The reaction was stopped by boiling at 100°C for 2 min, and cDNA was stored at −20°C until use.
Polymerase chain reaction
To carry out PCR, cDNA amplification was performed in a PCR master mix containing 1× PCR buffer (Promega, Madison, WI), 0.2 mM dNTP, 0.25 U Taq polymerase (Promega), and 50 ng sense and anti-sense primers. Reaction conditions consisted of 94°C for 30 s denaturation, 54°C for 30 s annealing, and 72°C for 1 min extension. Electrophoresis was performed in 1% agarose gels. After staining in ethidium bromide solution was achieved, the gel was observed under chemiluminescence Fluorchem SP (Alpha Innotech Corporation, San Leandro, CA). β-actin primers were used as a control for the efficiency of cDNA synthesis in each sample.
Extraction of mitochondria
Rat skeletal muscle mitochondria were isolated according Yao et al. (1994). Skeletal muscles were minced and homogenized in a medium containing 300 mM sucrose, 5 mM MOPS, 1 mM EGTA, 5 mM K2HPO4, and 0.1% BSA, pH 7.4 at 4°C. The homogenate was centrifuged at 1,500 g for 10 min. The supernatant was centrifuged for 5 min at 9,800 g and the mitochondrial pellet was washed and centrifuged twice in the same medium. The mitochondrial suspension was stored on ice before the CPT assay.
Assay of CPT-1 activity
CPT-1 activity was measured as the rate of decrease of palmitoyl-CoA assessed by spectrophotometry at 324 nm according to Hamdan et al. (2001) with slight modifications. Mitochondria were incubated for 2 min at 37°C in a medium containing 150 mM sucrose, 60 mM KCl, 25 mM Tris/HCl, 1mM EDTA, 0.1 mM 4,4′-dithiodipyridine and 1.3 mg/ml BSA in a final volume of 1 ml. The enzymatic reaction was initiated by adding palmitoyl-CoA (100 μM) and carnitine (400 μM) to generate palmitoylcarnitine and incubated at 37°C for 3 min.
Oil Red O staining
After drying for 30 min at room temperature, cryosections were fixed in ice cold 10% formalin for 5 min. After washing in distilled water, sections were placed in absolute propylene glycol for 5 min to avoid carrying water into Oil Red O (Sigma, St. Louis, MO). Sections were stained in Oil Red O solution for 8 min in a 60°C oven. The stained sections were washed in 85% propylene glycol solution for 5 min and rinsed in PBS. After washing, the sections were stained in Gill’s hematoxylin solution (Sigma) for 30 s and washed thoroughly in running water for 3 min. The sections were mounted with glycerin jelly. Light microscopy slides were observed and photographed using an Olympus BX50 microscope (Olympus, Tokyo, Japan).
Biochemical measurements
TG and cholesterol levels
TG and cholesterol levels were measured with assay kits, following the manufacturer’s protocols (Shinyang Chemical, Busan, South Korea). Briefly, serum sample (5 μl) was loaded and the reagent (200 μl) was added. This mixture was incubated at 37°C for 5 min. The optical density at 540 nm was then determined.
NEFA level
NEFA levels were measured with a NEFA assay kit (Daichi Pure Chemicals, Tokyo, Japan) according to the manufacturer’s protocol. Briefly, serum sample (7 μl) was loaded and the reagent I (150 μl) was added. After incubation at 37°C for 3 min, reagent II (300 μl) was added, and then this mixture was incubated at 37°C for 5 min. The optical density was measured at 540 nm.
Protein assay
Protein concentrations were measured using the method of Lowry et al. (1951) using BSA as a standard. Briefly, samples were loaded and Lowry reagent was added. After incubating at 37°C for 20 min, phenol reagent was added and this mixture was incubated at 37°C for 30 min. The optical density was measured at 620 nm.
Statistical analysis
For EMSA or Western blotting, one representative blot from triple independent experiments is shown. For the other assays, the results are expressed as mean ± SD (n = 6). The statistical significance of the difference between the groups was determined by one-factor analysis of variance (ANOVA) followed by the Fischer’s protected least significant difference (LSD) post hoc test. Values of P < 0.05 were considered statistically significant.
Results
CR counteracts decreased SREBP-1 and PPARγ activities in aged WAT
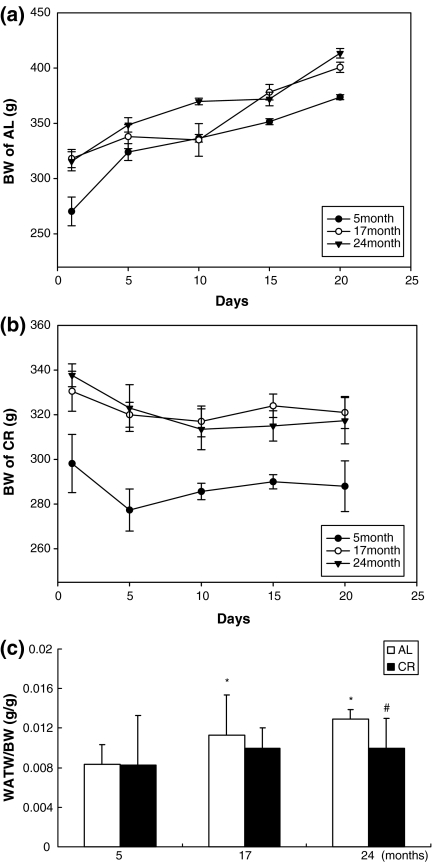
We investigated changes in body weight (BW) as a function of age and CR. AL rats gained weight during the 3-week period (Fig. 1a), while CR rats did not gain weight (Fig. 1b). The WAT weight (WATW)/BW ratio also increased with age in the AL group but was decreased significantly by CR, especially in 24-month-old rats (Fig. 1c).
Fig. 1.
a Body weight (BW) of ad libitum (AL) rats. b BW of calorie restriction (CR) rats. c White adipose tissue weight (WATW)/BW ratio in aged AL and CR rats; * P < 0.05 compared with 5-month-old AL rats, # P < 0.05 compared with each CR rat, respectively
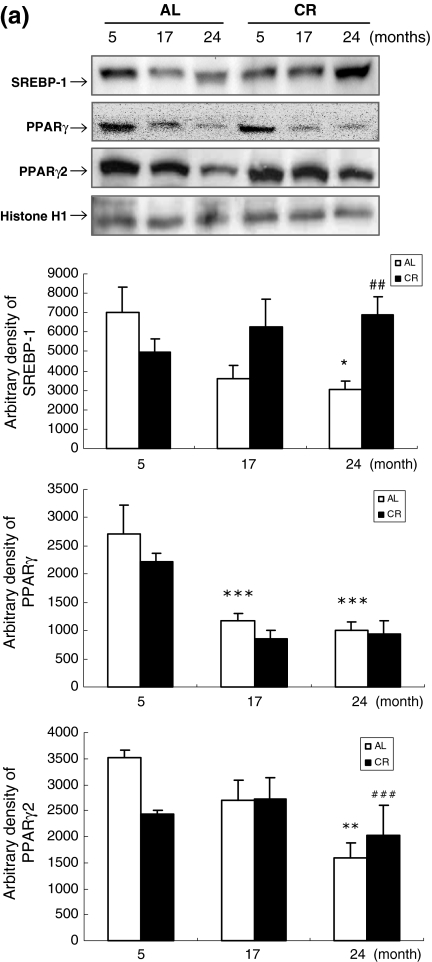
To determine the effect of aging and CR on adipogenesis in WAT, western blot analysis of WAT was performed using anti-SREBP-1, anti-PPARγ, and anti-PPARγ2 antibodies. SREBP-1, PPARγ, and PPARγ2 are preferentially observed in adipocytes and are related to adipocyte differentiation. The transcription factor activity of SREBP-1, PPARγ, and PPARγ2 decreased significantly with age, especially for 24-month-old AL rats. In contrast to AL rats, CR rats showed increased SREBP-1 and PPARγ2 expression (Fig. 2a). In addition to western blot analysis, EMSA was carried out using nuclear proteins from AL and CR rats to further investigate SREBP-1 and PPARγ. The results showed that the decreased SREBP-1 and PPARs DNA-binding activity observed with age was counteracted by CR (Fig. 2b,c; lanes 2–7). Furthermore, the binding specificity of SREBP-1 and PPARs was demonstrated using a 100-fold excess of an unlabeled oligonucleotide, which competed for binding (Fig. 2b,c; lane 8). These results indicate that CR can mitigate the decreased expression and activity of transcription factors related to adipogenesis in WAT during the aging process.
Fig. 2a–c.
Effects of age and CR on preadipocyte differentiation-related transcription factors in WAT. a Western blot analysis to detect sterol regulatory element-binding protein-1 (SREBP-1),and peroxisome proliferators-activated receptors (PPAR) γ and γ2 protein levels in WAT nuclear extracts (50 μg protein) from each group. Transcription factors SREBP-1, PPAR-γ, and -γ2 are involved in preadipocyte differentiation; these transcription factors decreased in aged AL rats but increased with age in CR rats. b,c Nuclear extracts for each group were prepared and assayed for SREBP-1 and PPAR induction by electrophoretic mobility shift assay (EMSA) as described in Materials and methods. *P < 0.05, ** P< 0.01, and *** P< 0.001 compared with 5-month-old AL rats; # P < 0.05, ## P < 0.01, ### P< 0.001 compared with each CR rat, respectively
Suppression of plasma NEFA, TG, and cholesterol by CR
Aged rats exhibited alterations in metabolism of lipids such as NEFA, TG, and cholesterol (Table 1). Plasma TG and cholesterol increased with age compared to the 5-month-old AL group, whereas CR significantly reduced TG and cholesterol levels at all older ages. Although NEFA levels in plasma are known to be influenced by many metabolic mediators, an increased plasma level could be related largely to reduced lipogenic activity and the adipose tissue lipid storage capacity. To obtain clues about age and CR effects on NEFA levels, the latter were measured with an assay kit. Plasma levels of NEFAs increased 8.46% and 30.31% in 17- and 24-month-old AL rats, respectively, compared with 5-month-old AL rats, suggesting that decreased expression and activity of lipogenic transcription factors influences the lipid storage capacity of aged WAT. In contrast, plasma NEFA levels in CR rats were 11.34%, 11.99%, and 18.76% in 5-, 17-, and 24-month-old rats, respectively (Table 1). Thus, CR clearly suppressed the age-related increase of plasma NEFAs observed in AL rats, which implies the possibility that, during aging, WAT adipocytes in CR rats are capable of storing lipids.
Table 1.
Plasma lipid parameters in aged ad libitum (AL) and calorie restriction (CR) rats. Data are represented as means ± SD of six rats. NEFA Non-esterified fatty acid
| 5 months | 17 months | 24 months | ||||
|---|---|---|---|---|---|---|
| AL | CR | AL | CR | AL | CR | |
| Plasma NEFA (μEq/l) | 461.64 ± 8.28 | 408.8 ± 2.35 # | 500.31 ± 1.25 | 410.05 ± 5.27 ## | 600.71 ± 3.41* | 500.03 ± 2.85 ## |
| Plasma triglycerides (mg/dl) | 190.15 ± 5.08 | 101.1 ± 3.33 # | 199.78 ± 8.86 | 137.81 ± 7.44 # | 262.49 ± 8.55* | 145.78 ± 3.15 ## |
| Plasma cholesterol (mg/dl) | 75.345 ± 0.78 | 74.34 ± 0.25 | 210.11 ± 4.47*** | 178.80 ± 3.97 ## | 227.64 ± 4.98*** | 187.75 ± 6.42 ## |
* P < 0.05, *** P < 0.001 compared with 5-month-old AL rats; # P < 0.05, ## P < 0.01 compared with each CR rat, respectively
Effects of age and CR on fatty acid β-oxidation in skeletal muscle
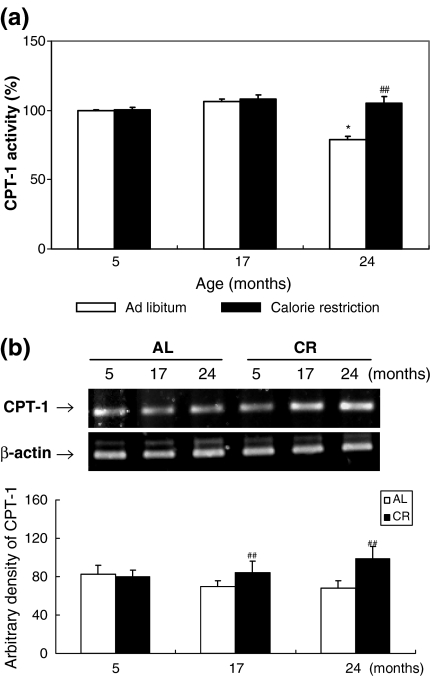
To explore the extent of fatty acid oxidation in muscle during aging, activity and mRNA levels of CPT-1 were examined. The enzyme CPT-1 plays a major role in the regulation of mitochondrial β-oxidation in all mammalian tissues. It catalyzes the first step of the transport of long-chain fatty acids from the cytosol into the mitochondrial matrix, where they further undergo β-oxidation (Hamdan et al. 2001). As shown in Fig. 3a, CPT-1 activity was decreased significantly in the skeletal muscle of 24-month-old AL rats compared with that of 5-month-old AL rats, and this effect was attenuated by CR. However, in these same animals we found no decrease in CPT-1 mRNA levels with age, and CR increased CPT-1 mRNA levels (Fig. 3b). The decreased CTP-1 activity observed in skeletal muscle of aged rats may be indicative of lower mitochondrial fatty acid oxidation, allowing lipid accumulation in aged skeletal muscle, but this effect is blunted by CR.
Fig. 3a,b.
Effects of aging and CR on carnitine palmitolytransferase-1 (CPT-1) activity in skeletal muscle. a Mitochondria were extracted and CPT-1 activity was assessed using spectrophotometry at 324 nm. b RT-PCR analysis of CPT-1 was performed on RNA isolated from AL and CR rats. β-actin primers were used in separate PCR reactions to control for the efficiency of cDNA synthesis in each sample. One representative result is shown from three experiments that yielded similar results. * P < 0.05 compared with 5-month-old AL rats, ## P < 0.01 compared with each CR rat, respectively
CR prevents muscle lipid accumulation during aging
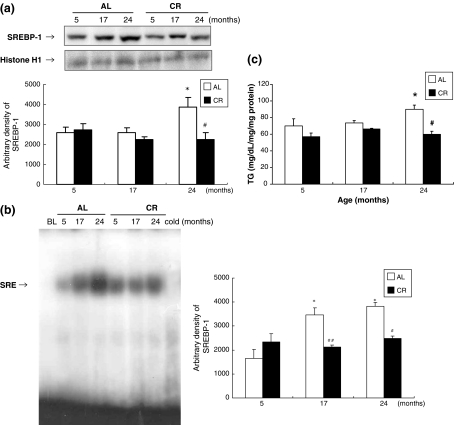
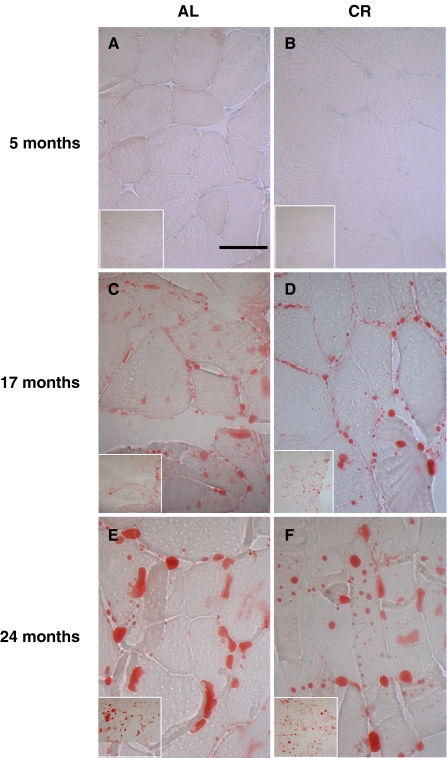
To investigate whether CR modulates lipid accumulation in aged skeletal muscle, we compared SREBP-1 expression and its activity along with the TG content of aged skeletal muscle. The results show that the lipogenic transcription factor SREBP-1 increased in aged AL rats, but this increase was inhibited by CR (Fig. 4a). To further investigate the results observed by western blot, EMSA was carried out on nuclear proteins from AL and CR rats. In the AL group, SREBP-1 DNA-binding activity increased with age, while the CR group did not show this age-related increase (Fig. 4b). In addition, we found that the TG level was 28.6% higher in 24-month-old AL rats compared to 5-month-old AL rats, and that 24-month-old CR rats had a significantly lower muscle TG level (Fig. 4c). To confirm the lipid accumulation in aged skeletal muscle, immunostaining was performed using the Oil Red O method. As shown in Fig. 5, lipid accumulation increased markedly in skeletal muscle with age, and CR attenuated this age-associated accumulation. These data are consistent with the SREBP-1 activity and TG content observed in aged skeletal muscle.
Fig. 4a–c.
Effects of aging and CR on lipid accumulation in skeletal muscle. a Western blot analysis to detect the level of SREBP-1 protein in nuclear extracts (50 μg protein) from each group. b Nuclear extracts for each group were prepared and assayed for SREBP-1 induction by EMSA as described in Materials and methods. c TG levels in aged skeletal muscle. One representative result is shown from three experiments that yielded similar results. * P < 0.05 compared with 5-month-old AL rats, # P < 0.05 compared with each CR rat, respectively
Fig. 5.
Oil Red O staining for detection of degree of lipid accumulation in skeletal muscle fibers. Skeletal muscle of six 24-month-old rats revealed significantly increased lipid accumulation compared with 5-month-old rats. Original magnification ×200 and ×400. Bar 50 μm
Effect of CR on inflammation in aged skeletal muscle
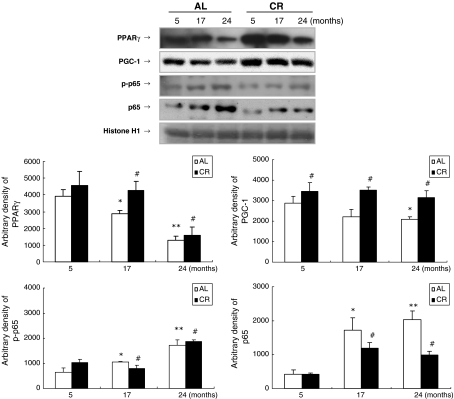
Because of the significant role of inflammation involving adipose tissue in aging, we investigated various pro-inflammatory transcription factors and genes in skeletal muscle with age. The levels of nuclear PPARγ and PPARγ coactivator-1 (PGC-1), an anti-inflammatory transcription factor and its coactivator, decreased significantly with age, while CR protected against this decrease (Fig. 6). Because PGC-1 increases mitochondrial biogenesis (Wang et al. 2008), decreased PGC-1 may be associated with reduced fatty acid oxidation in aged skeletal muscle. In contrast, nuclear translocation of pro-inflammatory NF-κB, especially p65, increased with age; CR prevented this change. Also, phosphorylation of the p65 subunit increased with age in skeletal muscle.
Fig. 6.
Effects of aging and CR on inflammatory transcription factors in skeletal muscle. Western blot analysis to detect PPARγ, PPARγ coactivator-1 (PGC-1), p-p65, and p65 protein levels was performed in nuclear extracts (50 μg protein) from AL and CR groups. One representative result is shown from three experiments that yielded similar results. ** P < 0.01 compared with 5-month-old AL rats, #P < 0.05 compared with each CR rat, respectively
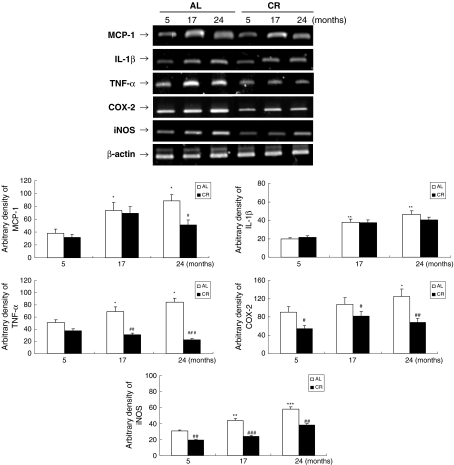
Furthermore, our findings show that the mRNA levels of NF-κB-dependent genes, monocyte chemotactic protein-1 (MCP-1), interleukin (IL)-1β, tumor necrosis factor (TNF)-α, cyclooxygenase (COX)-2, and inducible nitric oxygen synthase (iNOS), increased with age in the AL-fed animals but that the CR animals had lower levels of these NF-κB-dependent activities (Fig. 7). Increased MCP-1, especially, is related to increased adiposity and macrophage infiltration in skeletal muscle (Weisberg et al. 2003; Curat et al. 2004). Therefore, these results indicate that increased age-related inflammation is closely related to lipid accumulation, and that CR effectively modulates inflammation in aged skeletal muscle.
Fig. 7.
Effects of aging and CR on age-related inflammatory genes in skeletal muscle. RT-PCR analysis of monocyte chemotactic protein-1 (MCP-1), interleukin (IL)-1β, tumor necrosis factor (TNF-α), cyclooxygenase (COX-2), and inducible nitric oxygen synthase (iNOS) was performed on RNA isolated from AL and CR rats. β-actin primers were used in separate PCR reactions to control for the efficiency of cDNA synthesis in each sample. One representative result is shown from three experiments that yielded similar results. * P < 0.05, ** P < 0.01, *** P < 0.001 compared with 5-month-old AL rats; # P < 0.05, ## P < 0.01, ### P < 0.001 compared with each CR rat, respectively
Discussion
The aim of the present study was to investigate alterations in lipid distribution during aging and its modulation by CR. Substantial changes in body fat accumulation occur with aging (Imbeault et al. 2001), and lipid redistribution is shown to be one of the major age-related physiological changes that might underlie a wide range of negative health effects. In this context, an interesting view emerging is the role of central adipose tissue as a major lipid storage site that protects peripheral tissues such as muscle from lipid accumulation, which might cause lipotoxicity (Lee et al. 2001; Wang et al. 2008).
Data from the current study show that SREBP-1, PPARγ, and PPARγ2 activities decrease in aged WAT and that CR blunts these decreases. The significance of changes in these transcription factors is related to their key roles in regulating adipocyte differentiation (Fève 2005), and modulating the acquisition and maintenance of the adipocyte phenotype (MacDougald and Lane 1995).
Slawik and Vidal-Puig (2006) reported that preadipocyte differentiation capability decreases with age, which is considered the underlying reason for lipid “spillover” into various peripheral tissues like muscle, bone marrow, liver, and pancreas. According to these authors, a safe place to store lipids is likely in WAT, but its storage capacity may become saturated, partially because of decreased adipocyte numbers due to reduced preadipocyte differentiation (Slawik and Vidal-Puig 2006). Our data on decreased preadipocyte differentiation, increased plasma NEFA levels, and accumulated TG in muscle during aging are fully consistent with the “spillover” phenomenon.
At a molecular level, our data show that genes involved in adipogenesis decrease in activity with age in WAT. In particular, the activity of genes involved in PPARγ-dependent adipogenesis is shown to decline with age for AL animals but not for CR animals (Mueller et al. 2002). Linford et al. (2007) also reported that PPARγ gene expression is reduced with age, but is preserved with CR in WAT, as was similarly found in other tissues (Sung et al. 2004; Chung et al. 2008). Furthermore, Wang et al. (2008) recently demonstrated that decreased adipocyte storage capacity induces lipid accumulation in liver, heart, and skeletal muscle of the db/db-aP2-leptin receptor on an aP2 promoter (Lepr-b) transgenic mice and that ectopic lipid accumulation is related to metabolic syndrome, including T2D. The full-length transgene Lepr-b prevents the disappearance of adipocyte Lepr-b that normally accompanies overfeeding, and is therefore essential to block the antilipogenic autocrine action of leptin that prevents fat storage (Wang et al. 2008). These findings are consistent with our current observation of the decreased storage capacity of WAT, which is associated with reduced lipogenic transcription factors SREBP-1, PPARγ, and γ2 expression and activity during aging.
In the present study, CPT-1 activity decreased significantly with age in skeletal muscle, while CR prevented this decrease by maintaining a steady CPT-1 level in skeletal muscle, indicating increased fat deposit. Our morphometric analysis by Oil Red O staining confirmed the muscular lipid accumulation in aged muscle tissue. Thus, these results strongly suggest an age-related shift in lipid distribution patterns through the reduction of adipogenesis in WAT, and simultaneous increases in intramyocellular lipid accumulation.
The capacity for body cholesterol removal through the conversion of cholesterol to bile acids is also progressively decreased with age (Einarsson et al. 1985; Parini et al. 1999), and a decline in the activity of the rate-limiting enzyme in bile acid biosynthesis, cholesterol 7α-hydroxylase, has been demonstrated in the aging rat (Choi et al. 1987; Ståhlberg et al. 1991; Parini et al. 1999). CR rats exhibited a significant increase in plasma adiponectin accompanied by a decrease in plasma TG levels through modulation of key transcription target genes including fatty acid oxidation and energy combustion (Zhu et al. 2004). Therefore, our finding that CR rats showed a significant decrease in plasma TG and cholesterol may affect, to some extent, the protective effect of CR on intramyocellular lipid accumulation.
A recent report by Wang et al. (2008) on adipogenic capacity and its association with the susceptibility to T2D and metabolic syndrome reinforces the notion of spillover on the physiological and pathological consequences of increased peripheral adiposity with age.
One of the major pathological consequences of increased adiposity is its strong implication with chronic inflammation (Festa et al. 2001). Visceral adipose tissues, in particular, are main sources of various inflammatory adipokines, such as TNF-α, IL-6, IL-8, and PAI-1 (Sharma and Staels 2007), which have been shown to play causative roles in insulin resistance and metabolic syndrome (You et al. 2008). Data from our current study clearly showed that activity of the pro-inflammatory genes, MCP-1, IL-1β, TNF-α, COX-2, and iNOS, and transcription factors p65 and p-p65 increase significantly in aged skeletal muscle.
The most salient findings of the current study are the age-related changes in body lipid distribution with accumulated fat in muscle tissue, and the ability of CR to prevent these changes. The physiological and pathological consequences of lipid accumulation in muscle have been linked to insulin insensitivity in peripheral tissue in the absence of obesity (Gumbiner et al. 1992; Kim et al. 2004). A significant finding of the present study on CR is that a 3-week, short-term CR diet clearly was able to modulate age-related lipid redistribution through coordinated modulation of several of the key mediators involved in lipid metabolism.
Zhu et al. (2007) demonstrated that short-term and long-term CR significantly increases the expression of PPARγ, C/EBPβ, and Cdk-4 levels, and attenuates the age-related decline in C/EBPα expression in WAT, all of which have been documented as key effectors in adipogenic endeavors. Our results have revealed molecular clues to the mechanism of of blunted insulin sensitivity in relation to adipogenesis modulation (Dubois et al. 2006; McLaughlin et al. 2007). Our data provide a better understanding of the regulatory mechanisms underlying changes in body composition, insulin resistance, and inflammatory processes, all of which are key characteristics of the aging process.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (No. 2007-00376). We are thankful to the Aging Tissue Bank for supplying irradiated tissue.
References
- Bertrand HA, Anderson WR, Masoro EJ, Yu BP (1987) Action of food restriction on age-change in adipocyte lipolysis. J Gerontol 42:666–673 [DOI] [PubMed]
- Cartwright MJ, Tchkonia T, Kirkland JL (2007) Aging in adipocytes: Potential impact of inherent, depot-specific mechanisms. Exp Gerontol 42:463–471 doi:10.1016/j.exger.2007.03.003 [DOI] [PMC free article] [PubMed]
- Choi YS, Ide T, Sugano M (1987) Age-related changes in the regulation of cholesterol metabolism in rats. Exp Gerontol 22:339–349 doi:10.1016/0531-5565(87)90032-5 [DOI] [PubMed]
- Chung JH, Seo AY, Chung SW, Kim MK, Leeuwenburgh C, Yu BP, Chung HY (2008) Molecular mechanism of PPAR in the regulation of age-related inflammation. Ageing Res Rev 7:126–136 doi:10.1016/j.arr.2008.01.001 [DOI] [PubMed]
- Corcoran MP, Lamon-Fava S, Fielding RA (2007) Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr 85:662–677 [DOI] [PubMed]
- Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, Busse R, Bouloumir A (2004) From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes 53:1285–1292 doi:10.2337/diabetes.53.5.1285 [DOI] [PubMed]
- Djian P, Phillips M, Green H (1985) The activation of specific gene transcription in the adipose conversion of 3T3 cell. J Cell Physiol 124:554–556 doi:10.1002/jcp.1041240327 [DOI] [PubMed]
- Dubois SG, Heilbronn LK, Smith SR, Albu JB, Kelley DE, Ravussin E, Look AHEAD Adipose Research Group (2006) Decreased expression of adipogenic genes in obese subjects with type 2 diabetes. Obesity (Silver Spring) 14:1543–1552 doi:10.1038/oby.2006.178 [DOI] [PMC free article] [PubMed]
- Einarsson K, Nilsell K, Leijd B, Angelin B (1985) Influence of age on secretion of cholesterol and synthesis of bile acids by the liver. N Engl J Med 313:277–282 [DOI] [PubMed]
- Festa A, D’Agostino R Jr, Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, Haffner SM (2001) The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord 25:1407–1415 doi:10.1038/sj.ijo.0801792 [DOI] [PubMed]
- Fève B (2005) Adipogenesis: cellular and molecular aspects. Best Pract Res Clin Endocrinol Metab 19:483–499 doi:10.1016/j.beem.2005.07.007 [DOI] [PubMed]
- Gregerman RI (1994) Aging and hormone sensitive lipolysis: reconciling the literature. J Gerontol 49:B135–B139 [DOI] [PubMed]
- Gumbiner B, Thorburn AW, Ditzler TM, Bulacan F, Henry RR (1992) Role of impaired intracellular glucose metabolism in the insulin resistance of aging. Metabolism 41:1115–1121 doi:10.1016/0026-0495(92)90296-M [DOI] [PubMed]
- Hamdan M, Urien S, Louet HL, Tillement JP, Morin D (2001) Inhibition of mitochondrial carnitine palmitoyltransferase-1 by a trimetazidine derivative, S-15176. Pharmacol Res 44:99–104 doi:10.1006/phrs.2001.0829 [DOI] [PubMed]
- Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, Pfeiffer EF (1989) Promotion effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest 84:1663–1670 doi:10.1172/JCI114345 [DOI] [PMC free article] [PubMed]
- Hughes VA, Roubenoff R, Wood M, Frontera WR, Evans WJ, Fiatarone Singh MA (2004) Anthropometric assessment of 10-y changes in body composition in the elderly. Am J Clin Nutr 80:475–482 [DOI] [PubMed]
- Imbeault P, Vidal H, Tremblay A, Vega N, Nadeau A, Despres JP, Mauriege P (2001) Age-related differences in messenger ribonucleic acid expression of key proteins involved in adipose cell differentiation and metabolism. J Clin Endocrinol Metab 86:828–833 doi:10.1210/jc.86.2.828 [DOI] [PubMed]
- Karagiannides I, Tchkonia T, Dobson DE, Steppan CM, Cummins P, Chan G, Salvatori K, Hadzopoulou-Cladaras M, Kirkland JL (2001) Altered expression of C/EBP family members results in decreased adipogenesis with aging. Am J Physiol Regul Integr Comp Physiol 280:R1772–R1780 [DOI] [PubMed]
- Karagiannides I, Thomou T, Tchkonia T, Pirtskhalava T, Kypreos KE, Carwright A, Dalagiorgou G, Lash TL, Farmer SR, Timchenko NA, Kirkland JL (2006) Increased CUG triplet repeat-binding protein-1 predisposes to impaired adipogenesis with aging. J Biol Chem 281:23025–23033 doi:10.1074/jbc.M513187200 [DOI] [PubMed]
- Kelley DE (2005) Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest 115:1699–1702 doi:10.1172/JCI25758 [DOI] [PMC free article] [PubMed]
- Kerr LD (1995) Electrophoretic mobility shift assay. Methods Enzymol 254:619–632 doi:10.1016/0076-6879(95)54044-X [DOI] [PubMed]
- Kim HJ, Lee JS, Kim CK (2004) Effect of exercise training on muscle glucose transporter 4 protein and intramuscular lipid content in elderly men with impaired glucose tolerance. Eur J Appl Physiol 93:353–358 doi:10.1007/s00421-004-1214-2 [DOI] [PubMed]
- Kim JY, Jung KJ, Choi JS, Chung HY (2006) Modulation of the age-related nuclear factor-κB (NF-κB) pathway by hesperetin. Aging Cell 5:401–411 doi:10.1111/j.1474-9726.2006.00233.x [DOI] [PubMed]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 doi:10.1038/227680a0 [DOI] [PubMed]
- Lee Y, Wang MY, Kakuma T, Wang ZW, Babcock E, McCorkle K, Higa M, Zhou YT, Unger RH (2001) Liporegulation in diet-induced obesity. The antisteatotic role of hyperleptinemia. J Biol Chem 276:5629–5635 doi:10.1074/jbc.M008553200 [DOI] [PubMed]
- Liepa GU, Masoro EJ, Bertrand HA, Yu BP (1980) Food restriction as a modulator of age-related changes in serum lipids. Am J Physiol 238:E253–E257 [DOI] [PubMed]
- Linford NJ, Beyer RP, Gollahon K, Krajcik RA, Malloy VL, Demas V, Burmer GC, Rabinovitch PS (2007) Transcriptonal response to aging and caloric restriction in heart and adipose tissue. Aging Cell 6:673–688 doi:10.1111/j.1474-9726.2007.00319.x [DOI] [PubMed]
- Lowry DH, Rosebrough NJ, Farr AL, Randal RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275 [PubMed]
- MacDougald OA, Lane MD (1995) Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem 64:345–373 doi:10.1146/annurev.bi.64.070195.002021 [DOI] [PubMed]
- McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C, Reaven GM, Cushman SW (2007) Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia 50:1707–1715 doi:10.1007/s00125-007-0708-y [DOI] [PubMed]
- Mueller E, Drori S, Aiyer A, Yie J, Sarraf P, Chen H, Hauser S, Rosen ED, Ge K, Roeder RG, Spiegelman BM (2002) Genetic analysis of adipogenesis through peroxisome proliferators-activated receptor gamma isoforms. J Biol Chem 277:41925–41930 doi:10.1074/jbc.M206950200 [DOI] [PubMed]
- Parini P, Angelin, Rudling M (1999) Cholesterol and lipoprotein metabolism in aging reversal of hypercholesterolemia by growth hormone treatment in old rats. Arterioscler Thromb Vasc Biol 19:832–839 [DOI] [PubMed]
- Puissant C, Houdebine L (1990) An improvement of the single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Biotechnique 8:148–149 doi:10.1038/nbt0290-148 [DOI] [PubMed]
- Sharma AM, Staels B (2007) Peroxisome proliferators-activated receptorγ (PPARγ) and adipose tissue understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab 92:386–395 doi:10.1210/jc.2006-1268 [DOI] [PubMed]
- Shoelson SE, Herrero L, Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterology 132:2169–2180 doi:10.1053/j.gastro.2007.03.059 [DOI] [PubMed]
- Slawik M, Vidal-Puig AJ (2006) Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev 5:144–164 doi:10.1016/j.arr.2006.03.004 [DOI] [PubMed]
- Ståhlberg D, Angelin B, Einarsson K (1991) Age-related changes in the metabolism of cholesterol in rat liver microsomes. Lipids 26:349–352 doi:10.1007/BF02537197 [DOI] [PubMed]
- Sung B, Park S, Yu BP, Chung HY (2004) Modulation of PPAR in aging, inflammation, and calorie restriction. J Gerontol A Biol Sci Med Sci 59:997–1006 [DOI] [PubMed]
- Swierczynski J (2006) Leptin and age-related down-regulation of lipogenic enzymes genes expression in rat white adipose tissue. J Physiol Pharmacol 57(Suppl 6):85–102 [PubMed]
- Tucker MZ, Turcotte LP (2003) Aging is associated with elevated muscle triglyceride content and increased insulin-stimulated fatty acid uptake. Am J Physiol Endocrinol Metab 285:E827–E835 [DOI] [PubMed]
- van Herpen NA, Schrauwen-Hinderling (2008) Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav 94:231–241 doi:10.1016/j.physbeh.2007.11.049 [DOI] [PubMed]
- Wang MY, Grayburn P, Chen S, Ravazzola M, Orci L, Unger RH (2008) Adipogenic capacity and the susceptibility to type 2 diabetes and metabolic syndrome. Proc Natl Acad Sci USA 105:6139–6144 doi:10.1073/pnas.0801981105 [DOI] [PMC free article] [PubMed]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel FL, Ferrante AW Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830 [DOI] [PMC free article] [PubMed]
- Yang Y, Eggertsen G, Gafvels M, Andersson U, Einarsson C, Bjorkhem I, Chiang JYL (2004) Mechanisms of cholesterol and sterol regulatory element binding protein regulation of the sterol 12α-hydroxylase gene (CYP8B1). Biochem Biophys Res Commun 320:1204–1210 doi:10.1016/j.bbrc.2004.06.069 [DOI] [PubMed]
- Yao KW, Mao LF, Schulz H (1994) The relationship between mitochondrial activation and toxicity of some substituted carboxylic acids. Chem Biol Interact 90:225–234 doi:10.1016/0009-2797(94)90012-4 [DOI] [PubMed]
- You T, Nicklas BJ, Ding J, Penninx BW, Goodpaster BH, Bauer DC, Tylavsky FA, Harris TB, Kritchevsky SB (2008) The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. J Gerontol A Biol Sci Med Sci 63:414–419 [DOI] [PMC free article] [PubMed]
- Yu BP, Masoro EJ, Murata I, Bertrand HA, Lynd FT (1982) Life span study of SPF Fischer 344 male rats fed Ad Libitum or restricted diets: Longevity, growth, lean body mass and disease. J Gerontol 37:130–141 [DOI] [PubMed]
- Zhu M, Miura J, Lu LX, Bernier M, DeCabo R, Lane MA, Roth GS, Ingram DK (2004) Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol 39:1049–1059 [DOI] [PubMed]
- Zhu M, Lee GD, Ding L, Hu J, Qiu G, de Cabo R, Bernier M, Ingram DK, Zou S (2007) Adipogenic signaling in rat white adipose tissue: modulation by aging and calorie restriction. Exp Gerontol 42:733–744 doi:10.1016/j.exger.2007.05.011 [DOI] [PMC free article] [PubMed]