Abstract
Although anxiety and depression are not the core symptoms of Alzheimer’s Disease (AD), there are changes observed in mood in those with AD, as well as in the aging population. Anxiety and depression may be influenced by progesterone P4 and/or its neuroactive metabolites, dihydroprogesterone (DHP) and 5α-pregnan-3α-ol-20-one (3α,5α-THP). To begin to investigate progestogens’ role in AD, a double transgenic mouse model of early-onset familial AD that co-overexpresses mutant forms of amyloid precursor protein (APPswe) and presenilin 1 Δ exon 9 mutation was utilized. As such, the effects of long-term (from 6 to 12 months of age) administration of P4 to ovariectomized (ovx) wildtype and APPswe+PSEN1Δe9 mice for changes in affective behavior was investigated. APPswe+PSEN1Δ9 mutant mice had increased anxiety-like (i.e., increased emergence latencies, decreased time spent on the open quadrants of the elevated zero maze) and increased depressive-like behavior (i.e., increased time spent immobile) than did wildtype mice. Compared to vehicle-administration, P4 administration (which produced physiological circulating P4, DHP, and 3α,5α-THP levels, particularly in the wildtype mice) decreased depressant-like behavior in the forced swim test. These effects occurred independent of changes in general motor behavior/coordination, pain threshold, and plasma corticosterone levels. Thus, the APPswe+PSEN1Δ9 mutation alters affective behavior, and P4 treatment reversed depressive-like behavior.
Keywords: Allopregnanolone, Neurosteroid, Anxiety, Hippocampus, Neurodegeneration
Introduction
Alzheimer’s Disease (AD) is the most common form of neurodegeneration among the elderly (Evans et al. 1989) and may be influenced by sex steroids. AD is characterized by accumulation of β amyloid proteins into plaques and tangles and resulting behavioral dysfunctions in cognitive measures and neuropsychiatric symptoms, such as anxiety and depression. Women are 1.5–3 times more likely to have AD than are men (Barrett 1999). Progressive development of AD with aging occurs concomitant with a precipitous decline in ovarian steroids, estradiol (E2) and progesterone (P4), among women, and a decade-by-decade decline in androgens among men. It may be that some of these differences in prevalence of AD are related to differences in these steroids among women and men, and with aging (reviewed by Bernardi et al. 2004; Seeman 1997). In support of this idea, among subjects with AD, there are lower levels of a P4 metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP) in serum (Bernardi et al. 2000), and prefrontal cortex measured postmortem (Marx et al. 2006). Lower plasma 3α,5α-THP levels in those with AD or non-AD dementia, compared to controls, may be a biomarker for AD (Smith et al. 2006). Of interest is whether there are beneficial effects of P4 and/or its metabolites (“progestogens”) in situations of central nervous system (CNS) compromise as is seen with aging and AD.
Progestogens are trophic factors with a wide variety of functional effects throughout the lifespan (reviewed in Frye 2007). In the brain, P4 is converted by the 5α-reductase enzyme to dihydroprogesterone (DHP), which is then converted to 3α,5α-THP by the 3α-hydroxysteroid dehydrogenase enzyme. E2 enhances the effects of these enzymes and can increase central 3α,5α-THP levels (Cheng and Karavolas 1973; Frye and Rhodes 2005; Malendowicz 1976; Resko et al. 1986; Vongher and Frye 1999). There is evidence for beneficial effects of E2 and progestogens in models of compromised systems. For example, in in vitro studies, E2 and P4 reduce some effects of glutamate or Aβ exposure on measures of insult to hippocampal neurons (Goodman et al. 1996; Nilsen and Brinton 2002). Similarly, administration of E2, P4, or 3α,5α-THP can have beneficial effects in whole animal models of aging and neuronal compromise (i.e., seizure, cortical contusion, ischemia, and diabetic neuropathy models; Asbury et al. 1998; Cervantes et al. 2002; Charalampopoulos et al. 2006; Ciriza et al. 2004; Frye and Rhodes 2005; Frye and Walf 2008; Leonelli et al. 2007; Nilsen and Brinton 2002; Rhodes and Frye 2004; Roof et al. 1994; Sayeed et al. 2006). Progestogens can also decrease anxiety-like, fear, and depressive-like behavior among young and aged female rats or mice (Frye et al. 2004, 2006b; Frye and Rhodes 2006; Frye and Walf 2004; Martinez-Mota et al. 1999; Rodriguez-Landa et al. 2007). Together, these data substantiate further investigation of the effects of P4 administration for affective behavior in a whole animal model of AD.
Given that as many as 30–50% of women over the age of 85 have a dementia or AD (Bachman et al. 1992) and 30–40% of people with AD have depressive and/or psychotic symptoms (Wragg and Jeste 1989), investigating the role of progestogens in the related symptomology of these conditions is of interest. An approach taken to investigate the role of P4 and its metabolites was a double transgenic mouse model of familial AD that express a human presenilin 1 Δ exon 9 deletion mutation, which corresponds to a form of early-onset AD, and overexpression of a chimeric mouse/human amyloid precursor protein (APPswe; Borchelt et al. 1996a, 1996b, 1997). APPswe+PSEN1Δe9 mice start developing β-amyloid deposits between 5 and 6 months of age, which become substantial and more pronounced in female compared to male mice between 6 and 7 months of age (Burgess et al. 2006; Jankowsky et al. 2004). By 9 months of age, severe plaque deposition occurs within the cortex and hippocampus (Jankowsky et al. 2004). Given these characteristics, and that the primary risk factor for AD is age, in this study, we investigated the effects of P4 in a cohort of wildtype and APPswe+PSEN1Δe9 mice that were ovx at 6 months of age and replaced back with P4- or vehicle from 6 to 12 months of age. Mice were tested in anxiety and depression measures between 9 and 12 months of age. We hypothesized that: APPswe+PSEN1Δe9 mice would have increased anxiety-like and depressive-like behavior, compared to wildtype mice, and that P4 would reverse these effects.
Materials and methods
All experimentation was conducted in accordance with accepted standards of humane animal use, and methods that were utilized were pre-approved by the Institutional Animal Care and Use Committee at University at Albany–SUNY.
Subjects and housing
Female APPswe+PSEN1Δe9 (line 85) bigenic mice were obtained from Jackson Laboratory (Bar Harbor, ME). At Jackson Laboratory, this strain was maintained as hemizygotes by crossing the transgenics (originally obtained from D.R. Borchelt, Johns Hopkins University, Baltimore, MD) with mice on a B6C3F1/J background. Genotypes of mice were confirmed by PCR analysis of tail biopsies at Jackson Laboratory before mice were sent to our institution. Transgenic mice and their wildtype littermates were obtained at 6 months old as one cohort.
All mice were group-housed (4–5/cage) in cages containing woodchip shavings for bedding and one Nestlet. Cages were situated in a ventilated rack in a room in the Life Sciences Research Building Animal Care Facility at the University at Albany on a 12/12 h reversed light/dark cycle (lights off at 8:00 a.m.). Mice had continuous access to rodent chow and water in their homecages.
Ovariectomy and hormone administration
All mice were ovx under sodium pentobarbital anesthesia (80 mg/kg). Immediately following ovx, and when mice were still under anesthesia, pellets were subcutaneously implanted in the scruff of the neck. Mice were given a second pellet under anesthesia at 9 months of age so that mice were in the same experimental condition for 6 months. Pellets were placebo or P4 (25 mg, 90-day release), obtained from Innovative Research of America (Sarasota, FL).
General procedure
To investigate the effects of P4 on affective measures, mice were randomly assigned to be administered P4 or placebo vehicle at the beginning of the experiment. Mice were ovx at 6 months of age and administered a pellet containing vehicle or P4 under sodium pentobarbital anesthesia (80 mg/kg). Three months later, under anesthesia, ovx mice were administered a second pellet of the same drug that they received at time of ovx. At 9 months of age, mice were exposed to a 5-day handling procedure (described below) to habituate mice before behavioral testing began. Mice were tested once per week in the behavioral tasks described below. Mice were re-tested under the same experimental conditions in these tasks 4–6 weeks after they were originally tested. Data from both testing occasions is reported here. At the end of the study, blood was collected from mice so that plasma levels of corticosterone and progestogens could be determined. There were five transgenic mice in the P4 and vehicle groups, and six wildtype mice in each of the P4 group and vehicle groups. One transgenic mouse in the P4 group did not get tested twice in each measure and have plasma collected and, thus, data from this mouse were not included in the final analyses.
Handling procedure
Behavioral testing commenced following a 5-day handling procedure that was utilized to habituate mice to handling and behavioral observation by the experimenter (modified as per Frye et al. 2006b). On day 1, mice were picked up from their homecage, handled for 15 s, and returned to their homecage. On day 2, mice transferred from their homecage to a novel clean cage. On day 3, mice were weighed and then replaced to their homecage. On day 4, mice were transferred to another room via a cart. On day 5, mice were transferred to another room via a cart and placed in novel environment for 5 min.
Behavioral testing
AD is characterized by dysfunctions in measures of affect. As such, in the present experiment, mice were tested in several tasks (described below) to determine effects of mutation and P4-replacement on anxiety-like (Emergence, Elevated Zero Maze), and depression-like (Forced Swim Test) behavior. Given that performance in these tasks can be modulated by other factors, such as activity, coordination/balance, and/or responsiveness to aversive stimuli, control measures of general motor behavior (Open Field Activity), motor coordination/balance (Rotarod), and nociception (Tailflick) were also evaluated. Behavioral data were collected by trained observers and video-recorded with the aid of a video-camera and/or video-tracking system (Any-maze-Stoelting, Wood Dale, IL).
Emergence
For the emergence task, which is a modification of a dark-light chamber anxiety task (van Gaalen et al. 2002), mice were placed in a closed opaque cylinder (20 × 4 × 4 cm), secured in a brightly lit open field to prevent rolling. The latency for mice to emerge from the cylinder into the open field when its door is opened was used as an index of anxiety-like behavior, was recorded (max latency = 300 s).
Elevated zero maze
For the elevated zero maze task, mice were placed at the entrance of one of the two closed quadrants in the maze, which had black, 20 cm high walls made of Plexiglas. The maze is circular (40 cm in diameter with 5 cm wide runways) and elevated 70 cm above the ground. The total time spent in the closed and open quadrants was recorded for 5 min (as per Frye et al. 2006b). The total duration of time spent in the open quadrants is considered to reflect reduced anxiety-like behavior.
Forced swim test
Mice were placed in a glass cylinder (21.5 cm deep, 20.5 cm diameter) filled with 8 cm of 30°C tap water for 10 mins. During this time, the duration of immobility, when the mouse was floating and, neither paddling limbs, nor balancing on their tail, was recorded and utilized as an index of depressive behavior (Frye et al. 2004).
Open field task
Motor behavior of mice was assessed in a 39 × 39 × 30 cm open field that had a grid floor with a total of 16 equal squares delineated. An observer recorded the number of entries into the squares for 5 min (as per Frye et al. 2004). The total number of square entries made reflects the general motor activity of mice.
Rotarod
Motor coordination of mice was assessed using the Accurotor Roto-Rod Apparatus (AccuScan Instruments, Columbus, OH; Frye et al. 2006b). In this task, the rods were 3 cm in diameter and elevated 35 cm above the floor. Mice were first habituated to this task with three 30 s trials. One hour later, mice were tested with the rod rotating at constant speed of 20 rpm (Frye et al. 2006b). Mice had two trials in this testing session and the latency to fall from the rod was recorded (maximum latency = 180 s).
Tailflick
Pain thresholds for mice were determined using latency to move tail from a heat source (50°C; San Diego Instruments, San Diego, CA). Mice were gently held by the experimenter, and had their tail smoothed over the heat source so that it was flush to the surface, and the experimenter turned the heat source on. The latency for mice to move their tail from heat source was recorded by the observer for three consecutive trials, with a maximum latency of 10 s), and averaged (modified as per Frye et al. 2000). A longer latency indicates a higher pain threshold.
Tissue collection and dissection
Mice were euthanized by cervical dislocation, rapidly decapitated, and had tissues collected. Blood was collected via cardiac puncture and/or from the trunk following decapitation. Blood was collected in chilled eppendorfs containing 10 μl saturated EDTA solution and spun at 4°C at a speed of 3,000 g for 20 min and then stored at −20°C until radioimmunoassay of plasma. Immediately before radioimmunoassay, blood was spun at 4°C at a speed of 3,000 g for 10 min.
Radioimmunoassay for steroid hormones
Corticosterone, P4, DHP, and 3α,5α-THP concentrations in plasma were measured as described below, using previously reported methods (Frye and Bayon 1999). Corticosterone was extracted from plasma by heating samples at 60°C for 30 min. P4, DHP, and 3α,5α-THP were extracted from plasma with ether following incubation with distilled water and 800 counts per minute (cpm) of 3H steroid. After snap-freezing twice, test tubes containing steroid and ether were evaporated to dryness in a Savant. Dried down tubes were reconstituted with phosphate assay buffer to the original plasma volume immediately before set-up of radioimmunoassays. 3H corticosterone (NET 182: specific activity = 48.2 Ci/mmol), P4 (NET-208: specific activity = 47.5 Ci/mmol), and 3α,5α-THP (used for DHP and 3α,5α-THP, NET-1047: specific activity = 65.0 Ci/mmol), were purchased from Perkin Elmer (Boston, MA). The corticosterone antibody (#B3–163), obtained from Esoterix Endocrinology (Calabasas Hills, CA), which typically binds 40–60% of [3H]corticosterone, was used in a 1:20,000 dilution and bound 45% in the present study. The P4 antibody (P#337), obtained from G.D. Niswender (Colorado State University), when used in a 1:30,000 dilution typically binds between 30% and 50% of [3H]P4, and bound 48% in the present study. The DHP (X-947) and 3α,5α-THP antibodies (#921412–5), obtained from R. Purdy (Veterans Medical Affairs, La Jolla, CA), when used in a 1:5,000 dilution binds between 40–60% of [3H]3α,5α-THP and bound 47% in the present study. The range of the standard curves, prepared in duplicate, was 0–4 ng for corticosterone, and 0–8,000 pg for P4, DHP, and 3α,5α-THP. Standards were added to assay buffer followed by addition of the appropriate antibody (described above) and 3H steroid. Total assay volumes were 960 μl for corticosterone, 750 μl for P4, 950 μl for DHP, and 950 μl for 3α,5α-THP. All assays were incubated overnight at 4°C, except for corticosterone, which was incubated at room temperature for 60 min. Separation of bound and free steroid was done by rapidly adding dextran-coated charcoal to assay tubes and incubating for 20 min. Following incubation, tubes were centrifuged at 3,000 g for 20 min and the supernatant was decanted into a glass scintillation vial with 5 ml Scintiverse BD scintillation cocktail. Sample tube concentrations were calculated using the logit-log method (Rodbard and Hutt 1974), interpolation of the standards, and correction for recovery with Assay Zap. The inter- and intra-assay reliability coefficients were: 0.05 and 0.06 for corticosterone, 0.11 and 0.10 for P4, 0.11 and 0.09 for DHP, and 0.09 and 0.10 for 3α,5α-THP.
Statistical analyses
Two-way repeated measures analyses of variance (ANOVAs) tests were utilized to determine effects of genotype (wildtype vs APPswe+PSEN1Δe9) and hormone condition (vehicle vs P4) on behavioral measures on two test occasions. Two-way between subjects ANOVAs were utilized to determine effect of genotype and hormone condition for plasma steroid levels. Where appropriate, ANOVAs were followed by Fisher’s LSD post hoc tests to determine group differences. Significant main effects are reported when P < 0.05 and trends are considered when P < 0.10.
Results
Effects on anxiety-like and depression-like behavior
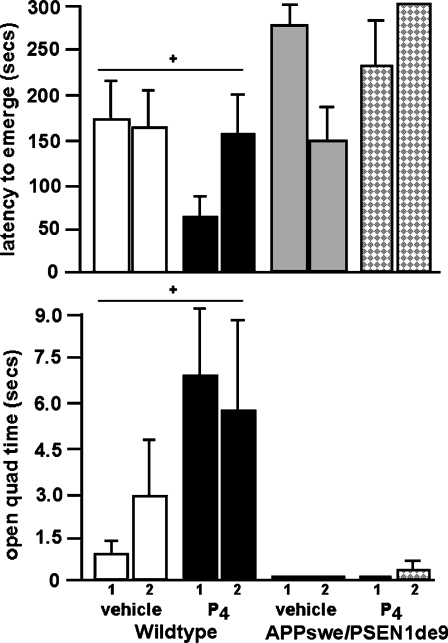
There were main effects of genotype for behavior in the emergence task [F1,34 = 6.6; P < 0.01; Fig. 1, top] and elevated zero maze [F1,34 = 5.3; P < 0.03; Fig. 1, bottom]. Post hoc tests revealed that, compared to wildtype mice, APPswe+PSEN1Δe9, mice had increased anxiety-like behavior, i.e., significantly longer latencies to emerge from the chamber and decreased time spent on the open quadrants of the elevated zero maze. There was neither an effect of P4 administration, nor repeated testing, on these measures. There were no significant interactions between these variables.
Fig. 1.
The latency [in seconds (mean ± SEM)] to emerge from the chamber (top) and time spent on the open quadrants of the zero maze (bottom). + Significant effect of genotype, P < 0.05
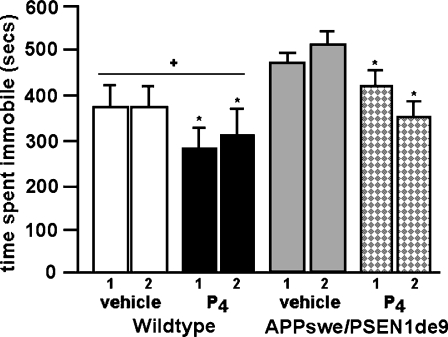
In the forced swim test of depression, there were main effects of genotype [F1,34 = 4.3; P < 0.04; Fig. 2] and hormone condition [F1,34 = 11.2; P < 0.01]. Post hoc tests revealed that APPswe+PSEN1Δe9 mice had increased depressive-like behavior, i.e., tended to spend more time immobile, than did wildtype mice. P4 administration significantly decreased immobility in this task. There was neither an effect of repeated testing, nor an interaction between variables, on this measure.
Fig. 2.
Mean time (in seconds; ± SEM) spent immobile in the forced swim test. + Significant effect of genotype, P < 0.05. * vs vehicle condition, P < 0.05
Effects on motor behavior, coordination, pain thresholds, and plasma corticosterone
Notably, these behavioral differences on affective measures were not accompanied by differences due to genotype, P4 condition, repeated testing, or an interaction between these variables, for coordination/balance (rotarod fall latency) or pain thresholds (tail flick latencies; see Table 1). There was also no effect of genotype, repeated testing, or any interactions between variables, for general motor activity in the open field (total entries); however, there was a main effect of hormone condition [F1,34 = 6.8; P < 0.01]. P4 administration decreased total entries in the open field compared to vehicle administration. APPswe+PSEN1Δe9 and wildtype mice had similar, basal levels of corticosterone (Table 2).
Table 1.
Effects of genotype and treatment for control behavioral measures on test 1 and 2. Motor behavior (open field entries), coordination (rotorod fall latency) and pain threshold responses (tail flick latency) of bigenic APPswe+PSEN1e9 mice and their wildtype counterparts administered vehicle or P4 via subcutaneous pellets (values are mean ± SEM)
| Total open field entries | Rotorod fall latency (s) | Tail flick latency (s) | ||||
|---|---|---|---|---|---|---|
| Condition | Test 1 | Test 2 | Test 1 | Test 2 | Test 1 | Test 2 |
| Wildtype vehicle | 91 ± 12 | 60 ± 11 | 5 ± 1 | 16 ± 11 | 6 ± 1 | 6 ± 1 |
| Wildtype P4 | 53 ± 14* | 75 ± 13* | 30 ± 16 | 16 ± 11 | 9 ± 1 | 7 ± 1 |
| APPswe+PSEN1 vehicle | 116 ± 43 | 103 ± 18 | 51 ± 33 | 8 ± 3 | 8 ± 1 | 7 ± 1 |
| APPswe+PSEN1 P4 | 54 ± 11* | 46 ± 16* | 7 ± 3 | 31 ± 24 | 8 ± 1 | 8 ± 1 |
*P < 0.05 (vs vehicle condition)
Table 2.
Effects of genotype and treatment for steroid levels. Plasma concentrations (mean ± sem)of corticosterone (CORT), progesterone (P4), dihydroprogesterone (DHP), and 5α-pregnan-3α-ol-20-one (3α,5α-THP) of wildtype and APPswe+PSEN1Δe9 mice administered vehicle or P4 via subcutaneous pellets
| Condition | CORT (ng/dl) | P4 (ng/ml) | DHP (ng/ml) | 3α,5α -THP (ng/ml) |
|---|---|---|---|---|
| Wildtype Vehicle (n = 6) | 3.4 ± 0.8 | 3.5 ± 1.6 | 6. ± 2.6 | 8.8 ± 1.2 |
| Wildtype P4 (n = 6) | 2.9 ± 0.5 | 28.2 ± 6.0* | 24.7 ± 5.9* | 16.2 ± 5.3* |
| APPswe+PSEN1 Vehicle (n = 5) | 3.4 ± 0.9 | 4.6 ± 1.6 | 5.6 ± 3.3 | 2.2 ± 0.8** |
| APPswe+PSEN1 P4 (n = 4) | 2.0 ± 1.1 | 27.5 ± 11.8* | 17.1 ± 7.5* | 11.0 ± 1.3*, ** |
*P < 0.05 (vs vehicle condition), **P ≤ 0.08 (vs wildtype)
Effects on plasma progestin levels
There was a main effect of hormone condition for P4 [F1,17 = 17.1; P < 0.01], DHP [F1,17 = 12.5; P < 0.01], and 3α,5α-THP [F1,17 = 6.2; P < 0.02] levels. P4 administration significantly increased plasma progestin levels in wildtype and APPswe+PSEN1Δe9 mice. There were no main effects of genotype for these measures, but there was a tendency for 3α,5α-THP levels to be lower in APPswe+PSEN1Δe9, compared to wildtype, mice [F1,17 = 3.3; P < 0.08].
Discussion
The present results partially supported our hypothesis that APPswe+PSEN1Δ9 mice would have increased anxiety-like and depressive-like behavior compared to wildtype mice, and that P4 administration would reverse some of these effects. In the emergence and elevated zero maze task, APPswe+PSEN1Δ9 mutant mice had increased anxiety-like behavior, characterized by increased emergence latencies and decreased time spent on the open quadrants, respectively, compared to wildtype mice. A similar pattern was observed in the forced swim test of depression behavior. Compared to wildtype mice, APPswe+PSEN1Δ9 mice spent more time immobile. In both APPswe+PSEN1Δ9 and wildtype mice, P4 decreased time spent immobile compared to that observed in mice that received placebo pellets. As expected, P4 administration increased P4, DHP, and 3α,5α-THP levels in plasma. There were no differences between groups for plasma corticosterone levels or control measures. Although the latencies to fall in the rotorod were short for all groups, suggesting that this task was difficult for these mice, there were also no differences due to genotype for total entries in the open field or tailflick latencies. Together, these results suggest that middle-aged mice with the APPswe+PSEN1Δ9 mutation demonstrate greater anxiety-like and depressive-like behavior, and that P4 treatment can decrease depressive-like behavior.
The present data confirm and extend findings from previously reported studies utilizing transgenic murine models of AD. For example, in these AD mice models, there are behavioral impairments in hippocampus-dependent learning tasks (Frye and Walf 2008; Lalonde et al. 2004; Reiserer et al. 2007) and alterations in anxiety/depression tasks (Lalonde et al. 2004; Lee et al. 2004). In the present study, we found that anxiety-like and depression-like behavior of APPswe+PSEN1Δe9 mice was increased compared to their wildtype counterparts in the emergence, elevated zero maze, and forced swim tasks. Together, these data confirm some of the reports of increased anxiety-like and depressive-like behavior with AD.
The present data confirm and extend previous findings to demonstrate the role of progestogens, particularly 3α,5α-THP, for AD and affective behavior. Although statistical analyses and interpretations of the present data may be somewhat limited due to small sample size, a different pattern of response to P4 in the wildtype and APPswe+PSEN1Δe9 is apparent in the two anxiety tasks that were utilized in the present study. In the emergence task, unlike in the elevated zero maze, P4 appears to increase anxiety-like behavior of the APPswe+PSEN1Δe9 mice, whereas P4 modestly decreases anxiety-like behavior of wildtype mice in both tasks. However, all mice that were tested spent a short duration in the open quadrants of the elevated zero maze. In a rat model of AD, rats that were administered chronic intracerebroventricular administration of β-amyloid (1–40) protein, demonstrated increased depressive behavior and decreased P4 levels in the hippocampus compared to controls (Urani et al. 2004). Rats with natural elevations in progestogens have decreased anxiety- and depressive-like behavior and this effect is mimicked by P4 administration to ovx rats or mice (Frye et al. 2000, 2004, 2006a; Frye and Rhodes 2006; Frye and Walf 2002; Martinez-Mota et al. 1999; Rodriguez-Landa et al. 2007). Attenuating metabolism of P4 to 3α,5α-THP in a 5α-reductase knockout model, or with a 5α-reductase inhibitor administered systemically or to the hippocampus/amygdala, increases anxiety-like and depressive-like behavior in rodents with natural elevations in P4 or those administered P4 (Frye and Walf 2002, 2004; Rhodes and Frye 2001; Walf et al. 2006). These data, in addition to the present findings that P4 administration decreased depressive-like behavior in wildtype and APPswe+PSEN1Δ9 mice, suggest that P4 and its metabolites may have beneficial effects on affective behavior in a healthy as well as a compromised system. It must be noted that the small sample sizes is a limitation of the present study. However, the present results, and previous studies of progestogens’ effects for anxiety and depression in AD and non-AD models, substantiate further investigation of the role and mechanisms of progestogens in murine models of AD.
Whether the effects observed are via actions of P4 or its metabolites is an interesting question as P4 and 3α,5α-THP have discrepant receptor targets. P4 binds with a high affinity to intracellular progestin receptors (PRs) (Brosens et al 2004; Iswari et al. 1986; Smith et al. 1974), while, in physiological concentrations, 3α,5α-THP is devoid of affinity for PRs (Rupprecht 2003). 3α,5α-THP can have rapid actions via some neurotransmitter receptors (e.g., GABAA, NMDA, dopamine; reviewed in Frye et al. 2006a). Indeed, in a mouse model of AD, in which there is overexpression of APPswe, no differences were observed in expression of PR in hippocampus using RT-PCR (von Arnim et al. 2006), suggesting that non-PR mechanisms may be important for the effects observed in the present. In our laboratory and many others, the role of 3α,5α-THP as a potent agonist of membrane GABAA/benzodiazepine receptor complexes (GBRs; Majewska et al. 1986; Wilson 1996), and its effects through actions involving other neurotransmitter receptors, such as dopamine and NMDARs, PRs, and their downstream effectors has been revealed (Frye et al. 2006a). Indeed withdrawal from progestogens alters GABA subunit expression and increases anxiety-like behavior in young adult rats (Gulinello et al. 2002; reviewed in Smith 2002). Identification of the receptor targets and/or signal transduction cascades underlying the observed effects of P4 are beyond the scope of this investigation, but will be elucidated in the future.
The clinical literature generally supports a role of endocrine factors altering AD and other neurodegenerative processes associated with aging. Some, but not all, studies have shown that steroid/E2-based therapies to postmenopausal women may attenuate decline in cognitive function, improve mood in AD, and decrease risk for developing AD (Asthana et al. 2001; Barrett-Connor and Kritz-Silverstein 1993; Brenner et al. 1994; Carlson et al. 2000; Fillit 2002; Fillit et al. 1986; Matthews et al. 1999; Paganini-Hill and Henderson 1994; Tang et al. 1996; Yaffe et al. 1998; Zandi et al. 2002). However, the therapeutic potential of hormone-based therapies for cognitive function/AD is under scrutiny given that reports from the Women's Health Initiative Memory Study (WHIMS), demonstrated that women administered E2, alone or in combination with progestogens, had greater cognitive impairment, and AD risk increased twofold, compared to the placebo group (Shumaker et al. 2003, 2004). Although basic science models generally support a beneficial role of P4, clinical studies such as these, call into question the potential beneficial role of progestogens. However, there were some methodological problems in the WHIMS trial. One may have been a long latency between steroid deprivation and subsequent replacement, which may attenuate the responsiveness to later progestin administration (Rubinow 2005). Data from animal models have demonstrated that beneficial effects of steroids may be dependent upon timing of replacement (Daniel et al. 2006). For this reason, in the present study, P4 was replaced at time of ovx. Another problem with the findings from clinical studies was that, in these women, hormone-based therapies were initiated many years post-menopause when there is a reduced capacity to form 3α,5α-THP from circulating P4 (Genazzani et al. 1998; de Wit et al. 2001). Indeed, an important consideration to investigate further is that the beneficial effects of progestogens may be dependent upon their capacity to be converted readily to 3α,5α-THP. Indeed, we have shown decrements in 3α,5α-THP levels in the hippocampus of these APPswe+PSEN1Δ9 mutant mice administered P4 compared to their wildtype counterparts (Frye and Walf 2008). In the present study, levels of DHP and 3α,5α-THP were lower in APPswe+PSEN1Δ9 mice, compared to wildtype mice, administered P4. Indeed, the ratio of conversion of P4 to DHP (as per Kellogg and Frye 1999), was 46% lower in APPswe+PSEN1Δ9 mutant mice compared to wildtype mice administered P4, whereas the conversion of DHP to 3α,5α-THP in the APPswe+PSEN1Δ9 mutant and wildtype mice administered P4 were comparable. These findings suggest that differences in 5α-reductase activity may underlie the phenotype observed. Given this possibility, one cannot discount possible effects of testosterone’s 5α-reduced products dihydrotestosterone and/or 3α-androstanediol, which may also play a role in age-related changes in affect and cognition (Janowsky 2006); however, these effects may be more evident in males. Finally, another possibility is that administration of steroids may have disorganizing effects in a compromised and more steroid-sensitive system, such as AD, following downregulation of steroids and their substrates as occurs with aging (Atwood et al. 2005; Webber et al. 2006, 2007). In the present study, these putative pathological effects of steroids with AD in middle-aged individuals were not observed in the present study, which may be related to the long-term administration P4 that produced physiological circulating progestin levels. Given that changes in mood/affect often occur before cognitive decline, and AD patients with major depression may be more cognitively impaired and disabled than AD patients without depression (Rovner et al. 1989), it is important to investigate these factors further in the future.
In summary, anxiety-like and depression-like behavior was increased in APPswe+PSEN1Δ9 mutant mice compared to their wildtype controls. P4 administration to mice between 9 and 12 months of age, irrespective of genotype, decreased depressive-like behavior compared to placebo administration. P4 administration increased P4, DHP, 3α,5α-THP levels in plasma, and there were modest decrements in the capacity for APPswe+PSEN1Δ9 mutant mice to covert P4 to its 5α-reduced metabolites. Thus, these data demonstrate that overexpression of APPswe and presenilin 1 Δe9 is associated with increased anxiety-like and depressive-like behavior, and that P4 can have salient effects to reduce depressive-like behavior in middle-aged individuals.
References
- Asbury ET, Fritts ME, Horton JE et al (1998) Progesterone facilitates the acquisition of avoidance learning and protects against subcortical neuronal death following prefrontal cortex ablation in the rat. Behav Brain Res 97:99–106. doi:10.1016/S0166-4328(98)00031-X [DOI] [PubMed]
- Asthana S, Baker LD, Craft S et al (2001) High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology 57:605–612 [DOI] [PubMed]
- Atwood CS, Meethal SV, Liu T et al (2005) Dysregulation of the hypothalamic-pituitary-gonadal axis with menopause and andropause promotes neurodegenerative senescence. J Neuropathol Exp Neurol 64:93–103 [DOI] [PubMed]
- Bachman DL, Wolf PA, Linn R et al (1992) Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology 42:115–119 [DOI] [PubMed]
- Barrett AM (1999) Probable Alzheimer's disease: gender-related issues. J Gend Specif Med 2:55–60 [PubMed]
- Barrett-Connor E, Kritz-Silverstein D (1993) Estrogen replacement therapy and cognitive function in older women. JAMA 269:2637–2641. doi:10.1001/jama.269.20.2637 [DOI] [PubMed]
- Bernardi F, Lanzone A, Cento RM et al (2000) Allopregnanolone and dehydroepiandrosterone response to corticotropin-releasing factor in patients suffering from Alzheimer's disease and vascular dementia. Eur J Endocrinol 142:466–471. doi:10.1530/eje.0.1420466 [DOI] [PubMed]
- Bernardi F, Pluchino N, Begliuomini S et al (2004) Disadaptive disorders in women: allopregnanolone, a sensitive steroid. Gynecol Endocrinol 19:344–353. doi:10.1080/09513590400018223 [DOI] [PubMed]
- Borchelt DR, Davis J, Fischer M et al (1996a) A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal 13:159–163 [DOI] [PubMed]
- Borchelt DR, Thinakaran G, Eckman CB et al (1996b) Familial Alzheimer's disease-linked presenilin 1 variants elevate Aβ1-42/1–40 ratio in vitro and in vivo. Neuron 17:1005–1013. doi:10.1016/S0896-6273(00)80230-5 [DOI] [PubMed]
- Borchelt DR, Ratovitski T, van Lare J et al (1997) Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 19:939–945. doi:10.1016/S0896-6273(00)80974-5 [DOI] [PubMed]
- Brenner DE, Kukull WA, Stergachis A et al (1994) Postmenopausal estrogen replacement therapy and the risk of Alzheimer's disease: a population-based case-control study. Am J Epidemiol 140:262–267 [DOI] [PubMed]
- Brosens JJ, Tullet J, Varshochi R et al (2004) Steroid receptor action. Best Pract Res Clin Obstet Gynaecol 18:265–283. doi:10.1016/j.bpobgyn.2004.01.006 [DOI] [PubMed]
- Burgess BL, McIsaac SA, Naus KE et al (2006) Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer's disease mouse models with abundant A β in plasma. Neurobiol Dis 24:114–127. doi:10.1016/j.nbd.2006.06.007 [DOI] [PubMed]
- Carlson LE, Sherwin BB, Chertkow HM (2000) Relationships between mood and estradiol (E2) levels in Alzheimer's disease (AD) patients. J Gerontol B Psychol Sci Soc Sci 55:47–53 [DOI] [PubMed]
- Cervantes M, Gonzalez-Vidal MD, Ruelas R et al (2002) Neuroprotective effects of progesterone on damage elicited by acute global cerebral ischemia in neurons of the caudate nucleus. Arch Med Res 33:6–14. doi:10.1016/S0188-4409(01)00347-2 [DOI] [PubMed]
- Charalampopoulos I, Alexaki VI, Tsatsanis C et al (2006) Neurosteroids as endogenous inhibitors of neuronal cell apoptosis in aging. Ann N Y Acad Sci 1088:139–152. doi:10.1196/annals.1366.003 [DOI] [PubMed]
- Cheng YJ, Karavolas HJ (1973) Conversion of progesterone to 5α-pregnane-3, 20-dione and 3α-hydroxy-5α-pregnan-20-one by rat medical basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology 93:1157–1162 [DOI] [PubMed]
- Ciriza I, Azcoitia I, Garcia-Segura LM (2004) Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J Neuroendocrinol 16:58–63. doi:10.1111/j.1365-2826.2004.01121.x [DOI] [PubMed]
- Daniel JM, Hulst JL, Berbling JL (2006) Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology 147:607–614. doi:10.1210/en.2005-0998 [DOI] [PubMed]
- de Wit H, Schmitt L, Purdy R et al (2001) Effects of acute progesterone administration in healthy postmenopausal women and normally-cycling women. Psychoneuroendocrinology 26:697–710. doi:10.1016/S0306-4530(01)00024-5 [DOI] [PubMed]
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO (1989) Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. JAMA 262:2551–2556 [DOI] [PubMed]
- Fillit HM (2002) The role of hormone replacement therapy in the prevention of Alzheimer disease. Arch Intern Med 162:1934–1942. doi:10.1001/archinte.162.17.1934 [DOI] [PubMed]
- Fillit H, Weinreb H, Cholst I et al (1986) Observations in a preliminary open trial of estradiol therapy for senile dementia-Alzheimer's type. Psychoneuroendocrinology 11:337–345. doi:10.1016/0306-4530(86)90019-3 [DOI] [PubMed]
- Frye CA (2007) Progestins influence motivation, reward, conditioning,stress, and/or response to drugs of abuse. Pharmacol Biochem Behav86:209–219 [DOI] [PMC free article] [PubMed]
- Frye CA, Bayon LE (1999) Mating stimuli influence endogenous variations in the neurosteroids 3α, 5α-THP and 3α-diol. J Neuroendocrinol 11:839–847. doi:10.1046/j.1365-2826.1999.00379.x [DOI] [PubMed]
- Frye CA, Rhodes ME (2005) Estrogen-priming can enhance progesterone's anti-seizure effects in part by increasing hippocampal levels of allopregnanolone. Pharmacol Biochem Behav 81:907–916 [DOI] [PubMed]
- Frye CA, Rhodes ME (2006) Infusions of 5α-pregnan-3α-ol-20-one(3α,5α-THP) to the ventral tegmental area, but not the substantianigra, enhance exploratory, anti-anxiety, social and sexual behavioursand concomitantly increase 3α,5α-THP concentrations in thehippocampus, diencephalon and cortex of ovariectomisedoestrogen-primed rats. J Neuroendocrinol 18:960–975 [DOI] [PubMed]
- Frye CA, Walf AA (2002) Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav 41:306–315. doi:10.1006/hbeh.2002.1763 [DOI] [PubMed]
- Frye CA, Walf AA (2004) Hippocampal 3α, 5α-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav 78:531–540. doi:10.1016/j.pbb.2004.03.024 [DOI] [PubMed]
- Frye CA, Walf AA (2008) Effects of progesterone administration and APPswe+PSEN1Deltae9 mutation for cognitive performance of mid-aged mice. Neurobiol Learn Mem 89:17–26 [DOI] [PubMed]
- Frye CA, Petralia SM, Rhodes ME (2000) Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α, 5α-THP. Pharmacol Biochem Behav 67:587–596. doi:10.1016/S0091-3057(00)00392-0 [DOI] [PubMed]
- Frye CA, Walf AA, Rhodes ME et al (2004) Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5α-reductase. Brain Res 1004:116–124. doi:10.1016/j.brainres.2004.01.020 [DOI] [PubMed]
- Frye CA, Rhodes ME, Petralia SM et al (2006a) 3α-hydroxy-5α-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience 138:1007–1014. doi:10.1016/j.neuroscience.2005.06.015 [DOI] [PMC free article] [PubMed]
- Frye CA, Sumida K, Dudek BC et al (2006b) Progesterone's effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology (Berl) 186:312–322. doi:10.1007/s00213-006-0309-3 [DOI] [PubMed]
- Genazzani AR, Petraglia F, Bernardi F et al (1998) Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab 83:2099–2103. doi:10.1210/jc.83.6.2099 [DOI] [PubMed]
- Goodman Y, Bruce AJ, Cheng B et al (1996) Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid β-peptide toxicity in hippocampal neurons. J Neurochem 66:1836–1844 [DOI] [PubMed]
- Gulinello M, Gong QH, Smith SS (2002) Progesterone withdrawal increases the α4 subunit of the GABA(A) receptor in male rats in association with anxiety and altered pharmacology - a comparison with female rats. Neuropharmacology 43:701–714. doi:10.1016/S0028-3908(02)00171-5 [DOI] [PMC free article] [PubMed]
- Iswari S, Colas AE, Karavolas HJ (1986) Binding of 5α-dihydroprogesterone and other progestins to female rat anterior pituitary nuclear extracts. Steroids 47:189–203. doi:10.1016/0039-128X(86)90088-7 [DOI] [PubMed]
- Janowsky JS (2006) The role of androgens in cognition and brain aging in men. Neuroscience 138:1015–1020. doi:10.1016/j.neuroscience.2005.09.007 [DOI] [PubMed]
- Jankowsky JL, Fadale DJ, Anderson J et al (2004) Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet 13:159–170. doi:10.1093/hmg/ddh019 [DOI] [PubMed]
- Kellogg CK, Frye CA (1999) Endogenous levels of 5α-reduced progestins and androgens in fetal vs. adult rat brains. Brain Res Dev Brain Res 115:17–24. doi:10.1016/S0165-3806(99)00041-3 [DOI] [PubMed]
- Lalonde R, Kim HD, Fukuchi K (2004) Exploratory activity, anxiety, and motor coordination in bigenic APPswe+PS1/DeltaE9 mice. Neurosci Lett 369:156–161. doi:10.1016/j.neulet.2004.07.069 [DOI] [PubMed]
- Lee KW, Lee SH, Kim H et al (2004) Progressive cognitive impairment and anxiety induction in the absence of plaque deposition in C57BL/6 inbred mice expressing transgenic amyloid precursor protein. J Neurosci Res 76:572–580. doi:10.1002/jnr.20127 [DOI] [PubMed]
- Leonelli E, Bianchi R, Cavaletti G et al (2007) Progesterone and its derivatives are neuroprotective agents in experimental diabetic neuropathy: a multimodal analysis. Neuroscience 144:1293–1304. doi:10.1016/j.neuroscience.2006.11.014 [DOI] [PubMed]
- Majewska MD, Harrison NL, Schwartz RD et al (1986) Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232:1004–1007. doi:10.1126/science.2422758 [DOI] [PubMed]
- Malendowicz LK (1976) Sex differences in adrenocortical structure and function. III. The effects of postpubertal gonadectomy and gonadal hormone replacement on adrenal cholesterol sidechain cleavage activity and on steroids biosynthesis by rat adrenal homogenates. Endokrinologie 67:26–35 [PubMed]
- Matthews K, Cauley J, Yaffe K et al (1999) Estrogen replacement therapy and cognitive decline in older community women. J Am Geriatr Soc 47:518–523 [DOI] [PubMed]
- Martinez-Mota L, Contreras CM, Saavedra M (1999) Progesterone reduces immobility in rats forced to swim. Arch Med Res 30:286–289. doi:10.1016/S0188-0128(99)00024-X [DOI] [PubMed]
- Marx CE, Trost WT, Shampine LJ et al (2006) The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer's disease. Biol Psychiatry 60:1287–1294. doi:10.1016/j.biopsych.2006.06.017 [DOI] [PubMed]
- Nilsen J, Brinton RD (2002) Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology 143:205–212. doi:10.1210/en.143.1.205 [DOI] [PubMed]
- Paganini-Hill A, Henderson VW (1994) Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol 140:256–261 [DOI] [PubMed]
- Reiserer RS, Harrison FE, Syverud DC et al (2007) Impaired spatial learning in the APPSwe+PSEN1DeltaE9 bigenic mouse model of Alzheimer's disease. Genes Brain Behav 6:54–65. doi:10.1111/j.1601-183X.2006.00221.x [DOI] [PubMed]
- Resko JA, Stadelman HL, Handa RJ (1986) Control of 5α-reduction of testosterone in neuroendocrine tissues of female rats. Biol Reprod 34:870–877. doi:10.1095/biolreprod34.5.870 [DOI] [PubMed]
- Rhodes ME, Frye CA (2001) Inhibiting progesterone metabolism in the hippocampus of rats in behavioral estrus decreases anxiolytic behaviors and enhances exploratory and antinociceptive behaviors. Cogn Affect Behav Neurosci 1:287–296. doi:10.3758/CABN.1.3.287 [DOI] [PubMed]
- Rhodes ME, Frye CA (2004) Progestins in the hippocampus of female rats have antiseizure effects in a pentylenetetrazole seizure model. Epilepsia 45:1531–1538. doi:10.1111/j.0013-9580.2004.16504.x [DOI] [PubMed]
- Rodbard D, Hutt DM (1974) Statistical analysis of radioimmunoassay and immunoradiometric assays: a generalized, weighted iterative, least squares method for logistic curve fitting. In: International Atomic Energy Agency (ed) Symposium on Radioimmunoassay and Related Procedures in Medicine. Uniput, New York, pp 209–233
- Rodriguez-Landa JF, Contreras CM, Bernal-Morales B et al (2007) Allopregnanolone reduces immobility in the forced swimming test and increases the firing rate of lateral septal neurons through actions on the GABAA receptor in the rat. J Psychopharmacol 21:76–84. doi:10.1177/0269881106064203 [DOI] [PubMed]
- Roof RL, Duvdevani R, Braswell L et al (1994) Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol 129:64–69. doi:10.1006/exnr.1994.1147 [DOI] [PubMed]
- Rovner BW, Broadhead J, Spencer M et al (1989) Depression and Alzheimer's disease. Am J Psychiatry 146:350–353 [DOI] [PubMed]
- Rubinow DR (2005) Reproductive steroids in context. Arch Women Ment Health 8:1–5. doi:10.1007/s00737-005-0065-0 [DOI] [PubMed]
- Rupprecht R (2003) Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology 28:139–168. doi:10.1016/S0306-4530(02)00064-1 [DOI] [PubMed]
- Sayeed I, Guo Q, Hoffman SW et al (2006) Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann Emerg Med 47:381–389. doi:10.1016/j.annemergmed.2005.12.011 [DOI] [PubMed]
- Seeman MV (1997) Psychopathology in women and men: focus on female hormones. Am J Psychiatry 154:1641–1647 [DOI] [PubMed]
- Shumaker SA, Legault C, Rapp SR et al (2003) Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2651–2662. doi:10.1001/jama.289.20.2651 [DOI] [PubMed]
- Shumaker SA, Legault C, Kuller L (2004) Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: women's health initiative memory study. JAMA 291:2947–2958. doi:10.1001/jama.291.24.2947 [DOI] [PubMed]
- Smith SS (2002) Withdrawal properties of a neuroactive steroid: implications for GABA(A) receptor gene regulation in the brain and anxiety behavior. Steroids 67:519–528. doi:10.1016/S0039-128X(01)00170-2 [DOI] [PubMed]
- Smith HE, Smith RG, Toft DO et al (1974) Binding of steroids to progesterone receptor proteins in chick oviduct and human uterus. J Biol Chem 249:5924–5932 [PubMed]
- Smith CD, Wekstein DR, Markesbery WR et al (2006) 3α, 5α-THP: a potential plasma neurosteroid biomarker in Alzheimer's disease and perhaps non-Alzheimer's dementia. Psychopharmacology (Berl) 186:481–485. doi:10.1007/s00213-005-0186-1 [DOI] [PubMed]
- Tang MX, Jacobs D, Stern Y et al (1996) Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet 348:429–432. doi:10.1016/S0140-6736(96)03356-9 [DOI] [PubMed]
- Urani A, Romieu P, Roman FJ et al (2004) Enhanced antidepressant efficacy of sigma1 receptor agonists in rats after chronic intracerebroventricular infusion of β-amyloid-(1–40) protein. Eur J Pharmacol 486:151–161. doi:10.1016/j.ejphar.2003.12.018 [DOI] [PubMed]
- van Gaalen MM, Stenzel-Poore MP, Holsboer F et al (2002) Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur J Neurosci 15:2007–2015. doi:10.1046/j.1460-9568.2002.02040.x [DOI] [PubMed]
- von Arnim CA, Verstege E, Etrich SM et al (2006) Mechanisms of hypoxic tolerance in presymptomatic APP23 transgenic mice. Mech Ageing Dev 127:109–114. doi:10.1016/j.mad.2005.09.025 [DOI] [PubMed]
- Vongher JM, Frye CA (1999) Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacol Biochem Behav 64:777–785. doi:10.1016/S0091-3057(99)00140-9 [DOI] [PubMed]
- Walf AA, Sumida K, Frye CA (2006) Inhibiting 5α-reductase in the amygdala attenuates antianxiety and antidepressive behavior of naturally-receptive and hormone-primed ovariectomized rats. Psychopharmacology (Berl) 186:302–311. doi:10.1007/s00213-005-0100-x [DOI] [PMC free article] [PubMed]
- Webber KM, Stocco DM, Casadesus G et al (2006) Steroidogenic acute regulatory protein (StAR): evidence of gonadotropin-induced steroidogenesis in Alzheimer Disease. Mol Neurodegener 1:14. doi:10.1186/1750-1326-1-14 [DOI] [PMC free article] [PubMed]
- Webber KM, Casadesus G, Atwood CS et al (2007) Gonadotropins: a cohesive gender-based etiology of Alzheimer disease. Mol Cell Endocrinol 260–262:271–275. doi:10.1016/j.mce.2006.01.018 [DOI] [PubMed]
- Wilson MA (1996) GABA physiology: modulation by benzodiazepines and hormones. Crit Rev Neurobiol 10:1–37 [DOI] [PubMed]
- Wragg RE, Jeste DV (1989) Overview of depression and psychosis in Alzheimer's disease. Am J Psychiatry 146:577–587 [DOI] [PubMed]
- Yaffe K, Sawaya G, Lieberburg I et al (1998) Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA 279:688–695. doi:10.1001/jama.279.9.688 [DOI] [PubMed]
- Zandi PP, Carlson MC, Plassman BL et al (2002) Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA 288:2123–2129. doi:10.1001/jama.288.17.2123 [DOI] [PubMed]