Abstract
The transcription rate of interleukin-6 (IL-6) can be reduced by the C-allele of a polymorphism (rs1800795) located in the 5′-flanking region of the IL-6 gene (NM_000600), and IL-6 plasma levels increase with age. We assembled an elderly Italian population [“The Treviso Longeva (Trelong) study”, age range 70–106 years, n = 668 subjects] and assessed rs1800795 genotype and plasma IL-6 concentrations. The rs1800795 genotype was also assessed in an independent Italian study (“Milan” study, age range 70–96, n = 245 subjects). To verify an age- or sex-specific effect of rs1800795 genotype we compared people younger (70–85) and older (85+) than 85 years of age. We found a significant reduction in the frequency of rs1800795 C/C genotype in 85+ men from the Trelong study, while in the Milan study this data did not reach significance. However, considering the two studies together, the frequency of the rs1800795 C/C genotype was significantly lower in 85+ than in 70–85 males (4.0% and 10.7%, respectively), while it remained unchanged in females. As for IL-6 plasma levels, after a multivariate analysis to control for confounders, a correlation between age and plasma IL-6 concentrations was revealed (P < 0.0001). An increase in circulating IL-6 levels in the entire 85+ group compared to the 70–85 group (P < 0.05, Tukey′s test) was also noticed. We suggest a sex-specific pattern for genetic variability linked to inflammatory response and longevity, consistent with the age-related increase in IL-6.
Keywords: IL-6, Longevity, Rs1800795, Immunosenescence, Inflammation, Sex
Introduction
Human longevity results from the complex interplay between genetics and environment. Throughout life, each of these factors contributes differently to lifespan and any single trait might be beneficial at one stage of life and detrimental in another. The genetic component of longevity appears to account for around 20–30% of lifespan, according to twin-based population studies (Herskind et al. 1996; vB Hjelmborg et al. 2006). Genes involved in basic cell pathways (stress response, DNA repair, microbial immunity and inflammation, metabolism and calorie restriction) are good candidates to modulate longevity. This aspect has been clarified in model organisms like Caenorhabditis elegans, Drosophila and the mouse under controlled environmental conditions (Antebi 2007; Herndon et al. 2002; Kim 2007).
Inflammation plays a major role in aging as it affects an individual’s capacity to counteract noxious events such as pathogen infection and cancer. An acute inflammatory response is beneficial for survival, but a chronic inflammatory state accompanies (and contributes to) age-associated diseases such as neurodegenerative disorders, autoimmunity and cancer (Caruso et al. 2004; Van Bodegom et al. 2007). A reliable marker of inflammation is interleukin-6 (IL-6), the circulating level of which is increased in chronic diseases and aging (Mastorakos and Ilias 2006; Jylhä et al. 2007). A single nucleotide polymorphism (SNP), named rs1800795 (consisting of a G → C transversion in position −174 from the transcription start site) located in the promoter region of the IL-6 gene (NM_000600) reduces IL-6 production at the transcriptional level (Fishman et al. 1998). The presence of the rs1800795 C-allele has been associated with a mild phenotype of inflammation-triggering pathologies (Hefler et al. 2005, Sawczenko et al. 2005). The role of rs1800795 in longevity has been explored in the Italian population with conflicting results (Bonafè et al. 2001; Capurso et al. 2004; Christiansen et al. 2004; Hurme et al. 2005). Increased IL-6 is generally considered a negative modulator of longevity, as it correlates with disability and mortality (Gruenewald et al. 2006; Jylhä et al. 2007).
We decided to measure plasma IL-6 and its modulation by rs1800795 in an elderly population from North-eastern Italy.
Materials and methods
Population recruitment
A comprehensive description of the “The Treviso Longeva” (Trelong) study has been reported elsewhere (Gallucci et al. 2007b). Briefly, starting from a list of about 14,000 Treviso inhabitants over 70 years of age, a population sample was built up, balanced for sex. The recruited sample size was of 668 independent subjects (311 males and 357 females). These enrolled participants were evaluated from the biologic, clinical and socio-economic point of view with a blood sample collection and an extensive structured interview. The study protocol, presenting the inclusion criteria, collecting procedure and questionnaire to be administered to all involved in the project, was submitted to and approved by the ethical committee of the National Institute on Research and Care of the Elderly (INRCA, Italy). This protocol includes an informed, written consent to be obtained from the participant, or from a legally responsible relative in case of mentally impaired subjects, for clinical and genetic studies.
Another cohort of aged people from Northern Italy composed of 245 subjects (119 males and 126 females, age range 70–96) was considered for the genetic analysis only (Milan study). No exclusion criteria other than age was applied. Also for this genetic study, which was approved by the local ethical committee, a written informed consent was obtained from the subject or a legally responsible relative.
Blood sampling and rs1800795 genotyping
Peripheral blood samples (30 mL) were collected by venipuncture; one aliquot was used to separate leukocytes, the other was mixed with sodium EDTA and centrifuged at 2,000 rpm for 10 min at 4°C to isolate the plasma fraction, which was divided into aliquots and stored at −80°C until required. From the enrolled population of 668 subjects, 590 gave their consent to blood collection and 587 plasma samples were successfully prepared.
Genomic DNA was extracted from leukocytes using a semi-automated nucleic acid extractor (AB6100, Applera, New York, NY), checked for quality using a UV-spectrophotometer (Eppendorf, Germany) and stored at 4°C. To assess the rs1800795 genotype, a gDNA aliquot (about 50 ng) was amplified by polymerase chain reaction (PCR) using the following primers: for 5′- ttg tca aga cat gcc aag tgc t-3′, rev 5′- gcc tca gag aca tct cca gtc c-3′. The resulting 190 bp PCR product was digested with 1 U Nla III (New England Biolabs, Hitchin, UK) and loaded on a capillary electrophoresis unit with standard reference markers for instrumental lining-up (Agilent Technologies, Santa Clara, CA). From the 590 blood samples of the Trelong study, 553 were successfully genotyped for rs1800795, while from the available 245 blood samples of the Milan study, 236 were genotyped for rs1800795.
Plasma IL-6 assay
Plasma IL-6 was assayed by a specific sandwich-type enzyme-linked immunosorbent assay (ELISA) (Ultra Sensitive ELISA Kit, BioSource, Camarillo, CA), according to the manufacturer′s instructions. The kit sensitivity was 0.10 pg/mL and intra-assay reproducibility was between 5–10% (%CV) . Each plasma sample was assessed at least in duplicate.
Statistical analysis
Frequency distributions were compared using “Goodness of fit” χ2-test, and means were compared by one-way analysis of variance (ANOVA) followed by Tukey′s or Dunnett′s post-hoc test. The limit of significance for genetic and biochemical analyses was set at α = 0.05. All calculations were done using StatView program ver. 5.0, except for the power calculation, which was performed using G*Power 3.03 (http://www.psycho.uni-duesseldorf.de/aap/projects/gpower/).
Results
Age and sex-related distribution of rs1800795
The demographic and basic clinical data of the population recruited in the Trelong study are summarised in Table 1. The sample was representative of the general Treviso population composition, ranging from 70 to 106 years of age. Males and females were represented equally (male-to-female ratio 0.87). The clinical data included smoking status (smoker/not smoker), the disease count index (DCI) and the Charlson comorbidity index (CCI) (Colinet et al. 2005).
Table 1.
Demographic and clinical data of the Trelong study. BMI Body mass index, DCI disease count index, CCI Charlson comorbidity index, SD standard deviation
| Age bracket (n) | No. of people (male:female) | |
|---|---|---|
| 70–74 (129) | 61:68 | |
| 75–79 (132) | 72:60 | |
| 80–84 (126) | 64:62 | |
| 85–89 (73) | 29:44 | |
| 90–94 (168) | 74:94 | |
| 95–99 (34) | 10:24 | |
| 100 + (6) | 1:5 | |
| Total (668) | 311:357 | |
| Quantitative parameters | Mean ± SD | |
| Age (years) | 84.0 ± 8.0 | |
| Platelets (n ×103 mm−3) | 235.0 ± 75.0 | |
| White cells (n ×103 mm−3) | 6.43 ± 1.70 | |
| Total cholesterol (mg / dL) | 214.0 ± 44.0 | |
| LDL cholesterol (mg / dL) | 136.0 ± 37.0 | |
| HDL cholesterol (mg / dL) | 56.0 ± 15.0 | |
| IgG (mg / dL) | 1,125 ± 412 | |
| BMI (Kg m−2) | 24.8 ± 4.1 | |
| Blood glucose (mg / dL) | 105 ± 33 | |
| DCI | 2.3 ± 1.6 | |
| CCI | 5.8 ± 2.0 | |
| Descriptive parameters | ||
| Smoking status | Yes (278); no (390) | |
The Trelong study also evaluated the class of prescribed medications in the elderly. The most frequent were cardiovascular drugs (36.9% of all prescriptions), followed by gastrointestinal/metabolic drugs (14.8%) and central nervous systems drugs (11.5%), while other prescribed drugs were below 10% (hormones, antibiotics, hematopoietic and anti-inflammatory drugs). We decided to perform our analysis on the population sample divided by 5-year intervals, or by comparing two groups: the 70–85 group and those 85+. This approach lead to a partition of the sample into proportions of two-thirds (under 85) and one-third (over 85). Males and females were represented equally in the 70–85 group, while in the 85+ group the male-to-female ratio was 0.67.
The genotypic distribution of rs1800795 in the entire population respected Hardy-Weinberg equilibrium (data not shown). We assessed the genotypic distribution of rs1800795 in our sample in 5-year groups. No significant difference came to light (data not shown) and the same was true when comparing the 70–85 and the 85+ groups (Table 2). However, when we divided the sample according to sex, we found a significant difference in genotypic distribution between 70–85 and 85+ males (P = 0.006, χ2-test), while the female group did not show any difference (Table 2). In the 85+ males, the homozygous C/C genotype dropped from 10.8% to 2.0%, and the heterozygous genotype increased from 39.2% to 50.0%. No difference was found for allelic frequencies (Table 2). In order to independently confirm this genetic data, we performed a study in another Italian cohort (Milan study). The C/C genotypic frequency in the 70–85 male group was 10.5%, while in the 85+ men it was 6.6%. For females, the C/C genotype frequency was 11.6% in the 70–85 and 12.6% in the 85+ group (Table 2). Considering the Trelong and the Milan study together, we found a significant reduction in the C/C frequency in 85+ males compared to 70–85 men (4.0% vs 10.7%) (Table 2). No difference came to light for females in the aggregated analysis (data not shown).
Table 2.
rs1800795 genotypic and allelic frequencies according to age and sex. M Men, F women
| Age bracket (n) | Genotype count (%) | Allele count (%) | P–value | ||||
|---|---|---|---|---|---|---|---|
| G/G | G/C | C/C | G/C | C/C | |||
| Trelong study | |||||||
| 70–85 (309) | 159 (51.4) | 125(40.5) | 25(8.1) | 443 (71.7) | 175 (28.3) | ||
| M (158) | 79 (50.0) | 62 (39.2) | 17 (10.8) | 220 (69.6) | 96 (30.4) | ||
| F (151) | 80 (53.0) | 63 (41.7) | 8 (5.3) | 223 (73.8) | 79 (26.2) | ||
| 85+ (244) | 118 (48.4) | 114 (46.7) | 12 (4.9) | 350 (71.7) | 138 (28.3) | ||
| M (98) | 47 (48.0) | 49 (50.0) | 2 (2.0)* | 143 (73.0) | 53 (27.0) | P= 0.006* | |
| F (146) | 71 (48.7) | 65 (44.5) | 10 (6.8) | 207 (70.9) | 85 (29.1) | ||
| Milan study | |||||||
| 70–85 (98) | 51 (52.0) | 36 (36.7) | 11 (11.3) | 138 (70.4) | 58 (29.6) | ||
| M (38) | 19 (50.0) | 15 (39.5) | 4 (10.5) | 53 (69.7) | 23 (30.3) | ||
| F (60) | 32 (53.3) | 21 (35.0) | 7 (11.6) | 85 (70.8) | 35 (29.2) | ||
| 85+ (138) | 68 (49.3) | 56 (40.6) | 14 (10.1) | 192 (69.6) | 84 (30.4) | ||
| M (77) | 36 (46.7) | 36 (46.7) | 5 (6.6)* | 108 (70.1) | 46 (29.9) | P = 0.2* | |
| F (61) | 32(52.4) | 21(35.0) | 8 (12.6) | 85 (69.7) | 37 (30.3) | ||
| Total (M) | |||||||
| 70–85 (196) | 98 (50.0) | 77 (39.3) | 21(10.7) | 273 (69.6) | 119 (30.4) | ||
| 85+ (175) | 83 (47.4) | 85 (48.6) | 7(4.0)* | 251 (71.7) | 99 (28.3) | P = 0.04* | |
* vs M 70–85, χ2-test
Plasma IL-6 concentration
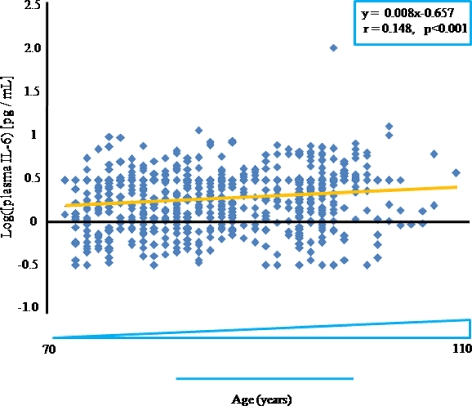
We measured plasma IL-6 concentrations in our sample and plotted the results (log transformed) according to age only. A total of 534 samples were above the lower sensitivity limit of the ELISA kit (0.1 pg/mL). IL-6 concentration increased with age, and simple linear regression showed a significant positive correlation between age and IL-6 level (r = 0.148, P < 0.001, sample size n = 534) (Fig 1).
Fig. 1.
Age-related increase in circulating IL-6. Scatter-plot showing log transformed individual plasma IL-6 levels measured by enzyme-linked immunosorbent assay (ELISA). The superimposed line shows the linear interpolation, the equation and statistical data of which are reported in the box. r Correlation coefficient
To control for confounders, we performed a multivariate analysis including age, sex, body mass index (BMI), smoking status, blood glucose, CCI (Colinet et al. 2005) and rs1800795 as covariates, and IL-6 plasma level (log transformed) as the dependent variable (Lubrano et al. 2005) (Table 3). We confirmed the association with age (P < 0.0001).
Table 3.
Multivariate linear model using log-transformed plasma IL-6 levels as the dependent variable. Total sample size: n = 534, R2 = 0.036
| F | P-value | |
|---|---|---|
| Age | 15.2 | <0.0001 |
| Sex | 1.1 | 0.27 |
| BMI | 0.01 | 1.0 |
| Smoking status | 0.1 | 0.77 |
| Blood glucose | 1.9 | 0.16 |
| CCI | 1.1 | 0.28 |
| rs1800795 genotypea | 1.3 | 0.31 |
aCalculated as C+ vs C−
When we divided the population into 5-year groups, an increase in plasma IL-6 mean level was evident, but this was significant in comparison to 70–75 people only in the extremely long-living subjects (96–100; Table 4). The ultracentenarians had an even higher plasma IL-6 level, but this did not reach significance in comparison to the 70–85 group due to small sample size (n = 5).
Table 4.
Age and sex-related IL-6 plasma concentrations
| Age bracket | Number of subjects | IL-6 (mean±SD) (pg/mL) |
|---|---|---|
| 70–75 | 100 | 1.33 ± 1.90 |
| 76–80 | 116 | 1.39 ± 1.47 |
| 81–85 | 93 | 1.68 ± 2.03 |
| 86–90 | 64 | 1.69 ± 1.82 |
| 91–95 | 128 | 1.95 ± 2.04 |
| 96–100 | 28 | 2.68 ± 3.60* |
| 100+ | 5 | 2.14 ± 2.23 |
| Total | 534 | |
| M | 250 | 1.60 ± 1.71 |
| F | 284 | 1.73 ± 2.24 |
*P < 0.01 vs 70–75, one-way ANOVA followed by Dunnett′s post-hoc test
To verify the sex effect on IL-6 level, we compared IL-6 level by sex over the entire population and found no difference (Table 4). The next step was to split our sample at 85 years of age and assess the sex-specific pattern (Table 5). The 70–85 group had less circulating IL-6 than the 85+ group (about 40% reduction), and this difference was more evident in the female group, where it reached significance (IL-6 mean ± SD: 1.43 ± 2.00 and 2.06 ± 2.43 pg/mL for women under and over 85, respectively). In 85+ males, an increased but not significant IL-6 level change was present in comparison to 70–85 men.
Table 5.
Sex effect on IL-6 concentrations in 85+ group
| Age bracket (n) | Plasma IL-6 (pg/mL) (mean±SD) |
|---|---|
| 70–85(309) | 1.45 ± 1.79 |
| M(161) | 1.47 ± 1.58 |
| F(148) | 1.43 ± 2.00 |
| 85 + (225) | 1.97 ± 2.24* |
| M(89) | 1.83 ± 1.91 |
| F(136) | 2.06 ± 2.43** |
*P < 0.05 vs 70–85; **P < 0.02 vs 70–85 F, one-way ANOVA followed by Tukey′s post-hoc test
Relationship between IL-6 level and rs1800795 genotype
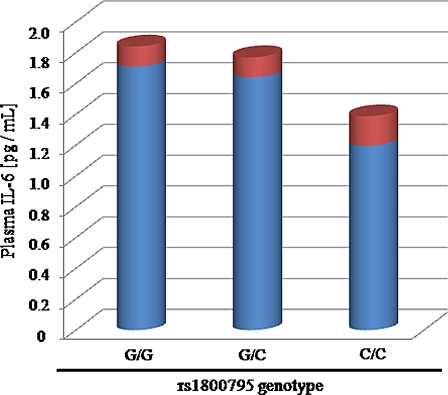
To verify a correlation between rs1800795 genotype and IL-6 level, we stratified the entire sample by rs1800795 genotype. A trend associating IL-6 concentration and rs1800795 genotype was evident, although the differences did not reach statistical significance (IL-6 mean ± SE : G/G 1.70 ± 0.13 pg/mL, n = 268 ; G/C 1.60 ± 0.13 pg/mL, n = 234 ; C/C 1.20 ± 0.19 pg/mL, n = 37 ; P> 0.05, one-way ANOVA) (Fig 2). We also tried to find a sex-specific effect on IL-6 circulating level after stratification of the whole sample by rs1800795 genotype and sex. No significant difference was found either for females or males, probably due to assay variability. In females, the C/C group had the lowest IL-6 levels (female IL-6 mean ± SE: G/G: 1.70 ± 0.17 pg/mL, n = 147; G/C: 1.60 ± 0.20 pg/mL, n = 127; C/C: 0.89 ± 0.20 pg/mL, n = 18). For men, this genotype-specific decrease was less evident (male IL-6 mean ± SE: G/G: 1.70 ± 0.19 pg/mL, n = 121; G/C: 1.60 ± 0.15 pg/mL, n = 111; C/C: 1.50 ± 0.3 pg/mL, n = 19).
Fig. 2.
Effect of rs1800795 genotype on IL-6 circulating level in the Trelong population. Plasma IL-6 was measured by ELISA as described in Materials and methods. Blue columns Mean plasma IL-6 concentration, red standard error. The observed trend did not reach statistical significance
Discussion
Human longevity is a very complex trait, influenced by genetic, sexual, behavioural and environmental factors. We addressed the question of whether the genetic variability due to rs1800795 polymorphism of the IL-6 promoter modulates human longevity, correlating these genetic data with IL-6 circulating levels. IL-6 is considered a reliable marker of mortality and disability, an aspect confirmed also by the Trelong study (Gallucci et al. 2007a). We recruited a sex-balanced and well-characterised group of people from Treviso (Italy) aged from 70 to 106 years (Gallucci et al. 2007b). The sample analysis was focussed on extremely long-living individuals (over 85 years) in comparison to normal living-subjects (between 70 and 85 years).
Genetic data on rs1800795 in the whole sample did not indicate any significant difference in genotypic or allelic frequencies according to age stratification. However, in 85+ males from the Trelong study we detected a very significant decrease in the C/C genotype, in contrast to women. We failed to replicate this data in an independent cohort (Milan study). The Milan study, involving 115 males, had 76% power to detect a difference in rs1800795 genotypic distribution between 70–85 and 85 + men with medium effect size (w = 0.32, calculated from the Trelong study as expected data) so it was not heavily underpowered. However, it is worth noting that also within the Milan study the 85 + males had the lowest C/C frequency (6.6%). In fact, the aggregated data analysis considering the Trelong and the Milan study together gave a significant difference between C/C genotype in 70–85 and 85+ males, even if the robustness of this data was weaker. This sex-specific effect of rs1800795 had already been reported in male centenarians from Central Italy, but in that case the C-allele was increased. A sex effect was also reported for polymorphisms of the IL-10 gene and of other cytokines, thus suggesting that men and women may have genetic differences in genes involved in immunity and inflammatory response (Giacconi et al. 2004; Cederholm et al. 2007; Lio et al. 2002; Barbieri et al. 2004). Our failure in replicating the association between the C-allele and male longevity might depend on the reduced number of centenarians in the Trelong study (n = 6, only one man), but also from regional variation. In fact, others did not find a correlation between the rs1800795 C-allele and longevity in a Sardinian population, thus suggesting a possible genetic variation in Italy from north to centre and south (Capurso et al. 2004; Pes et al. 2004). In females, we found no variation in C-allele frequency with age, but we do not rule out that a comparison with a younger population sample (<70 years) might have been successful in finding some difference.
The second part of our work investigated age- or sex-related modulation of plasma IL-6 concentration and correlation of the latter with rs1800795. We found a positive linear relation between plasma IL-6 and age, confirming that IL-6 increases through life, and in fact a significant difference was present between the 70–85 and the 85+ groups. The correlation between IL-6 and age was clearly confirmed also after controlling for potential confounders (sex, BMI, smoking status, blood glucose, CCI and rs1800795 genotype). Increased plasma IL-6 was present in both men and women, but reached significance only in females, probably due to individual variation combined with the smaller male sample size.
In verifying the correlation between IL-6 plasma level and rs1800795 genotype, we observed a clear trend associating the G/G genotype to a higher level of IL-6, while the C/C carriers had the lowest IL-6 levels. This observation was independent of sex, although C/C females had lower IL-6 than C/C males (IL-6 mean ± SE: 0.89 ± 0.20 pg/mL, n = 18 vs 1.50 ± 0.3 pg/mL, n = 19 for females and males, respectively), suggesting additional sex-specific mechanisms regulating IL-6 production. In short, we found that, in males, the age-related increase of IL-6 is explained, at least in part, by a decrease in the number of rs1800795 C/C carriers, and we did not associate the C-allele to an extended lifespan. For females, rs1800795 affects neither age-related IL-6 concentration nor longevity. The hypothesis that male longevity is more influenced by genetics has been found in the Trelong study for a IGF-1R polymorphism (D.A., personal observation) and is supported by other data showing that female longevity, in contrast, is more influenced by the environment (Franceschi et al. 2000a, 2000b).
Acknowledgements
This study was supported by grants from the Veneto Region, the Treviso Municipality, Treviso Province and Cassamarca Foundation. The support of Monzino Foundation (Milan, Italy) and “Centro Dino Ferrari” (Milan, Italy) is also acknowledged. We are grateful to all patients who kindly agreed to participate in this study and to J.D. Baggott for manuscript editing. L.P. is recipient of a fellowship from “Golgi Cenci Foundation”, Milan, Italy.
Contributor Information
Diego Albani, Phone: +39-02-39014594, FAX: +39-02-3546277, Email: albani@marionegri.it.
Maurizio Gallucci, Email: mgallucci@argeiricerca.it.
References
- Antebi A (2007) Genetics of aging in Caenorhabditis elegans. PLoS Genet 3:1565–1571. doi:10.1371/journal.pgen.0030129 [DOI] [PMC free article] [PubMed]
- Barbieri M, Bonafè M, Rizzo MR, Ragno E, Olivieri F, Marchegiani F, Franceschi C, Paolisso G (2004) Gender specific association of genetic variation in peroxisome proliferator-activated receptor (PPAR) gamma-2 with longevity. Exp Gerontol 39:1095–1100. doi:10.1016/j.exger.2004.03.034 [DOI] [PubMed]
- Bonafè M, Olivieri F, Cavallone L, Giovagnetti S, Mayegiani F, Cardelli M, Pieri C, Marra M, Antonicelli R, Lisa R, Rizzo MR, Paolisso G, Monti D, Franceschi C (2001) A gender-dependent genetic predisposition to produce high levels of IL-6 is detrimental for longevity. Eur J Immunol 31:2357–2361. doi:10.1002/1521-4141(200108) 31:8 < 2357::AID-IMMU2357 > 3.0.CO;2-X [DOI] [PubMed]
- Capurso C, Solfrizzi V, D′Introno A, Colacicco AM, Capurso SA, Semeraro C, Capurso A, Panza F (2004) Interleukin 6-174 G/C promoter gene polymorphism in centenarians: no evidence of association with human longevity or interaction with apolipoprotein E alleles. Exp Gerontol 39:1109–1114. doi:10.1016/j.exger.2004.03.037 [DOI] [PubMed]
- Caruso C, Lio D, Cavallone L, Franceschi C (2004) Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci 1028:1–13. doi:10.1196/annals.1322.001 [DOI] [PubMed]
- Cederholm T, Persson M, Andersson P, Stenvinkel P, Nordfors L, Madden J, Vedin I, Wretlind B, Grimble RF, Palmblad J (2007) Polymorphisms in cytokine genes influence long-term survival differently in elderly male and female patients. J Intern Med 262:215–223. doi:10.1111/j.1365-2796.2007.01803.x [DOI] [PubMed]
- Christiansen L, Bathum L, Andersen-Ranberg K, Jeune B, Christensen K (2004) Modest implication of interleukin-6 promoter polymorphisms in longevity. Mech Ageing Dev 125:391–395. doi:10.1016/j.mad.2004.03.004 [DOI] [PubMed]
- Colinet B, Jacot W, Bertrand D, Lacombe S, Bozonnat MC, Daures JP, Pujol JL (2005) A new simplified comorbidity score as a prognostic factor in non-small-cell lung cancer patients: description and comparison with the Charlson′s index. Br J Cancer 93:1098–1105. doi:10.1038/sj.bjc.6602836 [DOI] [PMC free article] [PubMed]
- Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P (1998) The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest 102:1369–1376. doi:10.1172/JCI2629 [DOI] [PMC free article] [PubMed]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000a) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254 [DOI] [PubMed]
- Franceschi C, Motta L, Valensin S, Rapisarda R, Franzone A, Berardelli M, Motta M, Monti D, Bonafè M, Ferrucci L, Deiana L, Pes GM, Carru C, Desole MS, Barbi C, Sartoni G, Gemelli C, Lescai F, Olivieri F, Marchegiani F, Cardelli M, Cavallone L, Gueresi P, Cossarizza A, Troiano L, Pini G, Sansoni P, Passeri G, Lisa R, Spazzafumo L, Amadio L, Giunta S, Stecconi R, Morresi R, Viticchi C, Mattace R, De Benedictis G, Baggio G (2000b) Do men and women follow different trajectories to reach extreme longevity? Italian Multicenter Study on Centenarians (IMUSCE) Aging (Milano) 12:77–84 [DOI] [PubMed]
- Gallucci M, Amici GP, Ongaro F, Gajo GB, De Angeli S, Forloni GL, Albani D, Prato F, Polito L, Zanardo A, Regini C (2007a) Associations of the plasma interleukin 6 (IL-6) levels with disability and mortality in the elderly in the Treviso Longeva (Trelong) study. Arch Gerontol Geriatr 44:193–198. doi:10.1016/j.archger.2007.01.026 [DOI] [PubMed]
- Gallucci M, Ongaro F, Bresolin F, Bernardi U, Salvato C, Minello A, Amici GP, Barasciutti E, Mazzuco S, Gajo GB, De Angeli S, Forloni GL, Albani D, Zanardo A, Regini C (2007b) The Treviso Longeva (Trelong) study: a biomedical, demographic, economic and social investigation on people 70 years and over in a typical town of North-East of Italy. Arch Gerontol Geriatr 44:173–192. doi:10.1016/j.archger.2007.01.025 [DOI] [PubMed]
- Giacconi R, Cipriano C, Albanese F, Boccoli G, Saba V, Olivieri F, Franceschi C, Mocchegiani E (2004) The -174G/C polymorphism of IL-6 is useful to screen old subjects at risk for atherosclerosis or to reach successful ageing. Exp Gerontol 39:621–628. doi:10.1016/j.exger.2003.12.013 [DOI] [PubMed]
- Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH (2006) Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci USA 103:14158–14163. doi:10.1073/pnas.0606215103 [DOI] [PMC free article] [PubMed]
- Hefler LA, Grimm C, Lantzsch T, Lampe D, Leodolter S, Koelbl H, Heinze G, Reinthaller A, Tong-Cacsire D, Tempfer C, Zeillinger R (2005) Interleukin-1 and interleukin-6 gene polymorphisms and the risk of breast cancer in caucasian women. Clin Cancer Res 11:5718–5721. doi:10.1158/1078-0432.CCR-05-0001 [DOI] [PubMed]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M (2002) Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419:808–814. doi:10.1038/nature01135 [DOI] [PubMed]
- Herskind A, McGue M, Holm N, Sørensen T, Harvald B, Vaupel J (1996) The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870-1900. Hum Genet 97:319–323. doi:10.1007/BF02185763 [DOI] [PubMed]
- Hurme M, Lehtimäki T, Jylhä M, Karhunen PJ, Hervonen A (2005) Interleukin-6-174G/C polymorphism and longevity: a follow-up study. Mech Ageing Dev 126:417–418. doi:10.1016/j.mad.2004.10.001 [DOI] [PubMed]
- Jylhä M, Paavilainen P, Lehtimäki T, Goebeler S, Karhunen PJ, Hervonen A, Hurme M (2007) Interleukin-1 receptor antagonist, interleukin-6, and C-reactive protein as predictors of mortality in nonagenarians: the vitality 90 + study. J Gerontol A Biol Sci Med Sci 62:1016–1021 [DOI] [PubMed]
- Kim SK (2007) Common aging pathways in worms, flies, mice and humans. J Exp Biol 210:1607–1612. doi:10.1242/jeb.004887 [DOI] [PubMed]
- Lio D, Scola L, Crivello A, Colonna-Romano G, Candore G, Bonafè M, Cavallone L, Franceschi C, Caruso C (2002) Gender-specific association between -1082 IL-10 promoter polymorphism and longevity. Genes Immun 3:30–33. doi:10.1038/sj.gene.6363827 [DOI] [PubMed]
- Lubrano V, Cocci F, Battaglia D, Papa A, Marraccini P, Zucchelli GC (2005) Usefulness of high-sensitivity IL-6 measurement for clinical characterization of patients with coronary artery disease. J Clin Lab Anal 19:110–114. doi:10.1002/jcla.20061 [DOI] [PMC free article] [PubMed]
- Mastorakos G, Ilias I (2006) Interleukin-6: a cytokine and/or a major modulator of the response to somatic stress. Ann N Y Acad Sci 1088:373–381. doi:10.1196/annals.1366.021 [DOI] [PubMed]
- Pes GM, Lio D, Carru C, Deiana L, Baggio G, Franceschi C, Ferrucci L, Oliveri F, Scola L, Crivello A, Candore G, Colonna-Romano G, Caruso C (2004) Association between longevity and cytokine gene polymorphisms. A study in Sardinian centenarians. Aging Clin Exp Res 16:244–248 [DOI] [PubMed]
- Sawczenko A, Azooz O, Paraszczuk J, Idestrom M, Croft NM, Savage MO, Ballinger AB, Sanderson IR (2005) Intestinal inflammation-induced growth retardation acts through IL-6 in rats and depends on the -174 IL-6 G/C polymorphism in children. Proc Natl Acad Sci USA 102:13260–13265. doi:10.1073/pnas.0503589102 [DOI] [PMC free article] [PubMed]
- Van Bodegom D, May L, Meij HJ, Westendorp RG (2007) Regulation of human life histories: the role of the inflammatory host response. Ann N Y Acad Sci 1100:84–97. doi:10.1196/annals.1395.007 [DOI] [PubMed]
- vB Hjelmborg J, Iachine I, Skytthe A, Vaupel JW, McGue M, Koskenvuo M, Kaprio J, Pedersen NL, Christensen K (2006) Genetic influence on human lifespan and longevity. Hum Genet 119:312–321 [DOI] [PubMed]