Abstract
AIM: To evaluate the accuracy of endoscopic ultrasound (EUS) in the staging of esophageal cancer.
METHODS: Only EUS studies confirmed by surgery were selected. Articles were searched in Medline and Pubmed. Two reviewers independently searched and extracted data. Meta-analysis of the accuracy of EUS was analyzed by calculating pooled estimates of sensitivity, specificity, likelihood ratios, and diagnostic odds ratio. Pooling was conducted by both the Mantel-Haenszel method (fixed effects model) and DerSimonian Laird method (random effects model). The heterogeneity of studies was tested using Cochran’s Q test based upon inverse variance weights.
RESULTS: Forty-nine studies (n = 2558) which met the inclusion criteria were included in this analysis. Pooled sensitivity and specificity of EUS to diagnose T1 was 81.6% (95% CI: 77.8-84.9) and 99.4% (95% CI: 99.0-99.7), respectively. To diagnose T4, EUS had a pooled sensitivity of 92.4% (95% CI: 89.2-95.0) and specificity of 97.4% (95% CI: 96.6-98.0). With Fine Needle Aspiration (FNA), sensitivity of EUS to diagnose N stage improved from 84.7% (95% CI: 82.9-86.4) to 96.7% (95% CI: 92.4-98.9). The P value for the χ2 test of heterogeneity for all pooled estimates was > 0.10.
CONCLUSION: EUS has excellent sensitivity and specificity in accurately diagnosing the TN stage of esophageal cancer. EUS performs better with advanced (T4) than early (T1) disease. FNA substantially improves the sensitivity and specificity of EUS in evaluating N stage disease. EUS should be strongly considered for staging esophageal cancer.
Keywords: Esophageal cancer, Cancer staging, Endoscopic ultrasound, TNM staging, Diagnostic accuracy
INTRODUCTION
Esophageal cancer is a devastating disease with a significant impact on patients’ lives and health-care systems world-wide. Esophageal cancer affects 1%-2% of people in the United States and up to 15% of people undergoing endoscopy for gastroesophageal reflux disease (GERD)[1]. The incidence of esophageal cancer is increasing in the USA, approximately 20.6% on average annually, despite a decrease in esophageal squamous cell cancer[2,3]. This increase is mostly due to a dramatic rise in esophageal adenocarcinoma, from 1.8 cases per 100 000 during 1987-1992 to 2.5 cases per 100 000 during 1992-1996[4]. From 1973 to 2002, esophageal adenocarcinoma has increased fourfold[5]. The impact of this disease is significant throughout the world due to its increasing incidence and significant mortality (5-year mortality rate > 80%)[6].
Based upon the increasing incidence and devastating consequences of esophageal adenocarcinoma, an increasing amount of resources has been evaluated and implemented in an effort to stage and treat this disease. Based upon the 1996 US national cancer database, the 5-year survival rate for esophageal cancer is as follows: stage 0 (TisN0M0) is 52%, stage I (T1N0M0) is 42%, stage II (T2N0M0 or T3N0M0) or (T1N1M0 or T2N1M0) is 29%, stage III (T3N1MO or T4NxM0) is 15%, and stage IV (TxNxM1) is 3%[7].
Staging of esophageal cancer is extremely important since it helps differentiate treatment options. To improve survival, many treatment modalities have been utilized for esophageal cancer, including surgery, radiotherapy, chemotherapy, and combinations of the aforementioned options[7]. For early disease, recent studies that have investigated endoscopic mucosal resection have shown a 5-year survival of 98%[8] and a low recurrence rate[9]. Although multiple treatment regimens exist and they overlap for each stage, the stage of disease is very important in guiding treatment and predicting outcomes.
Many staging modalities have been utilized for esophageal cancer, including chest CT, MRI, positron emission tomography (PET), and endoscopic ultrasound (EUS). CT and MRI lack the ability to differentiate layers of the esophageal mucosa. Thus, these modalities cannot accurately discern T stage of esophageal cancer. Chest CT provides important information regarding tumor size, lymph node involvement, and potential metastatic lesions. However, chest CT alone has a sensitivity of only 48% for mediastinal lymph node involvement[10]. MRI has been shown to be useful in preoperative evaluation and equally as accurate as CT in staging esophageal cancer; however, studies do vary[11]. MRI staging has been shown to have an accuracy of 40% with very low sensitivity and specificity[12,13]. For mediastinal lymph node involvement, thoracoscopic procedures for tissue biopsy carry a risk of complications in 25%-35% of cases[14,15]. An alternative to CT or MRI is PET. PET is a non-invasive test which has been shown to be beneficial in detection of metastatic disease (stage IV); however, detection of locoregional metastases is limited[13]. Due to limitations of CT, MRI, and PET, other modalities, such as EUS, have been initiated and reviewed.
EUS utilizes an echoendoscope that is passed directly into the esophagus, with the ability to visualize the individual histological layers of the esophagus[16]. This approach is particularly useful in evaluating invasion of local disease, especially esophageal cancer. EUS has been shown to detect more locoregional node involvement than CT or PET, with a higher sensitivity[17,18]. The accuracy of EUS to determine tumor depth has also been estimated to be quite accurate[18–20]. However, studies vary as to the accuracy of EUS in both the depth of local disease, nodal involvement, and the detection of distant metastases[21–24].
With EUS emerging as a very useful staging tool, its role in staging esophageal cancer continues to be addressed. Several studies have identified the potential benefits of EUS with esophageal cancer staging; however, results regarding the extent of its benefits have been inconsistent[52,72,82]. We conducted a meta-analysis to examine the role of EUS in the staging of esophageal cancer for loco-regional spread.
This meta-analysis and systematic review was written in accordance with the proposal for reporting by the QUOROM (Quality of Reporting of Meta-analyses) statement[25]. Since this study investigated diagnostic accuracy of a test, the study design for this meta-analysis and systematic review conformed to the guidelines of the Standards for Reporting of Diagnostic Accuracy (STARD) initiative[26].
MATERIALS AND METHODS
Study selection criteria
Only EUS studies confirmed by surgery or appropriate follow-up were selected. EUS criteria used for T staging were: T1, tumor invades the lamina propria or submucosa but not the muscularis propria; T2, tumor invades but does not extend beyond the muscularis propria; T3, tumor invades the peri-esophageal tissues but not adjacent organs; and T4, tumor invades adjacent structures. Nodal invasion was defined as invasion of mediastinal lymph nodes. From this pool, only studies from which a 2 × 2 table could be constructed for true-positive, false-negative, false-positive and true-negative values were included.
Data collection and extraction
Articles were searched in Medline, Pubmed, Ovid journals, CINAHL, ACP Journal Club, DARE, International Pharmaceutical Abstracts, Old Medline, Medline Non-indexed Citations, OVID Healthstar, and Cochrane Controlled Trials Registry. The search terms used were endoscopic ultrasound, EUS, ultrasound, endosonography, esophageal cancer, esophageal cancer, tumor staging, nodal invasion, staging, surgery, sensitivity, specificity, positive predictive value, and negative predictive value. 2 × 2 tables were constructed with the data extracted from each study. Two authors (SP and JR) independently searched and extracted the data. Any differences were resolved by mutual agreement.
Quality of studies
Clinical trial with a control arm can be assessed for the quality of the study. A number of criteria have been used to assess this quality of a study (e.g. randomization, selection bias of the arms in the study, concealment of allocation, and blinding of outcome)[27,28]. There is no consensus on how to assess studies without a control arm. Hence, these criteria do no apply to studies without a control arm[28]. Therefore, for this meta-analysis and systematic review, studies were selected based on completeness of data and inclusion criteria.
Statistical analysis
Meta-analysis for the accuracy of EUS in diagnosing the etiology of mediastinal lymphadenopathy was performed by calculating pooled estimates of sensitivity, specificity, likelihood ratios, and diagnostic odds ratios. EUS studies were grouped into periods of time to standardize the change in EUS technology and EUS criteria for lymph node involvement[29]. These periods of time were 1986-1994, 1995-1999 and 2000-2006. Pooling was conducted using the Mantel-Haenszel method (fixed effects model) and DerSimonian Laird method (random effects model). The confidence intervals (CIs) were calculated using the F distribution method[30]. Forrest plots were drawn to show the point estimates in each study, in relation to the summary pooled estimate. The width of the point estimates in the Forrest plots indicated the assigned weight for that study. For 0 values, 0.5 was added, as described by Cox[31]. The heterogeneity of the sensitivities and specificities was tested by applying the likelihood ratio test[32]. The heterogeneity of likelihood ratios and diagnostic odds ratios were tested using Cochran’s Q test, based upon inverse variance weights[33]. Heterogeneity among studies was also tested by using summary receiver operating characteristic (SROC) curves. SROC curves were used to calculate the area under the curve (AUC). The effect of publication and selection bias on the summary estimates was tested by the Egger[34] and Begg-Mazumdar[35] bias indicators. Also, funnel plots were constructed to evaluate potential publication bias using the standard error and diagnostic odds ratio[36,37].
RESULTS
An initial search identified 4130 reference articles, of these, 439 relevant articles were selected and reviewed. Forty-nine studies (n = 2558) which met the inclusion criteria were included in this analysis[10,18,20–24,38–40]. For T staging, there were 43 studies[10,18,20–24,39–72]. There were 44 studies for nodal staging[10,18,20–24,38–46,48–50,53,54,56–63,66–80], and of these, 4 used FNA for nodal staging[23,38,76,77]. Figure 1 shows the search results and Table 1 the characteristics for EUS studies included in this meta-analysis. All of the 49 studies included were published as full-text articles in peer-review journals. Not all studies had data for all the stages; we only used data for the available stage of esophageal cancer in a given paper. All the studies included used dedicated EUS machines. The calculated pooled estimates given are estimates calculated by the fixed effect model.
Figure 1.
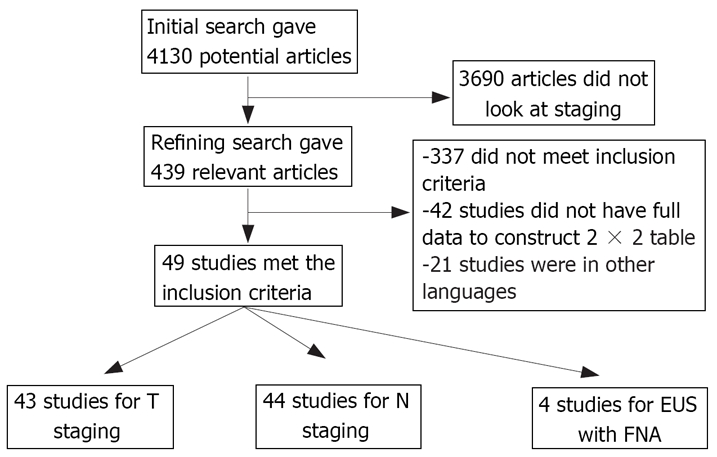
Search results.
Table 1.
Characteristics of studies included in this analysis
| Author | Year of publication | Type of enrolment | Confirmatory test | |
| 1 | Takemoto et al | 1986 | Consecutive | Surgery |
| 2 | Tio et al | 1986 | Prospective | Surgery |
| 3 | Murata et al | 1988 | Consecutive | Surgery |
| 4 | Tio et al | 1989 | Prospective | Surgery |
| 5 | Vilgrain et al | 1990 | Consecutive | Surgery |
| 6 | Botet et al | 1991 | Consecutive | Surgery |
| 7 | Tio et al | 1989 | Prospective | Surgery |
| 8 | Heintz et al | 1991 | Consecutive | Surgery |
| 9 | Rice et al | 1991 | Consecutive | Surgery |
| 10 | Ziegler et al | 1991 | Consecutive | Surgery |
| 11 | Tio et al | 1990 | Consecutive | Surgery |
| 12 | Fok et al | 1992 | Consecutive | Surgery |
| 13 | Rosch et al | 1992 | Consecutive | Surgery |
| 14 | Dittler et al | 1993 | Consecutive | Surgery |
| 15 | Grimm et al | 1993 | Prospective | Surgery |
| 16 | Hordijik et al | 1993 | Consecutive | Surgery |
| 17 | Yoshikane et al | 1993 | Consecutive | Surgery |
| 18 | Catalano et al | 1994 | Consecutive | Surgery |
| 19 | Greenberg et al | 1994 | Prospective | Surgery |
| 20 | Peters et al | 1994 | Consecutive | Surgery |
| 21 | Binmoeller et al | 1995 | Prospective | Surgery |
| 22 | Kallimanis et al | 1995 | Consecutive | Surgery |
| 23 | McLoughlin et al | 1995 | Consecutive | Surgery |
| 24 | Francois et al | 1996 | Consecutive | Surgery |
| 25 | Hasegawa et al | 1996 | Consecutive | Surgery |
| 26 | Holden et al | 1996 | Consecutive | Surgery |
| 27 | Hunerbein et al | 1996 | Consecutive | Surgery |
| 28 | Massari et al | 1996 | Prospective | Surgery |
| 29 | Natsugoe et al | 1996 | Consecutive | Surgery |
| 30 | Vikers et al | 1997 | Consecutive | Surgery |
| 31 | Shimizu et al | 1997 | Consecutive | Surgery |
| 32 | Pham et al | 1998 | Consecutive | Surgery |
| 33 | Vikers et al | 1998 | Prospective | Surgery |
| 34 | Browney et al | 1999 | Prospective | Surgery |
| 35 | Catalano et al | 1999 | Prospective | Surgery |
| 36 | Nishimaki et al | 1999 | Consecutive | Surgery |
| 37 | Salminen et al | 1999 | Consecutive | Surgery |
| 38 | Giovannini et al | 1999 | Prospective | Surgery |
| 39 | Krasna et al | 1999 | Consecutive | Surgery |
| 40 | Heidemann et al | 2000 | Consecutive | Surgery |
| 41 | Nesje et al | 2000 | Prospective | Surgery |
| 42 | Vazquez-Sequeiros et al | 2001 | Consecutive | Surgery |
| 43 | Wiersema et al | 2001 | Prospective | Surgery |
| 44 | Kienle et al | 2002 | Prospective | Surgery |
| 45 | Wakelin et al | 2002 | Consecutive | Surgery |
| 46 | Schwartz et al | 2002 | Consecutive | Surgery |
| 47 | Wu et al | 2003 | Prospective | Surgery |
| 48 | Shimoyama et al | 2004 | Consecutive | Surgery |
| 49 | DeWitt et al | 2005 | Prospective | Surgery |
Accuracy of EUS for T staging
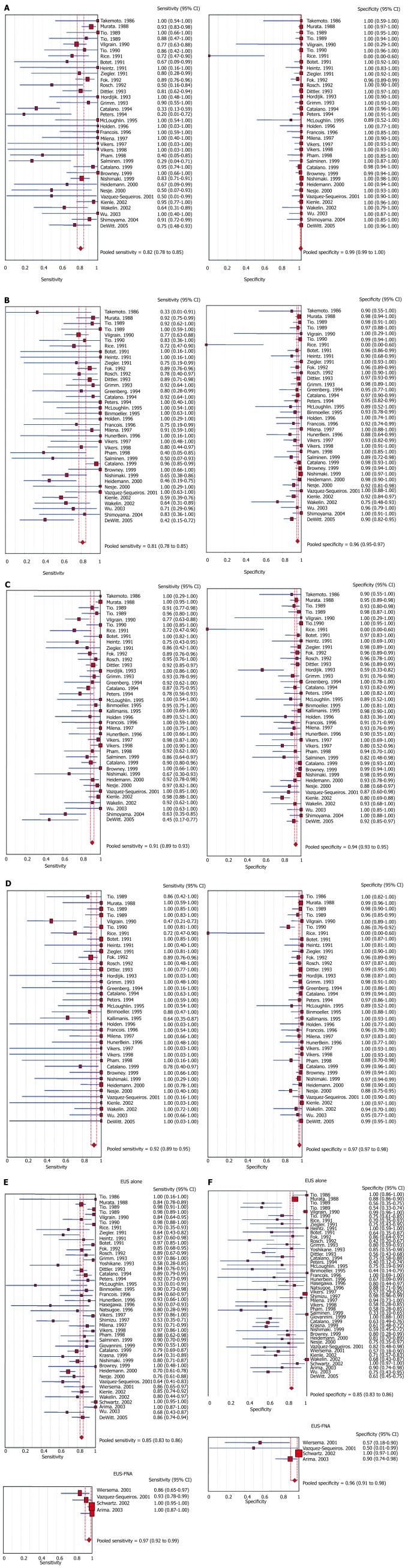
The pooled sensitivity and specificity of EUS to diagnose T1 stage cancer was 81.6% (95% CI: 77.8-84.9) and 99.4% (95% CI: 99.0-99.7), respectively. Figure 2A shows the sensitivity and specificity to diagnose T1 stage cancer in a Forrest plot. For T2 stage, EUS had a pooled sensitivity and specificity of 81.4% (95% CI: 77.5-84.8) and 96.3% (95% CI: 95.4-97.1), respectively. The Forrest plot in Figure 2B shows the sensitivity and specificity of EUS to diagnose T2 stage cancer. For T3 stage, EUS had a pooled sensitivity and specificity of 91.4% (95% CI: 89.5-93.0) and 94.4% (95% CI: 93.1-95.5), respectively. Figure 2C shows the ability of EUS to diagnose stage T3. To diagnose T4 stage cancer, EUS had a pooled sensitivity of 92.4% (95% CI: 89.2-95.0) and specificity of 97.4% (95% CI: 96.6-98.0). The sensitivity and specificity of EUS to diagnose T4 stage cancer from individual studies are shown as a Forrest plot in Figure 2D. A test of heterogeneity for all the pooled estimates for T stages had a P value > 0.10. All the pooled estimates calculated by fixed and random effect models were similar. Table 2 shows the pooled accuracy estimates of EUS for T stage esophageal cancer.
Figure 2.
A: Forrest plot showing sensitivity and specificity of EUS to diagnose T1 stage of esophageal cancer; B: Forrest plot showing sensitivity and specificity of EUS to diagnose T2 stage of esophageal cancer; C: Forrest plot showing sensitivity and specificity of EUS to diagnose T3 stage of esophageal cancer; D: Forrest plot showing sensitivity and specificity of EUS to diagnose T4 stage of esophageal cancer; E: Forrest plot showing sensitivity of EUS alone and EUS with FNA for N staging of esophageal cancer; F: Forrest plot showing specificity of EUS alone and EUS with FNA for N staging of esophageal cancer.
Table 2.
Accuracy of EUS with CIs to diagnose T stage in esophageal cancer
| Pooled sensitivity (%) | Pooled specificity (%) | Pooled LR+ | Pooled LR- | Pooled DOR | |
| T1 | 81.6 (77.8-84.9) | 99.4 (99.0-99.7) | 44.4 (15.5-127.4) | 0.2 (0.2-0.4) | 221.5 (118.5-413.9) |
| T2 | 81.4 (77.5-84.8) | 96.3 (95.4-97.1) | 16.6 (9.3-29.7) | 0.2 (0.2-0.3) | 90.7 (48.3-170.5) |
| T3 | 91.4 (89.5-93.0) | 94.4 (93.1-95.5) | 12.5 (7.7-20.3) | 0.1 (0.1-0.2) | 145.2 (90.3-233.4) |
| T4 | 92.4 (89.2-95.0) | 97.4 (96.6-98.0) | 25.4 (13.7-47.0) | 0.1 (0.1-0.2) | 250.0 (145.2-430.5) |
LR+: Positive likelihood ratio; LR-: Negative likelihood ratio; DOR: Diagnostic odds ratio.
Accuracy of EUS for N staging
With FNA, the sensitivity of EUS to diagnose N stage cancer improved from 84.7% (95% CI: 82.9-86.4) to 96.7% (95% CI: 92.4-98.9). Figure 2E depicts the sensitivity of EUS alone and EUS with FNA in diagnosing N stage cancer. The specificity of EUS improved from 84.6% (95% CI: 83.2-85.9) to 95.5% (95% CI: 91.0-98.2) with FNA. The Forrest plot in Figure 2F shows the specificity of EUS alone and EUS with FNA in diagnosing nodal invasion by esophageal cancer. The accuracy estimates of EUS alone and EUS with FNA are shown in Table 3. All the pooled estimates calculated by fixed and random effect models were similar. The P values for χ2 heterogeneity for all the pooled accuracy estimates were > 0.10.
Table 3.
Pooled estimate of accuracy of EUS alone and EUS-FNA in nodal staging of esophageal cancer with 95% CIs
| EUS | EUS-FNA | |
| Studies | 44 | 4 |
| Pooled sensitivity (%) | 84.7 (82.9-86.4) | 96.7 (92.4-98.9) |
| Pooled specificity (%) | 84.6 (83.2-85.9) | 95.5 (91.0-98.2) |
| Positive likelihood ratio | 3.3 (2.6-4.3) | 7.3 (0.9-54.3) |
| Negative likelihood ratio | 0.24 (0.9-0.3) | 0.05 (0.01-0.64) |
| Diagnostic odds ratio | 19.1 (12.7-28.5) | 164.5 (4.5-6027.7) |
Effect of technology
EUS studies were grouped into three periods of time to standardize the change in EUS technology and the change in EUS criteria for tumor staging. These periods were 1986-1994, 1995-1999 and 2000-2006. The pooled estimates of studies during these periods of time are shown in Table 4. The P value for χ2 heterogeneity for all the pooled accuracy estimates was > 0.10.
Table 4.
Accuracy of EUS with CIs to stage esophageal cancer over the past two decades
| Year | No. of studies | Pooled sensitivity (%) | Pooled specificity (%) | Pooled LR+ | Pooled LR- | Pooled DOR | |
| T1 | 1986-1944 | 17 | 80.4 (75.2-84.8) | 99.2 (98.4-99.7) | 41.5 (6.1-283.3) | 0.25 (0.14-0.43) | 181.9 (60.7-545.7) |
| 1995-1999 | 11 | 83.9 (76.0-90.0) | 99.4 (98.4-99.8) | 36.4 (18.5-71.6) | 0.21 (0.09-0.47) | 299.9 (107.8-834.1) | |
| 2000-2006 | 8 | 82.4 (72.6-89.8) | 100.0 (99.1-100.0) | 59.5 (22.0-161.1) | 0.27 (0.16-0.47) | 261.2 (81.4-838.0) | |
| T2 | 1986-1994 | 17 | 85.2 (80.2-89.4) | 96.8 (95.5-97.8) | 18.6 (5.9-58.6) | 0.19 (0.12-0.30) | 123.9 (47.7-322.0) |
| 1995-1999 | 13 | 86.8 (79.7-92.1) | 97.4 (95.8-98.5) | 16.9 (9.1-31.1) | 0.20 (0.11-0.38) | 139.5 (56.6-343.8) | |
| 2000-2006 | 8 | 62.9 (52.0-72.9) | 93.4 (90.4-95.6) | 8.3 (4.3-15.9) | 0.47 (0.34-0.64) | 24.7 (9.1-67.4) | |
| T3 | 1986-1994 | 18 | 90.8 (88.1-93.0) | 94.6 (92.6-96.2) | 13.9 (5.2-36.9) | 0.12 (0.07-0.19) | 157.7 (70.9-351.1) |
| 1995-1999 | 14 | 93.7 (90.0-96.3) | 96.4 (94.5-97.7) | 12.6 (7.6-20.9) | 0.11 (0.08-0.17) | 159.4 (77.9-326.2) | |
| 2000-2006 | 8 | 89.9 (84.5-93.9) | 90.0 (86.1-93.2) | 7.0 (4.6-10.8) | 0.11 (0.04-0.32) | 100.9 (33.5-303.9) | |
| T4 | 1986-1994 | 18 | 92.1 (87.9-95.2) | 96.9 (95.6-97.9) | 24.7 (8.4-72.7) | 0.09 (0.04-0.23) | 278.8 (97.2-799.9) |
| 1995-1999 | 14 | 89.2 (79.8-95.2) | 98.0 (96.7-98.96) | 22.2 (13.2-37.3) | 0.23 (0.15-0.36) | 227.1 (89.7-575.0) | |
| 2000-2006 | 8 | 100.0 (91.8-100.0) | 97.5 (95.4-98.8) | 20.2 (8.8-46.3) | 0.11 (0.04-0.29) | 272.6 (73.4-1013.2) | |
| N | 1986-1994 | 17 | 88.0 (85.4-90.2) | 85.2 (83.4-86.9) | 3.6 (2.4-5.4) | 0.2 (0.1-0.3) | 27.6 (14.6-52.4) |
| 1995-1999 | 17 | 82.6 (78.0-85.9) | 84.4 (81.6-86.9) | 3.0 (2.1-4.5) | 0.3 (0.2-0.4) | 14.8 (7.5-29.3) | |
| 2000-2005 | 10 | 81.6 (77.8-85.1) | 82.4 (78.2-86.1) | 3.4 (2.2-5.3) | 0.3 (0.2-0.4) | 14.9 (6.7-33.1) |
Bias estimates
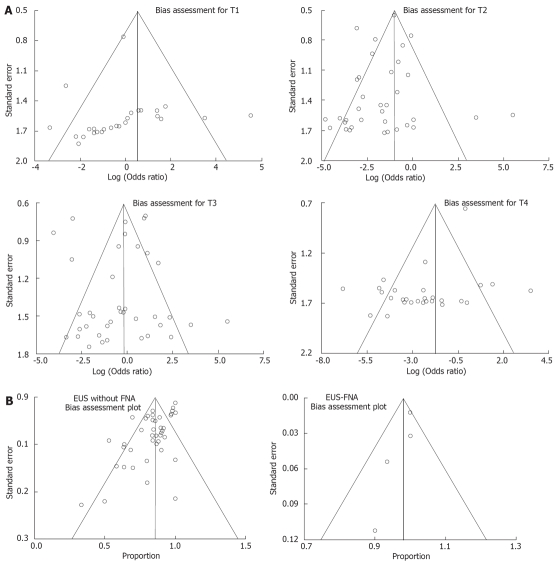
The publication bias calculated by the Begg-Mazumdar and Egger bias indicators for each stage of esophageal cancer invasion is shown in Table 5. The funnel plots to investigate the effect of publication bias on T stage is shown in Figure 3A. The effect of publication bias on N stage is shown in Figure 3B.
Table 5.
Bias indicators and AUC with the corresponding Q values for various cancer stages
| Begg-Mazumdar bias (Kendall's tau value, P) | Egger bias (95% CI, P) | AUC (SE) | Q (SE) | |
| T1 | -0.51, P = 0.01 | -0.48 (95% CI = -2.84 to 1.88, P = 0.68) | 0.97 (0.02) | 0.91 (0.02) |
| T2 | -0.14, P = 0.24 | -0.32 (95% CI = -1.74 to 1.10, P = 0.65) | 0.95 (0.02) | 0.89 (0.02) |
| T3 | -0.11, P = 0.32 | 0.33 (95% CI = -1.43 to 2.09, P = 0.70) | 0.97 (0.01) | 0.92 (0.01) |
| T4 | -0.07, P = 0.56 | -2.89 (95% CI = -5.35 to -0.44, P = 0.02) | 0.98 (0.01) | 0.93 (0.01) |
| N | -0.26, P = 0.01 | 0.29 (95% CI = -1.58 to 1.00, P = 0.69) | 0.91 (0.02) | 0.99 (0.02) |
Figure 3.
A: Funnel plots assessing bias for T staging; B: Funnel plots assessing bias for N staging.
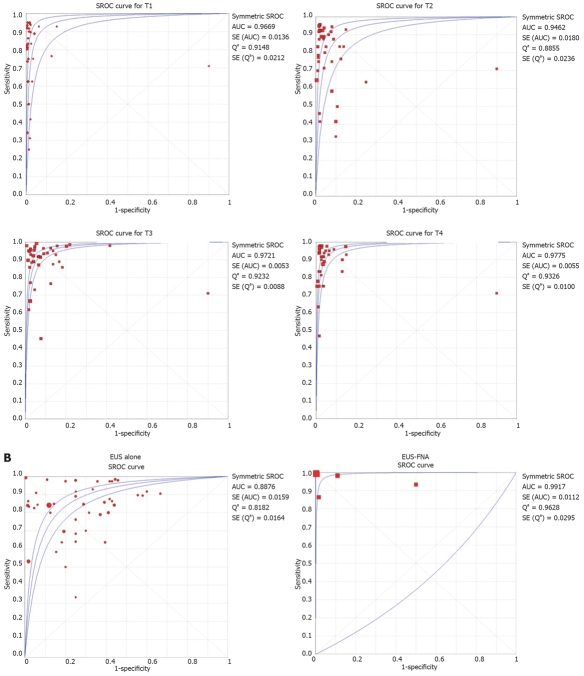
SROC curves were drawn for AUC and Q values. The AUC and Q values of EUS to diagnose various stages of esophageal cancer are shown in Table 5. SROC curves for T and N staging are shown in Figure 4A and B, respectively.
Figure 4.
A: SROC curves for various T stages of esophageal cancer; B: SROC curves for various N stages of esophageal cancer.
A subgroup analysis was performed by removing the studies in which the last or the first author was the same (e.g. Tio et al). This was done to make sure that the same data were not used by the studies, i.e. to avoid duplication. In the subgroup analysis, there was no significant change in the pooled estimates. Separate accuracy estimated for radial versus linear EUS technology could not be performed as the majority of the studies did not make a distinction or give separate accuracy values for radial or linear EUS technology.
DISCUSSION
This meta-analysis and systematic review shows that the pooled sensitivity of EUS for tumor invasion (T stage) is high (about 81%-90%), with it being higher for advanced disease than early disease. For all the T stages, the pooled specificity of EUS to diagnose depth of tumor invasion is very high (about 99%). Diagnostic odds ratio is defined as the odds of having a positive test in patients with a true anatomic stage of the disease when compared to patients who do not have the disease. EUS as a diagnostic test has a very high diagnostic odds ratio for T staging (about 250 times). For example, if EUS demonstrates that a patient has T1 stage disease, the odds of having the correct anatomic stage of T1 disease is 221 to 1. This helps physicians offer endoscopic treatment with confidence to patients with early disease[81–87]. Another way of looking at this is: if a small lesion is found to be esophageal cancer, then EUS is an excellent diagnostic test to examine the depth of tumor invasion, because of its very high sensitivity and specificity. The depth of tumor invasion can help decide if curative surgical or curative endoscopic mucosal resection or submucosal dissection can be offered to resect the lesion en bloc[81–87].
The positive likelihood ratio of a test is a gauge of how well it identifies a disease state. The higher the positive likelihood ratio, the better the test performs in identifying the true disease status. On the other hand, a negative likelihood ratio is a gauge of how well the test performs in excluding a disease state. The lower the negative likelihood ratio, the better the test performs in excluding a disease. For T staging, EUS has a high positive likelihood ratio for all T stages and a low negative likelihood ratio for T4 disease when compared to T1 disease. This indicates that EUS performs better in excluding T4 than T1 disease. Clinically, another viewpoint is: if EUS diagnoses T2 disease then the patient might still have anatomic T1 disease, but if EUS diagnoses T1 disease then the patient probably truly has anatomic T1 disease. This helps physicians offer surgical or endoscopic treatments with confidence if EUS diagnoses a patient with T1 esophageal cancer[81–87].
The major advantage of EUS is the ability to perform FNA during the procedure for tissue diagnosis. The procedure is, in comparison with other alternative options, safe, less invasive, and does not require general anesthesia or hospitalization[88]. The complication rate is extremely low (0.5%-2.3%), with several studies reporting no complications[75,76,88,89]. Other modalities using FNA, such as transbronchial CT or thoracoscopic procedures, cannot be used for the entire mediastinum[14,15,92–101]. EUS has the ability to image the aortopulmonary window, the subcarinal nodes, inferior mediastinum, and the entire posterior part of the mediastinum.
EUS as an imaging modality has high sensitivity and specificity to diagnose N stage esophageal cancer. This meta-analysis shows that FNA substantially improves the sensitivity (85% to 97%) and specificity (85% to 96%) of EUS in evaluating N stage esophageal cancer, therefore, EUS with FNA should be the diagnostic test of choice.
Over the last two decades, the specificity of EUS to diagnose T stage cancer has remained high. In addition, the sensitivity of EUS for T staging has improved, especially for early disease (T1), over the past two decades, which may represent improvement in imaging technology or training. For nodal staging, all the studies in which FNA was performed were from the most recent periods. The sensitivity and specificity of EUS alone to diagnose N stage cancer has not improved in the past two decades. Our meta-analysis demonstrates that the sensitivity and specificity of EUS markedly improved with FNA.
EUS as a diagnostic tool is not designed to detect distant metastasis, so this was not evaluated in this analysis.
Heterogeneity among different studies was determined by drawing SROC curves and finding the AUC, since different studies might use slightly different criteria for staging. An AUC of 1 for any test indicates that the test is excellent. SROC curves for EUS showed that AUC was very close to 1, which indicates that EUS is an excellent diagnostic test for staging esophageal cancer.
Studies with statistically significant results tend to be published and cited. Smaller studies may show larger treatment effects due to fewer case-mix differences (e.g. patients with only early or late disease) than larger trials. This can be estimated by bias indicators and construction of funnel plots. This publication and selection bias may affect the summary estimates. Also, bias among studies can affect the shape of the funnel plot. In this meta-analysis and systematic review, bias calculations using the Egger[35] and Begg-Mazumdar[36] bias indicators showed no statistically significant bias. Furthermore, funnel plot analyses showed no significant bias for EUS studies.
In conclusion, EUS has excellent sensitivity and specificity in accurately diagnosing T stage esophageal cancer. EUS performs better with advanced (T4) than early (T1) disease. FNA substantially improves the sensitivity and specificity of EUS in evaluating N stage esophageal cancers. EUS should be the test of choice for TN staging of esophageal cancer.
COMMENTS
Background
Prognosis and modality of treatment in patients with esophageal cancer depends on the staging of the tumor. The published data on the accuracy of endoscopic ultrasound (EUS) for staging esophageal cancer is varied. The aim of this meta-analysis and systematic review was to evaluate the accuracy of EUS in staging esophageal cancer.
Research frontiers
To date, there have been many studies on EUS in staging esophageal cancer, but no meta-analyses.
Innovations and breakthroughs
With EUS emerging as a very useful staging tool, its role in esophageal cancer continues to be addressed. Several studies have identified the potential benefits of EUS for esophageal cancer staging; however, results regarding the extent of its benefits have been inconsistent.
Applications
EUS has excellent sensitivity and specificity in accurately diagnosing TN stage of esophageal cancer. EUS performs better with advanced disease (T4) than early disease (T1). FNA substantially improves the sensitivity and specificity of EUS in evaluating N stage cancer. EUS should be strongly considered for staging esophageal cancer.
Terminology
EUS utilizes an echoendoscope which is passed directly into the esophagus, with the ability to visualize the individual histological layers of the esophagus. This approach is particularly useful in evaluating invasion of local disease, especially in esophageal cancer.
Peer review
This manuscript is well designed and prepared study finding an important conclusions. The most important point is that the real time PCR is significantly cheaper than the other commercial test.
Peer reviewer: Aydin Karabacakoglu, Dr, Assistant Professor, Department of Radiology, Meram Medical Faculty, Selcuk University, Konya 42080, Turkey
S- Editor Liu Y L- Editor Kerr C E- Editor Yin DH
References
- 1.Cossentino MJ, Wong RK. Barrett's esophagus and risk of esophageal adenocarcinoma. Semin Gastrointest Dis. 2003;14:128–135. [PubMed] [Google Scholar]
- 2.Bollschweiler E, Wolfgarten E, Gutschow C, Holscher AH. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92:549–555. doi: 10.1002/1097-0142(20010801)92:3<549::aid-cncr1354>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Blot WJ, McLaughlin JK. The changing epidemiology of esophageal cancer. Semin Oncol. 1999;26:2–8. [PubMed] [Google Scholar]
- 4.El-Serag HB, Mason AC, Petersen N, Key CR. Epidemiological differences between adenocarcinoma of the oesophagus and adenocarcinoma of the gastric cardia in the USA. Gut. 2002;50:368–372. doi: 10.1136/gut.50.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald RC. Review article: Barrett’s oesophagus and associated adenocarcinoma--a UK perspective. Aliment Pharmacol Ther. 2004;20 Suppl 8:45–49. doi: 10.1111/j.1365-2036.2004.02229.x. [DOI] [PubMed] [Google Scholar]
- 7.Daly JM, Karnell LH, Menck HR. National Cancer Data Base report on esophageal carcinoma. Cancer. 1996;78:1820–1828. doi: 10.1002/(sici)1097-0142(19961015)78:8<1820::aid-cncr25>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Ell C, May A, Pech O, Gossner L, Guenter E, Behrens A, Nachbar L, Huijsmans J, Vieth M, Stolte M. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer) Gastrointest Endosc. 2007;65:3–10. doi: 10.1016/j.gie.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 9.Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc. 2005;61:219–225. doi: 10.1016/s0016-5107(04)02756-7. [DOI] [PubMed] [Google Scholar]
- 10.Vickers J. Role of endoscopic ultrasound in the preoperative assessment of patients with oesophageal cancer. Ann R Coll Surg Engl. 1998;80:233–239. [PMC free article] [PubMed] [Google Scholar]
- 11.Takashima S, Takeuchi N, Shiozaki H, Kobayashi K, Morimoto S, Ikezoe J, Tomiyama N, Harada K, Shogen K, Kozuka T. Carcinoma of the esophagus: CT vs MR imaging in determining resectability. AJR Am J Roentgenol. 1991;156:297–302. doi: 10.2214/ajr.156.2.1898802. [DOI] [PubMed] [Google Scholar]
- 12.Quint LE, Glazer GM, Orringer MB. Esophageal imaging by MR and CT: study of normal anatomy and neoplasms. Radiology. 1985;156:727–731. doi: 10.1148/radiology.156.3.4023234. [DOI] [PubMed] [Google Scholar]
- 13.Lehr L, Rupp N, Siewert JR. Assessment of resectability of esophageal cancer by computed tomography and magnetic resonance imaging. Surgery. 1988;103:344–350. [PubMed] [Google Scholar]
- 14.Salazar AM, Westcott JL. The role of transthoracic needle biopsy for the diagnosis and staging of lung cancer. Clin Chest Med. 1993;14:99–110. [PubMed] [Google Scholar]
- 15.Gardner D, vanSonnenberg E, Dàgostino HB, et al. CT-guided transthoracic needle biopsy. Cardiovasc Intervent Radiol. 1991;14:17–23. doi: 10.1007/BF02635526. [DOI] [PubMed] [Google Scholar]
- 16.Wiersema MJ, Wiersema LM. High-resolution 25-megahertz ultrasonography of the gastrointestinal wall: histologic correlates. Gastrointest Endosc. 1993;39:499–504. doi: 10.1016/s0016-5107(93)70159-5. [DOI] [PubMed] [Google Scholar]
- 17.Pfau PR, Perlman SB, Stanko P, Frick TJ, Gopal DV, Said A, Zhang Z, Weigel T. The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest Endosc. 2007;65:377–384. doi: 10.1016/j.gie.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Grimm H, Binmoeller KF, Hamper K, Koch J, Henne-Bruns D, Soehendra N. Endosonography for preoperative locoregional staging of esophageal and gastric cancer. Endoscopy. 1993;25:224–230. doi: 10.1055/s-2007-1010297. [DOI] [PubMed] [Google Scholar]
- 19.Familiari P, Marchese M, Larghi A, Spada C, Costamagna G. Staging of esophageal carcinoma: endoscopic ultrasonography. Rays. 2005;30:357–362. [PubMed] [Google Scholar]
- 20.Rosch T, Lorenz R, Zenker K, von Wichert A, Dancygier H, Hofler H, Siewert JR, Classen M. Local staging and assessment of resectability in carcinoma of the esophagus, stomach, and duodenum by endoscopic ultrasonography. Gastrointest Endosc. 1992;38:460–467. doi: 10.1016/s0016-5107(92)70477-5. [DOI] [PubMed] [Google Scholar]
- 21.DeWitt J, Kesler K, Brooks JA, LeBlanc J, McHenry L, McGreevy K, Sherman S. Endoscopic ultrasound for esophageal and gastroesophageal junction cancer: Impact of increased use of primary neoadjuvant therapy on preoperative locoregional staging accuracy. Dis Esophagus. 2005;18:21–27. doi: 10.1111/j.1442-2050.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 22.Nesje LB, Svanes K, Viste A, Laerum OD, Odegaard S. Comparison of a linear miniature ultrasound probe and a radial-scanning echoendoscope in TN staging of esophageal cancer. Scand J Gastroenterol. 2000;35:997–1002. doi: 10.1080/003655200750023101. [DOI] [PubMed] [Google Scholar]
- 23.Vazquez-Sequeiros E, Norton ID, Clain JE, Wang KK, Affi A, Allen M, Deschamps C, Miller D, Salomao D, Wiersema MJ. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc. 2001;53:751–757. doi: 10.1067/mge.2001.112741. [DOI] [PubMed] [Google Scholar]
- 24.Wakelin SJ, Deans C, Crofts TJ, Allan PL, Plevris JN, Paterson-Brown S. A comparison of computerised tomography, laparoscopic ultrasound and endoscopic ultrasound in the preoperative staging of oesophago-gastric carcinoma. Eur J Radiol. 2002;41:161–167. doi: 10.1016/s0720-048x(01)00418-1. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 26.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. The Standards for Reporting of Diagnostic Accuracy Group. Croat Med J. 2003;44:635–638. [PubMed] [Google Scholar]
- 27.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 28.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 29.Puli SR, Singh S, Hagedorn CH, Reddy J, Olyaee M. Diagnostic accuracy of EUS for vascular invasion in pancreatic and periampullary cancers: a meta-analysis and systematic review. Gastrointest Endosc. 2007;65:788–797. doi: 10.1016/j.gie.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 30.Leemis LM, Trivedi KS. A Comparison of Approximate Interval Estimators for the Bernoulli Parameter. Am Stat. 1996;50:63–68. [Google Scholar]
- 31.Cox DR. The analysis of binary data. Vol. 50. Methuen: London; 1970. [Google Scholar]
- 32.Agresti A. Analysis of ordinal categorical data. Vol. 50. John Wileys & Sons: New York; 1984. [Google Scholar]
- 33.Deeks JJ. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323:157–162. doi: 10.1136/bmj.323.7305.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 35.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 36.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 38.Arima M, Tada M. Endoscopic ultrasound-guided fine needle aspiration biopsy in esophageal and mediastinal diseases: clinical indications and results. Digestive Endoscopy. 2003;15:93–99. [Google Scholar]
- 39.Binmoeller KF, Seifert H, Seitz U, Izbicki JR, Kida M, Soehendra N. Ultrasonic esophagoprobe for TNM staging of highly stenosing esophageal carcinoma. Gastrointest Endosc. 1995;41:547–552. doi: 10.1016/s0016-5107(95)70188-5. [DOI] [PubMed] [Google Scholar]
- 40.Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Urmacher C, Brennan MF. Preoperative staging of esophageal cancer: comparison of endoscopic US and dynamic CT. Radiology. 1991;181:419–425. doi: 10.1148/radiology.181.2.1924783. [DOI] [PubMed] [Google Scholar]
- 41.Bowrey DJ, Clark GW, Roberts SA, Maughan TS, Hawthorne AB, Williams GT, Carey PD. Endosonographic staging of 100 consecutive patients with esophageal carcinoma: introduction of the 8-mm esophagoprobe. Dis Esophagus. 1999;12:258–263. doi: 10.1046/j.1442-2050.1999.00071.x. [DOI] [PubMed] [Google Scholar]
- 42.Catalano MF, Alcocer E, Chak A, Nguyen CC, Raijman I, Geenen JE, Lahoti S, Sivak MV Jr. Evaluation of metastatic celiac axis lymph nodes in patients with esophageal carcinoma: accuracy of EUS. Gastrointest Endosc. 1999;50:352–356. doi: 10.1053/ge.1999.v50.98154. [DOI] [PubMed] [Google Scholar]
- 43.Catalano MF, Sivak MV Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442–446. doi: 10.1016/s0016-5107(94)70206-3. [DOI] [PubMed] [Google Scholar]
- 44.Dittler HJ, Siewert JR. Role of endoscopic ultrasonography in esophageal carcinoma. Endoscopy. 1993;25:156–161. doi: 10.1055/s-2007-1010275. [DOI] [PubMed] [Google Scholar]
- 45.Fok M, Cheng SW, Wong J. Endosonography in patient selection for surgical treatment of esophageal carcinoma. World J Surg. 1992;16:1098–103; discussion 103. doi: 10.1007/BF02067067. [DOI] [PubMed] [Google Scholar]
- 46.Francois E, Peroux J, Mouroux J, Chazalle M, Hastier P, Ferrero J, Simon J, Bourry J. Preoperative endosonographic staging of cancer of the cardia. Abdom Imaging. 1996;21:483–487. doi: 10.1007/s002619900109. [DOI] [PubMed] [Google Scholar]
- 47.Greenberg J, Durkin M, Van Drunen M, Aranha GV. Computed tomography or endoscopic ultrasonography in preoperative staging of gastric and esophageal tumors. Surgery. 1994;116:696–701; discussion 701-702. [PubMed] [Google Scholar]
- 48.Hasegawa N, Niwa Y, Arisawa T, Hase S, Goto H, Hayakawa T. Preoperative staging of superficial esophageal carcinoma: comparison of an ultrasound probe and standard endoscopic ultrasonography. Gastrointest Endosc. 1996;44:388–393. doi: 10.1016/s0016-5107(96)70086-x. [DOI] [PubMed] [Google Scholar]
- 49.Heidemann J, Schilling MK, Schmassmann A, Maurer CA, Buchler MW. Accuracy of endoscopic ultrasonography in preoperative staging of esophageal carcinoma. Dig Surg. 2000;17:219–224. doi: 10.1159/000018838. [DOI] [PubMed] [Google Scholar]
- 50.Heintz A, Hohne U, Schweden F, Junginger T. Preoperative detection of intrathoracic tumor spread of esophageal cancer: endosonography versus computed tomography. Surg Endosc. 1991;5:75–78. doi: 10.1007/BF00316841. [DOI] [PubMed] [Google Scholar]
- 51.Holden A, Mendelson R, Edmunds S. Pre-operative staging of gastro-oesophageal junction carcinoma: comparison of endoscopic ultrasound and computed tomography. Australas Radiol. 1996;40:206–212. doi: 10.1111/j.1440-1673.1996.tb00386.x. [DOI] [PubMed] [Google Scholar]
- 52.Hordijk ML, Zander H, van Blankenstein M, Tilanus HW. Influence of tumor stenosis on the accuracy of endosonography in preoperative T staging of esophageal cancer. Endoscopy. 1993;25:171–175. doi: 10.1055/s-2007-1010278. [DOI] [PubMed] [Google Scholar]
- 53.Hunerbein M, Dohmoto M, Rau B, Schlag PM. Endosono-graphy and endosonography-guided biopsy of upper-GI-tract tumors using a curved-array echoendoscope. Surg Endosc. 1996;10:1205–1209. doi: 10.1007/s004649900280. [DOI] [PubMed] [Google Scholar]
- 54.Massari M, Cioffi U, De Simone M, Lattuada E, Montorsi M, Segalin A, Bonavina L. Endoscopic ultrasonography for preoperative staging of esophageal carcinoma. Surg Laparosc Endosc. 1997;7:162–165. [PubMed] [Google Scholar]
- 55.Kallimanis GE, Gupta PK, al-Kawas FH, Tio LT, Benjamin SB, Bertagnolli ME, Nguyen CC, Gomes MN, Fleischer DE. Endoscopic ultrasound for staging esophageal cancer, with or without dilation, is clinically important and safe. Gastrointest Endosc. 1995;41:540–546. doi: 10.1016/s0016-5107(95)70187-7. [DOI] [PubMed] [Google Scholar]
- 56.Kienle P, Buhl K, Kuntz C, Dux M, Hartmann C, Axel B, Herfarth C, Lehnert T. Prospective comparison of endoscopy, endosonography and computed tomography for staging of tumours of the oesophagus and gastric cardia. Digestion. 2002;66:230–236. doi: 10.1159/000068360. [DOI] [PubMed] [Google Scholar]
- 57.McLoughlin RF, Cooperberg PL, Mathieson JR, Stordy SN, Halparin LS. High resolution endoluminal ultrasonography in the staging of esophageal carcinoma. J Ultrasound Med. 1995;14:725–730. doi: 10.7863/jum.1995.14.10.725. [DOI] [PubMed] [Google Scholar]
- 58.Murata Y, Suzuki S, Hashimoto H. Endoscopic ultrasono-graphy of the upper gastrointestinal tract. Surg Endosc. 1988;2:180–183. doi: 10.1007/BF02498796. [DOI] [PubMed] [Google Scholar]
- 59.Nishimaki T, Tanaka O, Ando N, Ide H, Watanabe H, Shinoda M, Takiyama W, Yamana H, Ishida K, Isono K, et al. Evaluation of the accuracy of preoperative staging in thoracic esophageal cancer. Ann Thorac Surg. 1999;68:2059–2064. doi: 10.1016/s0003-4975(99)01171-6. [DOI] [PubMed] [Google Scholar]
- 60.Peters JH, Hoeft SF, Heimbucher J, Bremner RM, DeMeester TR, Bremner CG, Clark GW, Kiyabu M, Parisky Y. Selection of patients for curative or palliative resection of esophageal cancer based on preoperative endoscopic ultrasonography. Arch Surg. 1994;129:534–539. doi: 10.1001/archsurg.1994.01420290080012. [DOI] [PubMed] [Google Scholar]
- 61.Pham T, Roach E, Falk GL, Chu J, Ngu MC, Jones DB. Staging of oesophageal carcinoma by endoscopic ultrasound: preliminary experience. Aust N Z J Surg. 1998;68:209–212. doi: 10.1111/j.1445-2197.1998.tb04748.x. [DOI] [PubMed] [Google Scholar]
- 62.Rice TW, Boyce GA, Sivak MV. Esophageal ultrasound and the preoperative staging of carcinoma of the esophagus. J Thorac Cardiovasc Surg. 1991;101:536–543; discussion 543-544. [PubMed] [Google Scholar]
- 63.Salminen JT, Farkkila MA, Ramo OJ, Toikkanen V, Simpanen J, Nuutinen H, Salo JA. Endoscopic ultrasonography in the preoperative staging of adenocarcinoma of the distal oesophagus and oesophagogastric junction. Scand J Gastroenterol. 1999;34:1178–1182. doi: 10.1080/003655299750024670. [DOI] [PubMed] [Google Scholar]
- 64.Shimoyama S, Yasuda H, Hashimoto M, Tatsutomi Y, Aoki F, Mafune K, Kaminishi M. Accuracy of linear-array EUS for preoperative staging of gastric cardia cancer. Gastrointest Endosc. 2004;60:50–55. doi: 10.1016/s0016-5107(04)01312-4. [DOI] [PubMed] [Google Scholar]
- 65.Takemoto T, Ito T, Aibe T, Okita K. Endoscopic ultrasono-graphy in the diagnosis of esophageal carcinoma, with particular regard to staging it for operability. Endoscopy. 1986;18 Suppl 3:22–25. doi: 10.1055/s-2007-1018437. [DOI] [PubMed] [Google Scholar]
- 66.Tio TL, Coene PP, den Hartog Jager FC, Tytgat GN. Preoperative TNM classification of esophageal carcinoma by endosonography. Hepatogastroenterology. 1990;37:376–381. [PubMed] [Google Scholar]
- 67.Tio TL, Coene PP, Schouwink MH, Tytgat GN. Esophago-gastric carcinoma: preoperative TNM classification with endosonography. Radiology. 1989;173:411–417. doi: 10.1148/radiology.173.2.2678255. [DOI] [PubMed] [Google Scholar]
- 68.Tio TL, Cohen P, Coene PP, Udding J, den Hartog Jager FC, Tytgat GN. Endosonography and computed tomography of esophageal carcinoma. Preoperative classification compared to the new (1987) TNM system. Gastroenterology. 1989;96:1478–1486. doi: 10.1016/0016-5085(89)90515-5. [DOI] [PubMed] [Google Scholar]
- 69.Tio TL, den Hartog Jager FC, Tytgat GN. The role of endoscopic ultrasonography in assessing local resectability of oesophagogastric malignancies. Accuracy, pitfalls, and predictability. Scand J Gastroenterol Suppl. 1986;123:78–86. doi: 10.3109/00365528609091867. [DOI] [PubMed] [Google Scholar]
- 70.Vickers J, Alderson D. Influence of luminal obstruction on oesophageal cancer staging using endoscopic ultrasonography. Br J Surg. 1998;85:999–1001. doi: 10.1046/j.1365-2168.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 71.Vilgrain V, Mompoint D, Palazzo L, Menu Y, Gayet B, Ollier P, Nahum H, Fekete F. Staging of esophageal carcinoma: comparison of results with endoscopic sonography and CT. AJR Am J Roentgenol. 1990;155:277–281. doi: 10.2214/ajr.155.2.2115251. [DOI] [PubMed] [Google Scholar]
- 72.Wu LF, Wang BZ, Feng JL, Cheng WR, Liu GR, Xu XH, Zheng ZC. Preoperative TN staging of esophageal cancer: comparison of miniprobe ultrasonography, spiral CT and MRI. World J Gastroenterol. 2003;9:219–224. doi: 10.3748/wjg.v9.i2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoshikane H, Tsukamoto Y, Niwa Y, Goto H, Hase S, Shimodaira M, Maruta S, Miyata A, Yoshida M. Superficial esophageal carcinoma: evaluation by endoscopic ultrasonography. Am J Gastroenterol. 1994;89:702–707. [PubMed] [Google Scholar]
- 74.Ziegler K, Sanft C, Zeitz M, Friedrich M, Stein H, Haring R, Riecken EO. Evaluation of endosonography in TN staging of oesophageal cancer. Gut. 1991;32:16–20. doi: 10.1136/gut.32.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giovannini M, Monges G, Seitz JF, Moutardier V, Bernardini D, Thomas P, Houvenaeghel G, Delpero JR, Giudicelli R, Fuentes P. Distant lymph node metastases in esophageal cancer: impact of endoscopic ultrasound-guided biopsy. Endoscopy. 1999;31:536–540. doi: 10.1055/s-1999-60. [DOI] [PubMed] [Google Scholar]
- 76.Wiersema MJ, Vazquez-Sequeiros E, Wiersema LM. Evaluation of mediastinal lymphadenopathy with endoscopic US-guided fine-needle aspiration biopsy. Radiology. 2001;219:252–257. doi: 10.1148/radiology.219.1.r01ap44252. [DOI] [PubMed] [Google Scholar]
- 77.Schwartz DA, Unni KK, Levy MJ, Clain JE, Wiersema MJ. The rate of false-positive results with EUS-guided fine-needle aspiration. Gastrointest Endosc. 2002;56:868–872. doi: 10.1067/mge.2002.129610. [DOI] [PubMed] [Google Scholar]
- 78.Shimizu Y, Tsukagoshi H, Fujita M, Hosokawa M, Kato M, Asaka M. Endoscopic ultrasonography for the detection of lymph node metastasis in superficial esophageal carcinoma. Dig Endosc. 1997;9:178–182. [Google Scholar]
- 79.Krasna MJ, Mao YS, Sonett J, Gamliel Z. The role of thoracoscopic staging of esophageal cancer patients. Eur J Cardiothorac Surg. 1999;16 Suppl 1:S31–S33. doi: 10.1016/s1010-7940(99)00180-3. [DOI] [PubMed] [Google Scholar]
- 80.Natsugoe S, Yoshinaka H, Morinaga T, Shimada M, Baba M, Fukumoto T, Stein HJ, Aikou T. Ultrasonographic detection of lymph-node metastases in superficial carcinoma of the esophagus. Endoscopy. 1996;28:674–679. doi: 10.1055/s-2007-1005575. [DOI] [PubMed] [Google Scholar]
- 81.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Shimizu Y, Oka M, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688–694. doi: 10.1016/j.cgh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 82.Shimoyama S, Imamura K, Takeshita Y, Tatsutomi Y, Yoshikawa A, Fujishiro M, Yahagi N. The useful combination of a higher frequency miniprobe and endoscopic submucosal dissection for the treatment of T1 esophageal cancer. Surg Endosc. 2006;20:434–438. doi: 10.1007/s00464-005-0144-3. [DOI] [PubMed] [Google Scholar]
- 83.Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67–S70. doi: 10.1016/s1542-3565(05)00291-0. [DOI] [PubMed] [Google Scholar]
- 84.Noguchi H, Naomoto Y, Kondo H, Haisa M, Yamatsuji T, Shigemitsu K, Aoki H, Isozaki H, Tanaka N. Evaluation of endoscopic mucosal resection for superficial esophageal carcinoma. Surg Laparosc Endosc Percutan Tech. 2000;10:343–350. [PubMed] [Google Scholar]
- 85.Higuchi K, Tanabe S, Koizumi W, Sasaki T, Nakatani K, Saigenji K, Kobayashi N, Mitomi H. Expansion of the indications for endoscopic mucosal resection in patients with superficial esophageal carcinoma. Endoscopy. 2007;39:36–40. doi: 10.1055/s-2006-945148. [DOI] [PubMed] [Google Scholar]
- 86.Ciocirlan M, Lapalus MG, Hervieu V, Souquet JC, Napoleon B, Scoazec JY, Lefort C, Saurin JC, Ponchon T. Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy. 2007;39:24–29. doi: 10.1055/s-2006-945182. [DOI] [PubMed] [Google Scholar]
- 87.Shimada H, Makuuchi H. Endoscopic treatment for esophageal cancer. Kyobu Geka. 2006;59:768–775. [PubMed] [Google Scholar]
- 88.Vilmann P. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of lymph nodes. Gastrointest Endosc. 1996;43:S24–S29. doi: 10.1016/s0016-5107(96)81510-0. [DOI] [PubMed] [Google Scholar]
- 89.Silvestri GA, Hoffman BJ, Bhutani MS, Hawes RH, Coppage L, Sanders-Cliette A, Reed CE. Endoscopic ultrasound with fine-needle aspiration in the diagnosis and staging of lung cancer. Ann Thorac Surg. 1996;61:1441–1445; discussion 1445-1446. doi: 10.1016/0003-4975(95)00052-6. [DOI] [PubMed] [Google Scholar]
- 90.Arita T, Kuramitsu T, Kawamura M, Matsumoto T, Matsunaga N, Sugi K, Esato K. Bronchogenic carcinoma: incidence of metastases to normal sized lymph nodes. Thorax. 1995;50:1267–1269. doi: 10.1136/thx.50.12.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Izbicki JR, Thetter O, Karg O, Kreusser T, Passlick B, Trupka A, Haussinger K, Woeckel W, Kenn RW, Wilker DK. Accuracy of computed tomographic scan and surgical assessment for staging of bronchial carcinoma. A prospective study. J Thorac Cardiovasc Surg. 1992;104:413–420. [PubMed] [Google Scholar]
- 92.McLoud TC, Bourgouin PM, Greenberg RW, Kosiuk JP, Templeton PA, Shepard JA, Moore EH, Wain JC, Mathisen DJ, Grillo HC. Bronchogenic carcinoma: analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology. 1992;182:319–323. doi: 10.1148/radiology.182.2.1732943. [DOI] [PubMed] [Google Scholar]
- 93.McKenna RJ Jr, Libshitz HI, Mountain CE, McMurtrey MJ. Roentgenographic evaluation of mediastinal nodes for preoperative assessment in lung cancer. Chest. 1985;88:206–210. doi: 10.1378/chest.88.2.206. [DOI] [PubMed] [Google Scholar]
- 94.Kondo D, Imaizumi M, Abe T, Naruke T, Suemasu K. Endoscopic ultrasound examination for mediastinal lymph node metastases of lung cancer. Chest. 1990;98:586–593. doi: 10.1378/chest.98.3.586. [DOI] [PubMed] [Google Scholar]
- 95.Harrow EM, Oldenburg FA Jr, Lingenfelter MS, Smith AM Jr. Transbronchial needle aspiration in clinical practice. A five-year experience. Chest. 1989;96:1268–1272. doi: 10.1378/chest.96.6.1268. [DOI] [PubMed] [Google Scholar]
- 96.Harrow EM, Wang KP. The staging of lung cancer by bronchoscopic transbronchial needle aspiration. Chest Surg Clin N Am. 1996;6:223–235. [PubMed] [Google Scholar]
- 97.Salazar AM, Westcott JL. The role of transthoracic needle biopsy for the diagnosis and staging of lung cancer. Clin Chest Med. 1993;14:99–110. [PubMed] [Google Scholar]
- 98.Gardner D, vanSonnenberg E, D'Agostino HB, Casola G, Taggart S, May S. CT-guided transthoracic needle biopsy. Cardiovasc Intervent Radiol. 1991;14:17–23. doi: 10.1007/BF02635526. [DOI] [PubMed] [Google Scholar]
- 99.Lopez L, Varela A, Freixinet J, Quevedo S, Lopez Pujol J, Rodriguez de Castro F, Salvatierra A. Extended cervical mediastinoscopy: prospective study of fifty cases. Ann Thorac Surg. 1994;57:555–557; discussion 557-558. doi: 10.1016/0003-4975(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 100.Barendregt WB, Deleu HW, Joosten HJ, Berg W, Janssen JP. The value of parasternal mediastinoscopy in staging bronchial carcinoma. Eur J Cardiothorac Surg. 1995;9:655–658. doi: 10.1016/s1010-7940(05)80113-7. [DOI] [PubMed] [Google Scholar]
- 101.Merav AD. The role of mediastinoscopy and anterior mediastinotomy in determining operability of lung cancer: a review of published questions and answers. Cancer Invest. 1991;9:439–442. doi: 10.3109/07357909109084642. [DOI] [PubMed] [Google Scholar]