Abstract
AIM: To investigate if the clinical efficacy of granulocytes and monocytes by adsorption (GMA) is associated with an increased frequency of peripheral regulatory T cells (Tregs), as these cells have proven to be successful in suppressing inflammatory bowel disease (IBD) in animal models.
METHODS: We report four cases of corticosteroid-dependent ulcerative colitis (UC) and two Crohn’s disease (CD) cases with severe cutaneous lesions who received GMA therapy. The frequency of CD4+ CD25high (Tregs) in peripheral blood was analyzed by flow cytometry and the expression of FoxP3 and TGF beta in purified CD4+ T cells was determined by real time PCR prior to and one month after the last apheresis session, and at the time of endoscopic and clinical assessing.
RESULTS: Increased expression of Fox P3 mRNA was found in all five patients who responded to cytapheresis with remission of clinical symptoms, mucosal inflammation and cutaneous lesions, and an increased frequency of circulating Tregs was found in four patients. These changes were not observed in the patient with UC who did no respond to GMA. Variations in TGF-β (mRNA) did not parallel that of FoxP3 mRNA.
CONCLUSION: The clinical efficacy of GMA on IBD and related extra intestinal manifestations was associated with an expansion of circulating CD4+ CD25+ Tregs and higher expression of FoxP3 in CD4+ T cells. Accordingly, an elevated CD4+ CD25+ FoxP3 may be a valuable index of remission in patients with IBD and other chronic relapsing-remitting inflammatory conditions during treatment with GMA.
Keywords: Adsorptive leukocytapheresis, Regulatory T cells, Inflammatory bowel diseases, Adacolumn, Granulocyte-monocyte adsorptive apheresis
INTRODUCTION
Granulocyte-monocyte adsorption apheresis (GMA) with the Adacolumn has shown efficacy in the treatment of inflammatory colitis in a series of studies, mainly performed by Japanese and European investigators[1,2]. AdacolumnR is a therapeutic extracorporeal device, which selectively adsorbs granulocytes and monocytes from peripheral blood. The selective adsorption is thought to be mediated by interactions of complement and Fc receptors on the granulocytes and monocytes, whereas complement factor and immunoglobulins adhere onto the surface of G-1 beads by ligand-receptor interactions[3]. Granulocytes and monocytes are believed to play a significant role in these and other autoimmune inflammatory diseases because of their capability to release a variety of mediators including active oxygen species and inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin β (IL1-β)[4,5]. This contention supports the use of GMA-apheresis and other therapeutic attempts to prevent damages caused by granulocytes and monocytes[6,7]. However, the clinical efficacy of cytapheresis cannot be fully explained by a simple transient removal of these cells.
CD4+ CD25high, FoxP3+ regulatory T cells (Tregs) play a major role in controlling the immune response by producing suppressive effects on activated immune cells. It is established that FoxP3, a forkhead/winged-helix transcription factor, is specifically expressed in Treg cells and is a master control protein for the generation of these cells[8]. Depletion of Tregs leads to autoimmunity in mice[9] and dysfunction of Tregs has been linked to human autoimmune diseases[10–13]. Immunosuppressive cytokines like IL-10 and TGF-β are required for the control of autoimmunity in many in vivo models, but suppression in vitro appears to be solely mediated by a cell-cell contact-dependent, cytokine independent mechanism[14]. Further, an inverse correlation has been reported between disease activity and frequency of peripheral Tregs in patients with inflammatory bowel disease (IBD)[15]. However, active IBD is not associated with a functional defect but with a contraction of the peripheral blood Treg pool and a moderate expansion in intestinal lesions[16]. Further, cultured gut-derived Tregs from patients with Crohn’s disease (CD) and healthy individuals suppress T cell proliferation and cytokine secretion[17]. Animal studies suggest that adoptive transfer of Tregs can reverse experimental colitis[18] and the long-term effects of ex vivo activated and transferred Treg cells are likely due to their ability to generate new cytokine-producing CD4+ Treg in vivo[19].
The involvement of TGF-β in Treg function remains controversial but it has been reported that TGF-β produced by CD4+ CD25+ T cells is involved in the suppressor activity of these cells, particularly in their ability to regulate intestinal inflammation[20]. There is evidence that TGF-β deficiency leads to development of autoimmune disease: TGF-β deficient mice and transgenic mice expressing the dominant negative form of TGF-β receptor are useful animal models for diabetes and IBD[21,22]. Suppressive activity of Tregs may be inhibited in TGF-β deficiency by acting on FoxP3. TGF-β is able to induce FoxP3 expression and subsequently a Treg phenotype in CD4+CD25- murine and human T cells, as well as a positive auto regulatory loop of TGF-β signaling by down-regulation of smad-7, an inhibitory protein for TGF-β signaling[23]. In addition, over expression of smad 7 that results in insensitivity to TGF-β has been associated with human IBD[24].
With the assumption of a role of Tregs in the prevention and potential cure of autoimmune inflammatory diseases, we aimed to ascertain whether the beneficial effects of GMA-apheresis correlate with an increase in CD4+ CD25high cell numbers and a higher expression of FoxP3 and TGF-β in peripheral blood CD4+ T cells in a small group of patients with ulcerative colitis or Crohn’s disease.
MATERIALS AND METHODS
Subjects
Four patients with ulcerative colitis (UC) and two patients with CD that were treated by selective GMA were included in this study. Main UC patient’s data are presented in Table 1. Steroid dependency was defined as the need for > 10 mg prednisolone daily for at least 4 wk to control clinical symptoms. Both of the two patients with CD had associated severe cutaneous conditions; patient 5, a 26 aged female was diagnosed with CD 7 years ago. This patient had ileocolonic lesions without fistulas and had previously been treated with steroids, 5-ASA, and azathioprine cycles without improvement. On August 2005, she had an ulcerative lesion in the left leg; diagnosis of pyoderma gangrenosum (PG) was made on clinical grounds and confirmed by a dermatologist. A skin biopsy was not required as there was no clinical doubt in the diagnosis. Patient 6 was a 45-year old female who was diagnosed to have CD with ileocolonic inflammation and has had several flares of uveitis and nodosum erythematosis. She was unsuccessfully treated with steroids and anti-TNF antibodies.
Table 1.
Ulcerative colitis patient’s data
| 1 | 2 | 3 | 4 | |
| Age (yr) | 37 | 42 | 38 | 36 |
| Sex | Male | Female | Female | Male |
| Disease’ location | Left side | Extensive colitis | Left side | Left side |
| Disease activity | Moderate-severe | Severe | Severe | Severe |
| Time from diagnosis (yr) | 2 | 10 | 8 | 22 |
| Truelove index | 13 | 17 | 14 | 15 |
| Previous therapy | Corticosteroids azathioprine | Corticosteroids azathioprine | Corticosteroids azathioprine | Corticosteroids azathioprine |
| Indication for GMA-apheresis | Steroid-dependent disease | Steroid-dependent disease | Steroid-dependent disease | Steroid-dependent disease |
| Cycles of GMA-apheresis | 10 | 5 | 7 | 5 |
| Response to GMA-apheresis | Remission | Response | Failure | Remission |
| Truelove index (after therapy) | 10 | 10 | 16 | 9 |
| Endoscopy | No inflammation | No inflammation | Bleeding ulcers | No inflammation |
| Recurrences | At 6 mo | At 6 mo | Non |
GMA: Granuocyte/monocyte apheresis.
Each patient received five cycles GMA-apheresis at weekly intervals (see below) and a clinical protocol including ileocolonoscopy was applied one month after the end of the therapy. Clinical outcome was assessed using the follow-up criteria: absence/near absence of clinical symptoms as remission; improvement of symptoms, (without increase in steroid dose) as response, and no change or worsening as failure. Disease activity was categorized as mild, moderate or severe, and relapse was defined as an increase in clinical symptoms, as judged by the treating physician. Blood samples were taken at entry and when efficacy was assessed to determine the frequency of peripheral regulatory CD4+ CD25high T cells and to quantify mRNAs for FoxP3 and TGF-β expressed in purified CD4+ T cells. Results from each individual patient were compared regarding to its clinical response to the therapy (Table 2).
Table 2.
Clinical and analytical data of Crohn disease’s patients
|
Patient 5 |
Patient 6 |
|||
| Pre apheresis | Post apheresis | Pre apheresis | Post apheresis | |
| CDA I | 241 | 161 | 236 | 156 |
| Cutaneous lesion | Pyoderma gangrenosum | Healing1 | Erythema nodosum | Healing1 |
| Hematocrit | 37.5 | 37.1 | 32.3 | 34 |
| Haemoglobin | 11.9 | 11.9 | 10.6 | 10.9 |
| GSV | 62 | 39 | 74 | 58 |
| C-reactive protein | 67 | 13 | 17 | 4 |
| Platelets × 103/mL | 241 | 161 | 236 | 151 |
Number of GMA-apheresis sessions to achieve the healing: 13 for PG, 5 for E N.
GMA procedures
Patient’s blood was passed through a column that is filled with specially designed cellulose acetate beads of 2 mm in diameter that absorb about 65% of granulocytes, 55% of monocytes and a very small fraction of lymphocytes[25]. The apheresis procedures were performed by a specially trained nurse at the outpatient gastrointestinal department. Adacolumn device (JIMRO, Takasaki, Japan) was used in all cases. The GMA sessions were performed at a flow rate of 20-30 mL/min for 60-90 min and the frequency and total number of apheresis sessions were typically 5 weekly sessions per patient. Non-adverse events or toxicity were observed.
Flow cytometry
Whole blood samples were stained with FITC (fluorescein) labeled anti-CD4 and APC conjugated anti-CD25 Abs; respective mouse isotype controls were employed; optimal dilution of antibodies were used and cells were washed and analyzed by flow cytometry in a FACScalibur cytometer and the CellQuest softward (Becton Dickinson). A live gate set around viable lymphocytes was used based on their forward scatter/side scatter (FCS/SCC) characteristics. For analysis of CD25high T cells, large activated cells as determined by FCS/SCC properties were excluded. Three cell populations were distinguished in CD4+ T cells, according to the surface expression of CD25: CD4+ CD25- “naive”; CD4+ CD25low-int “activated”, and CD4+ CD25high “regulatory” cells.
Cell isolation
Peripheral blood mononuclear cells (PBMC) were isolated from 20 mL freshly drawn blood by Ficoll density gradient centrifugation. CD4 positive cells were isolated from PBMC by positive selection using anti-CD4 human micro beads magnetic adhesion cell shorter (MACS) antibody (Miltenyi Biotech, Bergisch, Gladbach, Germany).
Quantitative PCR
CD4+ cell pellets were lysed in RLT buffer (Qiamp RNA blood mini kit (Qiagen, Germany) and total RNA was extracted. One microgram of total RNA was reverse transcribed using MMLV and random hexamers (Promega, USA) at 42°C one hour, and at 65°C 10 min. The cDNA obtained was stored at -20°C until PCR analysis.
FoxP3 and glucuronidase (GUS) as a housekeeping gene were analyzed by quantitative real time PCR performed with the Light Cycler Fast Start DNA Sybr green kit (Roche). FoxP3 and GUS primers and PCR conditions are described in[26] and[27] respectively. Reverse 3'primer are designed to span contiguous exons in order not to anneal to contaminating genomic DNA. To control for specificity of the amplification products, a melting curve analysis was performed and no unspecific products were observed. A standard curve of cDNA from CD4 cells from a donor with a high expression of FoxP3 was used. Arbitrary units were assigned to each dilution of cDNA. Relative FoxP3 expression values were calculated dividing the arbitrary units of Fox P3 by the GUS results in order to normalized RNA input. The result is expressed in % ratio.
TGF-β1 and human-glucuronyl-6-phosphato dehydrogenase (h-G6PDH) as a housekeeping gene were analyzed by quantitative real time PCR performed with Light Cycler Fast Start DNA hybridization probes kit (Roche). Primers and probes specific to TGF-β1 isoform were: Forward: exon 6: 5’-ggCTggAAgTggATCCACgA-3’ Reverse: 5’-gCAggAgCgCACGATCATgTT-3’; Probes: 5’-CTgCCCCTACATTTggAgCCTggAC-F-3’ 5’-LCRed640-gCAgTACAgCCA gCAgTACAgCAAggTCCTggCCCT-P-3’. Reverse 3’primer is designed to span contiguous exons in order not to anneal to contaminating genomic DNA. PCR conditions for both gene amplification were: 45 cycles of 3 segments: 95°C 10 s; 58°C 15 s and 72°C 15 s. A melting curve analysis was performed and no unspecific products were observed The copy number of TGF-β1 gene was calculated from a standard curve of a plasmid with the TGF-β1 PCR fragment cloned in the laboratory and h-G6PDH copies were determined with LC-h-G6PDH housekeeping gene set (Roche). Absolute quantification was expressed as ratio % for each cDNA.
RESULTS
Increase in peripheral Treg in patients with IBD who showed a favorable response to GMA
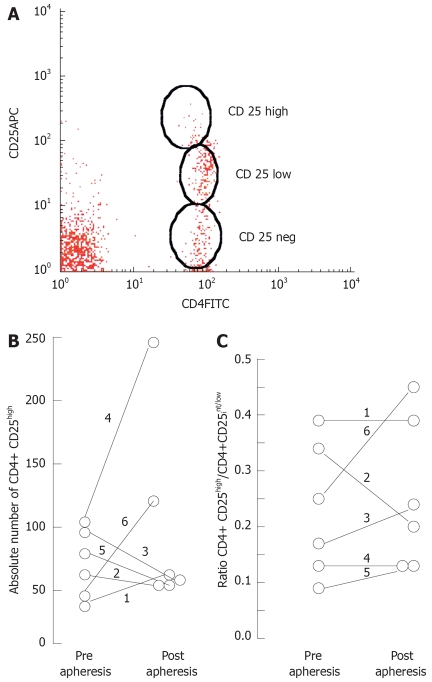
Regulatory T cells are usually identified by flow cytometry as CD4+ T cells expressing high level of the IL2-R α chain (CD25) at their surface (Figure 1). By using this criterion we found higher frequencies in samples obtained at the end of the GMA-apheresis sessions in three of five patients with IBD that responded favorably to the therapy, but a lower frequency in the one who failed to respond to this treatment. To exclude that changes in the frequency of Tregs reflect nonspecific effects of the therapy on other CD4+ T cell subsets, we also calculated the ratio CD4+ CD25high to CD4+ CD25low-int and compared the data in each individual case; higher ratio was found in one case, lower ratio in another, and no variation in the remaining four cases. Thus, a selective expansion of true Treg cells in the peripheral blood of treated patients could not be demonstrated. Human CD4+ CD25high Treg population is relatively indiscreet; the border line between CD25high and CD25low is often obscure, making it difficult determining the cellular subsets. Even carefully selected CD4+ CD25+ T cell clones do not represent a homogenous population of functional suppressor cells[28]. This fact may explain the differences in normal ranges described in the literature but it seems unlikely that its concerns the variations observed in this study.
Figure 1.
Variation of peripheral blood CD4+ CD25+ cells in patients treated by GMA-apheresis. A: Representative cytogram; B: Variation of T regs on each individual patient; C: Variation in the ratio CD4+ CD25high/CD4+ CD25low-int.
Clearer results were found by quantification of mRNA for FoxP3 expressed in purified CD4+ T cells by real time PCR. In contrast to most phenotypic markers of Tregs, the expression of FoxP3 seems to be crucial for their functional characteristics[29]. All five patients which showed successful response to Adacolumn therapy displayed increased levels of FoxP3 mRNA in blood CD4+ T cells after the GMA treatment, whereas diminished levels of FoxP3 transcripts were found in the one that did not respond to this therapy (Figure 2).
Figure 2.
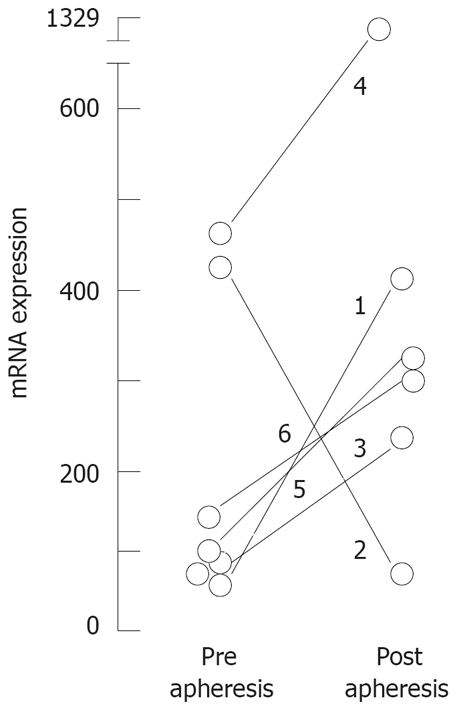
Variation of mRNA expression for FoxP3 in CD4+ T cells in matched samples from 6 patients treated by GMA-apheresis: Quantitative PCR was performed as described in methods; Results are expressed as FoxP3 expression relative to GUS expression per cent.
TGF-β expression in CD4+ T cells does not change after GMA
Next, we analyzed the expression of TGF-β in CD4+ T cells from GMA- treated patients and observed increased expression of TGF-β in post apheresis samples in four cases but this variation was not parallel to that observed for FoxP3 expression. The remaining two cases did not show variations in TGF-β and have had a different clinical response to GMA-apheresis (Figure 3).
Figure 3.
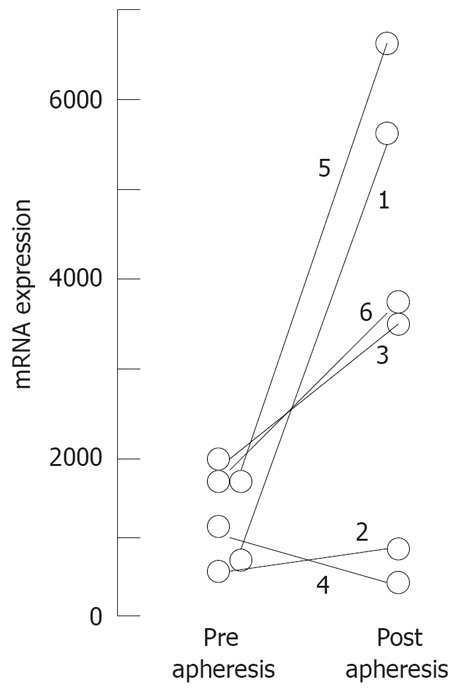
Variation of mRNA expression for TGF-beta in CD4+ T cells in matched samples from 6 patients treated by GMA-apheresis: Quantitative PCR was performed as described in methods; Results are expressed as TGF-beta 1 expression relative to GAPDH expression per cent.
Adacolumn therapy was successfully applied to healing cutaneous lesions related to inflammatory bowel disease
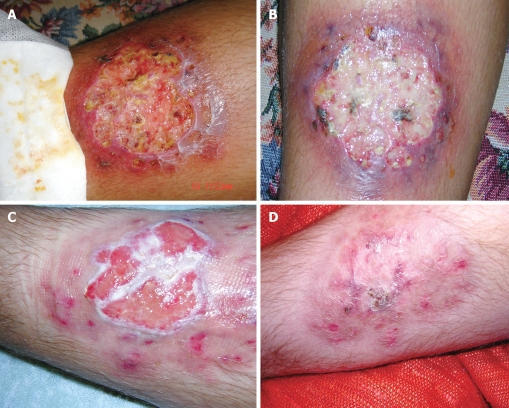
Both two patients with Crohn’s disease had severe cutaneous lesions that improved with the Adacolumn’s therapy; Figure 4 shows the complete healing of pyoderma gangrenosum, unsuccessfuly treated with steroids, after 13 sessions with GMA-apheresis. A successful response to GMA-apheresis was also observed in the case of erythematous nodosum associated with CD that was unsuccessfully treated with steroids and infliximab anti-TNF-α therapy. The effect of apheresis was transient as the cutaneous lesions recurred four months after the interruption of therapy and remitted with a new cycle of GMA-apheresis sessions.
Figure 4.
Improvement of pyode-rma gangrenosum in a patient with Crhon’ disease treated by GMA-aphersesis: pictures A-D, were taken at 1, 5, 8, and 13 apheresis sessions.
DISCUSSION
A current view is that a disregulated immunity in intestinal mucosa plays a pivotal role in the pathogenesis of IBD and that intestinal inflammation is perpetuated by inflammatory cytokines like IL-1β, TNF-α, IL6 and others[30]. Based on this perception, anti cytokine antibodies, notably anti TNF (infliximab) have been added to the conventional anti inflammatory drugs. Steroids, 5-aminosalicylic or sulphasalazine in combination with azathioprine and nutritional support for some patients are used as first-line medication for IBD. However, treatment failure has often been an indication for colostomy in steroid-refractory patients[31] and biologicals are dampened by serious concerns about their long-term efficacy and safety profiles[32,33]. The underlying rationale for GMA-apheresis is the fact that granulocytes and monocytes/macrophages (GMs) are a major source of inflammatory cytokines[34] and that selective removal of these cells, otherwise destined for migration to the intestine, reduce the intestinal inflammation. GMs removal is made by their adhesion to carrier beads through FcγR and complement receptors, but it is reported that Adacolumn increases peripheral blood lymphocytes, sparing prevailing cells and inducing an increase in de novo lymphocytes[35], largely attributable to an increase in CD4+ T cells. These changes on blood lymphocytes were also observed in our patients (data not shown). It is noteworthy that a low lymphocyte count has been associated with relapse of CD[36].
A selective increase of peripheral CD4+ Treg cells in patients treated with GMA has been suggested[37] and our results strongly support this opinion. We have observed higher frequency of Tregs and increased expression for FoxP3 mRNA in the blood of IBD patients treated with Adacolumn except in the one that did not respond to the therapy. Quantitative data showed expression differences in matched samples of purified CD4 T cells, obtained before and after a series of GMA. We think that these results contribute to enlighten the picture hence there is no published data at this time.
The position of GMA-apheresis in the medical therapy of ulcerative colitis has been recently reviewed[38]. According to the expressed opinion patients at any stage of their disease might benefit from this therapy and can avoid steroids and other drug based medications. In patients with steroid dependent UC up to 85% show efficacy with an excellent safety profile. Five from our six patients with active and severe IBD responded to GMA-apheresis therapy and the one that did no respond showed a further favorable response to the infliximab. Pyoderma gangrenosum (PG) is a chronic ulcerating skin condition that appears to be immune mediated, and approximately 30% of cases occur in association with IBD[39]. The mainstay treatment remains immunosuppression with corticosteroids and cyclosporine, and there is a number of reports of PG responding to anti TNF-α therapy[39]. We report the beneficial effect of GMA-apheresis therapy in a case of PG and a case of erythema nodosum, both associated with CD that did not respond to other therapies.
Thus, many patients with IBD likely benefit from GMA-apheresis therapy, potentially by an increase in frequency and function of CD4 Tregs that takes place in the action mechanism(s) of this procedure. The small number of patients in our study precludes to take out any statistical conclusion about this point but, independently of defining the actual mechanism of GMA-apheresis in IBD therapy, our results suggest that quantification of FoxP3 mRNA expressed in CD4 T cells of treated patients may be used as a valuable index of remission in IBD and perhaps in other chronic relapsing-remitting inflammatory conditions susceptible of this kind of therapy. If that were proved on a large series of GMA-treated patients, it could be helpful to predict the response to GMA and to support the rational use of this high-cost therapy. We also point out the fact that the patient that did not respond to GMA did respond to infliximab, and conversely, those which responded to GMA did not respond to other therapies. Thus, a tailor’s patient therapy could be considered in a future and good analytical criteria of response will be welcome.
COMMENTS
Background
Granulocyte/monocyte apheresis with Adacolumn seems to offer a safe and efficacious adjuvant treatment option in inflammatory bowel disease (IBD), rheumatoid arthritis and maybe in other inflammatory conditions, but the clinical efficacy cannot be explained by selective removal of these cells. Regulatory T cells play a major role in control of autoimmune diseases, and the FoxP3 transcription factor is specifically expressed in these cells and is crucial for their functional characteristics. Dysfunction of T regs it is suggested in patients with IBD and demonstrated in animal models of these pathologies
Innovations and breakthroughs
We report that the clinical efficacy of adsorptive cytapheresis in the treatment of inflammatory colitis is associated to an expansion of circulating T reg and increased expression of FoxP3. If that were proved in a large series of treated patients it could be helpful to make a valuable index of remission and to predict the response to this therapy.
Applications
All the findings of our study will provide a useful way for the treatment of inflammatory colitis.
Peer review
This is an interesting study. Authors showed that GMA induces increase of Treg and exerts anti-inflammatory response in IBD patients.
Peer reviewer: Akira Andoh, MD, Department of Internal Medicine, Shiga University of Medical Science, Seta Tukinowa, Otsu 520-2192, Japan
S- Editor Zhu WL L- Editor Kremer M E- Editor Ma WH
References
- 1.Shimoyama T, Sawada K, Hiwatashi N, Sawada T, Matsueda K, Munakata A, Asakura H, Tanaka T, Kasukawa R, Kimura K, et al. Safety and efficacy of granulocyte and monocyte adsorption apheresis in patients with active ulcerative colitis: a multicenter study. J Clin Apher. 2001;16:1–9. doi: 10.1002/jca.1000. [DOI] [PubMed] [Google Scholar]
- 2.Sawada K, Muto T, Shimoyama T, Satomi M, Sawada T, Nagawa H, Hiwatashi N, Asakura H, Hibi T. Multicenter randomized controlled trial for the treatment of ulcerative colitis with a leukocytapheresis column. Curr Pharm Des. 2003;9:307–321. doi: 10.2174/1381612033391928. [DOI] [PubMed] [Google Scholar]
- 3.Hiraishi K, Takeda Y, Shiobara N, Shibusawa H, Jimma F, Kashiwagi N, Saniabadi AR, Adachi M. Studies on the mechanisms of leukocyte adhesion to cellulose acetate beads: an in vitro model to assess the efficacy of cellulose acetate carrier-based granulocyte and monocyte adsorptive apheresis. Ther Apher Dial. 2003;7:334–340. doi: 10.1046/j.1526-0968.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 4.Grisham MB, Granger N. Mechanisms of neutrophil-mediated tissue injury. In: MacDermott RP, Stenson WF, editors. Inflammatory bowel disease. New York: Elsevier; 1992. pp. 225–239. [Google Scholar]
- 5.Bernard GR, Lucht WD, Niedermeyer ME, Snapper JR, Ogletree ML, Brigham KL. Effect of N-acetylcysteine on the pulmonary response to endotoxin in the awake sheep and upon in vitro granulocyte function. J Clin Invest. 1984;73:1772–1784. doi: 10.1172/JCI111386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vedder NB, Winn RK, Rice CL, Chi EY, Arfors KE, Harlan JM. A monoclonal antibody to the adherence-promoting leukocyte glycoprotein, CD18, reduces organ injury and improves survival from hemorrhagic shock and resuscitation in rabbits. J Clin Invest. 1988;81:939–944. doi: 10.1172/JCI113407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis LS, Kavanaugh AF, Nichols LA, Lipsky PE. Induction of persistent T cell hyporesponsiveness in vivo by monoclonal antibody to ICAM-1 in patients with rheumatoid arthritis. J Immunol. 1995;154:3525–3537. [PubMed] [Google Scholar]
- 8.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 9.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 10.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 11.Liu MF, Wang CR, Fung LL, Wu CR. Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol. 2004;59:198–202. doi: 10.1111/j.0300-9475.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 12.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Herrath M, Homann D. Introducing baselines for therapeutic use of regulatory T cells and cytokines in autoi-mmunity. Trends Immunol. 2003;24:540–545. doi: 10.1016/j.it.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi M, Nakamura K, Honda K, Kitamura Y, Mizutani T, Araki Y, Kabemura T, Chijiiwa Y, Harada N, Nawata H. An inverse correlation of human peripheral blood regulatory T cell frequency with the disease activity of ulcerative colitis. Dig Dis Sci. 2006;51:677–686. doi: 10.1007/s10620-006-3191-2. [DOI] [PubMed] [Google Scholar]
- 16.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 17.Kelsen J, Agnholt J, Hoffmann HJ, Romer JL, Hvas CL, Dahlerup JF. FoxP3(+)CD4(+)CD25(+) T cells with regulatory properties can be cultured from colonic mucosa of patients with Crohn’s disease. Clin Exp Immunol. 2005;141:549–557. doi: 10.1111/j.1365-2249.2005.02876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 19.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 21.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 24.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 25.Saniabadi AR, Hanai H, Takeuchi K, Umemura K, Nakashima M, Adachi T, Shima C, Bjarnason I, Lofberg R. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. 2003;7:48–59. doi: 10.1046/j.1526-0968.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 26.Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140:360–367. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E, Delabesse E, Macintyre E, Gottardi E, Saglio G, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia. 2003;17:2474–2486. doi: 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- 28.Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, Roncarolo MG. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med. 2002;196:1335–1346. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yagi S, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fuji S, et al. Crucial role of FoxP3 in the development and function of human CD regulatory T-cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 30.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 31.Kornbluth A, Marion JF, Salomon P, Janowitz HD. How effective is current medical therapy for severe ulcerative and Crohn’s colitis? An analytic review of selected trials. J Clin Gastroenterol. 1995;20:280–284. doi: 10.1097/00004836-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Atzeni F, Ardizzone S, Sarzi-Puttini P, Colombo E, Maconi G, De Portu S, Carrabba M, Bianchi Porro G. Autoantibody profile during short-term infliximab treatment for Crohn's disease: a prospective cohort study. Aliment Pharmacol Ther. 2005;22:453–461. doi: 10.1111/j.1365-2036.2005.02576.x. [DOI] [PubMed] [Google Scholar]
- 33.Melichar B, Bures J, Dedic K. Anorectal carcinoma after infliximab therapy in Crohn’s disease: report of a case. Dis Colon Rectum. 2006;49:1228–1233. doi: 10.1007/s10350-006-0647-6. [DOI] [PubMed] [Google Scholar]
- 34.Cassatella MA. The production of cytokines by polymo-rphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 35.Aoki A, Nakamura K, Yoshimatsu Y, Tsuda MD, Suzuki Y. Adacolumn selective leukocyte adsorption apheresis in patients with ulcerative colitis: clinical efficacy, effects on plasma IL-8 and the expression of Toll like receptors 2 on granulocytes. Dig Dis Sci. 2006;52:1326–1328. doi: 10.1007/s10620-006-9406-8. [DOI] [PubMed] [Google Scholar]
- 36.Heimann TM, Aufses AH Jr. The role of peripheral lymphocytes in the prediction of recurrence in Crohn’s disease. Surg Gynecol Obstet. 1985;160:295–298. [PubMed] [Google Scholar]
- 37.Hanai H. Positions of selective leukocytapheresis in the medical therapy of ulcerative colitis. World J Gastroenterol. 2006;12:7568–7577. doi: 10.3748/wjg.v12.i47.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Callen JP. Pyoderma gangrenosum. Lancet. 1998;351:581–585. doi: 10.1016/S0140-6736(97)10187-8. [DOI] [PubMed] [Google Scholar]
- 39.Brooklyn TN, Dunnill MG, Shetty A, Bowden JJ, Williams JD, Griffiths CE, Forbes A, Greenwood R, Probert CS. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505–509. doi: 10.1136/gut.2005.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]