Abstract
AIM: To investigate the effect of lifestyle intervention on non-alcoholic fatty liver disease (NAFLD) in Chinese obese children.
METHODS: Seventy-six obese children aged from 10 to 17 years with NAFLD were enrolled for a one-month intervention and divided randomly into three groups. Group1, consisting of 38 obese children, was an untreated control group without any intervention. Group 2, consisting of 19 obese children in summer camp, was strictly controlled only by life style intervention. Group 3, consisting of 19 obese children, received oral vitamin E therapy at a dose of 100 mg/d. The height, weight, fasting blood glucose (FBG), fasting serum insulin (FINS), plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), total cholesterol (TCHO) and homeostasis model assent-insulin resistance (HOMA-IR) were measured at baseline and after one month. All patients were underwent to an ultrasonographic study of the liver performed by one operator who was blinded to the groups.
RESULTS: The monitor indices of BMI, ALT, AST, TG, TCHO and HOMA-IR were successfully improved except in group 1. BMI and ALT in group 2 were reduced more significantly than in group 3 (2.44 ± 0.82 vs 1.45 ± 0.80, P = 0.001; 88.58 ± 39.99 vs 63.69 ± 27.05, P = 0.040, respectively).
CONCLUSION: Both a short-term lifestyle intervention and vitamin E therapy have an effect on NAFLD in obese children. Compared with vitamin E, lifestyle intervention is more effective. Therefore, lifestyle intervention should represent the first step in the management of children with NAFLD.
Keywords: Non-alcoholic fatty liver disease, Lifestyle intervention, Vitamin E, Obese, Children
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) tends to become epidemic in children worldwide in the latest decade. NAFLD is recognized as a cause of potentially progressive liver damage. The entire range of liver involvement in NAFLD can occur in children, such as hepatic macrovesicular steatosis without inflammation, steatosis with inflammation or fibrosis [which defined as non-alcoholic steatohepatitis (NASH)] and cirrhosis. NAFLD may be the hepatic aspect of the metabolic syndrome that comprises central obesity, insulin resistance, hypertension and dyslipidemia in adults and children[1]. The natural history of NAFLD is only partly known, the disease slowly progresses to cirrhosis and the initial assessment of NASH as being benign is not supported by the available evidences[2–8]. Insulin resistance has been shown to be the basic pathophysiological mechanism responsible for the fatty transformation of liver (first hit) as well as the second hit which leads to hepatocyte injury[9,10]. The gold standard of diagnosis is liver biopsy, but it is not frequently performed in the pediatric population. Liver ultrasonography (US), although not sensitive enough to assess liver fibrosis or inflammation, has a sensitivity of 89% and a specificity of 93% for detecting histological steatosis[11,12]. In the absence of liver biopsy, presumed NASH is conventionally diagnosed by classical ultrasonography together with an elevated serum level of alanine aminotransferase (ALT)[13].
Multiple therapeutic agents such as vitamin E, β-carotene, metformin, PPAR-gamma agonists and the lipase inhibitor orlistat have demonstrated to be useful in NAFLD in a series of small cases[14–18]. Lifestyle intervention (dietary restriction and exercise) has also improved the liver function of patients with NAFLD[19]. Conflicting data on the therapeutic efficacy of these drugs have been reported in the literature[14–18].
The aim of this study was to investigate the short-term effect of a lifestyle intervention on liver biochemistry and fasting insulin levels and compare with that of the vitamin E therapy in obese children with NAFLD.
MATERIALS AND METHODS
Seventy-six obese children, according to the criteria that a child was considered to be obese when the body mass index (BMI) exceeded the 95th BMI percentage for age and sex[20], were enrolled in this study. The age of the subjects ranged from 10 to 17 years (mean 13.7 ± 1.9 years). They were all obese children with liver fatty infiltration in ultrasonic appearance and abnormal liver function with higher alanine aminotransferase (ALT) by at least 1.5 times over the upper normal limit which was diagnosed as NASH[21]. They were divided randomly into three groups and the ultrasonography operator was blinded to the groups. Group 1 was an untreated control group, consisting of 38 obese children, who had not taken any medicine and lifestyle intervention. Group 2 had 19 obese children, taking no drug and treated only with strict lifestyle intervention at summer camp. Group 3, consisting of 19 obese children, was treated with vitamin E, while improving their behaviors and enjoying their lives freely by themselves at home. Patients who had positive markers for other liver diseases (hepatitis virus, TORCH, metabolic, genetic) or who had a history of alcohol intake were all excluded. Studies of the three groups were carried out at the same time, that of group 2 at the summer camp, and the others were done separately at home. They were all observed for one month, because the camp lasted only a month (Figure 1). The characteristics of the three groups are shown in Table 1.
Figure 1.
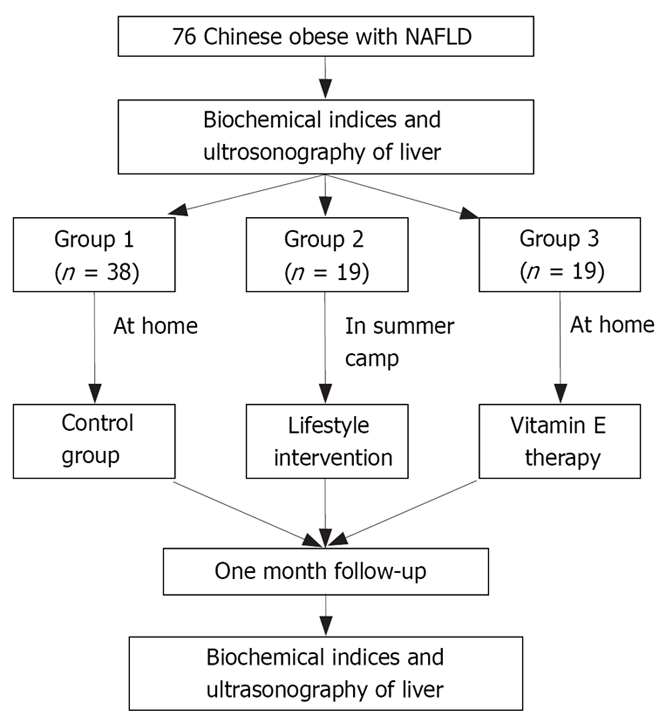
The dispositions of subjects. (76 obese children and adolescents with NAFLD aged between 10 and 17 years were enrolled in this study).
Table 1.
Baseline characteristics of the three groups
| Index | Group1 | Group2 | Group3 | F | P |
| (n = 38) | (n = 19) | (n = 19) | value | value | |
| Male/Female | 26/12 | 13/6 | 13/6 | 0.000 | 1.000 |
| Age (yr) | 14.04 ± 1.8 | 13.4 ± 2.5 | 13.4 ± 1.6 | 0.904 | 0.410 |
| BMI (kg/m2) | 29.81 ± 2.41 | 29.61 ± 1.48 | 29.36 ± 3.11 | 0.223 | 0.800 |
| ZBMI | 3.53 ± 1.17 | 3.02 ± 0.39 | 3.44 ± 1.57 | 1.268 | 0.287 |
| ALT (IU/L) | 144.77 ± 26.73 | 152.26 ± 49.30 | 139.98 ± 19.82 | 0.711 | 0.495 |
| AST (IU/L) | 86.63 ± 21.54 | 93.26 ± 38.94 | 78.55 ± 23.11 | 1.432 | 0.245 |
| TG (mmol/L) | 1.44 ± 0.35 | 1.38 ± 0.38 | 1.51 ± 0.33 | 0.698 | 0.501 |
| TCHO (mmol/L) | 4.70 ± 1.18 | 4.82 ± 0.91 | 4.61 ± 1.03 | 0.200 | 0.819 |
| FBG (mmol/L) | 4.26 ± 0.42 | 4.15 ± 0.39 | 4.22 ± 0.43 | 0.527 | 0.592 |
| FINS (IU/L) | 15.50 ± 2.10 | 15.54 ± 4.50 | 15.42 ± 1.10 | 0.011 | 0.989 |
| HOMA-IR | 2.93 ± 0.44 | 2.87 ± 0.88 | 2.89 ± 0.32 | 0.087 | 0.917 |
BMI: Body mass index; ALT: Plasma alanine aminotransferase; AST: Aspartate aminotransferase; TG: Triglyceride; TCHO: Total cholesterol; FBG: Fasting blood glucose; FINS: Fasting serum insulin; HOMA-IR: Homeostasis model assent-insulin resistance; Group 1: Receiving no intervention as control; Group 2: Taking no drug and treated only with lifestyle intervention at summer camp; Group 3: Taking vitamin E, and controlled lifestyle intervention at home.
Written informed consent was obtained from all participants and in case of minors, it was obtained from their parents. The study was conducted in accordance with the guidelines proposed in the Declaration of Helsinki and was approved by the Ethics Committee of the Children’s Hospital of Zhejiang University School of Medicine.
Lifestyle intervention
Nineteen patients in group 2 were strictly controlled by lifestyle intervention without any drug therapy. They took part in the summer camp without their parents. The Nuote Nutrient Center which was in charge of the camp, consisted of nutrient experts, physical experts and pediatricians. Physical exercises, including swimming, playing basketball and tabletennis, were taken freely for three hours of aerobic exercise each day. The diet management followed the principle of low-calorie [high in carbohydrate (50%) and low in fat (10%)] with the aim of a reduction in daily intake by 250 kcal. A total daily calorie intake was controlled from 1300 kcal to 1600 kcal based on the individual age. Two eggs and a bowl of soymilk were supplied at breakfast. Pork, egg, fish, shrimp, fresh vegetable, rice and corn were served at lunch and dinner. No beverage but mineral water was provided. They were requested to get up at 6: 30 O’clock in the morning and take aerobic physical exercise in the morning and afternoon. In the evening they did their homework and watched TV for an hour, then went to sleep at 21: 00 O’clock. The summer camp lasted one month. Nineteen patients in group 3 were controlled in lifestyle freely by themselves with total daily calorie intake and physical exercises (a low-intensity aerobic exercise to reach a 50%-60% maximum of their heart beat and maintained for 30 min, 2-3 times a week). Thirty-eight patients in group 1 did not receive any lifestyle intervention.
Drug therapeutic protocol
Patients in group 3 received vitamin E capsule at a dose of 100 mg/d for one month. Groups 1 and 2 received no drug treatment.
Monitoring indices
Indices of all patients, including the height, weight, fasting blood glucose (FBG), fasting serum insulin (FINS), plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG) and total cholesterol (TCHO), were measured in the morning after an overnight fast and repeated after a month. Fasting blood glucose was measured by the glucose oxidase method (Beijing North Biotechnology Invest, China) with intra-assay and inter-assay CV of 2.1% and 4.4%, respectively. Fasting serum insulin levels were determined by radioimmunity assay (Beijing North Biotechnology Invest, China). The intra-assay and inter-assay CV were 6.4% and 9.7%, respectively. ALT, AST, TG and TCHO were measured by routine laboratory test (BECKMAN Synchron Clinical System CX4, American) at the central laboratory of our unit. In this study, hypertransaminasemia (or elevation of serum ALT) was defined as serum ALT levels being raised by at least 1.5 times over the upper normal limit (normal range: < 50 IU/L). BMI = weight (kg)/height (m2). To compare BMI among different ages in both boys and girls, BMI Z-score was considered. The Z-score represents the number of s.d. above or below the mean value based on standardized tables for children[22]. Insulin resistance was assessed by homeostasis model assessment (HOMA-IR) based on serum fasting insulin and glucose concentrations. HOMA-IR = (FINS in mIU/L × FBG in mmol/L )/22.5. Our previous study proved that HOMA-IR was a valid insulin sensitivity index from OGTT parameters in obese children and adolescents[23].
All patients underwent an ultrasonographic study of the liver performed by one operator who was blinded to the groups. The apparatus used (LOGIC 500, GE corporation) was equipped with a convex 3.0-5.0 MHz probe. Longitudinal, subcostal, ascending, and oblique scans were performed. The ultrasonographic criteria of liver-kidney echo discrepancy, echo penetration into the deep portion of the liver, and clarity of liver blood vessel structures were used to diagnose fatty liver according to Graif M, et al[24].
Statistical analysis
Statistical analyses were conducted using SPSS software (Version 13.0). Pearson Chi-square was used to measure the enumeration data between subgroups. Quantitative data were presented as mean ± SD or median (range) and estimated by one-way ANOVA or paired-samples t test. Mann-Whitney test was used to evaluate the significance of skewed distributed data. A two-tailed P < 0.05 was considered statistically significant.
RESULTS
A total of 76 NASH patients were analyzed and observed for a month. The baseline characteristics of the three groups were not significantly different (P > 0.05) (Table 1). After one month, these characteristics in group 1 were not improved (P > 0.05) (Table 2).
Table 2.
Comparison of parameters among three groups by paired-samples t test
| Index | Group | Before intervention | After intervention | t value | P value |
| BMI | Group1 | 29.81 ± 2.41 | 29.83 ± 2.32 | -0.339 | 0.736 |
| Group2 | 29.61 ± 1.48 | 27.18 ± 1.83 | 12.892 | 0 | |
| Group3 | 29.37 ± 3.11 | 27.92 ± 3.29 | 8.034 | 0 | |
| ZBMI | Group1 | 3.53 ± 1.17 | 3.55 ± 1.16 | -1.765 | 0.086 |
| Group2 | 3.02 ± 0.39 | 2.15 ± 0.64 | 16.356 | 0 | |
| Group3 | 3.44 ± 1.57 | 2.57 ± 1.57 | 9.438 | 0 | |
| ALT | Group1 | 144.77 ± 26.73 | 144.82 ± 25.51 | -0.076 | 0.94 |
| Group2 | 152.26 ± 49.30 | 63.68 ± 23.38 | 9.654 | 0 | |
| Group3 | 139.97 ± 19.82 | 73.28 ± 10.11 | 13.219 | 0 | |
| AST | Group1 | 86.63 ± 21.54 | 85.73 ± 19.60 | 1.017 | 0.316 |
| Group2 | 93.26 ± 38.94 | 45.09 ± 19.18 | 6.699 | 0 | |
| Group3 | 78.55 ± 23.11 | 45.80 ± 6.66 | 6.9 | 0 | |
| TG | Group1 | 1.44 ± 0.35 | 1.46 ± 0.31 | -0.69 | 0.494 |
| Group2 | 1.38 ± 0.38 | 0.99 ± 0.37 | 3.851 | 0.001 | |
| Group3 | 1.51 ± 0.33 | 1.27 ± 0.28 | 4.6 | 0 | |
| THCO | Group1 | 4.70 ±1.18 | 4.69 ± 1.09 | 0.204 | 0.84 |
| Group2 | 4.83 ± 0.92 | 4.54 ± 0.98 | 2.783 | 0.012 | |
| Group3 | 4.61 ± 1.03 | 4.23 ± 0.82 | 2.24 | 0.038 | |
| FBG | Group1 | 4.26 ± 0.42 | 4.26 ± 0.32 | -0.046 | 0.964 |
| Group2 | 4.15 ± 0.39 | 4.13 ± 0.42 | 0.154 | 0.879 | |
| Group3 | 4.22 ± 0.43 | 4.08 ± 0.41 | 1.11 | 0.279 | |
| FINS | Group1 | 15.50 ± 2.10 | 15.71 ± 2.19 | -0.941 | 0.353 |
| Group2 | 15.54 ± 4.50 | 8.53 ± 4.08 | 4.322 | 0 | |
| Group3 | 15.42 ± 1.10 | 8.77 ± 2.46 | 10.26 | 0 | |
| HOMA-IR | Group1 | 2.93 ± 0.44 | 2.97 ± 0.51 | -0.886 | 0.382 |
| Group2 | 2.87 ± 0.88 | 1.63 ± 0.92 | 3.579 | 0.002 | |
| Group3 | 2.89 ± 0.32 | 1.62 ± 0.59 | 8.08 | 0 |
BMI: Body mass index; ALT: Plasma alanine aminotransferase; AST: aspartate aminotransferase; TG: Triglyceride; TCHO: Total cholesterol; FBG: Fasting blood glucose; FINS: Fasting serum insulin; HOMA-IR: Homeostasis model assent-insulin resistance.
In groups 2 and 3, all patients had declined BMI, ZBMI, ALT, AST, TG, TCHO, FINS and HOMA-IR after a month. There was more significant reduction in BMI and ALT levels after intervention in group 2 than in group 3 (U = 73.000, P = 0.001; U = 117.000, P = 0.040, respectively). No difference was found in the other indices between groups 2 and 3 (P > 0.05). Ten patients (52.63%) in group 2 had normal liver functions after the camping was completed.
Nine patients (47.37%) in group 3 became normal in liver functions in the end. The liver ultrasonography did not demonstrate any predominant changes after a one-month lifestyle intervention.
DISCUSSION
Non-alcoholic fatty liver disease affects a large proportion of the population. In our previous study, the prevalence of NAFLD was 22.41% among all obese subjects and the male is apt to obesity with NAFLD[1,25]. The pathogenesis of NAFLD has remained poorly understood since the earliest description of this disease. However, insulin resistance and oxidative stress play critical roles in the pathogenesis of non-alcoholic fatty liver disease. As yet no accepted drug treatment of NAFLD/NASH has been reported.
In this study, we found that the body weights of all the patients except control group were successfully reduced. Lifestyle intervention was associated with ALT improvement after a month. And it seemed that vitamin E therapy also improved the liver function. At the same time, lifestyle intervention proved to be more efficient than vitamin E therapy on BMI and ALT. The liver ultrasonography did not demonstrate any predominant change after a month intervention. It is likely that ultrasonography is not sensitive enough to detect an initial and short improvement in steatosis. Weight reduction will improve not only the liver condition but also the metabolic (insulin resistance) syndrome. Exercise is known to improve the sensitivity of muscle mass to insulin[26–28]. Furthermore, a recent clinical trial showed that exercise has modest therapeutic effect in reducing visceral fat and improving glucose intolerance[29]. These may partially explain the beneficial effect of lifestyle intervention in improving the hypertransaminasemia. Frequency and intensity of ideal exercise for treatment of NAFLD remains unknown. Suzuki A et al reported that patients with NAFLD should be encouraged to keep regular exercise and at least twice a week[30].
Vitamin E is frequently used among patients with NAFLD. The useful effect of vitamin E on inflammation and fibrosis among patients with NASH has been attributed to its potent antioxidant action. Oxidant stress has been cited as an important second hit in the pathogenesis of NASH, and obese children have been demonstrated to have significantly decreased serum levels of α-tocopherol[31–34]. In these studies, the dose of vitamin E was 300-1200 mg/d. In one study, vitamin E 300 mg/d was associated with fibrosis reversal[35]. However, Nobili et al reported that in 90 patients in biopsy-proven NAFLD children, a balanced calorie diet, physical exercise, and placebo or alpha-tocopherol 600 IU/d plus ascorbic acid 500 mg/d and a 12-mo double-blind placebo study found that vitamin E therapy had no effect on ALT or insulin resistance[36]. Kugelmans et al found that vitamin E improved insulin sensitivity and several of its associated parameters, including ALT levels in overweight otherwise healthy subjects but the effect of treatment was not sustained[37]. In our study, vitamin E was also found to improve insulin sensitivity and ALT level in a month therapy. So, we conclude that a short-time therapy with 100 mg vitamin E and lifestyle intervention may have an effect on ALT levels and insulin resistance in children with NAFLD.
Lifestyle intervention has been recommended for the treatment of NAFLD in obese children. Early intervention should attempt to increase physical activity while implementing dietary and other antiobesity measures. The emphasis should be laid on slow and modest reduction of body mass, not exceeding 2 pounds (1 kg)/wk, coupled with increased physical activity[20]. It is very encouraging that lifestyle intervention had short-term effect, but the long-term effect on NAFLD remains to be clarified. After the camping, the children and their monitors were assembled and taught how to do exercise and how to arrange daily diet. They were encouraged to have a low-intensity aerobic exercise such as playing basketball and table-tennis, quick walking, slow running or string jumping, etc to reach a 50%-60% maximum of their heart beat and maintain for at least 30 min 2-3 times a week. Diet was tailored based on individual preferences and balanced as hypocaloric diet (25-30 cal/kg per day, carbohydrate 50%-60%, fat 23%-30%, protein 15%-20%, fatty acid: two-thirds saturated, one-third unsaturated, ω6/ω3 ratio = 4:1).
In summary, our results suggest that simple lifestyle intervention with physical exercise and diet in children with NAFLD can lead to a significant improvement of liver function and insulin resistance. A short-term vitamin E therapy also has a effect on NAFLD in obese children. Compared with vitamin E therapy, lifestyle intervention is more effective. Therefore, lifestyle intervention should represent the first step in the management of children with NAFLD.
COMMENTS
Background
Non-alcoholic fatty liver disease (NAFLD) is likely to become epidemic in children worldwide in the latest decade. NAFLD is recognized as a cause of potentially progressive liver damage. The gold standard of diagnosis is liver biopsy but it is not frequently performed in the pediatric population. In the absence of liver biopsy, presumed NASH is conventionally diagnosed by classical ultrasonography together with an elevated serum level of alanine aminotransferase (ALT).
Research frontiers
Multiple therapeutic agents such as vitamin E, metformin, and lifestyle intervention have demonstrated to be useful in NAFLD. However, no accepted drug treatment of NAFLD/NASH has been reported.
Innovations and breakthroughs
Although the relation between diet and fat liver is known, it is the first report about lifestyle intervention on NAFLD in Chinese obese children. In this study, both a short-term lifestyle intervention and a short-term vitamin E therapy have an effect while lifestyle intervention is more effective than vitamin E.
Applications
Lifestyle intervention should represent the first step in the management of children with NAFLD.
Terminology
NAFLD encompasses a spectrum of disease ranging from simple hepatic steatosis to non-alcoholic steatohepatitis. The entire range of liver involvement characterizing NAFLD can occur in children, such as hepatic macrovesicular steatosis without inflammation, steatosis with inflammation or fibrosis (which defined as non-alcoholic steatohepatitis, NASH), and cirrhosis. NAFLD may be the hepatic aspect of the metabolic syndrome that comprises the central obesity, insulin resistance, hypertension and dyslipidemia in adults and children.
Peer review
This manuscript is of some interest because therapeutic intervention is limited to one month and the adherence to the program is really rigorous. This experiment may provide insights on the short-term modification of some indexes of NAFLD that probably anticipate the improvement of fatty liver.
Acknowledgments
We thank all children and their parents for participating in this project.
Supported by Science and Technology Department of Zhejiang Province of China, No. 2005C24001, No. 2004C30064
Peer reviewer: Amedeo Columbano, Professor, Dipartimento di Tossicologia, Sezione di Oncologia e Patologia Molecolare, Via Porcell 4, Cagliari 09124, Italy
S- Editor Zhu WL L- Editor Ma JY E- Editor Yin DH
References
- 1.Fu JF, Liang L, Zou CC, Hong F, Wang CL, Wang XM, Zhao ZY. Prevalence of the metabolic syndrome in Zhejiang Chinese obese children and adolescents and the effect of metformin combined with lifestyle intervention. Int J Obes (Lond) 2007;31:15–22. doi: 10.1038/sj.ijo.0803453. [DOI] [PubMed] [Google Scholar]
- 2.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 3.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 4.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042–2047. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell SH, Hespenheide EE. Subacute liver failure in obese women. Am J Gastroenterol. 2002;97:2058–2062. doi: 10.1111/j.1572-0241.2002.05922.x. [DOI] [PubMed] [Google Scholar]
- 6.Hui JM, Kench JG, Chitturi S, Sud A, Farrell GC, Byth K, Hall P, Khan M, George J. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38:420–427. doi: 10.1053/jhep.2003.50320. [DOI] [PubMed] [Google Scholar]
- 7.Fassio E, Alvarez E, Dominguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820–826. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 8.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 10.Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, Karim R, Lin R, Samarasinghe D, Liddle C, et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 11.Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, Kusano Y. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995;40:2002–2009. doi: 10.1007/BF02208670. [DOI] [PubMed] [Google Scholar]
- 12.Franzese A, Vajro P, Argenziano A, Puzziello A, Iannucci MP, Saviano MC, Brunetti F, Rubino A. Liver involvement in obese children. Ultrasonography and liver enzyme levels at diagnosis and during follow-up in an Italian population. Dig Dis Sci. 1997;42:1428–1432. doi: 10.1023/a:1018850223495. [DOI] [PubMed] [Google Scholar]
- 13.Stephen CH, Tri HL, Stacey MA. Non alcoholic steatohepatitis. In: Schiff ER, sorrell MF and Maddrey WC Schiff’s diseases of the liver (9th edition), editors. Philadelphia: Lippinott William &Wilkins; 2003. pp. 1261–1289. [Google Scholar]
- 14.Kawanaka M, Mahmood S, Niiyama G, Izumi A, Kamei A, Ikeda H, Suehiro M, Togawa K, Sasagawa T, Okita M, et al. Control of oxidative stress and reduction in biochemical markers by Vitamin E treatment in patients with nonalcoholic steatohepatitis: a pilot study. Hepatol Res. 2004;29:39–41. doi: 10.1016/j.hepres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 16.Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 17.Harrison SA, Ramrakhiani S, Brunt EM, Anbari MA, Cortese C, Bacon BR. Orlistat in the treatment of NASH: a case series. Am J Gastroenterol. 2003;98:926–930. doi: 10.1111/j.1572-0241.2003.07375.x. [DOI] [PubMed] [Google Scholar]
- 18.Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136:727–733. [PubMed] [Google Scholar]
- 19.Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, Powell EE. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413–419. doi: 10.1136/gut.2003.027581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Body mass index reference norm for screening overweight and obesity in Chinese children and adolescents. Zhonghua Liuxingbingxue Zazhi. 2004;25:97–102. [PubMed] [Google Scholar]
- 21.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 22.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang CL, Liang L, Fu JF, Hong F. Comparison of methods to detect insulin resistance in obese children and adolescents. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2005 Jul;34(4):316–319. doi: 10.3785/j.issn.1008-9292.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Graif M, Yanuka M, Baraz M, Blank A, Moshkovitz M, Kessler A, Gilat T, Weiss J, Walach E, Amazeen P, et al. Quantitative estimation of attenuation in ultrasound video images: correlation with histology in diffuse liver disease. Invest Radiol. 2000;35:319–324. doi: 10.1097/00004424-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Zou CC, Liang L, Hong F, Fu JF, Zhao ZY. Serum adiponectin, resistin levels and non-alcoholic fatty liver disease in obese children. Endocr J. 2005;52:519–524. doi: 10.1507/endocrj.52.519. [DOI] [PubMed] [Google Scholar]
- 26.Dela F, Mikines KJ, von Linstow M, Secher NH, Galbo H. Effect of training on insulin-mediated glucose uptake in human muscle. Am J Physiol. 1992;263:E1134–E1143. doi: 10.1152/ajpendo.2006.263.6.E1134. [DOI] [PubMed] [Google Scholar]
- 27.Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol. 1988;254:E248–E259. doi: 10.1152/ajpendo.1988.254.3.E248. [DOI] [PubMed] [Google Scholar]
- 28.Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- 29.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki A, Lindor K, St Saver J, Lymp J, Mendes F, Muto A, Okada T, Angulo P. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol. 2005;43:1060–1066. doi: 10.1016/j.jhep.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734–738. [PubMed] [Google Scholar]
- 32.Vajro P, Mandato C, Franzese A, Ciccimarra E, Lucariello S, Savoia M, Capuano G, Migliaro F. Vitamin E treatment in pediatric obesity-related liver disease: a randomized study. J Pediatr Gastroenterol Nutr. 2004;38:48–55. doi: 10.1097/00005176-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667–1672. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 34.Strauss RS. Comparison of serum concentrations of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and nonobese children (NHANES III). National Health and Nutrition Examination Survey. J Pediatr. 1999;134:160–165. doi: 10.1016/s0022-3476(99)70409-9. [DOI] [PubMed] [Google Scholar]
- 35.Executive summary of the third report of the National Cholesterol Enducation Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 36.Nobili V, Manco M, Devito R, Ciampalini P, Piemonte F, Marcellini M. Effect of vitamin E on aminotransferase levels and insulin resistance in children with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2006;24:1553–1561. doi: 10.1111/j.1365-2036.2006.03161.x. [DOI] [PubMed] [Google Scholar]
- 37.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–419. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]


