Abstract
AIM: To investigate the changes of histology and expression of MMP-2 and nm23-H1 in primary and metastatic gastric cancer.
METHODS: One hundred and seventy-seven gastric cancer patients with lymph node and/or distal metastasis between 1997 and 2001 were reviewed. Differences in histology of the primary and metastatic gastric cancer were assessed. MMP-2 and nm23-H1 immunoreactivity was compared in 44 patients with tumor infiltration to the serosa layer.
RESULTS: Poorly and moderately differentiated metastatic gastric cancer was found in 88.7% (157/177) and primary gastric cancer in 75.7% (134/177) of the patients. The histological type of metastatic gastric cancer that was not completely in accordance with the preponderant histology of primary gastric cancer was observed in 25 patients (14.1%). MMP-2 immunoreactivity in metastatic gastric cancer was significantly stronger than that in primary gastric cancer, while nm23-H1 immunoreactivity showed no difference in primary and metastatic gastric cancer.
CONCLUSION: Metastatic gastric cancer presents more aggressive histological morphology and higher MMP-2 immunoreactivity than primary gastric cancer. This heterogeneity may elicit a possible mechanism of gastric cancer metastasis.
Keywords: Heterogeneity, Gastric cancer, Nm23-H1, MMP-2, Histological change
INTRODUCTION
Gastric cancer, one of the most common malignant diseases in the world, has been shown to frequently metastasize. Certain studies have reported the possible mechanisms underlying its metastasis[1–3]. However, whether there is a histological difference between primary and metastatic gastric cancer is unclear and has been rarely reported.
Matrix metalloproteinases (MMPs) are defined as a family of enzymes which degrade extracellular membrane proteins, thus playing a significant role in tumor invasion and metastasis[4]. MMP-2 is one of the most extensively studied MMPs in the process of cancer. It was reported that elevated MMP-2 level is related to increased tumor metastasis and stage in the lung, breast, stomach and colon[5–8]. However, differences in MMP-2 expression between primary and metastatic gastric cancer have been rarely assessed.
The nm23 gene is a putative metastasis suppressor gene originally identified in metastatic murine melanoma cells[9]. Reduction in nm23 expression is related with a high incidence of lymph node metastasis or poor prognosis of gastric cancer[10,11]. However, there is no inverse relationship between nm23 expression and metastatic potential of gastric cancer[12]. Their relationship in gastric cancer remains controversial.
Our study was to investigate the differences in histology and expression of MMP-2 and nm23-H1 between primary and metastatic gastric cancer, and to elucidate the possible mechanism of tumor heterogeneity underlying gastric cancer metastasis.
MATERIALS AND METHODS
Patients and tissue specimens
Complete data and tissue specimens were obtained from 230 gastric cancer patients, who underwent resection of gastric cancer at Sir Run Run Shaw Hospital, Zhejiang University College of Medicine between June 1997 and January 2001. Among them, 177 patients including 124 males and 53 females, ranging in age from 20 years to 79 years with a mean age of 55.5 years, had pathologically confirmed lymph node and/or distal metastasis and were enrolled to assess the differences in histology between primary and metastatic gastric cancer. Their clinicopathological features are shown in Table 1. Disease stage was classified based on the 5th edition of the International Union against Cancer and the American Joint Committee for Cancer Staging.
Table 1.
Clinicopathologic data obtained from 177 gastric cancer patients
|
Patients |
||
| n | % | |
| Tumor size (cm) (mean ± SD) | 6.0 ± 2.9 | |
| Location | ||
| Upper or whole body | 40 | 22.6 |
| Lower or middle body | 137 | 77.4 |
| Gross type | ||
| Localized | 39 | 22.0 |
| Infiltrative | 138 | 78.0 |
| Depth of invasion | ||
| T1 | 25 | 14.1 |
| T2 | 86 | 48.6 |
| T3 | 44 | 24.9 |
| T4 | 22 | 12.4 |
| Retrieved lymph nodes (mean ± SD) | 22.4 ± 3.5 | |
| Stage | ||
| I | 6 | 3.4 |
| II | 29 | 16.4 |
| III | 88 | 49.7 |
| IV | 54 | 30.5 |
Forty-four patients, who had pathologically confirmed tumor infiltration to the serosal layer (24 pts, T3N1-3M0. 20 pts, T3NxM1), were recruited to evaluate the difference in MMP-2 and nm23-H1 immunoreactivity between primary and metastatic gastric cancer by immunohistochemistry.
Slides of tissue from primary and metastatic gastric cancer were observed by two pathologists. Following the criteria of World Health Organization (WHO), papillary adenocarcinoma was classified as well differentiated type, signet cell carcinoma and mucious adenocarcinoma as poorly differentiated type, tubular adenocarcinoma as well or moderately or poorly differentiated type.
We set the largest proportion of histological type of primary gastric cancer as the preponderant histological type. Thereby, percentage of the preponderant histological type of metastatic gastric cancer was as follows: -: lower than 5%; +: 5%-25%; ++: 25%-50%; +++: 50%-75%; ++++: higher than 75%. Comparison of changes in histology between primary and metastatic gastric cancer was made based on the percentages of their preponderant histological type.
Immunohistochemistry
Immunohistochemical study was performed using the following antibodies: anti-nm23-H1 protein (GE-213, monoclonal, 1:100; Manxin, Fuzhou, China) and anti-MMP-2 (CA-4001, monoclonal, 1:50; Manxin, Fuzhou, China). Four-μm thick sections of 10% formalin-fixed, paraffin-embedded gastric cancer tissue were cut, mounted on glass slides coated with 3-aminopropyltriehoxysilane, and air-dried overnight at 60°C. The sections were deparaffinized in xylene and rehydrated in ethanol. Endogenous peroxidase was blocked with methanol containing 3% hydrogen peroxidase for 25 min. For staining with anti-MMP-2, sections were pretreated with citrate buffer (0.01 mol/L, pH 6.0) and heated at 100°C in a microwave oven for 20 min. For staining with anti-nm23-H1, sections were pretreated with trypsin (0.5%, pH 7.4) for 20 min at room temperature. The sections were incubated with primary antibodies at 4°C overnight, stained with a streptavidin-biotin-peroxidase kit (Manxin, Fuzhou, China), and reacted in a solution containing 3, 3’-diaminobenzidine and peroxytrichloride substrate, and counterstained with hematoxylin. The provided sections known to react positively with nm23-H1 or MMP-2 (Manxin, Fuzhou, China) were used as a positive control. As a negative control, the primary antibody was deleted.
Evaluation
The immunoreactivity of each antibody was evaluated. MMP-2 and nm23-H1 immunoreactivity was graded as -: without or with immunoreactivity in less than 5% tumor cells; +: immunoreactivity in 5%-25% tumor cells; ++: immunoreactivity in 25%-50% tumor cells; +++: immunoreactivity in over 50% of tumor cells.
Statistical analysis
All statistical analyses were conducted using the statistical program SPSS 10.0 for windows (SPSS, Chicago, IL, USA). Differences in histological morphology and expression of MMP-2 and nm23-H1 between each group were analyzed by chi-square test or by Fisher’s exact test. P < 0.05 was considered statistically significant.
RESULTS
Histological changes
We observed different histological changes in primary and metastatic gastric cancer patients (Figure 1). Poorly and moderately differentiated metastatic gastric cancer was found in 88.7% (157/177) of the patients while primary gastric cancer in 75.7% (134/177) of the patients. The preponderant histological types of primary gastric cancer, graded as +++ and ++++, were more than those of metastatic lymph nodes (170 vs 138, P < 0.01). Moreover, the preponderant histological type of the metastatic lymph nodes in 14.1% patients (25/177) was not completely in accordance with that of primary gastric cancer (Table 2).
Figure 1.
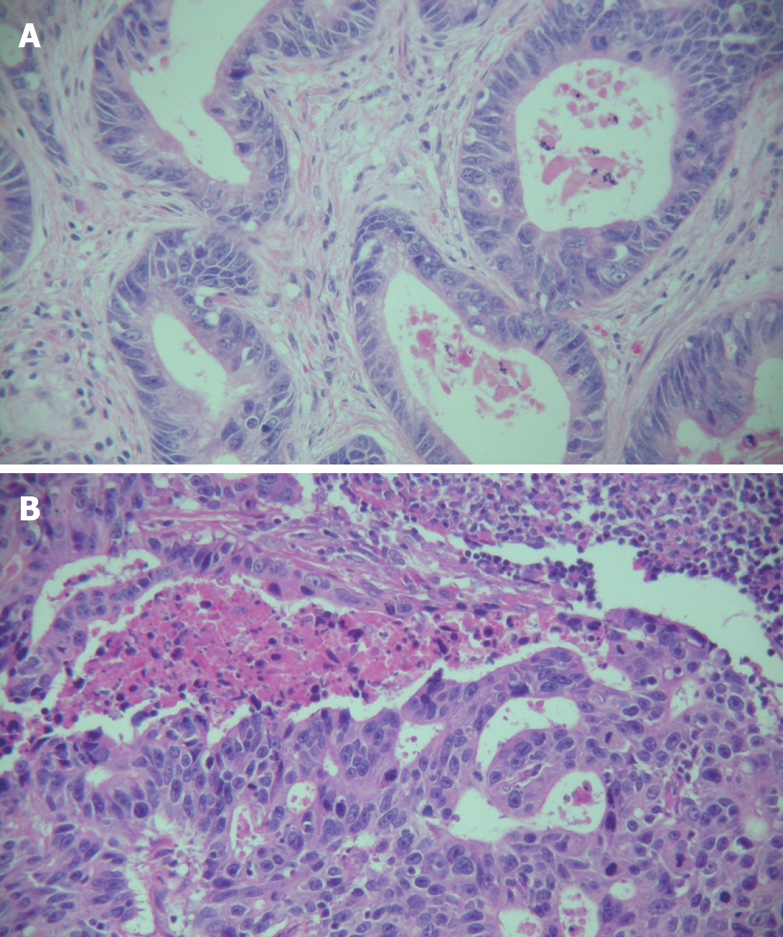
Histological changes in primary and metastatic gastric cancer (case 15) (× 200). A: Histological change in primary gastric cancer showing a well differentiated adenocarcinoma of the stomach with a glandular pattern; B: Histological changes in metastatic gastric cancer showing a moderately differentiated adenocarcinoma with a cribriform pattern.
Table 2.
Preponderant histological type of primary and metastatic lymph nodes in 177 gastric cancer patients
| Tumor type |
Grade |
P value | ||||
| - | + | ++ | +++ | ++++ | ||
| Primary lymph nodes | 0 | 0 | 7 | 69 | 101 | < 0.01 |
| Metastatic lymph nodes | 17 | 8 | 14 | 44 | 94 | |
MMP-2 immunoreactivity
MMP-2 immunoreactivity was significantly stronger in metastatic gastric cancer than in primary gastric cancer. For the 20 patients with distal metastasis, a different MMP-2 immunoreactivity was observed in primary and metastatic gastric cancer (Table 3). MMP-2 immunoreactivity was stronger in metastatic gastric cancer than in primary gastric cancer. However, the immunoreactivity was similar in metastatic lymph nodes and distal metastasis (Figure 2).
Table 3.
MMP-2 immunoreactivity in primary and metastatic T3 gastric cancer
| Patients (n) | Tumor type |
Grade |
P value | |||
| - | + | ++ | +++ | |||
| 44 T3NxMx | Primary lesion | 12 | 6 | 14 | 12 | 0.004 |
| Patients | Metastatic lymph node | 7 | 6 | 9 | 22 | |
| 20 T3NxM1 | Primary lesion | 6 | 3 | 7 | 4 | 0.019 |
| Patients | Metastatic lymph node | 4 | 3 | 4 | 9 | |
| Distal metastatic site | 3 | 5 | 2 | 10 | ||
Figure 2.
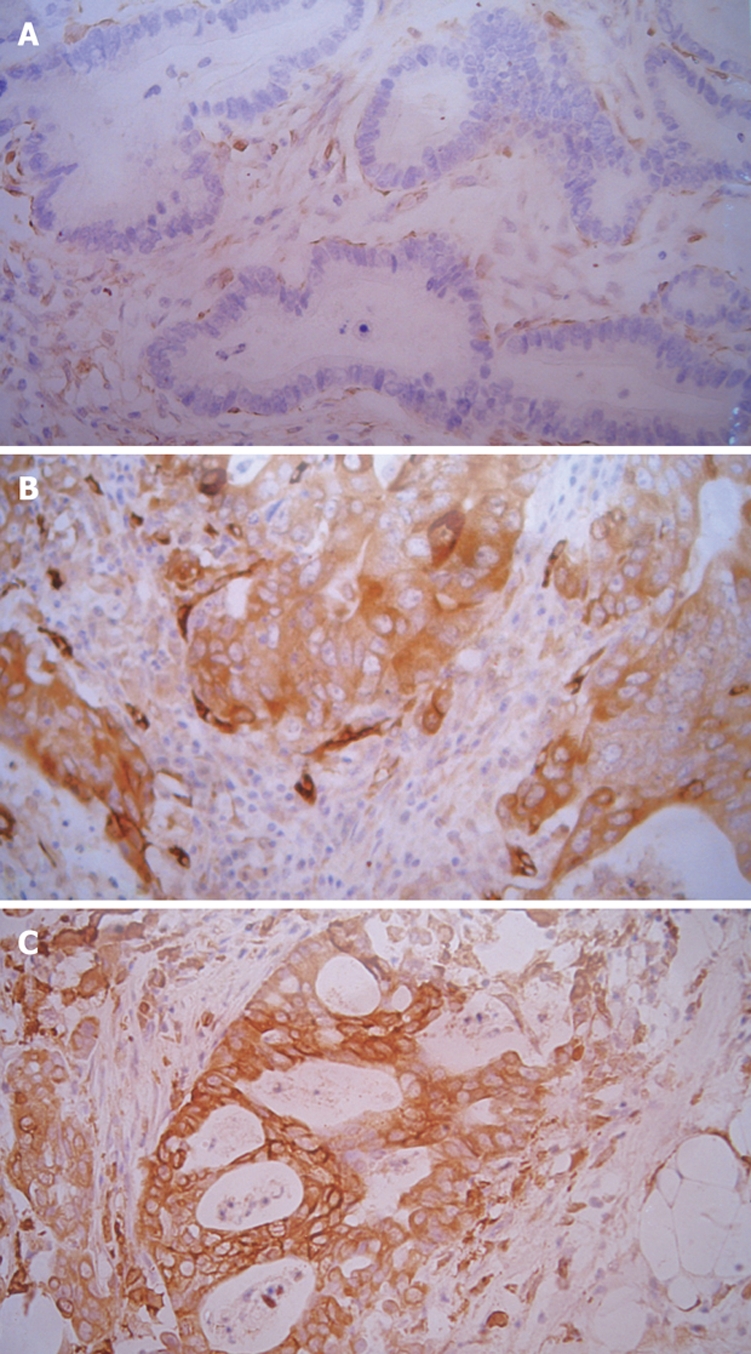
Immunohistochemistry of MMP-2 in gastric cancer (case 9) (× 200). A: Primary gastric cancer showing grade (-) immunoreactivity in MMP-2; B: Metastatic gastric cancer showing grade (+++) immunoreactivity; C: Distal metastatic gastric cancer showing grade (+++) immunoreactivity.
nm23-H1 immunoreactivity
There was no significant difference in nm23-H1 immunoreactivity between primary and metastatic gastric cancer. The immunoreactivity was quite similar in primary and metastatic lymph nodes and distal metastasis (Table 4).
Table 4.
nm23-H1 immunoreactivity in primary and metastatic T3 gastric cancer
| Patients (n) | Tumor type |
Grade |
P value | |||
| - | + | ++ | +++ | |||
| 44 T3NxMx | Primary lesion | 12 | 15 | 15 | 2 | 0.138 |
| Metastatic lymph node | 22 | 9 | 7 | 6 | ||
| 20 T3NxM1 | Primary lesion | 8 | 4 | 7 | 1 | 0.497 |
| Metastatic lymph node | 9 | 3 | 5 | 3 | ||
| Distal metastatic site | 10 | 3 | 5 | 2 | ||
DISCUSSION
Studies on intratumoral and intertumoral heterogeneity have provided valuable insights into the pathogenesis and progression of different tumors[13–15]. Although the concept of intratumoral heterogeneity of tumors has been generally accepted, studies on it in gastric cancer are scant. Previous reports focused mainly on comparison of molecular genetic alterations in each individual. However, this study investigated the tumor heterogeneity including histological morphology changes in primary and metastatic gastric cancer.
We observed different histological changes in primary and metastatic gastric cancer. Metastatic gastric cancer showed poorer differentiation. Meanwhile, the preponderant histological type of primary and metastatic gastric cancer was not completely identical. In the present study, the preponderant histological type of primary and metastatic gastric cancer site was different in 14.1% patients. These findings may imply that not all the histological types of primary gastric cancer have potential to metastasize, poorly differentiated cancer cells may play a significant role in lymph node metastasis, which may possibly explain why a small proportion of poorly differentiated cancer cells in primary gastric cancer may be preponderant in metastatic gastric cancer.
It is widely accepted that tumor may synchronously contain multiple histological types, reflecting different tumor differentiation and biological behavior. Most tumors may contain multiple cell clones with a diverse metastatic potential. Cell clones with a high metastatic potential are apt to metastasize to lymph nodes or distal organs[16,17]. This dynamic heterogeneity may give a possible explanation for the different changes in histology between primary and metastatic gastric cancer. Although the preponderant histological change in primary gastric cancer is generally considered a prognostic predictor, the other histological changes in primary gastric cancer, especially in poorly differentiated subclones, may be as important as the preponderant histological change for the prognosis of primary gastric cancer. However, few studies have been addressed this issue, further investigations are warranted.
Because of its ability to degrade the basement membrane, MMP-2 has been postulated as a potential marker of tumor progression and prognosis in different malignancies such as ovarian cancer, gastric cancer and lung carcinoma[8,18]. Schwartz et al[19] reported that MMP-2 is expressed in SK-GT1, SK-GT5 and SK-GT6 but not in SK-GT2 and SK-GT4 gastric cancer cell lines. Ji et al[20] reported that MMP-2 expression is significantly higher in advanced than in early gastric cancer patients[20]. These findings indicate that gastric cancer cells with a greater malignant and metastatic potential may secrete much more MMP-2 protein. Moreover, it has been shown that down-regulation of MMP or MMP-2 may inhibit tumor growth and metastasis, indicating that MMP-2 is correlated with gastric cancer invasion and metastasis[21,22]. In this study, MMP-2 immunoreactivity was significantly higher in metastatic than in primary gastric cancer. This is in agreement with previous reports and suggests that when cancer tends to become invasive, elevated MMP-2 may play a pivotal role in its metastasis.
It was reported that Nm23 is a metastasis suppressor gene[23]. However, its role in gastric cancer metastasis is controversial[24,25]. Nakayama et al[26] demonstrated that reduced expression of nm23 is associated with gastric cancer metastasis. Similar results have been reported by Hsu et al[27]. However, Wang et al[28] found that patients with a high nm23 expression are easy to develop distal metastasis and have a lower nm23 expression, displaying that nm23 may play a trivial role in inhibiting tumor metastasis. Yeung et al[29] reported that there is no difference in nm23 expression between primary and metastatic gastric cancer. Similar results were observed in the present study, suggesting that nm23 expression is not associated with gastric cancer metastasis. Further study is needed to find the definitive role of nm23 in cancer metastasis.
In conclusion, metastatic gastric cancer is more aggressive and has a higher expression in tumor genes than primary gastric cancer. This heterogeneity may elicit one of the possible mechanisms underlying gastric cancer metastasis.
COMMENTS
Background
Gastric cancer metastasis occurs frequently and its possible mechanism has not been well addressed. Intratumoral and intertumoral heterogeneity plays a significant role in tumor progression. However, studies on gastric cancer are rarely reported.
Research frontiers
The aim of this study was to evaluate the morphology and tumor metastatic gene heterogeneity in gastric cancer.
Innovations and breakthroughs
This study showed the changes in morphology and expression of MMP-2 of primary and metastatic gastric cancer.
Applications
The heterogeneity in gastric cancer may provide a clue to the possible mechanism of cancer invasion and metastasis.
Peer review
This is a nice study comparing primary and metastatic gastric cancer. The expression of MMP2, one of the known metalloproteases relating to tumor invasion and metastases, was stronger in metastatic than in primary gastric cancer. However, NM23-H1 expression did not change in primary and metastatic gastric cancer. Primary and metastatic gastric cancer were found to have different histological types.
Peer reviewer: Shingo Tsuji, Professor, Department of Internal Medicine and Therapeutics, Osaka University Graduate School of Medicine (A8), 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan
S- Editor Yang RH L- Editor Wang XL E- Editor Liu Y
References
- 1.Gentile A, Comoglio PM. Invasive growth: a genetic program. Int J Dev Biol. 2004;48:451–456. doi: 10.1387/ijdb.041799ag. [DOI] [PubMed] [Google Scholar]
- 2.Liotta LA, Kohn E. Cancer invasion and metastases. JAMA. 1990;263:1123–1126. [PubMed] [Google Scholar]
- 3.Schwartz GK. Invasion and metastases in gastric cancer: in vitro and in vivo models with clinical correlations. Semin Oncol. 1996;23:316–324. [PubMed] [Google Scholar]
- 4.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 5.Passlick B, Sienel W, Seen-Hibler R, Wockel W, Thetter O, Mutschler W, Pantel K. Overexpression of matrix metalloproteinase 2 predicts unfavorable outcome in early-stage non-small cell lung cancer. Clin Cancer Res. 2000;6:3944–3948. [PubMed] [Google Scholar]
- 6.Tryggvason K, Hoyhtya M, Pyke C. Type IV collagenases in invasive tumors. Breast Cancer Res Treat. 1993;24:209–218. doi: 10.1007/BF01833261. [DOI] [PubMed] [Google Scholar]
- 7.Nomura H, Sato H, Seiki M, Mai M, Okada Y. Expression of membrane-type matrix metalloproteinase in human gastric carcinomas. Cancer Res. 1995;55:3263–3266. [PubMed] [Google Scholar]
- 8.Wu ZY, Li JH, Zhan WH, He YL. Lymph node micrometastasis and its correlation with MMP-2 expression in gastric carcinoma. World J Gastroenterol. 2006;12:2941–2944. doi: 10.3748/wjg.v12.i18.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steeg PS, Bevilacqua G, Kopper L, Thorgeirsson UP, Talmadge JE, Liotta LA, Sobel ME. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst. 1988;80:200–204. doi: 10.1093/jnci/80.3.200. [DOI] [PubMed] [Google Scholar]
- 10.Barnes R, Masood S, Barker E, Rosengard AM, Coggin DL, Crowell T, King CR, Porter-Jordan K, Wargotz ES, Liotta LA. Low nm23 protein expression in infiltrating ductal breast carcinomas correlates with reduced patient survival. Am J Pathol. 1991;139:245–250. [PMC free article] [PubMed] [Google Scholar]
- 11.Ayhan A, Yasui W, Yokozaki H, Kitadai Y, Tahara E. Reduced expression of nm23 protein is associated with advanced tumor stage and distant metastases in human colorectal carcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63:213–218. doi: 10.1007/BF02899264. [DOI] [PubMed] [Google Scholar]
- 12.Charpin C, Garcia S, Bonnier P, Martini F, Andrac L, Horschowski N, Lavaut MN, Allasia C. Prognostic significance of Nm23/NDPK expression in breast carcinoma, assessed on 10-year follow-up by automated and quantitative immunocytochemical assays. J Pathol. 1998;184:401–407. doi: 10.1002/(SICI)1096-9896(199804)184:4<401::AID-PATH1220>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 13.Lichy JH, Dalbegue F, Zavar M, Washington C, Tsai MM, Sheng ZM, Taubenberger JK. Genetic heterogeneity in ductal carcinoma of the breast. Lab Invest. 2000;80:291–301. doi: 10.1038/labinvest.3780034. [DOI] [PubMed] [Google Scholar]
- 14.Yamasaki M, Takeshima Y, Fujii S, Matsuura M, Tagawa K, Inai K. Correlation between morphological heterogeneity and genetic alteration within one tumor in adenocarcinomas of the lung. Pathol Int. 2000;50:891–896. doi: 10.1046/j.1440-1827.2000.01134.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuukasjarvi T, Karhu R, Tanner M, Kahkonen M, Schaffer A, Nupponen N, Pennanen S, Kallioniemi A, Kallioniemi OP, Isola J. Genetic heterogeneity and clonal evolution underlying development of asynchronous metastasis in human breast cancer. Cancer Res. 1997;57:1597–1604. [PubMed] [Google Scholar]
- 16.Ling V, Chambers AF, Harris JF, Hill RP. Dynamic heterogeneity and metastasis. J Cell Physiol Suppl. 1984;3:99–103. doi: 10.1002/jcp.1041210412. [DOI] [PubMed] [Google Scholar]
- 17.Naito S, Walker SM, von Eschenbach AC, Fidler IJ. Evidence for metastatic heterogeneity of human renal cell carcinoma. Anticancer Res. 1988;8:1163–1167. [PubMed] [Google Scholar]
- 18.Kubben FJ, Sier CF, van Duijn W, Griffioen G, Hanemaaijer R, van de Velde CJ, van Krieken JH, Lamers CB, Verspaget HW. Matrix metalloproteinase-2 is a consistent prognostic factor in gastric cancer. Br J Cancer. 2006;94:1035–1040. doi: 10.1038/sj.bjc.6603041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz GK, Wang H, Lampen N, Altorki N, Kelsen D, Albino AP. Defining the invasive phenotype of proximal gastric cancer cells. Cancer. 1994;73:22–27. doi: 10.1002/1097-0142(19940101)73:1<22::aid-cncr2820730106>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Ji F, Chen YL, Jin EY, Wang WL, Yang ZL, Li YM. Relationship between matrix metalloproteinase-2 mRNA expression and clinicopathological and urokinase-type plasminogen activator system parameters and prognosis in human gastric cancer. World J Gastroenterol. 2005;11:3222–3226. doi: 10.3748/wjg.v11.i21.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Morisaki T, Matsunaga H, Sato N, Uchiyama A, Hashizume K, Nagumo F, Tadano J, Katano M. Protein-bound polysaccharide PSK inhibits tumor invasiveness by down-regulation of TGF-beta1 and MMPs. Clin Exp Metastasis. 2000;18:343–352. doi: 10.1023/a:1010897432244. [DOI] [PubMed] [Google Scholar]
- 22.Denkert C, Siegert A, Leclere A, Turzynski A, Hauptmann S. An inhibitor of stress-activated MAP-kinases reduces invasion and MMP-2 expression of malignant melanoma cells. Clin Exp Metastasis. 2002;19:79–85. doi: 10.1023/a:1013857325012. [DOI] [PubMed] [Google Scholar]
- 23.Gilles AM, Presecan E, Vonica A, Lascu I. Nucleoside diphosphate kinase from human erythrocytes. Structural characterization of the two polypeptide chains responsible for heterogeneity of the hexameric enzyme. J Biol Chem. 1991;266:8784–8789. [PubMed] [Google Scholar]
- 24.Seifert M, Welter C, Mehraein Y, Seitz G. Expression of the nm23 homologues nm23-H4, nm23-H6, and nm23-H7 in human gastric and colon cancer. J Pathol. 2005;205:623–632. doi: 10.1002/path.1724. [DOI] [PubMed] [Google Scholar]
- 25.Charpin C, Garcia S, Bonnier P, Martini F, Andrac L, Horschowski N, Lavaut MN, Allasia C. Prognostic significance of Nm23/NDPK expression in breast carcinoma, assessed on 10-year follow-up by automated and quantitative immunocytochemical assays. J Pathol. 1998;184:401–407. doi: 10.1002/(SICI)1096-9896(199804)184:4<401::AID-PATH1220>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama H, Yasui W, Yokozaki H, Tahara E. Reduced expression of nm23 is associated with metastasis of human gastric carcinomas. Jpn J Cancer Res. 1993;84:184–190. doi: 10.1111/j.1349-7006.1993.tb02853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu NY, Chow KC, Chen WJ, Lin CC, Chou FF, Chen CL. Expression of nm23 in the primary tumor and the metastatic regional lymph nodes of patients with gastric cardiac cancer. Clin Cancer Res. 1999;5:1752–1757. [PubMed] [Google Scholar]
- 28.Wang CS, Lin KH, Hsu YC, Hsueh S. Distant metastasis of gastric cancer is associated with elevated expression of the antimetastatic nm23 gene. Cancer Lett. 1998;128:23–29. doi: 10.1016/s0304-3835(98)00043-3. [DOI] [PubMed] [Google Scholar]
- 29.Yeung P, Lee CS, Marr P, Sarris M, Fenton-Lee D. Nm23 gene expression in gastric carcinoma: an immunohistochemical study. Aust N Z J Surg. 1998;68:180–182. doi: 10.1111/j.1445-2197.1998.tb04740.x. [DOI] [PubMed] [Google Scholar]


