Abstract
Objective
We retrospectively analyzed the surgical outcomes of 42 patients with growth hormone (GH)-secreting pituitary adenoma to evaluate the clinical manifestations and to determine which preoperative factors that significantly influence the remission.
Methods
Forty-two patients with GH-secreting pituitary adenoma underwent transsphenoidal surgery (TSS) between 1995 and 2007. The patient group included 23 women and 19 men, with a mean age of 40.2 (range 13-61) years, and a mean follow-up duration of 49.4 (range 3-178) months after the operation. For comparable radiological criteria, we classified parasellar growth into five grades according to the Knosp classification. We analyzed the surgical results of the patients according to the most recent stringent criteria for cure.
Results
The overall rate of endocrinological remission in the group of 42 patients after primary TSS was 64% (26 of 42). The remission rate was 67% (8 of 12) for microadenoma and 60% (18 of 30) for macroadenoma. The remission rate was 30% (3 of 10) for the group with cavernous sinus invasion and 72% (23 of 32) for the group with intact cavernous sinus. Cavernous sinus invasion in Knosp grade III and IV was significantly correlated with the remission rate. There was a significant relationship between preoperative mean GH concentration and early postoperative outcome, with most patients in remission having a lower preoperative GH concentration.
Conclusion
TSS is thought to be an effective primary treatment for GH-secreting pituitary adenomas according to the most recent criteria of cure. Because the remission rate in cases with cavernous sinus invasion is very low, early detection of the tumor before it extends into the cavernous sinus and a long-term endocrinological and radiological follow-up are necessary in order to improve the remission rate of acromegaly.
Keywords: Growth hormone-secreting pituitary adenoma, Cavernous sinus, Remission induction
INTRODUCTION
Acromegaly results from supraphysiological growth hormone (GH) release from pituitary somatotroph adenomas (99% of cases), resulting in persistently high circulating levels of GH. It has an annual incidence of three to four cases per 1 million people3) and a prevalence of approximately 60 per million. Overall, there appears to be no difference in race, gender or ethnicity among individuals affected with this condition. Several retrospective cohort studies suggest that mortality in acromegaly is at least twice that in the general population2,22,24,26). The cardiovascular effects such as hypertension, cardiomyopathy and valvular heart disease are associated with increased morbidity and premature mortality, and significant increases have been reported for both respiratory disorders and malignancies24).
The most recent consensus guidelines for the management of acromegaly suggest surgery as the first-line therapy, either alone or in combination with medical treatment, conventional radiotherapy and/or radiosurgery5,14,18,21). But, the cavernous sinus invasion renders the tumor surgically unresectable even in skilled hands, and remains one of the greatest challenges in neurosurgery. The residual tumor within the cavernous sinus can continue to cause endocrinological symptoms, which necessitates further treatment such as pharmacological therapy or radiotherapy. We analyzed the surgical results of 42 patients with GH-secreting pituitary adenoma focused especially on cases of cavernous sinus invasion.
MATERIALS AND METHODS
Forty-two patients with acromegaly among 259 pituitary tumors underwent transsphenoidal surgery (TSS) to remove a pituitary GH-secreting adenoma at our institution between May 1995 and December 2007. The initial medical records of the patients were reviewed in detail, and the diagnosis of acromegaly was confirmed by elevated GH (>5 ng/mL) and insulin-like growth factor-I (IGF-I) levels, elevated GH (>2 ng/mL) levels after oral glucose tolerant test (OGTT) and evidence of pituitary mass on magnetic resonance imaging scans. Postoperative and follow-up clinical, radiographic and laboratory data were collected from the medical records of the patients. In addition to the remission of GH oversecretion, postoperative pituitary function was assessed on the basis of an adrenocorticotropic hormone (250 mg) stimulation test, and the serum levels of thyroid-stimulating hormone, free thyroxine, prolactin, luteinizing hormone, follicle-stimulating hormone and testosterone/estradiol.
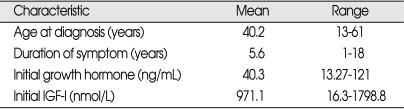
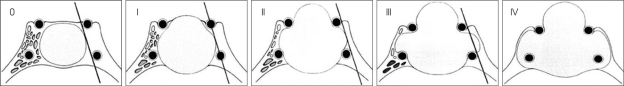
The clinical characteristics of the patients are shown in Table 1. The patient group included 23 women and 19 men, with a mean age of 40.2 (range, 13-61) years, and a mean follow-up duration of 49.4 (range, 3-178) months after the operation. The mean initial level of growth hormone was 40.3 (range, 13.27-121) ng/mL and the mean initial level of IGF-I was 971.1 (range, 16.3-1798.8) nmol/L. Eighteen patients suffered from diabetes mellitus. Sixteen patients had headache and twelve patients had visual disturbance. Prolactin levels were high in seven (16%) patients (Table 2). Tumors were classified as macroadenomas (≥10 mm) or microadenomas (<10 mm) on the basis of magnetic resonance (MR) or computerized tomography (CT) scanning. Thirty patients had macroadenomas and 12 patients had microadenomas. Further, we classified parasellar growth into five grades using the Knosp classification15). We regarded grades 0, I and II as non-invasive and grades III and IV as invasive (Fig. 1). The non-invasive group included 32 patients and the invasive group included 10 patients.
Table 1.
Preoperative characteristics of the 42 patients with acromegaly
Table 2.
Associated symptoms and signs of the patients with acromegaly
Fig. 1.
Knosp classification. Grade 0 represents the normal condition of the cavernous sinus space. The adenoma does not pass the tangent of medical aspects of supra- and intercavernous internal carotid artery (ICA). In grade I, the medial tangent is passed, but the extension does not go beyond the intercarotid line, which is the line drawn between the cross-sectional centers of the intra- and supracavernous ICA. Grade II is characterized by the tumor extending beyond the intercarotid line, but not extending beyond or tangent to the lateral aspects of the intra- and supracavernous ICA. Grade III is characterized by the tumor extending laterally to the lateral tangent of the intra-and supracavernous ICA. Grade IV is characterized by total encasement of the intracavernous carotid artery (From Knosp et al.15)).
Hormonal assessment was performed at 15 days, 3 months and 6 months postoperatively, and usually every year thereafter. The mean preoperative GH level was 40.3 (range, 13.27-121) ng/mL and the mean preoperative IGF-I level was 971.7 (range, 16.3-1798.8) nmol/L.
Endocrinological remission or cure involved the fulfilling of each of the following recent stringent criteria (depending on the availability at the time) : mean GH less than 2.5 ng/mL, GH value after OGTT of less than 1.0 ng/mL and a normal age-related IGF-I level1,7,18).
Data analysis was performed using SPSS 10.0 software (SAS Institute). The analysis of categorical variables was performed with Fisher's exact test or chi-square test. A probability p value of less than 0.05 was considered statistically significant.
RESULTS
The postoperative mean serum GH concentration (day-profile) decreased to 3.90 (range, 0.01-40.73) mg/L and 26 patients (64%) achieved a normal mean GH concentration. The overall rate of endocrinological remission in the group of 42 patients undergoing primary TSS was 64% (26 of 42). The remission rate was 67% (8 of 12) for microadenoma and 60% (18 of 30) for macroadenoma. The remission rate was 30% (3 of 10) for the group with cavernous sinus invasion and 72% (23 of 32) for the group with intact cavernous sinus. Cavernous sinus invasion of Knosp grade III and IV was significantly correlated with the remission rate (p<0.05).
Using logistic regression analysis, we did not find a significant relationship between early postoperative outcome and age, sex, or preoperative IGF-I level (Table 3). There was, however, a significant relationship between early preoperative mean GH concentration and postoperative outcome (p<0.05), with more patients in remission having a lower preoperative GH concentration.
Table 3.
Univariate analysis of factors affecting remission
No surgical mortality or serious morbidity was observed for the whole series, however, minor complications occurred in three patients. Postoperative diabetes insipidus was observed in one patient requiring medical therapy. A postoperative cerebrospinal fluid leakage occurred in one patient that required lumbar drainage without further morbidity. A case of aseptic meningitis was also observed.
DISCUSSION
We retrospectively analyzed the surgical results of TSS for acromegaly focusing on patients with biochemical and clinical follow-up data. Preoperative data such as age and sex distribution, tumor class and preoperative GH concentration were comparable with those from other studies1,3,14,21).
An endocrinological "cure" for acromegaly, as defined by the stringent criteria of biochemical remission10,21), was achieved in 64% of patients, similar to that reported in the most recent series16,19-21,25). TSS remains an effective treatment for acromegaly, whereas medical treatment and/or radiotherapy should be reserved for patients with persistent GH/IGF-I hypersecretion after surgery4). However, for patients with cavernous sinus invasion of Knosp grade III and IV15), invasive GH-secreting pituitary adenoma may be unresectable due to the involvement with structures of the cavernous sinus; the GH level can't be normalized, the surgical cure rate is low while the incidence of complications is high and the GH level must still be observed after the operation.
In general, adjuvant treatment is necessary in patients with remnant tumors without remission. But, there is debate surrounding the starting point of adjuvant treatment. Although the patient has not achieved a hormonal remission state, careful observation could be an alternative option if there are no hormonal symptoms6,26). Since their introduction into clinical use more than a decade ago, the octreotide long-acting release (LAR) have been considered primary or secondary medical therapy for acromegaly. In a recent study, it was found that there may be a role for pre-operative medical management if tumor shrinkage can be achieved prior to surgery. Surgical outcomes may also be improved by lowering preoperative GH and IGF-I levels4,5).
For residual tumor within the cavernous sinus area after medical or surgical treatment, gamma knife radiosurgery is a viable option. It is known from the present literature that although gamma knife radiosurgery can be effective in controlling the tumor volume of patients who are refractory to drug or surgical treatment, the biological cure rate is very low9,20,23,28). Studies have claimed that GH values were normalized in 62-83% of all acromegalic patients treated by fractionated radiotherapy over a 5-15 year period8,11). Reported complications included optic neuropathy and pituitary insufficiency17). Gamma knife radiosurgery is not only able to deliver a higher biological effective dose than fractionated radiotherapy, but it can do so without any of the usual associated complications9,17,23). It can thus be strongly argued that gamma knife radiosurgery should be considered before fractionated radiotherapy as an adjuvant therapy.
The exact effect of cavernous sinus invasion on pituitary adenoma is unknown. However, the presence of a sensitive molecular marker for tumor invasiveness will allow a more focused and cost-effective follow-up and long term management for these patients13). However, these markers can only provide limited predicting information. Recently, Isono et al.12) immunohistochemically examined the expression of leptin in pituitary adenomas and found that leptin expression correlated to the invasive potential of functioning adenomas. The polysialylated neural cell adhesion molecule was found strongly related to pituitary tumor invasion27). However, these factors have not been tested clinically, so further study is needed.
TSS of GH-secreting pituitary adenoma continues to be a safe and effective method for dealing with a large number of patients with acromegaly. The goal of overall management should be to provide the patient with the most effective means of long term control of this benign but potentially disabling disease.
The limitation of this study is being a retrospective study in small patients. Therefore, a prospective study in more patients will be necessary to further evaluate the factors affecting the remission rate of acromegaly. It is hoped that with advances in pharmacotherapy, surgical treatment may become even more focused, more precise and more effective over time.
CONCLUSION
This article gives an insight on the treatment of acromegaly, especially on adenomas invading the cavernous sinus. On the basis of recent remission criteria, our series demonstrate the good efficacy of TSS for acromegalic patients with microadenomas and non-invasive macroadenomas. In patients with larger tumors, cavernous sinus invasion or high preoperative GH levels, the remission rate is lower. Therefore, early detection of the tumor and a long-term endocrinological and radiological follow-up may improve the remission rate of acromegaly.
Acknowledgements
This work was supported by a grant from the Chunma medical research foundation, Korea, 2006.
References
- 1.Abbassioun K, Amirjamshidi M, Mehrazin A, Khalatbary I, Keynama M, Bokai H, et al. A prospective analysis of 151 cases of patients with acromegaly operated by one neurosurgeon : a follow-up of more than 23 years. Surg Neurol. 2006;66:26–31. doi: 10.1016/j.surneu.2005.11.063. discussion 31. [DOI] [PubMed] [Google Scholar]
- 2.Beauregard C, Truong U, Hardy J, Serri O. Long-term outcome and mortality after transsphenoidal adenomectomy for acromegaly. Clin Endocrinol (Oxf) 2003;58:86–91. doi: 10.1046/j.1365-2265.2003.01679.x. [DOI] [PubMed] [Google Scholar]
- 3.Bolanowski M, Zatonska K, Kaluzny M, Zielinski G, Bednarek-Tupikowska G, Bohdanowicz-Pawlak A, et al. A follow-up of 130 patients with acromegaly in a single centre. Neuro Endocrinol Lett. 2006;27:828–832. [PubMed] [Google Scholar]
- 4.Bush ZM, Vance ML. Management of acromegaly : is there a role for primary medical therapy? Rev Endocr Metab Disord. 2008;9:83–94. doi: 10.1007/s11154-007-9061-1. [DOI] [PubMed] [Google Scholar]
- 5.Carmichael JD, Bonert VS. Medical therapy : options and uses. Rev Endocr Metab Disord. 2008;9:71–81. doi: 10.1007/s11154-007-9068-7. [DOI] [PubMed] [Google Scholar]
- 6.Costa AC, Rossi A, Martinelli CE, Jr, Machado HR, Moreira AC. Assessment of disease activity in treated acromegalic patients using a sensitive GH assay : should we achieve strict normal GH levels for a biochemical cure? J Clin Endocrinol Metab. 2002;87:3142–3147. doi: 10.1210/jcem.87.7.8631. [DOI] [PubMed] [Google Scholar]
- 7.De P, Rees DA, Davies N, John R, Neal J, Mills RG, et al. Transsphenoidal surgery for acromegaly in wales : results based on stringent criteria of remission. J Clin Endocrinol Metab. 2003;88:3567–3572. doi: 10.1210/jc.2002-021822. [DOI] [PubMed] [Google Scholar]
- 8.Eastman RC, Gorden P, Glatstein E, Roth J. Radiation therapy of acromegaly. Endocrinol Metab Clin North Am. 1992;21:693–712. [PubMed] [Google Scholar]
- 9.Fukuoka S, Ito T, Takanashi M, Hojo A, Nakamura H. Gamma knife radiosurgery for growth hormone-secreting pituitary adenomas invading the cavernous sinus. Stereotact Funct Neurosurg. 2001;76:213–217. doi: 10.1159/000066721. [DOI] [PubMed] [Google Scholar]
- 10.Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, et al. Criteria for cure of acromegaly : a consensus statement. J Clin Endocrinol Metab. 2000;85:526–529. doi: 10.1210/jcem.85.2.6363. [DOI] [PubMed] [Google Scholar]
- 11.Goffman TE, Dewan R, Arakaki R, Gorden P, Oldfield EH, Glatstein E. Persistent or recurrent acromegaly. Long-term endocrinologic efficacy and neurologic safety of postsurgical radiation therapy. Cancer. 1992;69:271–275. doi: 10.1002/1097-0142(19920101)69:1<271::aid-cncr2820690145>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Isono M, Inoue R, Kamida T, Kobayashi H, Matsuyama J. Significance of leptin expression in invasive potential of pituitary adenomas. Clin Neurol Neurosurg. 2003;105:111–116. doi: 10.1016/s0303-8467(02)00129-4. [DOI] [PubMed] [Google Scholar]
- 13.Kawamoto H, Uozumi T, Kawamoto K, Arita K, Yano T, Hirohata T. Analysis of the growth rate and cavernous sinus invasion of pituitary adenomas. Acta Neurochir (Wien) 1995;136:37–43. doi: 10.1007/BF01411433. [DOI] [PubMed] [Google Scholar]
- 14.Kim IM, Yim MB, Lee CY. The outcome of transsphenoidal microsurgery for acromegaly. J Korean Neurosurg Soc. 2002;32:131–135. [Google Scholar]
- 15.Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space : a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993;33:610–617. doi: 10.1227/00006123-199310000-00008. discussion 617-618. [DOI] [PubMed] [Google Scholar]
- 16.Kreutzer J, Vance ML, Lopes MB, Laws ER., Jr Surgical management of GH-secreting pituitary adenomas : an outcome study using modern remission criteria. J Clin Endocrinol Metab. 2001;86:4072–4077. doi: 10.1210/jcem.86.9.7819. [DOI] [PubMed] [Google Scholar]
- 17.Landolt AM, Haller D, Lomax N, Scheib S, Schubiger O, Siegfried J, et al. Stereotactic radiosurgery for recurrent surgically treated acromegaly : comparison with fractionated radiotherapy. J Neurosurg. 1998;88:1002–1008. doi: 10.3171/jns.1998.88.6.1002. [DOI] [PubMed] [Google Scholar]
- 18.Ludecke DK, Abe T. Transsphenoidal microsurgery for newly diagnosed acromegaly : a personal view after more than 1,000 operations. Neuroendocrinology. 2006;83:230–239. doi: 10.1159/000095533. [DOI] [PubMed] [Google Scholar]
- 19.Minniti G, Jaffrain-Rea ML, Esposito V, Santoro A, Tamburrano G, Cantore G. Evolving criteria for post-operative biochemical remission of acromegaly : can we achieve a definitive cure? An audit of surgical results on a large series and a review of the literature. Endocr Relat Cancer. 2003;10:611–619. doi: 10.1677/erc.0.0100611. [DOI] [PubMed] [Google Scholar]
- 20.Newman CB. Medical therapy for acromegaly. Endocrinol Metab Clin North Am. 1999;28:171–190. doi: 10.1016/s0889-8529(05)70062-1. [DOI] [PubMed] [Google Scholar]
- 21.Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical 'cure'. Eur J Endocrinol. 2005;152:379–387. doi: 10.1530/eje.1.01863. [DOI] [PubMed] [Google Scholar]
- 22.Orme SM, McNally RJ, Cartwright RA, Belchetz PE United Kingdom Acromegaly Study Group. Mortality and cancer incidence in acromegaly : a retrospective cohort study. J Clin Endocrinol Metab. 1998;83:2730–2734. doi: 10.1210/jcem.83.8.5007. [DOI] [PubMed] [Google Scholar]
- 23.Petrovich Z, Yu C, Giannotta SL, Zee CS, Apuzzo ML. Gamma knife radiosurgery for pituitary adenoma : early results. Neurosurgery. 2003;53:51–59. doi: 10.1227/01.neu.0000068702.00330.47. discussion 59-61. [DOI] [PubMed] [Google Scholar]
- 24.Rajasoorya C, Holdaway IM, Wrightson P, Scott DJ, Ibbertson HK. Determinants of clinical outcome and survival in acromegaly. Clin Endocrinol (Oxf) 1994;41:95–102. doi: 10.1111/j.1365-2265.1994.tb03789.x. [DOI] [PubMed] [Google Scholar]
- 25.Shimon I, Cohen ZR, Ram Z, Hadani M. Transsphenoidal surgery for acromegaly : endocrinological follow-up of 98 patients. Neurosurgery. 2001;48:1239–1243. doi: 10.1097/00006123-200106000-00008. discussion 1244-1245. [DOI] [PubMed] [Google Scholar]
- 26.Swearingen B, Barker FG, 2nd, Katznelson L, Biller BM, Grinspoon S, Klibanski A, et al. Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab. 1998;83:3419–3426. doi: 10.1210/jcem.83.10.5222. [DOI] [PubMed] [Google Scholar]
- 27.Trouillas J, Daniel L, Guigard MP, Tong S, Gouvernet J, Jouanneau E, et al. Polysialylated neural cell adhesion molecules expressed in human pituitary tumors and related to extrasellar invasion. J Neurosurg. 2003;98:1084–1093. doi: 10.3171/jns.2003.98.5.1084. [DOI] [PubMed] [Google Scholar]
- 28.Zhang N, Pan L, Wang EM, Dai JZ, Wang BJ, Cai PW. Radiosurgery for growth hormone-producing pituitary adenomas. J Neurosurg. 2000;93(Suppl 3):6–9. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]