Abstract
Objective
Although there are several descriptions of this vessel, there is no detailed angiographic study of the accessory middle cerebral artery (AMCA) in Korea. We describe the angiographic characteristics of the cortical territory and origin of AMCA and discuss the clinical significance of this anomaly.
Methods
We searched for patients with AMCAs from a retrospective review of 1,250 conventional cerebral angiograms. We determined the origins, diameters and cortical territories of these AMCAs.
Results
Fifteen patients (15 of 1250 = 1.2%) had 16 AMCAs (one patient had bilateral AMCAs). AMCAs originated from the distal A1 in eleven cases, middle A1 in two, proximal A1 in two, and proximal A2 in one case. All AMCAs followed a course parallel to the main middle cerebral artery (MCA). All but three of these arteries were smaller than the main MCA. Thirteen of the smaller diameter AMCAs had cortical distribution to the orbito-frontal and prefrontal, and precentral areas. Three AMCAs had diameter as large as the main MCA. These three supplied the orbitofrontal, prefrontal, precentral, central and anterior-parietal arteries.
Conclusion
The AMCAs originated from A1 or A2. Most had smaller diameter than the main MCA. The AMCAs coursed along the horizontal portion of the MCA, but supplied the orbital surface, the anterior frontal lobe and sometimes wider cortical territory, including the precentral, central, anterior-parietal areas.
Keywords: Accessory middle cerebral artery, Cortical territory, Clinical implication
INTRODUCTION
A vascular neurosurgeon must know the normal anatomy of the basal arteries of the brain and their cortical distributions. The accessory middle cerebral artery (AMCA) is an anomalous vessel arising from the anterior cerebral artery (ACA) and running through the sylvian fissure along with the middle cerebral artery (MCA)24). Several reports described this vessel but there is no detailed analysis of angiographic features of the AMCA in Korea9,12). We describe the angiographic characteristics of the cortical territory and origin of the AMCA and discuss the clinical significance of this anomalous vessel.
MATERIALS AND METHODS
We retrospectively reviewed 1,250 conventional cerebral angiograms to find patients with an AMCA. Conventional cerebral angiography (Philips V-5000, Philips Medical System, Eindhoven, Netherlands) was performed on 744 patients between July 1, 1997 and August 31, 2006 in one hospital. Conventional cerebral angiography (Philips V-3000, Philips Medical System, Eindhoven, Netherlands) was performed on 506 patients between April 1, 2000 and September 30, 2006 in another hospital. The initial angiographic investigations were made for a variety of clinical reasons, including symptoms of cerebral ischemia, cerebral infarction, hemorrhagic contusion, intracerebral hemorrhage, headache and dizziness. The images were obtained either from a routine diagnostic study or at initial diagnosis during an interventional procedure. We subsequently evaluated all the angiograms for AMCAs. In patients exhibiting an AMCA, special attention was given to define the origin, diameter and cerebral territory of this vessel.
RESULTS
Fifteen patients (15 of 1250=1.2%) had 16 AMCAs (one patient (case 7) had bilateral AMCAs) (Table 1). AMCAs originated from distal A1 in eleven cases, middle A1 in two, proximal A1 in two and proximal A2 in one case. All AMCAs followed a parallel course with the main MCA. The size of the vessels is expressed as a relative value, with the diameter of the MCA at its proximal end defined as 1.0. The relative size of the proximal end of AMCAs ranged from 0.33 to 0.91 (0.57±0.21), whereas that of the proximal end of the ACA was 0.48 to 1.37 (0.95±0.31). All but three of these arteries had smaller caliber than the main MCA. Thirteen of the small-diameter AMCAs had a cortical distribution to the orbito-frontal, prefrontal, and precentral areas (Fig. 1). Three AMCAs (case 6, 7 right AMCA, 9) had diameters as large as the principal MCA; these supplied the orbito-frontal, prefrontal, precentral, central and anterior-parietal arteries (Fig. 2).
Table 1.
Summary of 15 patients with accessory middle cerebral artery
AMCA : accessory middle cerebral artery, ACA : anterior cerebral artery, MCA : middle cerebral artery, OF : orbito-frontal artery, PF : prefrontal artery, PC : precentral artery, C : central artery, AP : anterior-parietal artery, LT : left, RT : right, AN : aneurysm, SAH : subrachnoid hemorrhage, IVH : intraventricular hemorrhage, TIA : transient ischemic attack, AVF : arterio-venous fistula
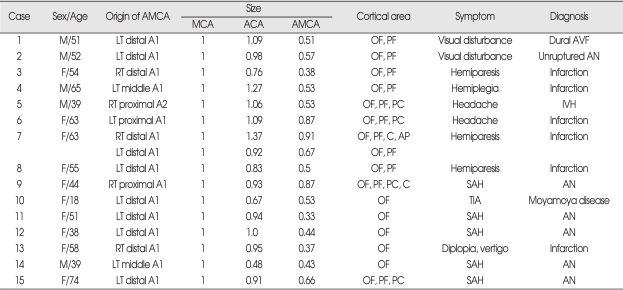
Fig. 1.
Left accessory middle cerebral artery with a smaller diameter than the main middle cerebral artery arising from the distal portion of the left A1. This vessel terminates in the orbito-frontal and prefrontal arteries (case 2). A : Antero-posterior view. B : Lateral view, arrow : accessory middle cerebral artery.
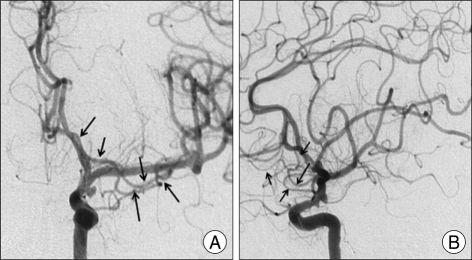
Fig. 2.
Right accessory middle cerebral artery with a diameter as large as that of the main middle cerebral artery originating from proximal right A1. This vessel terminates in the orbito-frontal, prefrontal, precentral, and central arteries (case 9). A : Antero-posterior. B : Lateral.
DISCUSSION
Origin and cortical distribution
Most AMCAs arise from the proximal portion (A1) of the ACA. Abanou et al.1) reported an incidence of AMCAs of 0.31% in their study of 6,000 angiograms. They classified AMCAs into three subtypes. Type 1 arises from the internal carotid artery proximal to its bifurcation into the middle and anterior cerebral arteries, type 2 originates from the proximal portion of the A1 segment of the ACA, and type 3 arises more distally along A1. In this classification, type 1 is actually a duplicated MCA. Uchino et al.26) described the origin of the AMCA in 68 cases, with only three originating from the A2 segment of the ACA and most arising from the A1 segment. Other authors have reported few cases in which an AMCA originates from the A2 segment of the ACA11,20,26). We also found a case of AMCAs originating from A2.
Most AMCAs are smaller than the main MCA. The AMCA may terminate within the sphenoid segment and may partially supply striated territories or even reach the insular and parietal cortical areas2,15). Several authors reported that an AMCA coursed within the sylvian fissure and supplied the territories of the orbito-frontal, prefrontal, precentral, and central arteries6,10,13). In ananatomical study of the variations in the MCA, Umansky et al.27) described two cases of an AMCA that fed the territories of the orbito-frontal artery and the precentral and central arteries. In our study, all but three of these arteries had smaller diameters than the main MCA. Thirteen small-diameter AMCAs had a cortical distribution to the orbito-frontal, prefrontal, and precentral areas. Three AMCAs with diameters as large as the main MCA supplied the orbito-frontal, prefrontal, precentral, central and anterior-parietal arteries.
Some authors suggested that the AMCAs form as a hypertrophy of the recurrent artery of Heubner (RAH)25). However, others argued this theory because of several reasons. First, perforating arteries rarely arise from an AMCA. Second, the RAH can exist with an AMCA. And third, the two vessels have different patterns of distribution13,22,23) Takahashi et al.23) showed that, along with the RAH, the AMCA phylogenetically represents the primitive vascular anastomosis to the pyriform cortex, with one or the other vessel usually predominating. The RAH normally takes over the main blood supply to the pyriform cortex with attrition in utero of any contribution from the AMCA, becoming rare in adults22). They proposed that the AMCA may be derived from various descendants of the anastomoses between the ACA and MCA over the tuberculum olfactorium, one of which is a normal predecessor of the RAH23). This may explain the developmental variants in these anastomoses, resulting in the formation of either an AMCA or a RAH. As the cerebral cortex grows, this communication normally disappears. The segment connected to the ACA becomes the RAH, and the segment connected to the MCA forms the lateral arteries. Phylogenetically, both the RAH and the MCA develop from the group of lateral striate arteries of the ACA, with a variable hemodynamic balance existing between these vessels7).
Clinical implications
Cerebral aneurysms arising from the origin of the AMCA have been reported. Recurrent blood flow in the AMCA may cause considerable stress at these arterial junctions3,18). The increased flow in the parent artery (A1) resulting from the presence of the AMCA may be the hemodynamic basis for the development of an aneurysm. Such an aneurysm may have a high rate of rupture18). An aneurysm of the AMCA was first reported in 197728), and so far there are total of only ten case reported in the literatre3-5,7,8,15,16,19,21,28). Eight of these patients had saccular aneurysms arising at the junction of the AMCA and the A1 segment. The orientation of these aneurysms was inferomedial in four cases and superomedial in other four cases. This medial orientation of the aneurysm may be a consequence of the flow direction in A1. One aneurysm originated from the trunk of the AMCA and was directed upward5). And last one is dissecting aneurysm of the AMCA and A119). In this study, we did not find any aneurysms arising from the AMCA.
Recognition of the presence of an AMCA requires special attention to the perforating arteries arising from this vessel that irrigate deep brain tissue in the ipsilateral pterional approach18). In a microanatomical study, Umansky et al.27) showed that in addition to the lateral striated perforating end arteries from the AMCA, the terminal cortical distribution was usually to orbito-frontal, precentral, and central vessels. Therefore, interruption of an AMCA will cause severe neurological deficits, particularly in the dominant hemisphere.
The clinical relevance of the AMCA requires pre- and intra-operative appreciation of the presence of this anomaly to avoid compromising the superficial and deep irrigation of the vessel. Despite its small size, the substantial collateral flow of the AMCA was demonstrated by Mueller et al.17).
Komiyama et al.14) reported that during MCA occlusion, the AMCA may be important in supplying collateral blood flow to the frontal lobe and basal ganglia via the perforating arteries.
CONCLUSION
In this study, 1.2% patients had 16 AMCAs. They originate from A1 or A2. The AMCAs course along the horizontal portion of the MCA and supply the orbital surface, the anterior frontal lobe and sometimes a wider cortical territory including precentral, central, and anterior-parietal areas. Most AMCAs have a smaller diameter than the main MCA. During MCA occlusion, the AMCA may be important in supplying collateral blood flow to the frontal lobe and basal ganglia via the perforating arteries. A saccular aneurysm may develop at the junction between the AMCA and the A1 segment.
Acknowledgements
This work was supported by research grant from Inje University.
References
- 1.Abanou A, Lasjaunias P, Manelfe C, Lopez-Ibor L. The accessory middle cerebral artery (AMCA). Diagnostic and therapeutic consequences. Anat Clin. 1984;6:305–309. doi: 10.1007/BF01654463. [DOI] [PubMed] [Google Scholar]
- 2.Crompton MR. The pathology of ruptured middle-cerebral aneurysms with special reference to the differences between the sexes. Lancet. 1962;2:421–425. doi: 10.1016/s0140-6736(62)90281-7. [DOI] [PubMed] [Google Scholar]
- 3.Fujiwara K, Saito K, Ebina T. Saccular aneurysm of the accessory middle cerebral artery--case report. Neurol Med Chir (Tokyo) 2003;43:31–34. doi: 10.2176/nmc.43.31. [DOI] [PubMed] [Google Scholar]
- 4.Fuwa I, Matsukado Y, Wada H. [Intracranial aneurysms associated with the accessory middle cerebral artery and duplication of the middle cerebral artery. Report of two cases.] Neurol Med Chir (Tokyo) 1984;24:207–211. doi: 10.2176/nmc.24.207. [DOI] [PubMed] [Google Scholar]
- 5.Georgopoulos CE, Papanikolaou PG, Vlachos KI, Atzemi-Moldow N, Hatzidakis GI. Ruptured aneurysm at the trunk of the accessory middle cerebral artery. Acta Neurochir (Wien) 1999;141:1233–1235. doi: 10.1007/s007010050424. [DOI] [PubMed] [Google Scholar]
- 6.Gibo H, Carver CC, Rhoton AL, Jr, Lenkey C, Mitchell RJ. Microsurgical anatomy of the middle cerebral artery. J Neurosurg. 1981;54:151–169. doi: 10.3171/jns.1981.54.2.0151. [DOI] [PubMed] [Google Scholar]
- 7.Han DH, Gwak HS, Chung CK. Aneurysm at the origin of accessory middle cerebral artery associated with middle cerebral artery aplasia : case report. Surg Neurol. 1994;42:388–391. doi: 10.1016/0090-3019(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 8.Handa J, Matsuda M, Okamoto K, Kidooka M. Association between accessory middle artery and cerebral aneurysm. Report of two cases. Acta Neurochir (Wien) 1982;64:151–157. doi: 10.1007/BF01405627. [DOI] [PubMed] [Google Scholar]
- 9.Hong JT, Huh PW, Chung DS, Kye DK, Cho KS, Kim DS. Accessory Middle Cerebral Artery. J Korean Neurosurg Soc. 1997;26:435–438. [Google Scholar]
- 10.Jain KK. Some Observations on the Anatomy of the Middle Cerebral Artery. Can J Surg. 1964;7:134–139. [PubMed] [Google Scholar]
- 11.Kahilogullari G, Ugur HC. Accessory middle cerebral artery originating from callosomarginal artery. Clin Anat. 2006;19:694–695. doi: 10.1002/ca.20371. [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Hur JW, Lee JW, Lee HK. Middle cerebral artery anomalies detected by conventional angiography and magnetic resonance angiography. J Korean Neurosurg Soc. 2005;37:263–267. [Google Scholar]
- 13.Komiyama M, Nakajima H, Nishikawa M, Yasui T. Middle cerebral artery variations : duplicated and accessory arteries. AJNR Am J Neuroradiol. 1998;19:45–49. [PMC free article] [PubMed] [Google Scholar]
- 14.Komiyama M, Nishikawa M, Yasui T. The accessory middle cerebral artery as a collateral blood supply. AJNR Am J Neuroradiol. 1997;18:587–590. [PMC free article] [PubMed] [Google Scholar]
- 15.Kuwabara S, Naitoh H. Ruptured aneurysm at the origin of the accessory middle cerebral artery : case report. Neurosurgery. 1990;26:320–322. doi: 10.1097/00006123-199002000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki S, Ito K, Ishii S. Aneurysm at the origin of the accessory middle cerebral artery. Surg Neurol. 1984;22:292–294. doi: 10.1016/0090-3019(84)90017-x. [DOI] [PubMed] [Google Scholar]
- 17.Mueller DP, Sato Y, Yuh WT. Accessory middle cerebral artery as a source of collateral blood flow. AJNR Am J Neuroradiol. 1991;12:1223–1224. [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura T, Houkin K, Saitoh H, Abe H. Aneurysm of the anterior communicating artery associated with accessory middle cerebral artery--case report. Neurol Med Chir (Tokyo) 1997;37:747–751. doi: 10.2176/nmc.37.747. [DOI] [PubMed] [Google Scholar]
- 19.Otawara Y, Suzuki M, Abe M, Tomizuka N, Ogawa A. Dissecting aneurysms of the anterior cerebral artery and accessory middle cerebral artery. Case report. Neurosurg Rev. 1997;20:145–148. doi: 10.1007/BF01138201. [DOI] [PubMed] [Google Scholar]
- 20.Reis CV, Zabramski JM, Safavi-Abbasi S, Hanel RA, Deshmukh P, Preul MC. The accessory middle cerebral artery : anatomic report. Neurosurgery. 2008;63:ONS10–ONS13. doi: 10.1227/01.neu.0000335005.99756.eb. discussion ONS13-ONS14. [DOI] [PubMed] [Google Scholar]
- 21.Sugita S, Yuge T, Miyagi J, Fujimura N, Shigemori M. Giant aneurysm at the origin of the accessory middle cerebral artery. Surg Neurol. 1995;44:128–130. doi: 10.1016/0090-3019(95)00128-x. [DOI] [PubMed] [Google Scholar]
- 22.Tacconi L, Johnston FG, Symon L. Accessory middle cerebral artery. Case report. J Neurosurg. 1995;83:916–918. doi: 10.3171/jns.1995.83.5.0916. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi S, Hoshino F, Uemura K, Takahashi A, Sakamoto K. Accessory middle cerebral artery : is it a variant form of the recurrent artery of Heubner? AJNR Am J Neuroradiol. 1989;10:563–568. [PMC free article] [PubMed] [Google Scholar]
- 24.Teal JS, Rumbaugh CL, Bergeron RT, Segall HD. Anomalies of the middle cerebral artery : accessory artery, duplication, and early bifurcation. Am J Roentgenol Radium Ther Nucl Med. 1973;118:567–575. doi: 10.2214/ajr.118.3.567. [DOI] [PubMed] [Google Scholar]
- 25.Tran-Dinh H. The accessory middle cerebral artery a variant of the recurrent artery of Heubner (A. centralis longa)? Acta Anat (Basel) 1986;126:167–171. [PubMed] [Google Scholar]
- 26.Uchino M, Kitajima S, Sakata Y, Honda M, Shibata I. Ruptured aneurysm at a duplicated middle cerebral artery with accessory middle cerebral artery. Acta Neurochir (Wien) 2004;146:1373–1374. doi: 10.1007/s00701-004-0353-x. discussion 1375. [DOI] [PubMed] [Google Scholar]
- 27.Umansky F, Dujovny M, Ausman JI, Diaz FG, Mirchandani HG. Anomalies and variations of the middle cerebral artery : a microanatomical study. Neurosurgery. 1988;22:1023–1027. doi: 10.1227/00006123-198806010-00008. [DOI] [PubMed] [Google Scholar]
- 28.Waga S, Kojima T, Morooka Y, Sakakura M. Aneurysm of the accessory middle cerebral artery. Surg Neurol. 1977;8:359–360. [PubMed] [Google Scholar]