Abstract
The stereotactic surgical target for dystonic tremor is the subject of ongoing debate. Targeting the subthalamic area using deep brain stimulation has been regaining interest as a therapy for various types of involuntary movements. We describe the efficacy of stimulation of the subthalamic area in a patient with intractable dystonic tremor. Excellent control without neurological complications was achieved. This case report demonstrates that the subthalamic area is a valuable target for the control of dystonic tremor.
Keywords: Subthalamic area, Dystonic tremor, Deep brain stimulation
INTRODUCTION
Dystonic tremor is a more irregular asymmetrical tremor than essential tremor and is associated with dystonia in the affected body1). Dystonia and tremor can be controlled by stimulating different subcortical nuclei3,9). At present, the most commonly used target for deep brain stimulation (DBS) therapy to treat dystonia and tremor is the globus pallidus internus (Gpi) and the ventral intermedius (Vim) nucleus, respectively; however, the optimal target for dystonic tremor remains unclear. Also, the value of thalamic target for severe proximal tremor is relatively limited2). Considering the subthalamic area as an anatomic target for movement disorders dates back to the 1960s, when campotomy demonstrated considerable benefits in selected patients4,7), but also carried a risk of neurological morbidity. With the advent of non-destructive DBS technology, targeting this region has been regaining interest as a therapy for various types of involuntary movements5,6,8). Only a few cases of subthalamic DBS treatment for dystonic tremor or severe proximal tremor have been reported2,6). The authors describe the efficacy of stimulation of the subthalamic area in a patient with conservatively intractable dystonic tremor including severe axial and proximal tremor.
CASE REPORT
A 28-year-old man with a 14-year history of secondary dystonia presented with involuntary movement of the neck, hands, and left foot, which was accompanied by a severe jerky irregular coarse tremor predominantly involving the head, neck and proximal right upper limb. The patient was assessed using the Burke-Fahn-Marsden dystonia rating scale and scored 82/120 on the movement scale and 19/30 on the disability scale; his rating was found to be 57/84 on the Fahn-Tolosa-Marin Tremor Rating scale. There was history of perinatal anoxia and seizure. Magnetic resonance imaging (MRI) showed structural abnormalities including mildly diffuse brain atrophy and dilatation of the third ventricle. He underwent implantation of bilateral DBS electrodes in the subthalamic area under local anesthesia. Electrode implantation was based on direct targeting using T2-weighted MRI and a Neurosurgery Simulator (Dimos, Korea) as surgical planning tools for targeting with refinement using microelectrode recording and stimulation. The length of anterior commissure-posterior commissure line was 27.3 mm. The width of the third ventricle was 10 mm. The initial planning target was 10.7 mm lateral of the midline, 5.6 mm posteior to the midcommissural point, and 3.6 mm below the commissural plane. Intraoperatively he reported visual blurring with stimulation at a frequency 130 Hz with a pulse duration of 60 µsec and an amplitude of 2.5 V. Therefore, the permanent electrode (Model 3387, Medtronic, Minneapolis, USA) was placed 2 mm more lateral on the left side and 2 mm more anterior and 1 mm more dorsal on the right side. The target was asymmetrically selected in the subthalamic area because of the fear of side effects (Fig. 1). After a 6-day test stimulation, the pulse generator was implanted in the infraclavicular area under general anesthesia. There was improvement in both the dystonia and tremor. There was a 80.5% (16/120) and a 89.5% (2/30) improvement on the movement scale and the disability scale of the Burke-Fahn-Marsden dystonia rating scale, respectively, in the stimulation-on state at 6 months post surgery compared with the baseline scale. Similarly, there was a 64.9% (20/84) improvement in the Fahn-Tolosa-Marin Tremor Rating scale. The neck pain associated with dystonic movement disappeared. Functional improvement in the use of the limbs was observed and this clinical improvement has remained stable for 18 months postoperatively. The dystonia and tremor were controlled by high-frequency stimulation of 130 Hz with a pulse duration of 60 µsec and an amplitude of 2.0 V on the right side and 2.5 V on the left side with bipolar stimulation across the distal two contacts (0-1+). The optimal stimulation effect was obtained in the subthalamic area. Side effects at this optimal stimulation were not seen. However, bilateral stimulation resulted in slurred speech and a sense of disequlibrium when walking at more than 3.2 volts at the bottom two contacts (0-1+). Stimulation-induced blurred vision occurred at 3.3 V on the right side and 3.8 V on the left side.
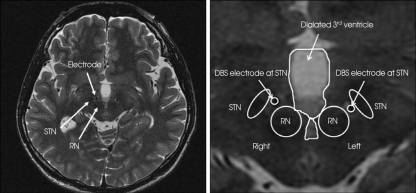
Fig. 1.
A postoperative axial T2-weighted magnetic resonance imaging scan showing the location of asymmetrically bilateral stimulation electrodes implanted in the subthalamic white matter, which is situated in the lateral to the red nucleus (RN) and medial to the subthalamic nucleus (STN).
DISCUSSION
The Vim target could be valuable option to improve tremor but not dystonia9). The Gpi is currently the most common target for treating dystonia but not the tremor component associated with it9). Historically, Mundinger et al.4) reported good results for treatment of essential tremor or spasmodic torticollis by making lesions in the zona incerta (ZI); however, there was an 8% incidence of postoperative complication. Recently, the subthalamic area has been revised as a surgical target for DBS for movement disorders. Velasco et al.8) noted that the prelemniscal radiation is an effective target for the alleviating of tremor and rigidity in patients with Parkinson's disease. Kitagawa et al.2) demonstrated the efficacy and safety of subthalamic DBS in two patients, one with severe refractory proximal essential tremor and one with dystonic tremor in which thalamotomy and intraoperative stimulation of the Vim nucleus are ineffective. The ZI and the prelemniscal radiation were selected as the surgical target. Plaha et al.6) reported good results using bilateral stimulation of the caudal ZI nucleus in a single patient with dystonic tremor.
Dystonic tremor seems to be generally accepted that tremor in the setting of obvious dystonia1). The pathophysiology of dystonic tremor suggests that perhaps there is an abnormality in two cotico-subcortical loops, the basal ganglia loop causing dystonia and the Vim-thalamocortical loop causing the tremor component3,10). The subthalamic area contains the ZI nucleus and the surrounding white matter tracts, including the prelemniscal radiation and the pallidothalmic tracts. The prelemniscal radiation contains cerebellar fibers as well as fibers from the ascending mesencephalic reticular formation. The ZI is a small diencephalic nucleus with widespread efferent connections; it sends efferent fibers to both the basal ganglia output nuclei and the Vim nucleus of the thalamus6). Therefore, the subthalamic area for stimulation has gained interest as a target for controlling various types of involuntary movement. However, the optimal targets within the subthalamic area are not known for various types of involuntary movements and they are particularly difficult to define from microrecording. Bilateral stimulation in the prelemiscal radiation frequently develped dysarthria and disequibrium8). Further studies need to be performed to identify the optimal targets and recording methods for electrophysiological confirmation in the subthalamic area for movement disorders.
CONCLUSION
This case report demonstrates that the subthalamic area is a valuable target for the control of dystonic tremor. Further studies are needed, however, to clarify recording method for electrophysiological confirmation and also to find the optimal target in the subthalamic area.
Fig. 2.
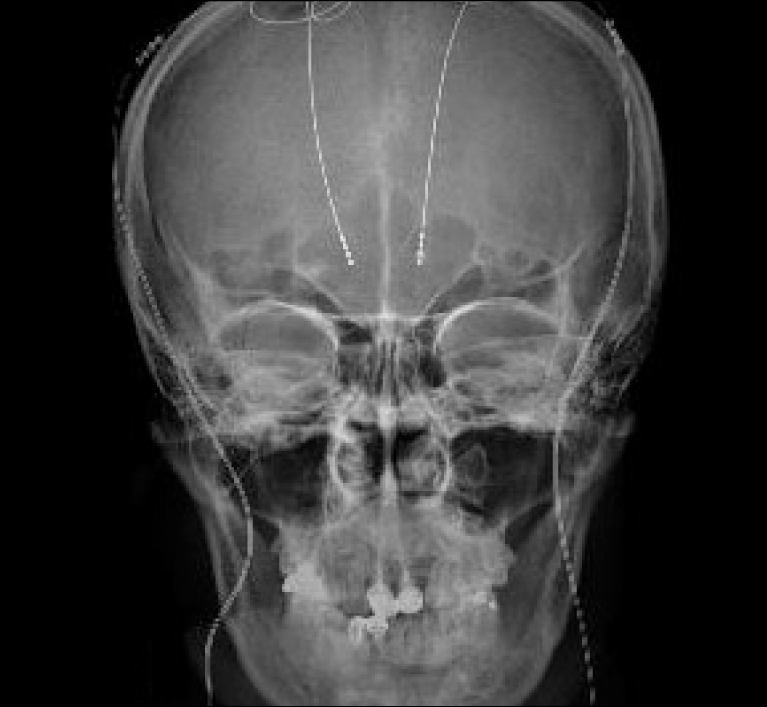
Postoperative antero-posterior radiograph showing the placed quadripolar permanent electrodes.
Acknowledgements
This work presented orally at the spring meeting of the Korean Society of Stereotactic and Functional Neurosurgery 2007.
References
- 1.Deuschl G. Dystonic tremor. Rev Neurol (Paris) 2003;159:900–905. [PubMed] [Google Scholar]
- 2.Kitagawa M, Murata J, Kikuchi S, Sawamura Y, Saito H, Sasaki H, et al. Deep brain stimulation of subthalamic area for severe proximal tremor. Neurology. 2000;55:114–116. doi: 10.1212/wnl.55.1.114. [DOI] [PubMed] [Google Scholar]
- 3.Molnar GF, Sailer A, Gunraj CA, Lang AE, Lozano AM, Chen R. Thalamic deep brain stimulation activates the cerebellothalamocortical pathway. Neurology. 2004;63:907–909. doi: 10.1212/01.wnl.0000137419.85535.c7. [DOI] [PubMed] [Google Scholar]
- 4.Mundinger F. Stereotaxic interventions on the zona incerta area for treatment of extrapyramidal motor disturbances and their results. Confin Neurol. 1965;26:222–230. doi: 10.1159/000104030. [DOI] [PubMed] [Google Scholar]
- 5.Murata J, Kitagawa M, Uesugi H, Saito H, Iwasaki Y, Kikuchi S, et al. Electrical stimulation of the posterior subthalamic area for the treatment of intractable proximal tremor. J Neurosurg. 2003;99:708–715. doi: 10.3171/jns.2003.99.4.0708. [DOI] [PubMed] [Google Scholar]
- 6.Plaha P, Khan S, Gill SS. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry. 2008;79:504–513. doi: 10.1136/jnnp.2006.112334. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel EA, Wycis HT, Szekely EG, Adams DJ, Flanagan M, Baird HW., 3rd Campotomy in various extrapyramidal disorders. J Neurosurg. 1963;20:871–884. doi: 10.3171/jns.1963.20.10.0871. [DOI] [PubMed] [Google Scholar]
- 8.Velasco F, Jimenez F, Perez ML, Carrillo-Ruiz JD, Velasco AL, Ceballos J, et al. Electrical stimulation of the prelemniscal radiation in the treatment of Parkinson's disease : an old target revised with new techniques. Neurosurgery. 2001;49:293–306. doi: 10.1097/00006123-200108000-00009. discussion 306-308. [DOI] [PubMed] [Google Scholar]
- 9.Vercueil L, Krack P, Pollak P. Results of deep brain stimulation for dystonia : a critical reappraisal. Mov Disord. 2002;17(Suppl 3):S89–S93. doi: 10.1002/mds.10148. [DOI] [PubMed] [Google Scholar]
- 10.Vitek JL. Pathophysiology of dystonia : a neuronal model. Mov Disord. 2002;17(Suppl 3):S49–S62. doi: 10.1002/mds.10142. [DOI] [PubMed] [Google Scholar]