Abstract
The goals of this study were to identify first-line drug resistance in new and previously treated tuberculosis (TB) cases and to determine risk factors for multidrug-resistant TB (MDR-TB) at a private referral center in Korea. All patients with culture-confirmed pulmonary TB over a 2-yr period between July 2002 and June 2004 were prospectively included in this study. In total, 637 patients were included; 512 (80.4%) were new cases, and 125 (19.6%) were previously treated cases. Resistance to at least one first-line drug was identified in 11.7% of new cases and 41.6% of previously treated cases. MDR-TB was detected in 3.9% of new cases and 27.2% of previously treated cases. The proportion of extensively drug-resistant TB among MDR-TB patients was 16.7% (9/54). Factors associated with MDR-TB included age under 45 yr, previous TB treatment, and the presence of cavitation on chest radiography. Rates of first-line drug resistance are high, particularly in previously treated patients, in the private sector in Korea. This underscores the need for an improved control program, coupled with early diagnosis of MDR-TB, to reduce the spread and development of resistance.
Keywords: Mycobacterium tuberculosis, Korea, Drug Resistance, Isoniazid, Rifampin
INTRODUCTION
Tuberculosis (TB) is the world's leading cause of death from a single infectious disease, and despite advances in chemotherapy policy, the prevalence of TB remains high. Furthermore, resistance to anti-TB drugs is an increasing problem in many parts of the world, and the identification of the drug-resistance rate and risk factors is critical for controlling drug-resistant TB (1). Patients with multidrug-resistant TB (MDR-TB) are difficult to cure, and treatment is much more toxic and expensive (2-6).
In Korea, TB remains a major public health threat and an economic burden. Official data reported 35,269 new cases (73.0/100,000 population) in 2005 (7). In Korea, a complex cooperative relationship exists between the public and private sectors in the treatment of TB (8). The public sector is composed of approximately 250 National Tuberculosis Program (NTP) health centers and two national TB hospitals. The private sector includes a wide variety of physicians, ranging from general practitioners in private practice to pulmonary specialists at referral hospitals.
Previous studies of drug-resistant TB in Korea have been conducted mainly in the public sector (9-12), where according to the results of a recent national survey, MDR-TB strains occurred in 2.7% of new cases and 14.0% of previously treated cases (12). However, few data have been collected regarding the drug resistance of Mycobacterium tuberculosis in the private sector, where at least two-thirds of TB patients in Korea were treated (7).
The goals of this study were to identify first-line anti-TB drug resistance in new and previously treated patients and to determine the risk factors for MDR-TB at a private referral center in Korea.
MATERIALS AND METHODS
Patients
All patients with culture-confirmed pulmonary TB diagnosed at the Samsung Medical Center (a 1,250-bed referral hospital in Seoul, Korea) over a 2-yr period between July 2002 and June 2004 were prospectively included in this study. During the 2-yr study period, M. tuberculosis was isolated from 743 patients. Of these, 106 were excluded from this study: the patients who had already started treatment for TB before the beginning of the study period (n=29), pediatric patients who were less than 15 yr of age (n=7), and extrapulmonary TB cases (n=70). Thus, 637 patients with culture-confirmed pulmonary TB were included in this study.
Laboratory methods
Clinical specimens were stained using the Ziehl-Neelsen method (13). The results of sputum smear on microscopy were reported semiquantitatively, and a positive smear was defined as one with more than one acid-fast bacillus (AFB) per 100 in a field under high-power (13). Clinical specimens were cultured on 3% Ogawa medium. The identity of M. tuberculosis cultures was confirmed using a combination of growth rate, colony morphology, and pigmentation, and with the aid of a commercial DNA probe (Gen-Probe Amplified Mycobacterium Tuberculosis Direct Test; Gen-Probe Inc. San Diego, CA, U.S.A.). All isolates of M. tuberculosis were referred to the Korean Institute of Tuberculosis for drug susceptibility testing. When multiple isolates were obtained from the same patient, only the first isolate was used in this study.
The drug susceptibility of the M. tuberculosis isolates was determined by the absolute concentration method, using Lowenstein-Jensen medium (9). The drugs and their critical concentrations for resistance were as follows: isoniazid (INH), 0.2 µg/mL; rifampin (RFP), 40 µg/mL; streptomycin (SM), 10 µg/mL; and ethambutol (EMB), 2 µg/mL. Pyrazinamide (PZA) susceptibility was determined by a pyrazinamidase test.
Definitions
Patients were classified into two groups according to their treatment history at the time of diagnosis: new cases, which included patients who had never received anti-TB treatment or who had received treatment for <4 weeks, and previously treated cases, which included patients who had taken anti-TB drugs for at least 4 weeks (14).
Initial resistance was defined as the presence of drug-resistant M. tuberculosis strains in new cases. Acquired resistance was defined as the presence of drug-resistant M. tuberculosis strains in patients who were reported to have received anti-TB treatment for >4 weeks. Any drug resistance was defined as resistance to any drug. Monoresistance was defined as resistance to only one of the five first-line drugs (INH, RFP, PZA, SM, and EMB). MDR was defined as resistance to at least INH and RFP.
MDR-TB cases were further analyzed to determine the extent of drug resistance. Extensively drug-resistant TB (XDR-TB) was defined as resistance to at least RFP and INH (which is MDR-TB by definition), in addition to any fluoroquinolone, and to at least one of the three following injectable drugs used in anti-TB treatment: capreomycin, kanamycin, and amikacin (15). The second-line drugs and their critical concentrations for resistance were as follows: kanamycin 40 µg/mL; capreomycin 40 µg/mL; prothionamide 40 µg/mL; cycloserine 30 µg/mL; para-aminosalicylic acid 1.0 µg/mL; and ofloxacin 2.0 µg/mL.
Statistical analysis
The overall results are expressed as percentages and absolute frequencies for qualitative variables and as mean results for quantitative variables. We compared the clinical and radiographic findings between patients with MDR-TB and those with non-MDR-TB. Frequencies were analyzed using the chi-square test or the Fisher's exact test, as appropriate. Multivariate analysis was conducted with a logistic regression model to determine independent risk factors for MDR-TB. A p value of less than 0.05 was considered significant. Calculations were performed using the SPSS statistical program (SPSS version 10.0; SPSS Inc. Chicago, IL, U.S.A.).
RESULTS
Characteristics of patients
In total, 637 patients newly diagnosed with pulmonary TB between July 2002 and June 2004 were included. Of the 637 patients, 512 (80.4%) were new cases and 125 (19.6%) were previously treated cases. The mean age of the patients was 48 yr (range, 15-92 yr), and 372 (58%) were male (Table 1).
Table 1.
Characteristics of the study population (n=637)
TB, tuberculosis; AFB, acid-fast bacilli.
Drug resistance of M. tuberculosis
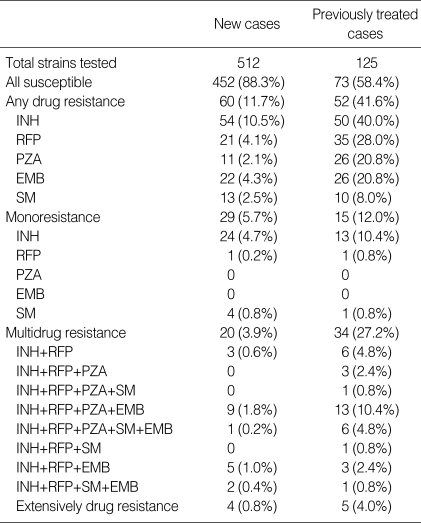
Of the M. tuberculosis strains isolated from the 637 patients, 525 (82.4%) were susceptible to all first-line drugs tested, and 112 (17.6%) were resistant to at least one drug. The different resistance profiles of the patients are shown in Table 2.
Table 2.
Resistance to first-line drugs among Mycobacterium tuberculosis strains
INH, isoniazid; RFP, rifampicin; PZA, pyrazinamide; SM, streptomycin; EMB, ethambutol.
According to the previous treatment status, isolates from 452 (88.3%) of the 512 new cases were susceptible to all first-line drugs, whereas this percentage was 58.4% for previously treated patients (73/125). Fifty-four strains from new cases and 50 from previously treated patients were resistant to INH (10.5% and 40.0%, respectively). Twenty cases of MDR-TB were observed among new patients (3.9%) and 34 among previously treated patients (27.2%). Nine patients (16.7%, 9/54) with MDR-TB were XDR-TB.
Risk factor analysis of patients with MDR-TB
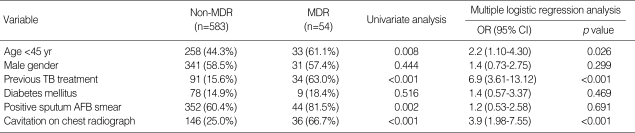
Patients with MDR-TB (61%) were more likely to be under 45 yr of age than non-MDR-TB patients (44%; p=0.008). MDR-TB cases (63.0%) were also more likely to have received previous TB treatment than non-MDR-TB (15.6%) cases (p<0.001). In addition, patients with MDR-TB were more likely to have had a higher percentage of sputum smear results that were positive for AFB (81.5%) and to have exhibited cavitation on chest radiography (66.7%) compared to patients with non-MDR-TB (60.4% and 25.0%, respectively; p=0.002 and p<0.001, respectively).
Multivariate analysis showed that a younger age (<45 yr old; OR, 2.2; 95% CI, 1.10-4.30; p=0.026), previous TB treatment (OR, 6.9; 95% CI, 3.61-13.12; p<0.001), and presence of cavitation on chest radiography (OR, 3.9; 95% CI, 1.98-7.55; p<0.001) were significant independent risk factors for MDR-TB (Table 3).
Table 3.
Variables associated with multidrug-resistant tuberculosis
MDR, multidrug-resistant; OR, odds ratio; 95% CI, 95% confidence interval; TB, tuberculosis; AFB, acid-fast bacilli.
DISCUSSION
The objective of this study, which consecutively enrolled all patients with newly diagnosed culture-confirmed pulmonary TB during a 2-yr study period, was to identify first-line drug resistance in the new and previously treated patients and to determine risk factors for MDR-TB at a private referral hospital in Korea. The major findings in this study were the followings. The rate of MDR-TB in the private referral hospital was high, especially for the previously treated cases. In addition, age under 45 yr, previous TB treatment, and presence of cavitation were independent risk factors for MDR-TB.
Knowing the true drug resistance rates in a region or country is essential for developing appropriate treatment strategies. According to the results of a recent national survey in Korea, the percentage of MDR strains was 2.7% in new cases and 14.0% in previously treated cases (12). However, this survey was conducted only in TB patients who had been treated in the public sector. In Korea, the number of TB cases treated in the private sector has been continuously increasing. In the last Nationwide Tuberculosis Prevalence Survey from 1995, 53% of patients were reported to have received treatment in NTP health centers and 47% in the private sector (16). By contrast, 66% of patients with TB were treated in the private sector and 34% in the NTP health centers in recent years (7). Therefore, it is imperative to evaluate the drug resistance rate of M. tuberculosis in the private sector in Korea.
In Korea, a few small retrospective studies on the drug resistance of M. tuberculosis have been conducted in the private sector, reporting rates of MDR-TB up to 7.0% in new patients and 38.1% in previously treated patients (17-20). Previous studies had several limitations. First, the drug susceptibility test was not recommended to new cases by the NTP program in Korea until recent years. Therefore, the retrospective study of patients who had drug susceptibility test results could overestimate the drug resistance rates. Second, prevalence surveys of TB may overrepresent cases with longer duration of positive culture results, thereby overrepresenting patients with MDR-TB, because MDR-TB tends to be associated with a longer duration of positive culture results than drug-susceptible TB.
This prospective study over a 2-yr period included newly diagnosed cases of pulmonary TB, excluding prevalent cases. In our study, the rate of primary resistance to any drugs and the rate of MDR-TB in new patients were 11.7% and 3.9%, respectively. These initial resistance figures are similar to the mean figures in the public sector (12.8% and 2.7%, respectively) (12). By contrast, the rate of acquired resistance to any drug and the rate of MDR-TB in previously treated patients in our study were 41.6% and 27.2%, respectively. These values are significantly higher than those reported in the public sector (27.7% and 14.0%, respectively) (12). These differences could be explained by the higher proportion of treatment failure cases among previously treated patients in private tertiary referral hospitals, while the majority of previously treated patients in public sector are cases of relapse due to drug-susceptible bacilli (8).
This study demonstrated that 16.7% (9/54) of MDR-TB cases were XDR-TB. In March 2006, a report from the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) drew international attention to the emergence of XDR-TB (21). In this report, XDR-TB was defined as resistance to at least three of the six classes of available second-line drugs (aminoglycosides, polypeptides, fluoroquinolones, thioamides, cycloserine, and para-aminosalicylic acid) in addition to MDR-TB (21). The proportion with XDR-TB among MDR-TB cases in Korea was 15.4% in this report (21). Recently, WHO revised laboratory case definition for XDR-TB. XDR-TB was defined as resistance to at least RFP and INH, in addition to any fluoroquinolone, and to at least one of the three following injectable drugs used in anti-TB treatment: capreomycin, kanamycin, and amikacin (15). The proportion of XDR-TB among MDR-TB cases in our study (16.7%) was similar to that in the previous report (15.4%) (21), although the definition of XDR-TB has been changed.
In addition, our study found that previous history of TB treatment, younger age (<45 yr old), and presence of cavitation on radiography were significant risk factors for MDR-TB. In line with other reports (22-24), previous TB treatment was the strongest risk factor for MDR-TB in our study (OR, 6.9; 95% CI, 3.61-13.12).
An association was observed between MDR-TB and age under 45 yr, as has been noted in previous studies (24-26). This result could reflect the year in which effective anti-TB drugs such as RFP were introduced. In Korea, RFP-based regimens were first applied in the private sector during the 1980s, and the current 6-month, four-drug regimen became standard in the NTP in 1990. Elderly patients have acquired the organisms in the past, when the circulating bacilli were susceptible, whereas young patients have acquired the bacilli more recently, when the bacilli were more likely to be resistant.
The presence of cavitation on chest radiography was also an independent risk factor for MDR-TB in our study. Although some previous studies suggest that cavitation on chest radiography is a risk factor for MDR-TB (27, 28), a possible explanation is still elusive.
This study has several limitations. Among those with previous TB treatment, a lack of information on the susceptibility of the isolate from the initial TB episode precluded ascertaining whether drug resistance was attributable to inadequate or incomplete treatment, or was the results of primary infection with a resistant strain. In addition, the potential association between drug resistance and treatment length could not be examined, as the timing and duration of previous treatments were not well documented in the patients' medical records or recollected by patients (29).
In summary, the drug-resistance rate of pulmonary TB, especially MDR-TB, is higher in the previously treated patients at a private referral hospital than in those in the public sector in Korea. Younger age (<45 yr old), previous TB treatment, and the presence of cavitation on chest radiography were independent risk factors of MDR-TB. In addition, a significant proportion of MDR-TB cases was XDR-TB. This underscores the need for an improved control program, coupled with early diagnosis of MDR-TB, in the private sector in Korea, especially in high-risk patients, to reduce the spread and development of resistance.
Footnotes
This work was supported by the Samsung Biomedical Research Institute grant (# SBRI C-A6-402-1).
References
- 1.Espinal MA, Kim SJ, Suarez PG, Kam KM, Khomenko AG, Migliori GB, Baez J, Kochi A, Dye C, Raviglione MC. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283:2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 2.Colebunders R, Apers L, Shamputa IC. Treatment of multidrug-resistant tuberculosis. Lancet. 2004;363:1240. doi: 10.1016/S0140-6736(04)15970-9. [DOI] [PubMed] [Google Scholar]
- 3.Timperi R, Han LL, Sloutsky A, Becerra MC, Nardell EA, Salazar JJ, Smith-Fawzi MC. Drug resistance profiles of Mycobacterium tuberculosis isolates: five years' experience and insight into treatment strategies for MDR-TB in Lima, Peru. Int J Tuberc Lung Dis. 2005;9:175–180. [PubMed] [Google Scholar]
- 4.Ferrara G, Richeldi L, Bugiani M, Cirillo D, Besozzi G, Nutini S, Casali L, Fiorentini F, Codecasa LR, Migliori GB. Management of multidrug-resistant tuberculosis in Italy. Int J Tuberc Lung Dis. 2005;9:507–513. [PubMed] [Google Scholar]
- 5.Torun T, Gungor G, Ozmen I, Bolukbasi Y, Maden E, Bicakci B, Atac G, Sevim T, Tahaoglu K. Side effects associated with the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9:1373–1377. [PubMed] [Google Scholar]
- 6.Kang YA, Choi YJ, Cho YJ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. Cost of treatment for multidrug-resistant tuberculosis in South Korea. Respirology. 2006;11:793–798. doi: 10.1111/j.1440-1843.2006.00948.x. [DOI] [PubMed] [Google Scholar]
- 7.Korean Center for Disease Control and Prevention. Annual report on the notified tuberculosis patients in Korea (2005.1-2005.12) 2006. [Google Scholar]
- 8.Seung KJ, Bai GH, Kim SJ, Lew WJ, Park SK, Kim JY. The treatment of tuberculosis in South Korea. Int J Tuberc Lung Dis. 2003;7:912–919. [PubMed] [Google Scholar]
- 9.Kim SJ, Bai GH, Hong YP. Drug-resistant tuberculosis in Korea, 1994. Int J Tuberc Lung Dis. 1997;1:302–308. [PubMed] [Google Scholar]
- 10.Pablos-Mendez A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, Bustreo F, Cohn DL, Lambregts-van Weezenbeek CS, Kim SJ, Chaulet P, Nunn P. Global surveillance for antituberculosis-drug resistance, 1994-1997. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N Engl J Med. 1998;338:1641–1649. doi: 10.1056/NEJM199806043382301. [DOI] [PubMed] [Google Scholar]
- 11.Espinal MA, Laszlo A, Simonsen L, Boulahbal F, Kim SJ, Reniero A, Hoffner S, Rieder HL, Binkin N, Dye C, Williams R, Raviglione MC. Global trends in resistance to antituberculosis drugs. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N Engl J Med. 2001;344:1294–1303. doi: 10.1056/NEJM200104263441706. [DOI] [PubMed] [Google Scholar]
- 12.Bai GH, Park YK, Choi YW, Bai JI, Kim HJ, Chang CL, Lee JK, Kim SJ. Trend of anti-tuberculosis drug resistance in Korea, 1994-2004. Int J Tuberc Lung Dis. 2007;11:571–576. [PubMed] [Google Scholar]
- 13.American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Treatment of tuberculosis: guidelines for national programmes. 2003. [Google Scholar]
- 15.World Health Organization. Report of the meeting of the WHO Global Task Force on XDR-TB. 2006. [Google Scholar]
- 16.Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998;2:27–36. [PubMed] [Google Scholar]
- 17.Kim JH, Kim JH, Jang TW, Jung MH. Drug-resistant pulmonary tuberculosis in Kosin Medical Center. Tuber Respir Dis. 1995;42:831–837. [Google Scholar]
- 18.Kim SY, Jeong SS, Kim KW, Shin KS, Park SG, Kim AK, Cho HJ, Kim JO. Drug-resistant pulmonary tuberculosis in a tertiary referral hospital in Korea. Korean J Intern Med. 1999;14:27–31. doi: 10.3904/kjim.1999.14.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JH, Chang JH. Drug-resistant tuberculosis in a tertiary referral teaching hospital of Korea. Korean J Intern Med. 2001;16:173–179. doi: 10.3904/kjim.2001.16.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DK, Kim MO, Kim TH, Sohn JW, Yoon HJ, Shin DH, Park SS. The prevalence and risk factors of drug resistance pulmonary tuberculosis investigated at one university hospital in Seoul. Tuber Respir Dis. 2005;58:243–247. [Google Scholar]
- 21.Centers for Disease Control and Prevention. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs--worldwide, 2000-2004. MMWR Morb Mortal Wkly Rep. 2006;55:301–305. [PubMed] [Google Scholar]
- 22.Arevalo M, Solera J, Cebrian D, Bartolome J, Robles P. Risk factors associated with drug-resistant Mycobacterium tuberculosis in Castilla-la-Mancha (Spain) Eur Respir J. 1996;9:274–278. doi: 10.1183/09031936.96.09020274. [DOI] [PubMed] [Google Scholar]
- 23.Ruddy M, Balabanova Y, Graham C, Fedorin I, Malomanova N, Elisarova E, Kuznetznov S, Gusarova G, Zakharova S, Melentyev A, Krukova E, Golishevskaya V, Erokhin V, Dorozhkova I, Drobniewski F. Rates of drug resistance and risk factor analysis in civilian and prison patients with tuberculosis in Samara Region, Russia. Thorax. 2005;60:130–135. doi: 10.1136/thx.2004.026922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faustini A, Hall AJ, Perucci CA. Risk factors for multi-drug resistant tuberculosis in Europe: a systematic review. Thorax. 2006;61:158–163. doi: 10.1136/thx.2005.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Djuretic T, Herbert J, Drobniewski F, Yates M, Smith EG, Magee JG, Williams R, Flanagan P, Watt B, Rayner A, Crowe M, Chadwick MV, Middleton AM, Watson JM. Antibiotic resistant tuberculosis in the United Kingdom: 1993-1999. Thorax. 2002;57:477–482. doi: 10.1136/thorax.57.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kordy FN, Al-Thawadi S, Alrajhi AA. Drug resistance patterns of Mycobacterium tuberculosis in Riyadh, Saudi Arabia. Int J Tuberc Lung Dis. 2004;8:1007–1011. [PubMed] [Google Scholar]
- 27.Harrow EM, Rangel JM, Arriega JM, Cohen I, Regil Ruiz MI, De-Riemer K, Small PM. Epidemiology and clinical consequences of drug-resistant tuberculosis in a Guatemalan hospital. Chest. 1998;113:1452–1458. doi: 10.1378/chest.113.6.1452. [DOI] [PubMed] [Google Scholar]
- 28.Sharma SK, Turaga KK, Balamurugan A, Saha PK, Pandey RM, Jain NK, Katoch VM, Mehra NK. Clinical and genetic risk factors for the development of multi-drug resistant tuberculosis in non-HIV infected patients at a tertiary care center in India: a case-control study. Infect Genet Evol. 2003;3:183–188. doi: 10.1016/s1567-1348(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 29.Espinal MA, Laserson K, Camacho M, Fusheng Z, Kim SJ, Tlali RE, Smith I, Suarez P, Antunes ML, George AG, Martin-Casabona N, Simelane P, Weyer K, Binkin N, Raviglione MC. Determinants of drug-resistant tuberculosis: analysis of 11 countries. Int J Tuberc Lung Dis. 2001;5:887–893. [PubMed] [Google Scholar]