Abstract
Non-typhoidal Salmonella (NTS) is an important commensal microorganism. The purpose of this study was to determine the epidemiological relation between NTS isolates from livestock and NTS isolates from human by analyzing antimicrobial susceptibilities and performing molecular typing. We determined the serotypes of 36 human clinical isolates and 64 livestock isolates, performed antimicrobial susceptibility testing against 8 antibiotics, and determined the molecular types of isolated NTS spp. by pulsed field gel electrophoresis (PFGE). In human isolates, S. enteritidis was the most common serotype (17 isolates; 47.2%) and S. typhimurium the second most (8 isolates; 22.2%). In livestock isolates, S. typhimurium was the most common serotype (15 isolates; 23.44%), and S. enteritidis was the second most (14 isolates; 21.88%). Ampicillin and tetracycline resistance were 50% (32/64 isolates) each among broiler-chicken NTS isolates. No human or livestock NTS isolates showed resistance to ciprofloxacin, TMP-SMX, or ceftriaxone. However, 19.4% (7/36) and 46.8% (30/64) of the human and livestock NTS isolates were resistant to nalidixic acid (MIC ≥16 mg/mL), respectively. The presence of the three identical PFGE molecular types from human and broiler-chicken NTS isolates suggests the possibility of transmission from livestock to humans.
Keywords: Salmonella Infections, Salmonella Enteritidis, Salmonella Typhimurium, Epidemiology
INTRODUCTION
Non-typhoidal Salmonella (NTS) spp. is an important cause of infection in both human and animals. Although food-borne diseases caused by NTS are representative of NTS infections in human, bacteremia and other invasive diseases are also caused by NTS.
Several studies have documented that farm animals are the main reservoir for NTS in developed countries. In the US and Europe, outbreaks of gastroenteritis caused by Salmonella enterica serotype Enteritidis have been considered an important public health problem since 1990 (1). Nearly 1.4 million cases of Salmonellosis occur every year in the United States (2). Investigations of outbreaks and sporadic cases have indicated repeatedly that the most common sources of S. enteritidis infection are undercooked or raw eggs, contaminated poultry, beef, pork, eggs, and milk (3, 4). For instance, in the U.S.A., it is estimated that over 95% of NTS infections are related to food-borne transmission (2).
NTS infection has emerged as an important public health problem in Korea since 1995, when the nation-wide school meal program started. However, with increasingly stringent food safety measures, salmonella have shown a tendency to decrease, i.e., from 2,242 isolates in 1999 to 902 isolates in 2002 in Korea (5). Recently, however, it has been reported that the prevalence of Salmonella spp. in food, especially in poultry products (up to 2.2%) is high in Korea (6). Investigations have shown that the prevalence of S. enteritidis-infected chicken and eggs in retail outlets is 18.5% (7), and have suggested that infected raw eggs or chickens could be major sourceies of Salmonellosis in Korea, but there has been no further studies.
In these days, multi-drug resistant (MDR) salmonella is an emerging problem because of the wide-spread use of antibiotics in veterinary medicine and animal husbandry, and as growth promoters in livestock (8). According to a previous report, the frequency of MDR S. typhimurium DT104 was 28% among total S. typhimurium isolates in US (9). The frequency of DT104 in all isolated S. typhimurium was 10.8% and 2.5% in 1998, and 1999, respectively in Korea (10).
The purpose of this study was to identify the presence of DT104 among NTS isolated from broiler chicken which is the main animal reservoir of Salmonellosis and among isolates from patients of southwestern Seoul, Korea in 2003. The antimicrobial resistance pattern was examined to understand the seriousness of antimicrobial resistance. In addition, the most common serotype and molecular type were investigated from the NTS isolated from the patients of the same area and broiler chicken to verify the relation between human and livestock isolates.
MATERIALS AND METHODS
Human isolates
A total of 36 representative human NTS isolates were collected from patients with clinical NTS infections who visited Korea University Guro Hospital that was located in Southwestern area of Seoul, between October 2002 and August 2003.
Sample collection from livestock
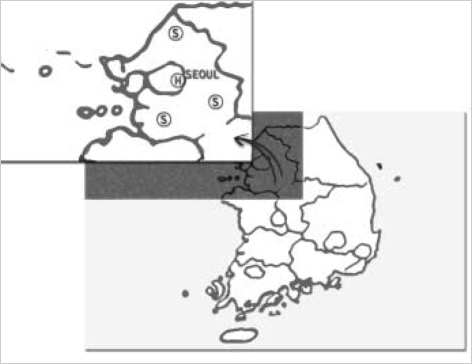
Form March 2003 to August 2003, a total of 300 samples from broiler-chickens originating from a number of different farms those are located in suburbs of the southwest area of Seoul were collected from three representative slaughter houses those were also located in the same area (Fig. 1). However, individual farms were not identified. Five times in total, 20 chickens each were sampled from each slaughter house. Sampling was done in a random systematic manner with every fifth bird in the processing line being sampled. Entire ceca were removed from the slaughter and dispatched to a microbiology laboratory, where their contents were removed. Sample materials were pre-enriched in buffered peptone water (35℃ for 20 hr) and selectively enriched by inoculation on Modified Semisolid Rapparport Vassiliadis Medium at 41.5℃ for 24 hr. Pure cultures were obtained by streaking on blood agar and MacConkey agar plates. Slide agglutination test using polyvalent Salmonella antiserum (Difco, Detroit, IM, U.S.A.) was used for preliminary identification of isolates.
Fig. 1.
Locations of study hospital (H) and three slaughter-houses (S) in the southwest area of Seoul and its suburbs.
Serotyping
All isolates were confirmed as Salmonella, and then serotyped (11). Serotyping was performed at the Korean Institute of Health & Environment, Seoul, Korea.
Antimicrobial susceptibility testing
Antimicrobial susceptibility tests were performed on Muller-Hinton agar (Oxoid, U.S.A.) by using the agar dilution method (12). Minimal inhibitory concentrations (MIC) were determined according to the National Committee for Clinical Laboratory Standards (NCCLS) (13) guidelines. Escherichia coli ATCC 25922 was used for as a quality control standard.
The following antibiotics were tested: ampicillin, gentamicin, kanamycin, ciprofloxacin, tetracycline, trimethoprim-sulfamethoxazole (TMP-SMX), chloramphenicol, nalidixic acid, and ceftriaxone.
Pulsed field gel electrophoresis (PFGE)
The clonality of Salmonella was analyzed by PFGE. Following the previously described methods (14, 15), genomic DNA was prepared by embedding cells of Salmonella isolates in agarose plugs and lysing the cells using lysozyme, sacrosyl, and deoxycholate. Xba I (TaKaRa, Shiga, Japan) was used to digest the DNA for each isolate. Electrophoresis to separate fragments by size was carried out using the CHEF-DR III system (BioRad, Richmond, CA, U.S.A.). The PFGE pulsing and running conditions were an initial 3 sec to a final 30 sec for 23 hr and at 6 V/cm at 14℃ Bacteriophage lambda concatamers (New England Biolabs, Hitchin, U.K.) were used as molecular size markers. Following electrophoresis, the gel was stained with ethidium bromide, visualized under UV light and photographed. Clustering analysis was based on unweighted-pair group method of arithmetic average (UPGMA) and was performed with Gel-Compar software (version 4, Applied Maths).
RESULTS
Human NTS: clinical characteristics of patients and serotypes
Thirty-six human NTS were isolated from October 2002 to August 2003 at Korea University Guro Hospital. These isolates were from sporadic cases seeking medical attention, who were living in the southwest Seoul and neighboring areas.
It was difficult to incriminate any particular common food source, and moreover, there were not any travel histories, and therefore we could assume that the cases had developed in the Guro district and its neighboring, southwest area of Seoul.
Among 36 human isolates, 32 NTS were isolated from stools. The other NTS sources were blood (3 isolates) and urine (1 isolate). There were 22 men and 14 women, with a median age of 29 yr. Most of the patients did not have any underlying diseases, such as diabetes mellitus, malignancy, renal dysfunction, abnormal liver function, peptic ulcer disease, or cardiovascular disease. One patient had breast cancer.
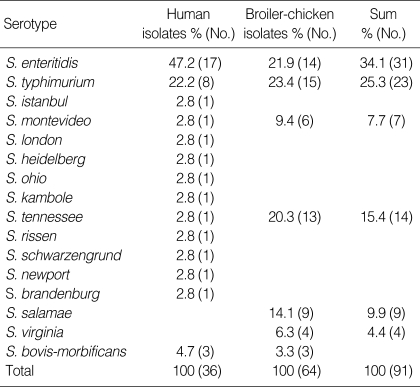
Serotypes of human NTS isolates are listed in Table 1. The main Salmonella serovars were S. enteritidis (17; 47.2%) and S. typhimurium (8; 22.2%). Other serotypes such as S. brandenburg (1), S. heidelberg (1), and S. istanbul (1) were isolated in small numbers.
Table 1.
Distribution of the human and livestock Salmonella by serotype
Broiler-chicken NTS: characteristics and serotypes
Of the 300 cecal samples from freshly slaughtered broiler-chicken, NTS were isolated from 21.3% (n=64) of the slaughtered chicken between March 2003 to August 2003. S. typhimurium was the most common serotype (15 isolates; 23.44%), S. enteritidis (14 isolates; 21.88%) and S. tennessee (13 isolates; 20.31%) were the second and third most common (Table 1).
Antimicrobial susceptibility
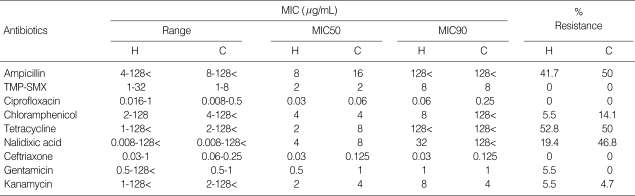
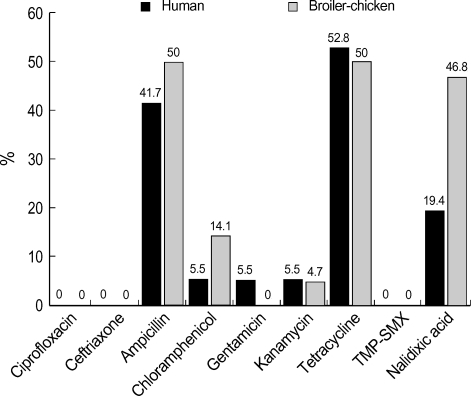
The antimicrobial susceptibilities of the NTS isolates are shown in Table 2. Of the 36 human NTS isolates, 25% (9 isolates) were susceptible to all tested antimicrobial agents. However, only 4.7% (3 isolates) were susceptible to all tested antibiotics among 64 livestock NTS isolates. The remaining strains were non-susceptible to one or more of the antimicrobial agents tested. The proportions of resistance to tetracycline and ampicillin were remarkable in both groups: 52.8% (19 isolates) and 41.7% (15 isolates) of 36 human NTS were resistant to tetracycline and ampicillin and 50.0% (32 isolates) to each antibiotic among 64 livestock NTS isolates (Fig. 2). The prevalence of dual resistance to ampicillin and tetracycline were 38.9% (14 of 36 isolates) and 54.7% (35 of 64 isolates) in human and livestock NTS isolates, respectively.
Table 2.
Minimal inhibitory concentration (MIC)s of NTS isolates against major antibiotics
H, human isolates (N=36); C, broiler-chicken (N=64), TMP-SMX, trimethoprim-sulfamethoxazole.
Fig. 2.
Antimicrobial resistance (%) of human and broiler-chicken NTS isolates.
No human or livestock isolate showed resistance to ciprofloxacin, TMP-SMX, or ceftriaxone. Although all our NTS isolates were susceptible to ciprofloxacin, 19.4% (7 of 36 isolates) and 46.8% (30 of 64 isolates) of the human and livestock NTS isolates were resistant to nalidixic acid (MIC ≥16 mg/mL), respectively.
Even though 8.3% (3 of 36 isolates) and 17.2% (11 of 64 isolates) of the human and livestock NTS isolates demonstrated resistance to three or more antibiotics, we could not find out DT104-like strain by antibiotic susceptibility test. Overall, the level of resistance in human NTS was similar to that of livestock in Korea except against chloramphenicol.
Pulsed field gel electrophoresis
In order to compare the clonality of NTS isolated from human and broiler-chicken of the Southwest area of Seoul and its suburb area, we typed all the 54 isolates of S. enteritidis, S. typhimurium; major serotypes of our study, by PFGE and XbaI digestion.
S. enteritidis was the most common isolate regardless of NTS source or isolation date (Fig. 3A), there was one predominant PFGE type, A0, and this profile was further differentiated into 2 subtypes (A0 and A1) with a 1-3-bands difference (F=0.8-1.0). It was shown that 87.1% of the total strains sampled (27 of 31) had very closely related profiles, indicating the homogenicity and clonality of S. enteritidis strains. They showed as high as 94% similarity in dendrogam analysis. Other than PFGE type A, two different PFGE types were observed among S. enteritidis isolates: B (6.5%, 2 isolates) and C (6.5%, 2 isolates). Unique B type was associated with two strains isolated from one bacteremic patient with breast cancer and from one patient with diarrhea. Type C was observed in strains, one from other diarrhea patient, and the other from broiler chicken.
Fig. 3.
Dendrogram showing cluster analysis of PFGE Xba-I patterns from 54 strains of S. enteritidis and S. typhimurium generated with GelCompar by the UPGMA method. The different PFPs and number of strains tested are indicated.
(A) S. enteritidis, (B) S. typhimurium.
The second most common isolate was S. typhimurium and also regardless of the source, 21 isolates (7 human isolates and 14 chicken isolates) from the total of 23 isolates (8 human isolates and 15 chicken isolates) were type D in PGFE, and 91.3% of the total isolates showed very closely related profiles. In the dendrogram analysis it was divided into two parts; a main cluster (PFGE type D) showing 90% similarity and 2 unique strains (one from a patient with diarrhea and the other from broiler-chicken), PFGE type E, L (Fig. 3B).
As a result, regardless of the source, both S. typhimurium and S. enteritidis showed limited PFGE pattern diversity during the study period for each serotype. Moreover, a relatively unique PFGE pattern could be observed for each serotype.
DISCUSSION
Non-typhoidal Salmonella represent an important public health problem in many parts of the world, including Korea. As found in the U.S.A. and Europe, domestic animals are the major reservoir and foods of animal origin are the major vehicles of NTS infection in human (16, 17). This may also be applicable for the NTS we studied. We found that the distribution of NTS serovars among livestock from slaughterhouses were similar to those in humans. Both human and livestock isolates were predominantly S. enteritidis and S. typhimurium, although more diverse serotypes were isolated from broiler-chicken. We could infer that infected broiler-chicken is the major infection source of human Salmonellosis.
Almost all of the human and broiler-chicken isolates showed resistance to more than one antibiotics, suggesting that antimicrobial resistance is widespread in both human and livestock. As noted in another study (6), Salmonella isolated from livestock showed resistance to ampicillin (50%) and tetracycline (50%), which reflects the wide use of tetracycline, ampicillin, streptomycin, and nitrofurantoin in the Korean broiler industry.
In this study, no isolates showed resistance to both ciprofloxacin and ceftriaxone either among the human or broiler-chicken isolates. However, 7 human (19.4%) and 30 (46.9%) broiler-chicken NTS isolates were resistant to nalidixic acid (MIC ≥16 mg/mL) and showed reduced susceptibility to ciprofloxacin (MIC ≥0.125 mg/mL). The incidence of nalidixic acid resistance among broiler-chicken NTS (46.8%) was significantly higher than those from human NTS (19.4%) in this study (p=0.006). The higher rate of nalidixic acid resistance among broiler-chicken isolates compared to human would be related to the widespread use of enrofloxacin in commercial farms; susceptibility of NTS to enrofloxacin reported to be decreasing since late 1990s (18).
This result is important because there are several reports of treatment failures in humans with short-course fluoroquinolone therapy for Salmonella infections caused by strains with reduced fluoroquinolone susceptibility (19-22). Since fluoroquinolones are the drugs of choice for extraintestinal and serious intestinal complications involving Salmonellosis, fluoroquinolone resistance has the potential to cause serious therapeutic problems. An outbreak of quinolone-resistant bacteria has been reported from livestock to human (23). Thus, the use of fluoroquinolones in livestock should be restricted.
Although incidences of MDR NTS in human and in livestock were low in our study, the use of antibiotics in animal husbandry is one of the possible causes of increasing MDR Salmonella and drug-resistant Salmonella in livestock. Moreover, MDR Salmonella and drug-resistant Salmonella could be transmitted from livestock to human (24). Also, humanto-human transmission of MDR Salmonella has been implicated in a series of hospital outbreaks in Europe, North Africa, the Middle East, and in Southeast Asia (25-27). So, the continuous surveillance of antibiotic resistant NTS in human and animal sources remains a first approach to the detection of possible outbreaks of MDR NTS in Korea.
Dendrogram analysis of XbaI-digested genomic DNA separated by PFGE showed that certain common, dominant PFGE groups are present among NTS from humans and broiler-chicken, namely, type A for S. enteritidis, type D for S. typhimurium. Therefore, we presume that these human NTS serovars are related to corresponding serovars from animal sources, and that a few dominant strains are largely responsible for NTS infection in human and in broiler-chicken. Such molecular typing data allow us to elucidate the clonal relationship of the strains and would be useful for tracing the source of NTS infection in humans.
Based on our serotyping and molecular typing results, we found that common types of NTS have already spread between human and livestock in Korea. Coincidental isolation of the same Salmonella serovars with indistinguishable molecular type from human and livestock underlines the importance of zoonotic relationships (28).
The limitations of this study are as follows: 1) human isolates were restricted to the southwest area of Seoul; 2) the number of human isolates was small; and 3) although cross contamination in the slaughterhouse was eliminated to the best, same serotype or genotype NTS might have been isolated due to cross-infection among chickens in the slaughterhouse.
However, since the area of detection of patients and the region of chicken farms and slaughterhouse match, the comparison of the relation between the patient isolates and chicken isolates would not be a serious problem.
In conclusion, breeding chicken in a clean environment, slaughtering food animals in hygienic spaces, and a greater adherence to food processing standards are also required to prevent the intra- and interspecies transmission of NTS. Furthermore, the more prudent use of antibiotics is recommended for humans, in veterinary medicine, and in animal husbandry, which will be the key to reduce transmission of antimicrobial resistant NTS.
Footnotes
Technology Development Program for Agriculture and Forestry (201102-03-2-HD120), Ministry of Agriculture and Forestry, Republic of Korea.
References
- 1.Rodrigue DC, Tauxe RV, Rowe B. International increase in Salmonella enteritidis: a new pandemic? Epidemiol Infect. 1990;105:21–27. doi: 10.1017/s0950268800047609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St Louis ME, Morse DL, Potter ME, DeMelfi TM, Guzewich JJ, Tauxe RV, Blake PA. The emergence of grade A eggs as a major source of Salmonella enteritidis infections. New implications for the control of salmonellosis. JAMA. 1988;259:2103–2107. [PubMed] [Google Scholar]
- 4.Trepka MJ, Archer JR, Altekruse SF, Proctor ME, Davis JP. An increase in sporadic and outbreak-associated Salmonella enteritidis infections in Wisconsin: the role of eggs. J Infect Dis. 1999;180:1214–1219. doi: 10.1086/314984. [DOI] [PubMed] [Google Scholar]
- 5.Numbers of culture-confirmed Salmonella serovars isolates, 1996-2002. CDMR. Korean National Institute of Health. [Accessed 26 March 2003]. available: http://www.dis.mohw.go.kr.
- 6.Chung YH, Kim SY, Chang YH. Prevalence and antibiotic susceptibility of Salmonella isolated from foods in Korea from 1993 to 2001. J Food Prot. 2003;66:1154–1157. doi: 10.4315/0362-028x-66.7.1154. [DOI] [PubMed] [Google Scholar]
- 7.Chang YH. Prevalence of Salmonella spp. in poultry broilers and shell eggs in Korea. J Food Prot. 2000;63:655–658. doi: 10.4315/0362-028x-63.5.655. [DOI] [PubMed] [Google Scholar]
- 8.Fey PD, Safranek TJ, Rupp ME, Dunne EF, Ribot E, Iwen PC, Bradford PA, Angulo FJ, Hinrichs SH. Ceftriaxone-resistant Salmonella infection acquired by a child from cattle. N Engl J Med. 2000;342:1242–1249. doi: 10.1056/NEJM200004273421703. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control. NARMS 2000 Annual Report Part 2. Antimicrobial resistance of Salmonella isolates, 1996-2000; pp. 50–52. [Google Scholar]
- 10.Park MS, Kang YH, Lee SJ, Song CY, Lee BK. Characteristics of epidemic multidrug-resistant Salmonella enterica Serovar Typhimurium DT 104 strains first isolated in Korea. Korean J Infect Dis. 2002;34:1–8. [Google Scholar]
- 11.Khakhria R, Woodward D, Johnson WM, Poppe C. Salmonella isolated from humans, animals and other sources in Canada, 1983-92. Epidemiol Infect. 1997;119:15–23. doi: 10.1017/s0950268897007577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poppe C, McFadden KA, Demczuk WH. Drug resistance, plasmids, biotypes and susceptibility to bacteriophages of Salmonella isolated from poultry in Canada. Int J Food Microbiol. 1996;30:325–344. doi: 10.1016/0168-1605(96)00960-9. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically-Sixth edition: Approved Standard. Wayne, Pa, USA: NCCLS; 2003. p. M7-A6. [Google Scholar]
- 14.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsen JE, Brown DJ, Skov MN, Christensen JP. Bacterial typing methods suitable for epidemiological analysis. Applications in investigations of salmonellosis among livestock. Vet Q. 1993;15:125–135. doi: 10.1080/01652176.1993.9694390. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed R, Soule G, Demczuk WH, Clark C, Khakhria R, Ratnam S, Marshall S, Ng LK, Woodward DL, Johnson WM, Rodgers FG. Epidemiologic typing of Salmonella enterica serotype Enteritidis in a Canada-wide outbreak of gastroenteritis due to contaminated cheese. J Clin Microbiol. 2000;38:2403–2406. doi: 10.1128/jcm.38.6.2403-2406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Threlfall EJ. Epidemic Salmonella typhimurium DT 104-a truly international multiresistant clone. J Antimicrob Chemother. 2000;46:7–10. doi: 10.1093/jac/46.1.7. [DOI] [PubMed] [Google Scholar]
- 18.Lee YJ, Kim KS, Kwon YK, Tak RB. Biochemical characteristics and antimicrobials susceptibility of Salmonella gallinarum isolated in Korea. J Vet Sci. 2003;4:161–166. [PubMed] [Google Scholar]
- 19.Brown JC, Thomson CJ, Amyes SG. Mutations of the gyrA gene of clinical isolates of Salmonella typhimurium and three other Salmonella species leading to decreased susceptibilities to 4-quinolone drugs. J Antimicrob Chemother. 1996;37:351–356. doi: 10.1093/jac/37.2.351. [DOI] [PubMed] [Google Scholar]
- 20.Launay O, Nguyen Van JC, Buu-Hoi A, Acar JF. Typhoid fever due to a Salmonella typhi strain of reduced susceptibility to fluoroquinolones. Clin Microbiol Infect. 1997;3:541–543. doi: 10.1111/j.1469-0691.1997.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 21.Le Lostec Z, Fegueux S, Jouve P, Cheron M, Mornet P, Boisivon A. Reduced susceptibility to quinolones in Salmonella typhi acquired in Europe: a clinical feature of treatment. Clin Microbiol Infect. 1997;3:576–577. doi: 10.1111/j.1469-0691.1997.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 22.Vasallo FJ, Martin-Rabadan P, Alcala L, Garcia-Lechuz JM, Rodriquez-Creixems M, Bouza E. Failure of ciprofloxacin therapy for invasive nontyphoidal salmonellosis. Clin Infect Dis. 1998;26:535–536. doi: 10.1086/517087. [DOI] [PubMed] [Google Scholar]
- 23.Molbak K, Baggesen DL, Aarestrup FM, Ebbesen JM, Engberg J, Frydendahl K, Gerner-Smidt P, Petersen AM, Wegener HC. An outbreak of multidrug-resistant, quinolone-resistant Salmonella enterica serotype Typhimurium DT104. N Engl J Med. 1999;341:1420–1425. doi: 10.1056/NEJM199911043411902. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Multidrug-resistant Salmonella serotype Typhimurium-United States. MMWR Morb Mortal Wkly Rep. 1997;46:308–310. [PubMed] [Google Scholar]
- 25.Anderson ES, Threlfall EJ, Carr JM, McConnell MM, Smith HR. Clonal distribution of resistance plasmid-carrying Salmonella typhimurium, mainly in the Middle East. J Hyg (Lond) 1977;79:425–448. doi: 10.1017/s0022172400053286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben Hassen A, Bejaoui M, Hichri A, Lakhoua R, Ben Redjeb S. Non-typhoid Salmonella in pediatric patients in Tunis (Hospital Charles-Nicolle) from 1980 to 1991. Bull Soc Pathol Exot. 1993;86:190–194. [PubMed] [Google Scholar]
- 27.Frost JA, Rowe B, Ward LR, Threlfall EJ. Characterization of resistance plasmids and carried phages in an epidemic clone of multiresistant Salmonella typhimurium in India. J Hyg (Lond) 1982;88:193–204. doi: 10.1017/s0022172400070066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cody SH, Abbott SL, Marfin AA, Schulz B, Wagner P, Robbins K, Mohle-Boetani JC, Vugia DJ. Two outbreaks of multidrug-resistant Salmonella serotype typhimurium DT104 infections linked to raw-milk cheese in Northern California. JAMA. 1999;281:1805–1810. doi: 10.1001/jama.281.19.1805. [DOI] [PubMed] [Google Scholar]