Abstract
Diagnosis of Mycoplasma pneumoniae infection is important due to its variable clinical manifestations and absence of response to beta-lactams. Introduction of enzyme immunoassays (EIAs) for serologic diagnosis of M. pneumoniae has made it possible to separate the analyses of specific IgG and IgM antibodies. We compared four different commercial EIAs, ImmunoWELL IgG, IgM (GenBio), Medac IgG, IgA, IgM (Medac), Platelia IgG, IgM (Sanofi Pasteur), and Ridascreen IgG, IgA, IgM (r-Biopharm) with indirect particle agglutination assay (PA), Serodia-MycoII (Fujirebio). We tested 91 specimens from 73 pediatric patients (2-17 yr) hospitalized at a tertiary-care hospital between December 2005 and January 2006. The measurements of IgM EIAs were correlated with PA titers (Spearman's correlation coefficient, from 0.89 to 0.92) with high concordance rates, ranging from 82.4% to 92.3%. However, some negative IgM-EIA results in PA-positive specimens indicated that serial samplings with convalescent sera would be necessary to confirm M. pneumoniae infection.
Keywords: Mycoplasma Pneumoniae, Particle Agglutination, Immunoenzyme Techniques
INTRODUCTION
Mycoplasma pneumoniae infections may be manifested in upper respiratory tract, lower respiratory tract, or both, presenting sore throat, hoarseness, fever, chills, cough, coryza, malaise, wheezing, dyspnea, progression to bronchopneumonia or lobar pneumonia requiring hospitalization, and extrapulmonary symptoms (1). This broad spectrum of symptoms cannot be differentiated from symptoms of the infections caused by other bacteria or viruses. The specific diagnosis M. pneumoniae infection is important because treatment with β-lactam antibiotics is ineffective, whereas treatment with macrolides or tetracyclines may markedly reduce the duration of illness (2). However, reference laboratory methods for the diagnosis of M. pneumoniae infection have not been established.
Culture is time-consuming and relatively insensitive. The introduction of polymerase chain reaction (PCR) for detection of M. pneumoniae in respiratory tract specimens has lessened the importance of culture, enabling rapid and sensitive detection. However, PCR cannot differentiate colonization from infection nor can detect organisms in the convalescent phase (3-5).
Despite its drawbacks, for the use in immunosuppressed persons who are unable to mount an antibody response, serologic diagnosis of M. pneumoniae infections has long been the cornerstone of diagnosis and epidemiologic study (1). The complement fixation (CF) test was the standard serologic method for the diagnosis of M. pneumoniae infection. The CF test, using a glycolipid antigen, provides non-specific reactions and therefore lacks sensitivity (6). Alternative formats adapted for commercial serologic assays include indirect immunofluorescence assay (IFA), particle agglutination (PA) assay, and enzyme-linked immunoassay (EIA). IFAs for M. pneumoniae provide accurate, quantitative serological data, but their interpretation is subjective and a fluorescence microscope is necessary (1). The PA assay is the most widely used method in Korea because it is easy to perform and give quantitative results with acceptable sensitivity. However, the ambiguity in the interpretation of agglutination, non-specific reactions, and inability to discriminate between IgG and IgM are drawbacks of the PA assay for the diagnosis of M. pneumoniae infection (7). Thus there is a need for EIA that can detect IgG and IgM separately to distinguish current from past infections. A few different EIA kits are now available in Korea, and some institutions have introduced them recently. Changes in testing methods from PA to EIA could be confusing to clinicians because of differences between PA titer and EIA units; however, there is no available data for the Korean patients. We analyzed the performance of four commercial EIA kits sold in Korea and correlated the results with PA assay results.
MATERIALS AND METHODS
Subjects and study design
Ninety-one sera from 73 children were requested for M. pneumoniae antibody assay in the Department of Laboratory Medicine from 1 December 2005 to 13 January 2006. The age of study subjects ranged from 17 months to 17 yr (mean 5.3 yr), and 39 (53.4%) were male. They were admitted at he Sanggye Paik Hospital, a tertiary-care hospital in Seoul and were tested with a PA assay and four EIAs on the same day. The medical records were reviewed, retrospectively. The serum samples were drawn 5-15 days after the onset of their respiratory or other symptoms. The patients were divided into four groups based on their respiratory manifestation. Group I comprised 37 patients with pneumonia proven by abnormal chest radiographs. Group II comprised 14 patients with upper or lower respiratory infections including nasopharyngitis, bronchitis, croup, and bronchiolitis with normal chest radiographs. Group III comprised 17 patients who complained of aggravation of wheezing or dyspnea, with an underlying diagnosis of asthma, without signs of other respiratory infections. Group IV comprised 5 patients with extrapulmonary symptoms including: infectious mononucleosis proven by Epstein Barr virus IgM anti-VCA (viral capsid antigen) (1 patient), glomerulonephritis of unknown cause (2 patients), and Henoch-Schönlein purpura (HSP) (2 patients). The positive rates of M. pneumoniae antibodies were evaluated in each group with different serologic assays. If a patient had two or more results, the higher value was selected for the positive rates.
Particle agglutination assay
The Serodia-MycoII (Fujirebio Inc., Tokyo, Japan) test was performed according to the manufacturer's instructions. This is a semi-quantitative agglutination assay using gelatin particles sensitized with a crude antigen mixture of M. pneumoniae (Mac strain). Using the serum diluent supplied, serum samples were diluted serially giving final dilutions of 1:40 to 1:20,480. After 3-hr incubation at room temperature, buttons or compact, smooth rings of particles in the bottom of the wells were read as negative agglutination patterns and a more extensive ring as positive. The manufacturer recommended that titers of 1:40 or more be regarded as positive.
Enzyme immunoassays
Four different commercially available EIA kits containing all reagents were evaluated using the protocols supplied. The results were interpreted by the cutoff values established by the manufacturer; each run was validated with a negative and positive control included in the kits. Each manufacturer recommended retests after two or three weeks in cases with equivocal results because they might indicate the onset of or the resolution of infections. However, for simplicity of results, the equivocal ranges were considered to be negative in this study.
ImmunoWELL™ M. pneumoniae IgG-, IgM-EIA (GenBio, San Diego, CA, U.S.A.; purchased from DOW Biomedica) is an indirect EIA, using a purified glycolipid of M. pneumoniae (strain FH) as the antigen. The ImmunoWELL IgM test utilizes an IgG absorbent to eliminate interference due to rheumatoid factors or residual human IgG antibodies. The quantities of IgG and IgM antibodies in the serum were calculated as the ratio of the sample absorbance value to calibrator absorbance value multiplied by the assigned value in U/mL of the calibrator given in the insert of each kit. The results were expressed as an arbitrary unit (AU/mL). The cutoff of IgG antibody was 200 AU/mL, and the equivocal range for IgM antibody was 770-950 AU/mL.
M. pneumoniae IgG-, IgA-, IgM- ELISA Medac (Medac GmbH, Wedel, German; purchased from Naroo Ditech, Inc.) uses a recombinant antigen mixture of M. pneumoniae. The results of IgG and IgA antibodies were expressed in AU/mL on the basis of a standard curve with one-point calibration; the equivocal ranges were 9-11 AU/mL. After absorption of IgG antibody or rheumatoid factor, semiquantitative IgM antibody was measured as a ratio of sample optical density (OD) to cutoff OD calculated from negative control measurements. The ratio 0.9 to 1.1 was the equivocal range.
Platelia™ M. pneumoniae IgG-, IgM-EIA (Sanofi Diagnostica Pasteur, Marnes la Coquette, France; purchased from Bio-Rad Laboratories) detects anti-M. pneumoniae antibodies by indirect EIA. The microplate was coated with a solubilized ultrasonicate of an M. pneumoniae culture containing enriched P1 cytadhesin and other membrane proteins. The specimen arbitrary unit (AU) values of IgG antibodies were determined from the calibration curve and were interpreted as follows. A value of <10 AU/mL for a single serum sample was considered insignificant, a value of 10 to 19 AU/mL was considered low, a value of 20 to 39 AU/mL was considered moderate, and a value of >40 AU/mL was considered high. IgM-EIA is a double-sandwich immunocapture EIA using a microplate coated with human anti-µ chain antibodies. The presence of specific IgM antibody in the specimen was determined by a ratio comparing the OD of the serum to the mean OD of cutoff controls; a ratio >1.0 was regarded as positive. The presence of IgM or high IgG was regarded as recent or current infection according to the manufacture's instructions.
Ridascreen® M. pneumoniae IgG-, IgA-, IgM-EIA (r-Bio-pharm, Darmstadt, Germany; purchased from Asan Pharmaceuticals) is a semiquantitative microtiter EIA for the detection of M. pneumoniae IgG, IgM, or IgA antibodies. The results were expressed in AU/mL on the basis of a calibration curve and a lot-dependent correction factor. The equivocal ranges were: 23-31 AU/mL for IgG, 39-50 AU/mL for IgA, and 50-71 AU/mL for IgM.
Statistics
Degrees of agreements were evaluated with concordance rates (%) and kappa values. Concordance rates were the proportion of sera with concurrent positive or negative results in both assays. The correlation between the PA titer and EIA quantitative values were evaluated using the Spearman correlations.
RESULTS
Positive rates of M. pneumoniae antibody in patient groups
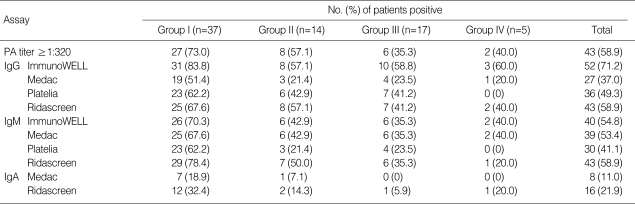
Overall IgG positive rates were variable with the different IgG EIAs and ranged from 37.0% to 71.2%. The positive rates of IgM antibodies showed a narrower range from 41.1% to 58.9%. Group I patients, patients with pneumonia proven by abnormal chest radiographs, showed the highest number of IgM-positive samples, which ranged from 62.2% to 78.4% (Table 1).
Table 1.
Positive rates of M. pneumoniae antibody according to patient groups
Group I, patients with radiograph-proven pneumonia; Group II, upper or lower respiratory infections with normal chest radiographs; Group III, aggravation of wheezing or dyspnea in underlying asthma; Group IV, extrapulmonary symptoms.
Two patients in group I were diagnosed as viral pneumonia; each patient with adenovirus pneumonia and respiratory syncytial virus pneumonia showed a low PA titer and negative IgM EIAs. Five patients with extrapulmonary symptoms (group IV) were included, and 3 of them had positive results with one or more test method. A patient with infectious mononucleosis by Ebstein-Barr virus showed a positive result with Medac IgM, equivocal results with Immuno-WELL and Ridascreen IgM, and a negative result with Platelia IgM. A patient with glomerulonephritis showed a PA titer of 1:160 and positive ImmunoWELL IgM result. A HSP patient had a PA titer of 1:320 and positive IgM results from EIAs except for Platelia IgM EIA.
Agreements between PA and four EIAs
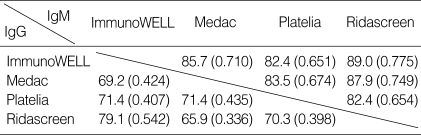
Degrees of agreements among 4 kinds of EIAs were evaluated (Table 2). IgM-specific EIAs showed higher agreements than IgG-specific EIAs. ImmunoWELL and Ridascreen had highest agreements (IgM, 89.0% of concordance rate, 0.775 of kappa value; IgG, 79.1% of concordance rate, 0.542 of kappa value).
Table 2.
Percent concordant rates (kappa value) among 4 kinds of IgG- and IgM-specific enzyme immunoassays for anti-M. pneumoniae
Right upper areas are values of IgM. Left lower areas are those of IgG.
Degrees of agreements between PA and each EIA were evaluated based on each of PA titer, 1:40, 1:80, 1:160, 1:320, and 1:640. Three kinds of IgM-specific EIA had highest agreements at PA titer 1:320 (concordance rate/kappa value, 92.3%/0.839 for Medac; 91.2%/0.812 for Ridascreen; and 86.8%/0.729 for ImmunoWELL). Platelia IgM EIA had better agreement with PA titer 1:640 (86.8%; kappa value, 0.737) than with PA 1:320 (82.4%; kappa value, 0.656).
Comparison of IgG-specific EIAs with PA assays showed a wide range of concordance rates. Each of IgG-specific EIAs had highest agreement with PA assay at the different titer; ImmunoWELL and Ridascreen had highest agreement with PA titer, 1:160 (91.2%; kappa value, 0.774 and 74.7%; kappa value, 0.446, respectively), Platelia did with PA titer, 1:320 (76.9%; kappa value, 0.528), and Medac did with PA titer, 1:640 (74.7%; kappa value, 0.498).
Medac IgA EIAs showed positive results in 12 specimens and Ridascreen IgA EIA did in 19 specimens; 5 specimens showed positive results with both IgA EIAs (76.9% of agreement; kappa value, 0.192).
Correlations between EIA quantitative results and PA titers
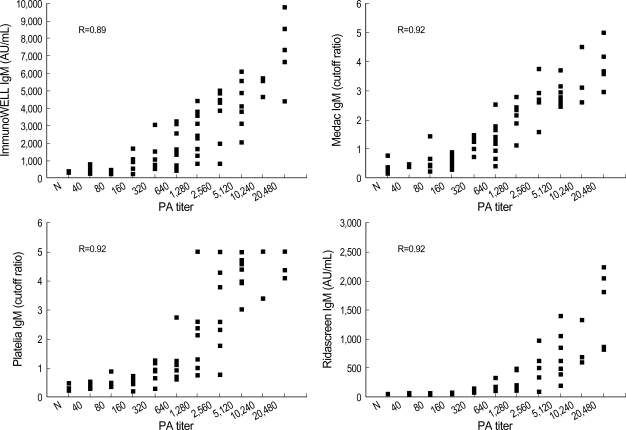
To examine the distribution of quantitative values, the results of each EIA were compared to titers of PA assay. There were significant correlations between the PA titers and the results of each IgM EIA (Spearman's correlation coefficient, from 0.89 to 0.92) (Fig. 1). The results of IgG EIAs showed no significant correlation with PA titers (coefficients of Spearman correlation, 0.51 for ImmunoWELL; 0.67 for Medac and Platelia; 0.42 for Ridascreen). Correlations among EIAs were more significant for IgM EIAs (coefficients of Spearman correlation, ranging from 0.860 to 0.947) than IgG EIAs (ranging from 0.488 to 0.808).
Fig. 1.
Spearman correlation of M. pneumoniae IgM titers obtained by various IgM assays in relation to particle agglutination assay titers. All correlations were significant (p<0.001).
Comparisons of PA titers and EIA results
All eight specimens with negative PA results and 18 specimens with a PA titer ≥1:5,120 showed concordant results with all IgM assays.
Nineteen specimens had PA titer 1:1,280 or 1:2,560, and 3 of them (15.8%) showed negative IgM results; a 5-yr-old child with bronchitis had a PA titer of 1:2,560 and high IgG but negative IgM results with Platelia (IgG, 100 AU/mL) and ImmunoWELL (IgG, 3,589 AU/mL). Another 5-yr-old child with a PA titer 1:1,280 and equivocal IgM result by ImmunoWELL showed a positive conversion in a few days. The third, a 7-yr-old boy with nasopharyngitis had a PA titer of 1:1,280 and equivocal or negative results with either Medac or Platelia IgM assays.
Twenty-one specimens from 16 patients had PA titers of 1:640 or 1:320. Among them, 8 specimens (38.1%) from 5 patients had no detectable IgM antibodies in two or more EIAs, and 6 specimens (28.6%) were IgM-negative only with the Platelia EIA. All of them had no significant change of PA titers in serial specimens. Among the six cases with negative Platelia IgM results, one case had pneumonia with a high IgG value (96 AU/mL, suggesting high risk), another case was diagnosed as HSP without follow-up specimen, and the remaining four cases were patients with pneumonia or asthma.
Twenty-two patients (25 specimens) presented with PA titers from 1:40 to 1:160. Most of these cases had negative IgM EIAs, and some had positive IgG EIAs. ImmunoWELL IgM EIA detected IgM antibodies from two patients with a PA titer of 1:160 that manifested clinically as pneumonia and glomerulonephritis, respectively. Ridascreen IgM EIA detected IgM antibodies from two patients with pneumonia with a PA titer of 1:160 and another with 1:40. Medac IgM EIA detected IgM from a patient with croup and a PA titer of 1:80.
The review of medical record for 73 patients revealed that 43 patients had the paired PA tests at an interval of 5 to 15 days. Nine patients presented PA titer changes of four-fold or more and, 14 specimens from them were included in this comparison study. All of them showed positive IgM antibodies with 4 kinds of EIAs, and 2 of them exhibited the seroconversions of IgG antibodies with 2 EIAs.
DISCUSSION
It is well known that M. pneumoniae causes 40% or more cases of community-acquired pneumonia and as many as 18% of cases requiring hospitalization in children (1). Recent epidemiologic studies indicated that M. pneumoniae are frequently involved as causative agents in upper or lower respiratory tract infections including common colds, pharyngitis, bronchitis, wheezing, and extrapulmonary disease in children aged less than 5 yr as well as in school ages and throughout the year with periodic epidemics at 3-7 yr intervals (8). M. pneumoniae had been isolated from the respiratory tract of 20-50% of asthmatic patients with acute exacerbations and patients with chronic stable asthma (8). Principi et al. evaluated the incidences of acute M. pneumoniae infection in 613 Italian children aged 2-14 yr with community-acquired lower respiratory infections. EIA for M. pneumoniae-specific IgG/IgM antibodies indicated that M. pneumoniae was the causative agent in 25.6% of acute bronchitis (ranged from 19.3 to 52.9% over age groups), 28.0% of wheezing (22.6% to 50.0%), and 33.9% of pneumonia with infiltration on chest radiograph (20.0% to 54.6%) (9). They also reported that 24.2% of pharyngitis were due to M. pneumoniae (8). Layani-Milon et al. reported that a PCR-hybridization assay in nasal swab detected M. pneumoniae from 7.3% of children with influenza-like upper respiratory infections, ranging from 2.0 to 10.1% over the five period (10). Our patients who were diagnosed as pneumonia with abnormal chest radiograph (group I) showed higher IgM positive rates than previous reports; ranging from 62.2% to 78.4% with 4 kinds of EIAs (Table 1). Platelia detected IgM antibodies from 21.4% of group II patients (upper or lower respiratory infection except pneumonia with infiltrations) and 23.5% of group III patients (acute exacerbation of asthma), and other 3 IgM EIAs detected IgM antibodies more frequently compared with Platelia in groups II and III or than previous reports in group II. Because our study was performed on 73 children within 2 winter-months for the purpose of comparison of methods, the possibility of M. pneumoniae epidemics and the small number of study participants could explain the higher positive rates. The positive or equivocal reactions of IgM EIAs in a patient with infectious mononucleosis suggested false positive activity due to heterophil antibodies. More specimens need to be evaluated for the heterophil antibody reactions to fully understand these findings. A patient with glomerulonephritis and a patient with HSP had a positive reaction in one or more IgM EIAs. These cases could not be discriminated from true M. pneumoniae infections; in fact, there have been many cases reported as glomerulonephritis and other systemic manifestations in patients with M. pneumoniae (11, 12).
The PA titer for the best sensitivity and specificity for the diagnosis of M. pneumoniae infections was controversial in previous reports. A comparison study of PA titer, culture, and PCR indicated a PA titer ≥1:160 had the greatest diagnostic power in a child with respiratory symptoms based on good receiver-operating characteristic areas under the curve (13). Other investigators suggested a PA titer ≥1:640 (14, 15). Choi et al. evaluated the mycoplasma antibody titer from 177 healthy children with the PA assay used in our study; 46.3% of healthy children were PA-positive, and their titers were 1:320 or less (15). In our series, Serodia MycoII showed high sensitivity presenting positive results in 83 of 94 specimens (91.2%) with the manufacturer's cutoff, 1:40. However, most of 25 specimens with PA titers ≤1:160 were negative for IgM antibodies with EIAs but positive for IgG antibodies. We evaluated the agreements of EIAs with each titer of PA and 3 of 4 IgM EIAs had the best concordance with PA titer 1:320. They showed good concordance rates, 82.4% to 92.3% and their quantitative results were significantly correlated. However, IgG EIAs demonstrated a lower concordance and no significant correlations with PA titers. These observations supported the manufacturer's claim that Serodia MycoII detects exclusively M. pneumoniae-specific IgM. However, some cases suggested that IgG antibodies resulted PA-positive reactions, especially at low PA titers.
A few specimens in our study showed IgM negativity in spite of high PA titers (≥1:1,280) and similar findings were observed in previous reports (7). Our cases showed high IgG titers in the same specimen or positive conversions of IgM antibodies in follow-up specimens. Yoon et al. reported positive rates of M. pneumoniae antibodies reached a peak from 7th to 9th day and went down after 16th day (16). Some specimens in our series were obtained 10 days or more after the onset of symptoms and follow-up specimens at an interval less than 7 days. It could not be ruled out that late sampling times decreased the IgM EIA positive rates and increased the IgG EIA positive rates in PA-positive specimens (17). The first blood specimen within 10 days of the onset of illness and convalescent serum at intervals of 7 to 10 days are important for demonstrating a significant rise in antibody titers (17, 18). For Platelia IgG EIA, the manufacturer's guidelines indicated that high IgG values (>40 AU/mL) could be interpreted as a current infection and it compensated for the relatively low sensitivity of the Platelia IgM assay. Use of EIAs enabled the simultaneous testing of specific IgG and IgM antibodies, and it was helpful for assessing some IgM negative- and IgG positive cases as past infections, especially for the patients with low PA titers. However, in the clinical situation of a single negative IgM EIA result, another specimen for paired evaluation was supposed to be necessary to establish the diagnosis of current infection of M. pneumoniae. This comparison study included 34 paired sera from 16 patients, and two of them showed positive conversions of IgG antibodies. Further studies with large numbers of paired sera might show the possibility of IgG EIAs as a supplementary role for confirming current infections of M. pneumoniae.
Nineteen patients (20.9%) had IgA antibodies with one or two available IgA EIA kits, and all of them had positive IgM results suggesting the specificities of IgA EIAs for current M. pneumoniae infections. However, their clinical usefulness was hampered by low positive rates in our pediatric patients. Granstrom et al. reported that IgA EIA was useful for early diagnosis of acute infection in adults due to its earlier response compared to IgM and occasional lack of IgM formation in adults (19). They reported that specific IgA antibodies developed more regularly and more rapidly than IgM antibodies, and IgA titers also started to decrease earlier than IgM antibodies or the late-peaking IgG responses. All of our patients with positive IgA results had high IgM titers, suggesting early phases of infections. Late sampling was considered as a possible cause of low positive rates of IgA antibodies in our patients. Presentations in post-acute stages of infections at our tertiary-care hospital was thought to have caused missing the early phase to obtain IgA-positive result.
An assay format other than serologic methods was not performed for the determination of the specificity and sensitivity. Even though there is no reference method for the diagnosis of M. pneumoniae infection, a study with a different method, such as PCR or culture, would provide more informative data.
In conclusion, IgM EIAs for the detection of M. pneumoniae infection showed good agreements with the PA assay, and their quantitative results were well correlated. Confirmation of M. pneumoniae infection in a case with negative IgM antibodies required a serial sampling with convalescent serum.
References
- 1.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Block S, Hedrick J, Hammerschlag MR, Cassell GH, Craft JC. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr Infect Dis J. 1995;14:471–477. doi: 10.1097/00006454-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Williamson J, Marmion BP, Worswick DA, Kok TW, Tannock G, Herd R, Harris RJ. Laboratory diagnosis of Mycoplasma pneumoniae infection. 4. Antigen capture and PCR-gene amplification for detection of the Mycoplasma: problems of clinical correlation. Epidemiol Infect. 1992;109:519–537. doi: 10.1017/s0950268800050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorigo-Zetsma JW, Zaat SA, Wertheim-van Dillen PM, Spanjaard L, Rijntjes J, van Waveren G, Jensen JS, Angulo AF, Dankert J. Comparison of PCR, culture, and serological tests for diagnosis of Mycoplasma pneumoniae respiratory tract infection in children. J Clin Microbiol. 1999;37:14–17. doi: 10.1128/jcm.37.1.14-17.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tjhie JH, van Kuppeveld FJ, Roosendaal R, Melchers WJ, Gordijn R, MacLaren DM, Walboomers JM, Meijer CJ, van den Brule AJ. Direct PCR enables detection of Mycoplasma pneumoniae in patients with respiratory tract infections. J Clin Microbiol. 1994;32:11–16. doi: 10.1128/jcm.32.1.11-16.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs E. Serological diagnosis of Mycoplasma pneumoniae infections: a critical review of current procedures. Clin Infect Dis. 1993;17(Suppl 1):79–82. doi: 10.1093/clinids/17.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- 7.Barker CE, Sillis M, Wreghitt TG. Evaluation of Serodia Myco II particle agglutination test for detecting Mycoplasma pneumoniae antibody: comparison with mu-capture ELISA and indirect immuno-fluorescence. J Clin Pathol. 1990;43:163–165. doi: 10.1136/jcp.43.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Principi N, Esposito S. Emerging role of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric respiratory-tract infections. Lancet Infect Dis. 2001;1:334–344. doi: 10.1016/S1473-3099(01)00147-5. [DOI] [PubMed] [Google Scholar]
- 9.Principi N, Esposito S, Blasi F, Allegra L Mowgli study group. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin Infect Dis. 2001;32:1281–1289. doi: 10.1086/319981. [DOI] [PubMed] [Google Scholar]
- 10.Layani-Milon MP, Gras I, Valette M, Luciani J, Stagnara J, Aymard M, Lina B. Incidence of upper respiratory tract Mycoplasma pneumoniae infections among outpatients in Rhone-Alpes, France, during five successive winter periods. J Clin Microbiol. 1999;37:1721–1726. doi: 10.1128/jcm.37.6.1721-1726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koletsky RJ, Weinstein AJ. Fulminant Mycoplasma pneumoniae infection. Report of a fatal case, and a review of the literature. Am Rev Respir Dis. 1980;122:491–496. doi: 10.1164/arrd.1980.122.3.491. [DOI] [PubMed] [Google Scholar]
- 12.Vitullo BB, O'Regan S, de Chadarevian JP, Kaplan BS. Mycoplasma pneumonia associated with acute glomerulonephritis. Nephron. 1978;21:284–288. doi: 10.1159/000181405. [DOI] [PubMed] [Google Scholar]
- 13.Lee EY, Lee DJ, Lee JA, Kim SW, Chang MW. Comparison of PCR, culture and serologic tests for diagnosis of Mycoplasma pneumoniae infection. Pediatr Allergy Respir Dis. 2005;15:359–367. [Google Scholar]
- 14.Kim CK, Chung CY, Kim JS, Kim WS, Park Y, Koh YY. Late abnormal findings on high-resolution computed tomography after Mycoplasma pneumonia. Pediatrics. 2000;105:372–378. doi: 10.1542/peds.105.2.372. [DOI] [PubMed] [Google Scholar]
- 15.Choi SK, Jung JA, Kim KH, Kim GH. Study of seroprevalence of antimycoplasma antibody in healthy children and its diagnostic value. J Korean Pediatr Soc. 1998;41:489–497. [Google Scholar]
- 16.Yoon SH, Jung JK, Oh MH. Cold Agglutinin and Mycoplasma Antibody Titers in Children with Mycoplasma pneumoniae Pneumonia During Recent 5 Years. J Korean Pediatr Soc. 1996;39:943–952. [Google Scholar]
- 17.Daxboeck F, Krause R, Wenisch C. Laboratory diagnosis of Mycoplasma pneumoniae infection. Clin Microbiol Infect. 2003;9:263–273. doi: 10.1046/j.1469-0691.2003.00590.x. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Yoo Y, Kim DK, Kang H, Koh YY. Distributions of antibody titers to Mycoplasma pneumoniae in Korean children in 2000-2003. J Korean Med Sci. 2005;20:542–547. doi: 10.3346/jkms.2005.20.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granstrom M, Holme T, Sjogren AM, Ortqvist A, Kalin M. The role of IgA determination by ELISA in the early serodiagnosis of Mycoplasma pneumoniae infection, in relation to IgG and mu-capture IgM methods. J Med Microbiol. 1994;40:288–292. doi: 10.1099/00222615-40-4-288. [DOI] [PubMed] [Google Scholar]