Abstract
Because additive effects of inhaled corticosteroids and long-acting anticholinergics are unclear, we undertook this study to compare the efficacy of tiotropium alone and tiotropium plus budesonide in patients with chronic obstructive pulmonary disease. The study subjects were randomized to receive either tiotropium 18 µg once daily with or without budesonide 200 µg twice daily for 6 weeks. The efficacy variables were changes in trough forced expiratory volume in one second (FEV1), St. George's Respiratory Questionnaire (SGRQ), 6-minute walk distance (6MWD), and use of rescue medication. One hundred patients were randomized and 81 completed the study. The mean age was 64.0 yr, and the mean FEV1 was 39.7% predicted. Compared with tiotropium alone (N=40), the tiotropium/budesonide combination (N=41) was related to an improvement in the SGRQ total score (tiotropium -2.8 units and tiotropium/budesonide -5.6 units, p=0.003). 6MWD was improved by 13.5 m in the tiotropium group and by 22.5 m in the tiotropium/budesonide group (p=0.031). Changes in trough FEV1 and the use of rescue medication were similar between two groups. In conclusion, compared with tiotropium alone, the tiotropium/budesonide combination was related to an improved health-related quality of life. These data support that low-dose budesonide may enhance the efficacy of tiotropium.
Keywords: Pulmonary Disease, Chronic Obstructive; Budesonide; Tiotropium; Quality of Life; Exercise; Spirometry
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a disease state characterized by an airflow limitation that is not fully reversible and an insidiously progressive impairment in health-related quality of life (HRQoL). Airflow limitation is usually both progressive and associated with an abnormal inflammatory response of the lungs to noxious particles or gases. Inhaled bronchodilator therapy is the mainstay treatment for COPD. When symptoms persist and are not adequately controlled by short-acting bronchodilators, recent guidelines recommend regular treatment with mono- or combination therapies of long-acting bronchodilators (1, 2).
Previous clinical trials of long-acting anticholinergics (tiotropium) have uniformly demonstrated a beneficial effect in terms of improving forced expiratory volume in one second (FEV1) and reducing exacerbation rates compared with placebo or ipratropium (3-9). Tiotropium also improves HRQoL compared with placebo or ipratropium (4, 5, 7, 10, 11). Recent studies have also revealed that combination therapy with tiotropium and long-acting β2-agonists (LABA) achieved a greater improvement in FEV1 than the individual component alone (12-14).
However, the role of inhaled corticosteroids (ICS) in the management of COPD is less certain (15, 16). Although the use of ICS remains one of the most controversial issues in COPD pharmacotherapy, it is estimated that 41 to 50% of patients with stable COPD receive ICS (17, 18). Moreover, ICS was recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD workshop) for symptomatic COPD patients with a FEV1 of <50% predicted (Stage III: Severe COPD and Stage IV: Very Severe COPD) and repeated exacerbations (1). This treatment has been shown to reduce the frequency of exacerbations and to improve health status, whereas withdrawal from ICS treatment can lead to exacerbations in some patients (19). Moreover, the combination of ICS and LABA is more effective than the individual components in stabilizing lung function or improving HRQoL (20-24). However, the effects of long-acting anti-cholinergics with and without ICS on lung function and HRQoL has not been reported as yet.
Previous animal studies suggested that corticosteroids may enhance the effects of anticholinergics by influencing the differential expression of M2 and M3 muscarinic receptors in airway smooth muscles (25, 26). The present study was designed to evaluate the efficacy of tiotropium/budesonide against tiotropium alone in patients with severe-to-very severe COPD according to the GOLD criteria (1). The efficacy variables were trough FEV1, HRQoL, exercise capacity, and use of rescue medication.
MATERIALS AND METHODS
Study design and subjects
The study was a randomized, prospective, open-label design and was performed at a single center (Kangnam General Hospital, Yongin, Korea). The study was approved by the hospital's medical ethics committee, and written informed consent was obtained from all patients before any study procedure was undertaken.
Patients with a clinical diagnosis of COPD (according to the GOLD guidelines) (1) and relatively stable airway obstruction with a postbronchodilator FEV1 of <50% predicted and a ratio of FEV1 to forced vital capacity (FVC) of <70% were prospectively enrolled. Patients had to be at least 40 yr old, current or previous smokers (≥10 pack-years), and all had experienced at least one episode of COPD exacerbation within 2 yr before the first clinic visit.
Patients with any of the followings were excluded: a history of asthma, allergic rhinitis, atopy, or an elevated blood eosinophil count, a significant disease other than COPD, a severe tuberculosis-sequelae (more than one lobe), or a recent history of myocardial infarction, heart failure, or cardiac arrhythmia requiring medication. In addition, patients were excluded if they were receiving oxygen therapy or had experienced any respiratory infection or COPD exacerbation during the 6-week period prior to screening. Patients with known hypersensitivity to anticholinergic drugs, known symptomatic prostatic hypertrophy, or narrow-angle glaucoma were also excluded.
Study procedures
The study had a run-in period of one week and a treatment period of 6 weeks, and involved four scheduled visits to clinics, i.e., at the start of the run-in period (visit 1), at the start of treatment (visit 2), and after 3 and 6 weeks of treatment (visits 3 and 4). Following the screening visit (visit 1), eligible patients entered one-week run-in period to ensure clinical stability (i.e., no exacerbations). At the start of the runin period, all inhaled short-acting anticholinergics, LABA, and ICS were withdrawn. The patients continued to take permitted COPD medications in stable doses, including methylxanthines, mucolytics, oral steroids (at a dose of <10 mg per day prednisolone or equivalent), and short-acting inhaled β2-agonists (salbutamol) for acute symptom relief. Patients who successfully completed this phase entered the 6-week treatment period. At the end of the run-in period (visit 2), eligible patients were randomly assigned using random number table to treatment with tiotropium (SpirivaR, Boehringer Ingelheim; Ingelheim am Rhein, Germany) 18 µg once daily or treatment with tiotropium 18 µg once daily plus budesonide (PulmicortR, AstraZeneca, Korea) 200 µg twice daily. Tiotropium was supplied as a dry powder capsule and was inhaled through a HandiHalerR device. Budesonide was inhaled from a TurbuhalerR, a dry powder inhaler system. No other inhaled medications were permitted except for salbutamol Metered Dose Inhalers (100 mg per actuation) as needed for acute symptom relief.
Measurements
At the start of the run-in period, patients underwent a medical examination, laboratory testing, and 12-lead echocardiography. During the treatment period, all patients completed a daily diary card and recorded their symptoms, the inhalation of study medications, and the number of puffs of rescue salbutamol administered.
Spirometry was conducted at the start of run-in period (visit 1) and at the end of the run-in period (visit 2) and after 6-week treatment period (visit 4). Measurements were performed using a spirometer (CPFS/D, MedGraphics; St. Paul, MN, U.S.A.) that met the American Thoracic Society (ATS) criteria (27). The highest FEV1 and FVC values obtained from three technically adequate measurements were retained. Baseline FEV1 was defined as FEV1 on the morning of the randomization visit (visit 2) prior to the allocation of study medication. Follow-up FEV1 was determined during visit 4 and was defined as the measurement taken after 6-week treatment of the study medication. During visit 4, the morning dose of study medication was withheld and spirometry was performed between 9AM to 10 AM to measure trough levels because study medication was inhaled on the previous day in the morning (approximately 9-10 AM for tiotropium and budesonide) and in the evening (approximately 9-10 PM for budesonide). Trough FEV1 difference was defined as the change in trough FEV1 before (visit 2) and after (visit 4) 6-week treatment.
HRQoL was determined using the Korean version of the St George's Respiratory Questionnaire (SGRQ) and was applied at the end of the run-in period (visit 2) and after 6-week treatment (visit 4). The SGRQ is a disease-specific instrument that contains 50 items in three subscales (symptoms, activity, and impacts) (28). Total SGRQ scores are calculated from the responses obtained to all 50 items, and a lower score represents an improvement. For SGRQ total scores, a difference of ≥4 units was considered clinically meaningful (29). Previous study validated that the Korean version of SGRQ is well correlated with the dyspnea scale in patients with COPD (30).
To determine exercise capacity, a 6-minute walk test (6MWT) was conducted according to the ATS guidelines (31), at the end of the run-in period (visit 2) and after 6-week treatment (visit 4). According to a previous study, the clinically meaningful level of increase in 6-minute walk distance (6MWD) was at least 54 m (32).
At each scheduled visit, details of clinical status and adverse events, including exacerbations and withdrawals, were recorded. An exacerbation was defined as an increase in symptoms requiring either a course of oral corticosteroids or antibiotics or hospital admission. These changes in medication were made at the investigator's discretion. Patients were excluded from the analyses if less than 80% of the scheduled study medication had been taken.
Statistical analysis
The present study was a preliminary study, and was the first known comparison study of tiotropium once daily and iotropium once daily plus budesonide twice daily. In view of the lack of previous research, no statistical hypotheses were drawn and no formal sample size calculation was made. The use of a preliminary study in clinical research is a wellestablished scientific procedure because only through the of a preliminary study can statisticians clarify data distributions and determine appropriate sample sizes for fullscale clinical trials.
Descriptive data for continuous variables are presented as mean±standard error of the mean (SEM). T-tests were used to compare changes between baseline and subsequent measurements for each treatment group. Chi-squared or Fisher's exact test were used to compare categorical variables. All patients with available on-treatment data were included in the analyses. Statistical significance was considered at p<0.05, and statistical analyses were performed using SPSS for Windows Release 13.0 (SPSS; Chicago, IL, U.S.A.).
RESULTS
The clinical characteristics of enrolled patients
Enrollment began in September 2005, and follow-up concluded in March 2006. We assessed eligibility criteria in 148 potential study participants. Among these potential study participants, 37 subjects did not meet eligibility criteria, and 11 subjects declined to participate in the study. We randomly assigned the 100 patients who met all eligibility criteria: 50 to tiotropium and 50 to tiotropium plus budesonide. Of these 100 patients randomized, 81 completed the study and 19 were excluded from final analysis. The reasons for withdrawal were as follows: exacerbation of COPD (three patients in the tiotropium group and two patients in the tiotropium/budesonide group), pneumonia (one patient in the tiotropium/bedesonide group), refusal of further involvement in the study (three patients in the tiotropium group and one patient in the tiotropium/budesonide group), poor medication compliance (<80% of scheduled study medications; two patients in the tiotropium group and two patients in the tiotropium/budesonide group), and unavailability for follow-up (two patients in the tiotropium group and three patients in the tiotropium/budesonide group).
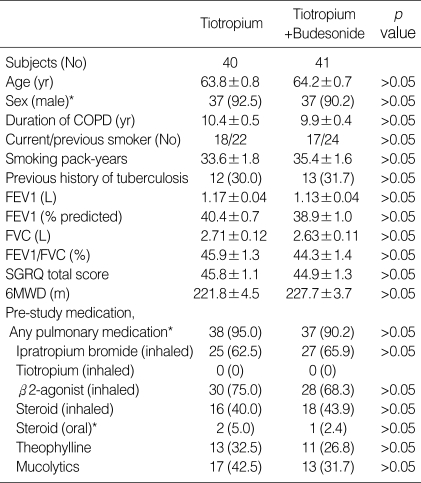
Patients' demographic and baseline characteristics are summarized in Table 1. There were no significant differences in demographic and baseline data, including smoking history, lung function, SGRQ total score, and 6MWD. The mean age of study subjects was 64.0±0.7 yr, with 91% being men. Over 90% of study subjects previously received at least one pulmonary medication. However, previous medication use was not different between the two groups, and no one was previously treated with tiotropium before the randomization. Although a total of 25 patients had a past medical history of tuberculosis (12 in the tiotropium group and 13 in the tiotropium/budesonide group, p>0.05), sequelae were generally minimal (not more than one lobe).
Table 1.
Demographic data and baseline characteristics of study patients
Data are presented as mean±SEM or No (%).
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; SGRQ, St. George's Respiratory Questionnaire; 6MWD, 6-minute walk distance.
*, Fisher's exact test.
Spirometry
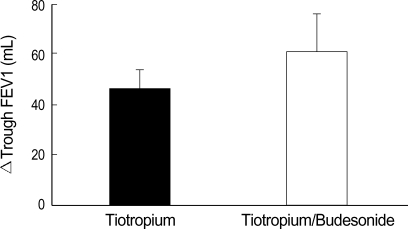
The mean FEV1 of study subjects was 1.15±0.04 L (39.7±0.9% predicted). The mean FEV1 on the morning of the randomization visit (visit 2) was 1.17±0.04 L (40.4±0.7% predicted) in the tiotropium group and 1.13±0.04 L (38.9±1.0% predicted) in the tiotropium/budesonide group (p>0.05) (Table 1). Trough FEV1 differences before (visit 2) and after (visit 4) 6-week treatment were not different between the tiotropium group (N=40, 46.4±10.4 mL) and the tiotropium/budesonide group (N=41, 61.0±14.5 mL) (p>0.05) (Fig. 1). Next, subgroup analyses were performed in order to investigate differences in treatment response. In the tiotropium/budesonide combination group, age, smoking status/history, baseline FEV1, and history of pulmonary tuberculosis did not affect trough FEV1 differences.
Fig. 1.
Mean trough FEV1 differences before and after 6 weeks of treatment in tiotropium (46.4±10.4 mL) and tiotropium/budesonide (61.0±14.5 mL) groups (p>0.05). Δ, change in; FEV1, forced expiratory volume in one second.
HRQoL
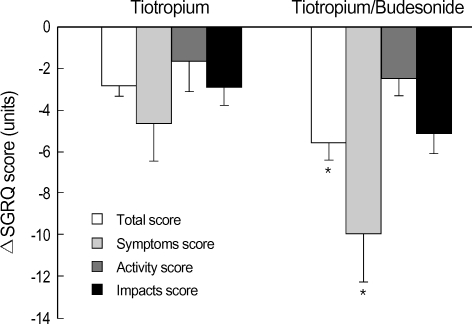
After 6-week treatment, mean improvements in SGRQ total scores were -2.8±0.5 units in the tiotropium group (N=40) and -5.6±0.7 units in the tiotropium/budesonide group (N=41) (p=0.003) (Fig. 2), giving a mean difference of -2.8 units. In addition, the proportions of patients who achieved a clinically meaningful change (at least 4 units) in SGRQ total score were 42.5% (17/40) in the tiotropium group and 73.2% (30/41) in the tiotropium/budesonide group (p=0.007), giving a mean difference of 30.7%. Among three subscale scores, symptoms scores were significantly improved in the tiotropium/budesonide group (-10.0±2.3 units) compared with the tiotropium group (-4.7±1.9 units) (p=0.002) (Fig. 2). However, activity and impacts scores were not different between the two groups (p>0.05). In the subgroup analyses of tiotropium/budesoinde combination, age, smoking status/history, baseline FEV1, and history of pulmonary tuberculosis were not different between improvement group (decrease in SGRQ total score ≥4, N=30) and no improvement group (decrease in SGRQ total score <4, N=11).
Fig. 2.
Mean differences in SGRQ total and subscale scores (symptoms, activity, and impact scores) before and after 6-week treatment in tiotropium and tiotropium/budesonide groups. Δ, change in; SGRQ, St George's Respiratory Questionnaire.
*, p<0.05 for the group comparison.
6MWT
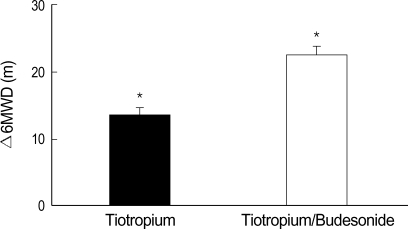
Of the 81 study subjects, 76 were able to perform the follow-up 6MWT after 6-week treatment. Five subjects declined to participate in the follow-up 6MWT (two in the tiotropium group and three in the tiotropium/budesonide group). 6MWD was improved by 13.5±1.9 m in the tiotropium group (N=38) and by 22.5±2.4 m in the tiotropium/budesonide group (N=38) (p=0.031). However, the proportions of patients who achieved a clinically meaningful increase (at least 54 m) in 6MWD were similar between the tiotropium group (8/38, 21.1%) and the tiotropium/budesonide group (11/38, 28.9%) (p>0.05) (Fig. 3). In the subgroup analyses of tiotropium/budesoinde combination, age, smoking status/history, baseline FEV1, and history of pulmonary tuberculosis were not different between the improvement group (increase in 6MWD ≥54 m, N=11) and the no improvement group (increase in 6MWD <54 m, N=27).
Fig. 3.
Mean 6MWD differences before and after 6 weeks of treatment in tiotropium (13.5±1.9 m) and tiotropium/budesonide groups (22.5±2.4 m). Δ, change in; 6MWD, 6-minute walk distance.
*, p<0.05 for the group comparison.
Rescue medication
Patients recorded numbers of "as needed" puffs of salbutamol. Total puffs during the 6-week treatment were not different between the two groups (the tiotropium group, N=39, 59.8±3.2 puffs; the tiotropium/budesonide group, N=41, 47.4±2.0 puffs) (p=0.089).
Adverse events
The proportions of patients who experienced an incident of mouth dryness were similar in the two groups (the tiotropium group, 5.0%; the tiotropium/budesonide group, 4.8%). However, more patients experienced hoarseness in the tiotropium/budesonide group (tiotropium/budesonide 4.8%; tiotropium 0%).
DISCUSSION
In this 6-week study, the tiotropium/budesonide combination was found to improve SGRQ total and symptoms scores compared with tiotropium alone. Moreover, combination treatment was found to be related to a higher proportion of clinically meaningful improvement (at least 4 units) in the SGRQ total score compared with tiotropium alone. Although changes in trough FEV1 and the use of rescue medication were not significant for the two groups, tiotropium/budesonide increased mean 6MWD by 9 m versus tiotropium alone, and this was statistically significant (p=0.031). However, this result does not meet the previously recommended clinically meaningful level of increase in 6MWD (31). In one study of 112 patients with stable COPD, the smallest difference in 6MWD associated with a noticeable clinical difference in the patient's perception of exercise performance was a mean of 54 m (32).
Although some studies showed that high-dose regimens of ICS were more effective in slowing the decline of lung function (33), the combination of formoterol with low or medium doses of inhaled budesonide (320 or 640 µg per day) were also associated with improvement in lung function and reduction in severe exacerbations compared to individual component alone in other studies (22, 24). Therefore, we chose a relatively low dose of inhaled budesonide (400 µg per day) rather than medium or high doses. However, the low dose of ICS in this study may explain the lack of differences of trough FEV1 and modest improvement in 6MWD.
In present study we tried to compare the efficacy of tiotropium/budesonide against tiotropium, but some study subjects had a previous history of pulmonary medication including short-acting inhaled anticholinergics (64.2%), ICS (42.0%) and oral theophylline (29.6%) before screening visits. However, further data analyses revealed that changes in trough FEV1, SGRQ total score, and 6MWD were not different according to the previous medication of short-acting inhaled anticholinergics, ICS, or theophylline. However, we cannot completely exclude the possibility of the aggravation of HRQoL in the tiotropium group because of cessation of ICS (43.9%). The COPE study revealed that the discontinuation of fluticasone propionate in patients with COPD is associated with a higher risk of exacerbation and of a significant deterioration in aspects of HRQoL (19).
Contrary to previous studies (4-7, 9-11, 13, 14), the clinical effects of tiotropium alone were not conspicuous in this study. This discrepancy may be attributed to different study periods and populations, i.e., FEV1 of <60-70% predicted in the previous studies (4-7, 9-11, 13, 14) vs. FEV1 of <50% predicted in the present study. In previous studies designed to evaluate the efficacy of tiotropium, investigators allowed continued ICS use, and therefore, in those studies, the concomitant use of ICS may have acted as a confounding variable (ICS use, 42.1-88.7% of study subjects) (5-7, 9, 13, 14), since corticosteroids may enhance the effects of anticholinergics by influencing the differential expression of M2 and M3 muscarinic receptors (25, 26). Moreover, the concomitant use of ICS and tiotropium was not definitively described in some studies (4, 10, 11). Due to these limitations, previous data about the efficacy of tiotropium may be biased by concomitant use of ICS, and the present study also supported that ICS may enhance the efficacy of tiotropium.
A total of 5 among 100 enrolled subjects (5%) experienced acute exacerbation over the 6-week period, which corresponded to previous studies (27.9% over 6 month and 42.5% over 1 yr) (8, 34). However, the short duration in the present study was a limitation to evaluate the effect of budesonide combination on the frequencies of acute exacerbation.
In the present study, a total of 25 patients had a medical history of tuberculosis (12 in the tiotropium group and 13 in the tiotropium/budesonide group). Subgroup analyses revealed no significant relationship according to the presence or absence of post-tuberculosis sequelae with respect to changes in trough FEV1, 6MWD, and SGRQ total scores.
Although the low compliance can be a real limitation of inhaler therapy, the combination of tiotropium and budesonide did not increase the total frequencies of poor medication compliance, follow-up loss or refusal of further involvement in the study compared with tiotropium alone (7% for tiotropium/budesonide and 6% for tiotropium).
However, the present study has several limitations; the study was open-labeled rather than placebo-controlled and was performed at a single center. Moreover, it was conducted over a relatively short period. Due to these limitations, the results of the present study cannot be generalized to the whole COPD patient population. Nevertheless, this study is the first to evaluate the effects of the tiotropium/budesonide combination in patients with severe to very severe COPD. To confirm the additive effects of ICS and tiotropium, further investigation is required with higher doses of ICS for a long-term period.
In summary, the 6-week combination treatment of tiotropium and budesonide was found to improve SGRQ total scores, symptoms scores, and 6MWD compared with tiotropium alone. However, tiotropium/budesonide combination was not related to the increase in trough FEV1 differences or reduction of the salbutamol rescue medication.
Footnotes
This work was supported by a grant of the Korean Health 21 R & D Project, Ministry of Health & Welfare, Republic of Korea (0412-CR03-0704-0001).
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. NHLBI/WHO workshop report. Bethesda: National Heart, Lung and Blood Institute; 2001. [accessed: 23 Sep 2006]. Update of the management sections. http://www.goldcopd.com. [Google Scholar]
- 2.Celli BR, MacNee W ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 3.Disse B, Speck GA, Rominger KL, Witek TJ, Jr, Hammer R. Tiotropium (Spiriva): mechanistical considerations and clinical profile in obstructive lung disease. Life Sci. 1999;64:457–464. doi: 10.1016/s0024-3205(98)00588-8. [DOI] [PubMed] [Google Scholar]
- 4.Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax. 2003;58:399–404. doi: 10.1136/thorax.58.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donohue JF, van Noord JA, Bateman ED, Langley SJ, Lee A, Witek TJ, Jr, Kesten S, Towse L. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest. 2002;122:47–55. doi: 10.1378/chest.122.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Vincken W, van Noord JA, Greefhorst AP, Bantje TA, Kesten S, Korducki L, Cornelissen PJ Dutch/Belgian Tiotropium Study Group. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J. 2002;19:209–216. doi: 10.1183/09031936.02.00238702. [DOI] [PubMed] [Google Scholar]
- 7.Casaburi R, Mahler DA, Jones PW, Wanner A, San PG, ZuWallack RL, Menjoge SS, Serby CW, Witek T., Jr A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217–224. doi: 10.1183/09031936.02.00269802. [DOI] [PubMed] [Google Scholar]
- 8.Niewoehner DE, Rice K, Cote C, Paulson D, Cooper JA, Jr, Korducki L, Cassino C, Kesten S. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005;143:317–326. doi: 10.7326/0003-4819-143-5-200509060-00007. [DOI] [PubMed] [Google Scholar]
- 9.van Noord JA, Bantje TA, Eland ME, Korducki L, Cornelissen PJ. A randomised controlled comparison of tiotropium and ipratropium in the treatment of chronic obstructive pulmonary disease. The Dutch Tiotropium Study Group. Thorax. 2000;55:289–294. doi: 10.1136/thorax.55.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oostenbrink JB, Rutten-van Molken MP, Al MJ, Van Noord JA, Vincken W. One-year cost-effectiveness of tiotropium versus ipratropium to treat chronic obstructive pulmonary disease. Eur Respir J. 2004;23:241–249. doi: 10.1183/09031936.03.00083703. [DOI] [PubMed] [Google Scholar]
- 11.Casaburi R, Kukafka D, Cooper CB, Witek TJ, Jr, Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest. 2005;127:809–817. doi: 10.1378/chest.127.3.809. [DOI] [PubMed] [Google Scholar]
- 12.Cazzola M, Centanni S, Santus P, Verga M, Mondoni M, di Marco F, Matera MG. The functional impact of adding salmeterol and tiotropium in patients with stable COPD. Respir Med. 2004;98:1214–1221. doi: 10.1016/j.rmed.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 13.van Noord JA, Aumann JL, Janssens E, Smeets JJ, Verhaert J, Disse B, Mueller A, Cornelissen PJ. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J. 2005;26:214–222. doi: 10.1183/09031936.05.00140404. [DOI] [PubMed] [Google Scholar]
- 14.van Noord JA, Aumann JL, Janssens E, Verhaert J, Smeets JJ, Mueller A, Cornelissen PJ. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest. 2006;129:509–517. doi: 10.1378/chest.129.3.509. [DOI] [PubMed] [Google Scholar]
- 15.Barnes PJ. Inhaled corticosteroids are not beneficial in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:342–344. doi: 10.1164/ajrccm.161.2.16125_2. [DOI] [PubMed] [Google Scholar]
- 16.Highland KB. Inhaled corticosteroids in chronic obstructive pulmonary disease: is there a long-term benefit? Curr Opin Pulm Med. 2004;10:113–119. doi: 10.1097/00063198-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Miravitlles M, Mayordomo C, Artes M, Sanchez-Agudo L, Nicolau F, Segu JL. Treatment of chronic obstructive pulmonary disease and its exacerbations in general practice. EOLO Group. Estudio Observacional de la Limitacion Obstructiva al Flujo aEreo. Respir Med. 1999;93:173–179. doi: 10.1016/s0954-6111(99)90004-5. [DOI] [PubMed] [Google Scholar]
- 18.Van Andel AE, Reisner C, Menjoge SS, Witek TJ. Analysis of inhaled corticosteroid and oral theophylline use among patients with stable COPD from 1987 to 1995. Chest. 1999;115:703–707. doi: 10.1378/chest.115.3.703. [DOI] [PubMed] [Google Scholar]
- 19.van der Valk P, Monninkhof E, van der Palen J, Zielhuis G, van Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. Am J Respir Crit Care Med. 2002;166:1358–1363. doi: 10.1164/rccm.200206-512OC. [DOI] [PubMed] [Google Scholar]
- 20.Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, Shah T. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:1084–1091. doi: 10.1164/rccm.2112055. [DOI] [PubMed] [Google Scholar]
- 21.Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, Anderson J, Maden C TRial of Inhaled STeroids ANd long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 22.Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, Peterson S, Olsson H. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- 23.Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, Shah T. The efficacy and safety of fluticasone propionate (250 microg)/ salmeterol (50 microg) combined in the Diskus inhaler for the treatment of COPD. Chest. 2003;124:834–843. doi: 10.1378/chest.124.3.834. [DOI] [PubMed] [Google Scholar]
- 24.Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22:912–919. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- 25.Emala CW, Clancy J, Hirshman CA. Glucocorticoid treatment decreases muscarinic receptor expression in canine airway smooth muscle. Am J Physiol. 1997;272:L745–L751. doi: 10.1152/ajplung.1997.272.4.L745. [DOI] [PubMed] [Google Scholar]
- 26.Jacoby DB, Yost BL, Kumaravel B, Chan-Li Y, Xiao HQ, Kawashima K, Fryer AD. Glucocorticoid treatment increases inhibitory m(2) muscarinic receptor expression and function in the airways. Am J Respir Cell Mol Biol. 2001;24:485–491. doi: 10.1165/ajrcmb.24.4.4379. [DOI] [PubMed] [Google Scholar]
- 27.American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 28.Jones PW, Quirk FH, Baveystock CM. The St Georges Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- 29.Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax. 2001;56:880–887. doi: 10.1136/thorax.56.11.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim EJ, Park JH, Yoon SJ, Lee SJ, Cha SI, Park JY, Jung TH, Kim CH. Relationship between dyspnea and disease severity, quality of life, and social factors in patients with chronic obstructive pulmonary disease. Tuberc Respir Dis. 2006;60:397–403. [Google Scholar]
- 31.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 32.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155:1278–1282. doi: 10.1164/ajrccm.155.4.9105067. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland ER, Allmers H, Ayas NT, Venn AJ, Martin RJ. Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta-analysis. Thorax. 2003;58:937–941. doi: 10.1136/thorax.58.11.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dusser D, Bravo ML, Iacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur Respir J. 2006;27:547–555. doi: 10.1183/09031936.06.00062705. [DOI] [PubMed] [Google Scholar]