Abstract
The purpose of this study was to determine whether the levels of soluble fms-like tyrosine kinase-1 (sFlt-1) and placenta growth factor (PlGF) are altered during the second trimester in the plasma of women who subsequently develop preeclampsia. We performed a case-control study to compare the levels of sFlt-1 and PlGF in the preeclamptic (n=46) and normal pregnant women (n=100). The maternal plasma levels of sFlt-1 and PlGF were measured by enzyme-linked immunosorbent assay. The sFlt-1 levels were significantly higher in the preeclamptic women than in normal controls (p<0.001), while the PlGF levels were significantly lower (p<0.001). In normal controls, sFlt-1 levels were positively correlated (r=0.27, p=0.008), whereas, in the preeclamptic women, those were negatively correlated with the PlGF levels (r=-0.423, p=0.005). Furthermore, the log[sFlt-1/PlGF] ratio was significantly higher in the preeclamptic women than in normal controls (p<0.001). The receiver operating characteristic curve revealed a specificity of 78% with a diagnostic sensitivity of 80.4%; the optimal cut-off value of the log[sFlt-1/PlGF] ratio was 1.4 (95% CI 0.756-0.910, p<0.001). Preeclampsia showed a strong association with increased levels of sFlt-1 and decreased levels of PlGF in the second trimester maternal plasma. Accordingly, the sFlt-1/PlGF ratio may provide early prediction of subsequent development of preeclampsia.
Keywords: Pre-Eclampsia, Fms-like Tyrosine Kinase 1, Placental Growth Factor, Biological Markers
INTRODUCTION
Preeclampsia, a pregnancy-specific syndrome affecting about 5% to 10% of pregnancies, is characterized by the presence of hypertension and proteinuria. This syndrome is one of the leading causes of maternal and fetal morbidity and mortality worldwide and occurs only in the presence of a placenta and remits dramatically after the placenta has been delivered. Although the exact pathogenesis of preeclampsia remains unclear, it has been suggested that endothelial dysfunction may play a central role in the development of preeclampsia. Impaired trophoblastic invasion of the maternal placental bed is considered to be the initial event of endothelial dysfunction in preeclampsia. Consequently, reduction of uteroplacental blood perfusion by shallow implantation results in local placental hypoxia. A hypoxic/ischemic placenta may then release placental factors into the maternal circulation eventually causing endothelial dysfunction, which leads to the main clinical symptoms of preeclampsia (including hypertension and proteinuria).
A role of the balance between soluble fms-like tyrosine kinase (sFlt-1) and placental growth factor (PlGF) in the pathogenesis of preeclampsia has been proposed by several investigators (1-4). sFlt-1 is a secreted splice variant of Flt-1 that antagonizes vascular endothelial growth factor (VEGF) and PlGF by binding, and prevents their interaction with endothelial receptors on the cell surface (5). PlGF is a secreted dimeric glycoprotein that possesses potent angiogenic and mitogenic activities capable of inducing the proliferation, migration, and activation of endothelial cells (6, 7). It has been recently shown that increased sFlt-1 occurs approximately five to six weeks before the onset of clinical symptoms of preeclampsia (1, 2), whereas decreased PlGF levels are observed prior to 20 weeks of gestation in women who subsequently develop preeclampsia (3, 4). Moreover, administration of exogenous sFlt-1 to pregnant rats leads to reduced PlGF, hypertension, proteinuria, and glomerular endothelial injury (8). Therefore, the pathogenesis of preeclampsia may involve an imbalance of angiogenic factors, and the measurement of sFlt-1 in combination with PlGF may distinguish women who subsequently develop preeclampsia from women who remain normotensive during pregnancy (8).
In this study we investigated whether the second trimester plasma levels of sFlt-1 and PlGF were altered in women who subsequently developed preeclampsia.
MATERIALS AND METHODS
Subjects
Subjects were recruited from the Obstetrics and Gynecology Department at Cheil General Hospital between Octerber 2001 and December 2003. The study subjects included 46 women who subsequently developed preeclampsia (17 with mild preecalmpsia and 29 with severe preeclampsia) and 100 women with normal pregnancies who were matched for race, maternal age, and gestational age at the time of blood sampling. Preeclampsia was defined as hypertension (systolic blood pressure ≥140 mmHg and diastolic blood pressure ≥90 mmHg after 20 weeks gestation) and proteinuria (≥300 mg in a 24 hr urine collection or ≥1+ on dipstick testing). Exclusion criteria included major congenital anomalies, fetal chromosomal abnormalities, multiple pregnancies, chronic hypertension, diabetes mellitus, and renal disease. Control subjects were selected randomly from women who were normotensive and without proteinuria throughout pregnancy and who delivered a healthy neonate at term without significant medical or obstetric complications such as; chronic hypertension, diabetes, renal insufficiency, congenital anomalies or fetal demise. This study was approved by the Ethics Committee at Cheil General Hospital, and written informed consent was obtained from all enrolled women.
Samples and immunoassay
Maternal blood was obtained at 14 to 23 weeks of gestation and was collected into heparinized vacutainer tubes. Maternal plasma samples were isolated by 2,500 g centrifugation for 10 min. Aliquots of maternal plasma were stored at -70℃ until analysis. Levels of sFlt-1 and PlGF in maternal plasma were measured with a commercially available enzyme-linked immunosorbent assay (ELISA, R&D System, Minneapolis, MN, U.S.A.) according to the manufacture's instructions. All samples were examined in duplicate, and the mean values of individual samples were used for statistical analysis. The minimal detectable levels in the assays of sFlt-1 and PlGF were 5.0 and 7.0 pg/mL, respectively. The inter- and intra-assay coefficients of variation were 3.8% and 7.0% for sFlt-1 and 3.6% and 11.0% for PlGF, respectively.
Statistical analysis
All data are presented as the median (range) and number (percentage). Data not normally distributed were analyzed by nonparametric tests. A comparison of continuous variables between the two groups was performed using the Student's t test or Mann-Whitney U test, and proportions were compared with the χ2 test or Fisher's exact test. Spearman's rank correlation was used to assess the relationship between the two variables. Receiver operating characteristic (ROC) analysis was performed to assess the best cut-off value and discriminating capacity of the log[sFlt-1/PlGF] ratios in the second trimester plasma for predicting women who subsequently developed preeclampsia. Sensitivity, specificity, and odds ratio (OR) with 95% confidence intervals (CI) were calculated in order to consider the predictive efficacy of the log[sFlt-1/PlGF] ratio. Statistical analysis was performed with the Statistical Package for Social Sciences version 10.0 (SPSS Inc., Chicago, IL, U.S.A.). A p value <0.05 was considered statistically significant.
RESULTS
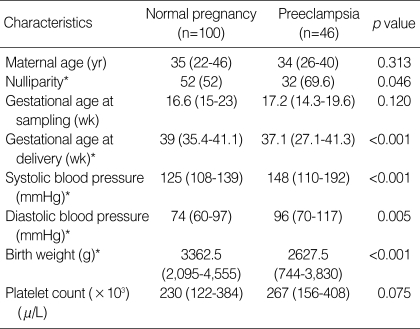
The clinical characteristics of the study population are shown in Table 1. There were no significant differences in maternal age, gestational age at blood sampling, or platelet count between the preeclamptic and normal pregnant women. As expected, systolic and diastolic blood pressures were significantly higher in the preeclamptic women than in normal controls. By contrast, nulliparity, gestational age at delivery, and birth weight were lower in the preeclamptic women than in the normal controls.
Table 1.
Clinical characteristics of the study population
Values are expressed as median (range) or number (%).
*Statistically significant, p<0.05.
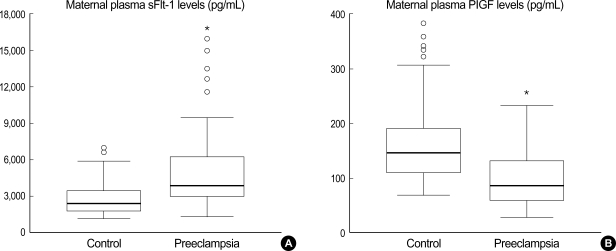
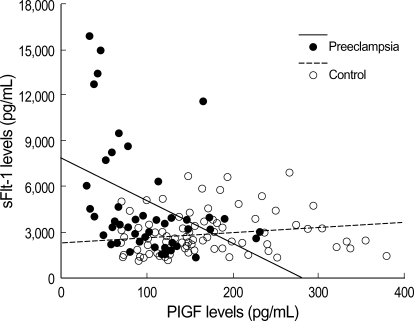
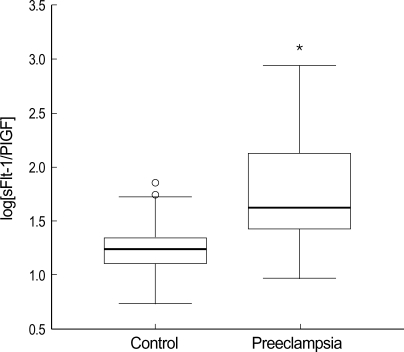
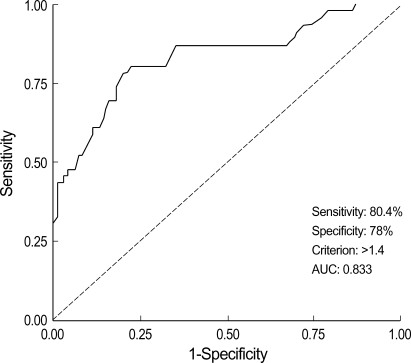
Maternal plasma sFlt-1 levels were significantly higher in the preeclamptic women than in normal controls (median 3,861, range 1,389-15,915 vs. median 2,353, range 1,071-6,898, p<0.001) (Fig. 1A). The levels of maternal plasma PlGF levels were significantly lower in the preeclamptic women than in normal controls (median 86, range 29-232 vs. median 146, range 68-380, p<0.001) (Fig. 1B). Fig. 2 presents the correlation between the plasma sFlt-1 and PlGF levels in the two groups. In the preeclamptic women, there was a significant negative correlation between the plasma sFlt-1 and PlGF levels (r=-0.423, p=0.005), whereas there was a significant positive correlation between these variables in normal controls (r=0.270, p=0.008). We also evaluated the ratio of log[sFlt-1/PlGF] in the maternal plasma of the preeclamptic women and normal controls. The plasma log[sFlt-1/PlGF] ratio was significantly higher in the preeclamptic women than in normal controls (median 1.6, range 1.0-2.9 vs. median 1.2, range 0.5-1.9, p<0.001) (Fig. 3). The maternal plasma log[sFlt-1/PlGF] ratio with the cut-off value of 1.4 provided the best combination with 80.4% sensitivity and 78% specificity (area under the curve [95% CI]: 0.833 [0.756-0.910], p<0.001) (Fig. 4). Women with the maternal plasma log[sFlt-1/PlGF] ratio of > 1.4 had an increased risk of subsequently developing preeclampsia (OR [95% CI]: 17.0 [7.3-39.5], p<0.001).
Fig. 1.
Box plots indicating levels of sFlt-1 (A) and PlGF (B) in maternal plasma between normal controls and preeclamptic women. Boxes denote the interquartile range with the upper and lower horizontal edges representing the 75th and 25th percentiles, respectively. The central horizontal lines represent the medians. The vertical whiskers above and below the boxes represent the range of outlying data points up to 1.5 times the interquartile range, and the circles beyond the whiskers represent severe outliers. *Statistically significant, p<0.05.
Fig. 2.
Correlation between sFlt-1 and PlGF levels in maternal plasma of the preeclamptic women (●) and normal controls (○). The solid and dashed lines indicate the regression lines for the preeclamptic women and normal controls, respectively.
Fig. 3.
Box plots indicating ratios of the log[sFlt-1/PlGF] in maternal plasma between normal controls and preeclamptic women. Boxes denote the interquartile range with the upper and lower horizontal edges representing the 75th and 25th percentiles, respectively. The central horizontal lines represent the medians. The vertical whiskers above and below the boxes represent the range of outlying data points up to 1.5 times the interquartile range, and the circles beyond the whiskers represent severe outliers. *Statistically significant, p<0.05.
Fig. 4.
Receiver operating characteristic curve (ROC) showing the ability of the maternal plasma log[sFlt-1/PlGF] to differentiate preeclampsia from normal pregnancies. AUC, area under the curve.
DISCUSSION
We found a decreased level of PlGF and an increased level of sFlt-1 in the second trimester plasma of women who subsequently developed preeclampsia compared to normal pregnant women. Moreover, PlGF levels of the normal controls were positively correlated, while those of the preeclamptic women were negatively correlated with sFlt-1 levels. Our data also revealed that the sFlt-1/PlGF ratio in the preeclamptic women was significantly higher compared to normal controls. We speculated that preeclampsia may be secondary to endothelial dysfunction caused by the imbalance of circulating angiogenic factors of placental origin, such as sFlt-1 and PlGF. Further analysis will be required to clarify the regulation of angiogenic factors and their secretion in the women with preeclampsia as well as the role of angiogenic factors in the pathophysiology of preeclampsia.
Our data regarding both sFlt-1 and PlGF levels are consistent with the previous studies of Chaiworapongsa et al. (9) and Koga et al. (10) who found elevated plasma/serum concentrations of sVEGFR-1 in patients with preeclampsia at the time of diagnosis. In addition, the placentas from preeclamptic women have been shown to produce higher concentrations of sFlt-1 in vitro compared to normal controls (11, 12). Excess placental sFlt-1 may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia (3, 8). Several studies have documented that beginning in the early second trimester, and as early as 10 to 11 weeks of gestation, the plasma/serum PlGF concentration in women who go on to develop preeclampsia is lower than in normotensive controls (13-16).
The results of current studies suggest that the identification of high concentrations of sFlt-1 combined with low concentrations of PlGF may be used to predict the development of preeclampsia several weeks in advance of clinical onset of symptoms (1, 3, 17). Levine et al. (1) reported that the increase of sFlt-1 corresponds to a decrease of free PlGF in the serum of patients with preeclampsia resulting in endothelial dysfunction. Their results revealed that preeclampsia is associated with increased sFlt-1 concentrations in pregnant women five weeks before clinical disease and also with decreased PlGF from 13 to 16 weeks of gestation (1). Furthermore, the combination of first-trimester maternal serum concentrations of sFlt-1 (high) and PlGF (low) clearly identifies women at high risk of subsequent preeclampsia (3). The combination of both serum PlGF and sFlt-1 distinguished women who subsequently developed preeclampsia from those who subsequently developed gestational hypertension, delivered SGA (small for gestational age) newborns or completed a normal-term pregnancy (17).
Some investigators have suggested that the sFlt-1/PlGF ratio may be predictive of preeclampsia risk (1). In the present study, we evaluated the preeclampsia risk in relationship to the sFlt-1/PlGF ratio. In agreement with previous reports, preeclamptic women in our study had a higher sFlt-1/PlGF ratio compared to the normal controls. In addition, a recent study has demonstrated that severe preeclampsia is associated with increased urinary output of sFlt-1 and a decreased output of PlGF at the time of clinical manifestation (18). Therefore, rapid noninvasive screening of hypertensive women based on the Flt-1/PlGF ratio may be used to define the severity of preeclampsia (18). These findings suggest that alteration of sFlt-1 and PlGF levels may be important in the pathophysiology of preeclampsia.
The mechanisms responsible for the alternation of maternal plasma sFlt-1 and PlGF concentrations in women with preeclampsia remain unknown. In vitro studies have shown that PlGF is decreased (19) and sFlt-1 is increased (20) in trophoblast cells under conditions of reduced oxygen tension. It appears that PlGF deficiency and sFlt-1 excess may result from placental hypoxia associated with incomplete remodeling of maternal spiral arteries. The incompletely remodeled arteries offer persistently high resistance to uterine artery blood flow, and they may be predisposed to vascular rupture in the placental bed, especially after the onset of hypertension (21, 22). However, there is still not enough evidence to support the notion that altered levels of these angiogenic factors in maternal plasma of preeclamptic women are the consequence or the cause of a placentation deficit. Furthermore, the specific role of sFlt-1 and PlGF in the physiologic and pathologic development of placentation requires further investigation.
In summary, our results showed that sFlt-1 levels were increased and PlGF levels were decreased in second trimester plasma of women who subsequently developed preeclampsia compared to the normal pregnant women, and that a negative correlation existed between the circulating sFlt-1 and PlGF levels in the preeclamptic women. We also found that sFlt-1/PlGF ratios were significantly higher in women with preeclampsia than in normal controls. Although sFlt-1 and PlGF have been shown to be associated with preeclampsia and may be potential markers for the early prediction of preeclampsia before clinical manifestations are identified, further prospective, large-scale, longitudinal studies are essential to determine the usefulness of sFlt-1 and PlGF in predicting preeclampsia.
Footnotes
This study was supported by Grant No. KRF-2005-041-E00222 of the Korea Research Foundation.
References
- 1.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 2.McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol. 2004;191:1240–1246. doi: 10.1016/j.ajog.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 4.Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188:177–182. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 5.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y, Chen A, An SS, Ji RW, Davidson D, Llinas M. Kringle 5 of plasminogen is a novel inhibitor of endothelial cell growth. J Biol Chem. 1997;272:22924–22928. doi: 10.1074/jbc.272.36.22924. [DOI] [PubMed] [Google Scholar]
- 7.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- 8.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee Kim Y, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Am J Obstet Gynecol. 2004;190:1541–1547. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 10.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160:1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helske S, Vuorela P, Carpen O, Hornig C, Weich H, Halmesmaki E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol Hum Reprod. 2001;7:205–210. doi: 10.1093/molehr/7.2.205. [DOI] [PubMed] [Google Scholar]
- 13.Su YN, Lee CN, Cheng WF, Shau WY, Chow SN, Hsieh FJ. Decreased maternal serum placenta growth factor in early second trimester and preeclampsia. Obstet Gynecol. 2001;97:898–904. doi: 10.1016/s0029-7844(01)01341-2. [DOI] [PubMed] [Google Scholar]
- 14.Chappell LC, Seed PT, Briley A, Kelly FJ, Hunt BJ, Charnock-Jones DS, Mallet AI, Poston L. A longitudinal study of biochemical variables in women at risk of preeclampsia. Am J Obstet Gynecol. 2002;187:127–136. doi: 10.1067/mob.2002.122969. [DOI] [PubMed] [Google Scholar]
- 15.Polliotti BM, Fry AG, Saller DN, Mooney RA, Cox C, Miller RK. Second-trimester maternal serum placental growth factor and vascular endothelial growth factor for predicting severe, early-onset preeclampsia. Obstet Gynecol. 2003;101:1266–1274. doi: 10.1016/s0029-7844(03)00338-7. [DOI] [PubMed] [Google Scholar]
- 16.Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertens Pregnancy. 2004;23:101–111. doi: 10.1081/PRG-120028286. [DOI] [PubMed] [Google Scholar]
- 17.Bersinger NA, Odegard RA. Second- and third-trimester serum levels of placental proteins in preeclampsia and small-for-gestational age pregnancies. Acta Obstet Gynecol Scand. 2004;83:37–45. [PubMed] [Google Scholar]
- 18.Buhimschi CS, Norwitz ER, Funai E, Richman S, Guller S, Lockwood CJ, Buhimschi IA. Urinary angiogenic factors cluster hypertensive disorders and identify women with severe preeclampsia. Am J Obstet Gynecol. 2005;192:734–741. doi: 10.1016/j.ajog.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 19.Shore VH, Wang TH, Wang CL, Torry RJ, Caudle MR, Torry DS. Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta. 1997;18:657–665. doi: 10.1016/s0143-4004(97)90007-2. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen--a review. Placenta. 2000;21(Suppl A):S16–S24. doi: 10.1053/plac.1999.0524. [DOI] [PubMed] [Google Scholar]
- 21.Eskes TK. Abruptio placentae. A "classic" dedicated to Elizabeth Ramsey. Eur J Obstet Gynecol Reprod Biol. 1997;75:63–70. doi: 10.1016/s0301-2115(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 22.Dommisse J, Tiltman AJ. Placental bed biopsies in placental abruption. Br J Obstet Gynaecol. 1992;99:651–654. doi: 10.1111/j.1471-0528.1992.tb13848.x. [DOI] [PubMed] [Google Scholar]