Abstract
The motivational effects of drugs play a key role during the transition from casual use to abuse and dependence. Ethanol reinforcement has been successfully studied through Pavlovian and operant conditioning in adult rats and mice genetically selected for their ready acceptance of ethanol. Another model for studying ethanol reinforcement is the immature (preweanling) rat, which consumes ethanol and exhibits the capacity to process tactile, odor and taste cues and transfer information between different sensorial modalities. This review describes the motivational effects of ethanol in preweanling, heterogeneous non-selected rats. Preweanlings exhibit ethanol-mediated conditioned taste avoidance and conditioned place aversion. Ethanol's appetitive effects, however, are evident when using first- and second-order conditioning and operant procedures. Ethanol also devalues the motivational representation of aversive stimuli, suggesting early negative reinforcement. It seems that preweanlings are highly sensitive not only to the aversive motivational effects of ethanol but also to its positive and negative (anti-anxiety) reinforcement potential. The review underscores the advantages of using a developing rat to evaluate alcohol's motivational effects.
Keywords: infant rat, ethanol reinforcement, operant learning, appetitive conditioning, aversive conditioning, anxiolytic effects, ontogeny, conditioned taste aversion, conditioned place preference, early ethanol exposure, second order conditioning
1. Introduction
1.1. Preliminary considerations
Ethanol is arguably the most consumed psychoactive drug in the world. Controlled drinking may be common, but for many individuals ethanol consumption leads to severe psychiatric disorders, with estimates of lifetime prevalence of alcohol dependence reaching 20% and 8% of men and women in the United States, respectively (Enoch, 2003). Genetic factors have been traditionally considered as the main determinants of ethanol affinity (Tyndale, 2003). The level of heritability for alcohol dependence has been claimed to be approximately 50% (Ducci and Goldman, 2008; Enoch, 2003). Indeed, a positive family history for alcoholism is strongly associated with later alcohol abuse or dependence. Male adults with a family history of alcoholism (in first-degree and either second- or third-degree relatives) exhibit a three-fold greater incidence of alcoholism than subjects without a family history of alcoholism (Dawson et al., 1992). However, a family history of alcoholism has long been acknowledged to encompass the shared influences of both genetic and environmental influences (National Institute on Alcohol Abuse and Alcoholism, 1993). In the past two decades, the paramount role usually ascribed to genetics in alcohol research has been somewhat tempered by epidemiological and experimental preclinical research indicating that early prenatal or postnatal ethanol experience is significantly associated with later responsiveness to the drug (for a review, see Spear and Molina, 2005). Additionally, ethanol initiation during certain ontogenetic stages, notably adolescence, constitutes a risk factor for the development of later problems with the drug. Specifically, people who begin drinking at age 15 are four-times more likely to become alcohol-dependent than those who start at age 21 (Grant and Dawson, 1997). Ethanol intake usually begins during adolescence, with a decrease in the average age of initiation in the United States from 17.8 years in 1987 to 15.9 years in 1996 (Windle, 2003). A recent study suggests that the peak year for alcohol initiation is even earlier (13-14 years, Faden, 2006). Heavy drinking in this population is also widespread; with 30% of 12th graders reporting that they had been drunk at least once in the last 30 days (Johnston et al., 2007). The insights derived from this and related research (Spear & Molina, 2005; Abate et al., 2008) have added to the conceptualization of alcohol abuse and dependence, which now is considered a developmental disorder with etiological onset at childhood and adolescence (National Institute on Alcohol Abuse and Alcoholism, 2008).
Therefore, understanding the experiential factors that can interact with genetic predisposition to promote high alcohol consumption is important. Ethanol is a complex psychopharmacological agent that exerts a wide array of behavioral effects. Ethanol is a nutrient rich in calories (7 kcal/g; Molina et al., 2007) with a distinctive flavor and taste characterized by a combination of sweet and bitter qualities. These orosensory features can serve as signals (conditioned stimulus, CS) for the upcoming presence of biologically relevant situations (unconditioned stimulus, US) (Molina et al., 1986). For example, contingent experience with the scent of alcohol and aversive stimulation resulted in conditioned avoidance toward the ethanol odor and reduced ethanol intake measured in 21-day-old rats (Serwakta et al., 1986). Ethanol also can act as an interoceptive context that, when present during the acquisition and retrieval phases of a given learning situation, regulates the storage and expression of memories. State dependency mediated by ethanol has been reported in infant, adolescent, and adult rats (Fernandez-Vidal et al., 2003; Hunt et at., 1990; Bruins Slot et al., 1999).
Caloric, orosensory, and state-dependent properties of ethanol play important roles in the regulation of alcohol-seeking and consumption. Initial acceptance of oral ethanol in humans and in non-initiated genetically heterogeneous rats is modulated by these species' apparent dislike for the flavor of ethanol. Ethanol's sensory features have been proposed to constitute a “taste barrier,” precluding substantial intake of the drug (Pautassi et al., 2008b). Ethanol intake in two-bottle choice tests decreases sharply as ethanol concentration increases. If faced with a forced choice between water and a relatively low concentration of ethanol (1 to 5%), heterogeneous non-selected rats may show a modest preference for the drug, but ethanol consumption decreases dramatically as higher concentrations are employed (Kiefer et al., 1987; Samson et al., 1988). Consistent with the hypothesis of a “taste barrier,” Kiefer et al. (2005) found that naive rats displayed aversive orofacial reactions when intraorally stimulated with ethanol.
However, beyond caloric or sensory effects, environmental vulnerability to alcohol abuse is mostly influenced by the balance between the contrasting motivational effects of ethanol (Lynch and Carroll, 2001; Roma et al., 2008). Rather than being peripheral constructs, motivational concepts are at the core of all major modern theories of drug abuse and dependence. The compulsive pattern of drug-seeking and -taking found in dependent subjects has been suggested to result from the sensitization of a motivational system responsible for providing incentive salience to environmental stimuli (Robinson and Berridge, 2004; Berridge and Robinson, 1998). Koob and Le Moal (2001, 2008) proposed that drug dependence involves a deviation from the normal reward set point of the organism. Alcohol abuse and dependence are no exceptions to these considerations—motivational properties of ethanol represent critical factors in the modulation of drug-seeking and -taking. Appetitive and aversive consequences of ethanol increase and decrease, respectively, the subsequent probability of such ethanol-focused behaviors (Cunningham, 1998). These factors seem to be particularly important for understanding why certain individuals progress rapidly from controlled use of alcohol to abuse and dependence, while others continue controlled drinking despite continuous exposure to the drug. These subpopulations, consisting of controlled users and those that abuse the drug, may exhibit differential sensitivity to the motivational effects of ethanol. For example, some Asian subjects exhibit an impaired ability to metabolize acetaldehyde, a toxic by-product of ethanol metabolism, because of the lack of one or more alleles for aldehyde dehydrogenase. These persons have increased sensitivity to the aversive peripheral effects of ethanol, a fact that could explain their lower rates of alcohol abuse and dependence (Duranceaux et al., 2006). In contrast, subjects who are at risk for developing alcohol dependence exhibit aldehyde dehydrogenase allelic variations as well as a differential pattern of heart rate activation when given ethanol (Conrod et al., 1997, 1998; Montano et al., 2006; Mulligan et al., 2003). Ethanol-induced changes in heart rate are considered an index of the affective effects of ethanol.
In summary, the balance between the contrasting motivational effects of ethanol is thought to contribute, among several and varied causes, to the progression from moderate consumption to the compulsive pattern of alcohol-seeking behavior observed in alcohol dependence (Robinson and Berridge, 2004; Stewart and Badiani, 1993). Early exposure to alcohol seems to further accelerate this progression (Abate et al., 2008). Therefore, elucidating the motivational reactivity to ethanol early in ontogeny should provide helpful insights into the understanding of the development of alcohol abuse and dependence (Cunningham et al., 2000a).
1.2. Ethanol-mediated reinforcement
Ethanol exerts positive and negative reinforcing effects as well as acute aversive effects. The latter include peripheral physiological alterations, such as gastrointestinal disturbance (Adinoff et al., 1998) and autonomic reactions (e.g., facial flushing; Duranceaux et al., 2006), as well as central nervous system effects possibly related to the release of endogenous κ opioid ligands (Marinelli et al., 2000; Shippenberg et al., 2007). A significant proportion of ethanol's reinforcing consequences involve pleasurable postingestive effects mediated by the activation of reward centers in the midbrain (Tupala and Tiihonen, 2008). Indeed, ethanol-mediated positive reinforcement has often been considered the hallmark mechanism underlying the initiation and maintenance of alcohol intake (National Institute on Alcohol Abuse and Alcoholism, 1993). Systemic ethanol enhances the central release of dopamine and endogenous ligands for μ and δ opioid receptors, neurotransmitter systems involved in the hedonic assessment of drugs and other stimuli (Ericson et al., 2003). Ethanol also has the ability to ameliorate negative central states such as anxiety or dysphoria (Wilson et al., 2004). These negative reinforcing effects also play a role in shaping alcohol-seeking and consumption. Recent work has suggested that baseline anxiety and stress vulnerability increase after several years of heavy ethanol consumption (Koob, 2006). Under this framework, these changes would be associated with a shift in motivational control from positive to negative ethanol-mediated reinforcement. Hence, alcohol use may become predominantly motivated not by ethanol's rewarding effects but rather by the ability of the drug to counteract stress and relieve anxiety or depressed mood states (Koob, 2006; Koob and Le Moal, 2008).
In fact, despite mediation by different central mechanisms, positive and negative ethanol reinforcement share similar behavioral consequences. Both sources of reinforcement (i) increase the probability of behaviors directed at gaining access to ethanol and (ii) induce conditioned preferences to stimuli predicting these consequences (Abate et al., 2008).
Ethanol's motivational effects serve as an effective US, supporting the acquisition of associative learning (Cunningham et al., 2000a). Ethanol's postabsorptive effects can act as an aversive US, decreasing preference for stimuli paired with administration of the drug. Conditioned taste aversion (CTA) has proven to be one of the most reliable and sensitive paradigms for assessing the aversive effects of ethanol. Rats or mice given access to a gustatory stimulus (conditioned stimulus) followed by ethanol subsequently exhibit avoidance of the drug-related taste. Conditioned place preference (CPP) has been perhaps the paradigm most widely employed for assessing ethanol-mediated motivational learning (Schechter and Calcagnetti, 1998; Tzschentke, 1998; 2007). In this paradigm, animals (generally, rats or mice) are exposed to a distinctive chamber while experiencing the effects of ethanol intoxication. The drug-paired chamber contains visual, tactile, or odor cues. On alternate sessions, the animal could also receive saline administration paired with an alternative context. Testing is usually conducted by placing the animal in a two-compartment apparatus containing the cues signaling the drug effects as well as those previously paired with saline administration. The time spent in the ethanol-paired compartment is considered an index of conditioned preference mediated by the drug treatment. Pairings between ethanol's pharmacological consequences and visual or tactile stimuli can result in appetitive memories measured as increased preference for these CSs in the CPP paradigm (Cunningham et al., 2000a). Anti-anxiety effects of ethanol also are inferred by the drug's facilitatory effects in approach-avoidance tasks, such as the elevated plus maze or light-dark transition test (Hitzemann, 2000), or Pavlovian tasks (Pautassi et al., 2005a, 2006).
1.3. Ethanol reinforcement in mature rats and mice
Understanding ethanol reinforcement and the factors that inhibit or exacerbate these motivational effects is required for understanding the mechanisms underlying seeking and self-administration processes. Ethanol reinforcement has been studied with great benefit in adult rats and mice (Cunningham et al., 2000a).
Mature subjects from both species readily express ethanol-mediated CTA (Cunningham et al., 1988, 1992b), particularly after three or four conditioning trials and when employing moderate to high ethanol doses as the US in rats (1.2-3.0 g/kg; Cappell et al., 1973; Sherman et al., 1983) and mice (2.0-4.0 g/kg; Risinger and Cunningham, 1995; Risinger and Boyce, 2002). Lower doses (0.4-1.0 g/kg) do not appear to induce reliable CTA in mature rats (Sherman et al., 1983) or mice (Aragon and Amit, 1993). One of the earliest examples of ethanol-mediated CTA can be seen in the work by Berman and Cannon (1974). Although they employed an extremely long pre-exposure phase (35 days) and an ethanol-beer mixture as US, this pioneering work defined characteristics of ethanol-induced CTA that are yet to be challenged. These researchers gave rats varying levels of non-reinforced ethanol exposure prior to pairings of saccharin and postabsorptive consequences of ethanol [2.0, 4.0, or 6.0 g/kg, intragastric (i.g.)]. Ethanol-mediated CTA was attenuated, but not eliminated, by previous experience with the drug. The CTA was dose-dependent (i.e., better learning with higher doses), emerged after two conditioning trials, and was inversely related to the amount of previous ethanol experience. These results were replicated on several occasions (e.g., Cannon et al., 1975). Furthermore, Davies and Parker (1990) observed that previous ethanol experience not only reduced ethanol-mediated CTA but also suppressed the otherwise strong conditioned disgust reaction (e.g., gaping, chin rubbing, paw treading, limb flicking) observed when rats were intraorally (i.o.) stimulated with the ethanol-related taste. Among the mechanisms likely underlying ethanol's ability to induce CTA, two have received compelling empirical support: drug-induced hypothermia (Cunningham et al., 1988, 1992b) and induction of emesis-like effects by activation of chemoreceptors in the brainstem (Thiele et al., 1998).
Mice easily learn to prefer a place paired with ethanol's pharmacological effects. The prototypical protocol involves a few training sessions in which an ethanol injection (0.5-4.0 g/kg) is followed by exposure to a distinctive set of environmental cues (CS+; Cunningham et al., 2006a). Interspersed between CS+/ethanol trials, mice receive vehicle and are exposed to an alternate set of cues (CS−). This protocol typically yields a monotonic dose-response pattern, with higher doses resulting in greater learned preferences (Groblewski et al., 2008). The finding of reliable ethanol-mediated CPP in mice has proven to be an invaluable asset in the search for the mechanisms underlying ethanol reinforcement. Among many other factors, this paradigm has aided in analyzing the role of brain structures (e.g., ventral tegmental area and nucleus accumbens; Bechtholt and Cunningham, 2005), pharmacological treatments such as baclofen (Bechtholt and Cunningham, 2005), N-methyl-d-aspartate (NMDA) ligands (Boyce-Rustay and Cunningham, 2004), and dopamine receptor antagonists (Cunningham et al., 1992a), genetic background (Cunningham et al., 2000b), and behavioral variables (e.g., apparatus bias; Cunningham et al., 2003) associated with the acquisition or expression of ethanol reinforcement. Furthermore, the same paradigm can be employed after minimal variations in timing to assess ethanol-mediated conditioned place aversion (CPA). The typical outcome is CPP when mice are subjected to CS exposure 5 min after ethanol administration (4 g/kg, intragastric administration) but CPA if ethanol is infused just before exposure to the CS (Cunningham et al., 2002a).
Duration of exposure to CS is another important variable that can affect the strength of CPP or CPA. It seems that a short trial duration is more effective to induce CPP than longer trials. In an intriguing study, DBA/2J mice were given ethanol (2g/kg) and immediately exposed to a CS+ until 5, 10 or 30 minutes after ethanol administration. As expected, all mice expressed CPP. The magnitude of this conditioned preference, however, was inversely proportional to trial duration (Cunningham & Prather, 1992). A subsequent study (dose: 3.0 g/kg: Risinger & Cunningham, 1992) observed similar CPP when using the 5 min trial but found signs of conditioned aversion when the trial lasted until post-administration time 60 min. These results suggest that ethanol's rewarding effects may be more clearly observed during the early phase of the intoxication, when blood alcohol levels are rising. This possibility will be discussed later.
The expression of ethanol-mediated CPP in the rat has proven difficult. Heterogeneous (i.e., non-genetically selected) rats generally do not exhibit appetitive learning following pairings of visual/tactile cues and postabsorptive effects of alcohol. Conditioned place aversion appears to be the most common outcome of such associative process. These aversions are easily established when the ethanol dose exceeds 1 g/kg (Cunningham and Niehus, 1988; Cunningham et al., 1993; Gauvin and Holloway, 1992; Schechter and Krimmer, 1992). Lower doses rarely exert significant aversive or appetitive conditioning (Stewart and Grupp, 1985; van der Kooy et al., 1983; Fidler et al., 2004). The conditions under which ethanol induces CPP in adult rats are still unclear. Studies that achieved alcohol-mediated CPP in heterogeneous rats employed very extensive training and pre-exposure procedures (Bozarth, 1990; Peana et al., 2008; Reid et al., 1985) or concurrent presentation of other reinforcers (Marglin et al., 1988). The employment of genetically selected rats (e.g., Marchigian Sardinian alcohol-preferring; Ciccocioppo et al., 1999b) has also yielded some success.
A more general obstacle in the analysis of ethanol's reinforcing properties has been the non-selected rodent's natural disinclination to drink ethanol as adults. Heterogeneous, nonselected rats and mice may drink ethanol when offered at low concentrations or under conditions with restricted access to fluids or food. However, consumption decreases sharply in unrestricted situations and particularly when the ethanol concentration reached or exceeded approximately 5% v/v (Kiefer et al., 1987; Ponce et al., 2004). This problem has been solved in part by genetic selection (Crabbe et al., 2006) and also through the development of initiation procedures, notably the sucrose-fading procedure (Samson, 1986), that overcome the apparent “taste barrier” represented by the unlearned dislike of ethanol's chemosensory properties.
1.4. The infant rat as a model for testing ethanol reinforcement and the effects of early ethanol exposure
Another vehicle for studying ethanol reinforcement is the immature, preweanling rat [first 3 postnatal weeks, postnatal days (PD) 1 to 21]. An analysis of early sensitivity to ethanol in neonates and infants serves as a fast, economical, and highly analytical model of the effects of the drug in humans. The immature heterogeneous rat readily consumes ethanol at rates comparable to genetically selected adults, without the need for initiation procedures. Specifically, if given access to oral ethanol in conditions defined as “independent feeding” (Hall, 1990), 2- to 3-week-old infant rats will consume enough 25% v/v ethanol in 20 min to increase their blood alcohol levels (BALs) to approximately 300 mg% (Truxell and Spear, 2004; also see Sanders and Spear, 2007). Generally, consumption of ethanol surpasses that of water by PD6, reaches its maximum by PD12-14, then declines monotonically throughout ontogeny, with the level of intoxication nearing adult levels (i.e., 30-50 mg%) by the end of the third postnatal week (Truxell et al., 2007; Doremus et al., 2005). High affinity for ethanol can also be observed even earlier in development. Neonatal rats consumed similar levels of ethanol and saccharin when the solution was offered through a device resembling the maternal nipple (Petrov et al., 2001; Varlinskaya et al., 1999; see section 3.3.1). The clear shift between high to low ethanol acceptance through ontogeny does not happen in a neuro-developmental vacuum. As will be discussed later, neurotransmitter systems change in their function as the animal ages. These ontogenetic shifts in neurochemical function, viewed along side the dramatic change in ethanol acceptance, may allow for the study of possible mechanisms involved in ethanol's acceptance.
Infancy in the rat appears to provide a useful preparation for the analysis of ethanol reinforcement. An obvious advantage of using an infant model is its logistical ease (i.e., easy manipulation, brevity of training and testing procedures, short housing associated with lower costs, etc.). The infant rat also has the capacity to process tactile, odor and taste cues and transfer information between different sensory modalities (Spear and Molina, 1987). These information processing capabilities allow testing ethanol's effects through complex and highly analytical learning preparations, such as second-order conditioning (see section 3.3.2).
The usefulness of a developmental approach is not restricted to the understanding of ethanol ingestion and reinforcement. The ontogenetic strategy has helped to understand more general phenomena, such as the role of hippocampus in conditioned fear (Stanton et al., 2000; Ivkovich et al., 2000), the differences between trace and long-delay fear conditioning (Hunt and Richardson, 2007) or the role of specific sets of proteins in the expression of social behavior (Garcia et al., 2009). In all these cases the strategy was to correlate a given outcome with corresponding changes in the normal neural pattern of development. For instance, Garcia and coworkers (2009) found that the ontogenetic differentiation from nurse to forager worker in the honeybee is correlated with differential protein expression. In regards to ethanol, there is an intriguing correlation between the affinity for this drug and the ontogeny of certain neurotransmitters, notably γ-aminobutyric acid-A (GABAA). Although on PD4 genetically heterozygous infant rats drank less ethanol (5% v/v) than water, this pattern reversed by PD6 and by PD8, when ethanol ingestion was twice that of water ingestion (Sanders and Spear, 2007). The GABA system initially has an excitatory function that switches to inhibitory by about postnatal day 9 (depending on brain area; Rivera et al., 1999). It could be hypothesized that these developmental changes in GABA function are linked to the patterns of ethanol consumption observed in infant rats. This serves only as an example, and confirmation of this hypothesis is still pending, but the study by Sanders and Spear (2007) underscores the value of ontogenetic analysis to assess affinity for ethanol and, potentially, ethanol reinforcement.
The study of ethanol-mediated learning in the immature rat may provide further benefits by clarifying the effect of early prenatal or postnatal experience with alcohol on later affinity for the drug, particularly during adolescence. Regardless of a family history of alcoholism, humans with earlier alcohol initiation are more likely to become alcohol-dependent than those with a later onset of drinking (Grant, 1998; also see Rhee et al., 2003). Alcohol exposure, however, can also begin in the womb. A longitudinal evaluation of the offspring of 439 parents assessed the relative weight of prenatal and genetic factors upon alcohol use by age 14 and 21. Prenatal alcohol was a direct and significant predictor of alcohol abuse during adolescence and early adulthood, even after adjusting for a family history of alcoholism (Baer et al., 1998, 2003). Similar results were observed in studies that employed alternative methodological approaches (e.g., assessment of adoptees with or without prenatal alcohol exposure; Yates et al., 1998). Furthermore, cultural practices promote early contact with ethanol not only during pregnancy (Chambers et al., 2005; Morris et al., 2008) but also through milk during breastfeeding (Chien et al., 2005, 2008; Menella, 2001; Mennella and Beauchamp, 1993) and routine medical procedures (Croce, 1987; Fildes, 1986; Flores-Huerta et al., 1992).
A full experimental analysis of the relationship between early prenatal and postnatal experience with alcohol and heightened affinity for the drug in humans is limited by the obvious ethical and legal constraints regulating experimental behavioral research in infancy, childhood and adolescence (Witt, 1994). During the last two decades, however, employment of developmental rat models has provided a valuable tool for the analysis of these and related phenomena (Spear and Varlinskaya, 2005). In rats, contamination of the amniotic fluid by ethanol before partum results in neonatal memories for the sensory attributes of ethanol (Molina et al., 1995). Moreover, infant rats exposed to moderate doses of ethanol during late gestation exhibit a differential pattern of responsiveness when stimulated with ethanol odor and, perhaps more important, heightened ethanol consumption (Dominguez et al., 1998). Chronic daily administration of ethanol throughout the preweanling period (PD6-12) also leads to heightened ethanol consumption when assessed during infancy (PD15; Lopez and Molina, 1999) or adulthood (PD120; Hayashi and Tadokoro, 1985). These results, combined with several other animal studies (e.g., Abate et al., 2000, 2002, 2004; Arias and Chotro, 2005, 2006b; Bond and Di Giusto, 1976; Chotro and Arias, 2003; Pepino et al, 2004; Ponce et al., 2004; Randall et al., 1983) indicate increased ethanol ingestion after early exposure to ethanol. A recent review of this literature (Chotro et al., 2007) evaluated 22 studies that explored the effect of maternal ethanol intoxication during gestation on later ethanol intake by the offspring. Only four of these studies failed to report significant effects, and Chotro et al. (2007) pinpointed procedural and statistical issues that could underlie the lack of significant results. Interestingly, all of the remaining 18 studies reported increased ethanol intake in infancy, adolescence, or adulthood after in uterus ethanol experience.
With regard to the mechanisms underlying this association, Chotro et al. (2007) discussed potential ethanol-induced teratology and alterations in neurohormonal responses that may be related to heightened consumption of the drug during adolescence or adulthood. However, the evidence for these possibilities was scarce or only correlational. On the other hand, Chotro et al. (2007) found substantial support for the hypothesis that heightened postnatal preference for ethanol can result from early chemosensory learning about ethanol. Maternal intoxication with ethanol results in fetal exposure to both chemosensory and toxic effects of the drug (Szeto, 1989). An associative account for the effects of early ethanol exposure was proposed by Spear and Molina (2005). They suggested that, in addition to simple passive pre-exposure effects, associative conditioning is likely to occur in the fetus or infant when it is exposed to ethanol. The young organism learns that chemosensory properties of ethanol (e.g., taste, flavor) or other stimuli present in the context of ethanol administration predict the positive rewarding postabsorptive effects of the drug. This process may result in increased seeking or consumption of the drug later in life.
An enormous body of clinical and preclinical research as well as intriguing hypotheses regarding the mechanisms underlying the link between early ethanol exposure and later alcohol abuse has been amassed in reviews by Spear and Molina (2005), Chotro et al. (2007) and Abate et al. (2008). However, little has been described concerning how and if ethanol exerts pharmacologically reinforcing effects in developing organisms. Clearly, for these hypotheses to be tested, further analyses of early associative conditioning through ethanol's motivational properties need to be conducted. The present review may help fill this gap by describing our current knowledge concerning the sensitivity of preweanling rats to the appetitive, aversive, and negative reinforcing consequences of ethanol.
1.5. General aims
The current review discusses the motivational properties of ethanol assessed in preweanling rats (PD0-21; P0 is date of birth). One of the purposes of this review is to describe our current understanding of how the rat pup learns about ethanol's hedonic properties. Given the long-term difficulty of testing ethanol reinforcement in normal genetically heterozygous adults, what are the prospects for testing ethanol conditioned reinforcement in the normal developing genetically heterozygous rat? The present review discusses potential answers to this question. It should be noticed that the review will not focus on the ontogeny of ethanol intake nor the consequences of ethanol in uterus. Studies on ethanol intake and prenatal exposure will be briefly discussed in some sections when they provide relevant information for the understanding of ethanol reinforcement (for more information on ethanol intake in infant rats, see Chotro et al., 2007; Kozlov et al., 2008; Truxell and Spear, 2004; Truxell et al., 2007).
This article is guided by the hypothesis that considerable evidence has been accumulated in the past decade to conclude that infant rats are highly sensitive to not only the aversive motivational properties of postabsorptive ethanol but also its positive reinforcing and negative reinforcing (anti-anxiety) effects.
2. Conditioned aversion mediated by ethanol in the preweanling rat
Ethanol-mediated CTA has been observed in 10-day-old or older rats. Although adults usually require a rather extensive training phase, one-trial ethanol-mediated CTA is common in infants (Abate et al., 2001; Arias and Chotro, 2006b; Molina and Chotro, 1989; Hunt and Spear, 1989; Pueta et al., 2005), although two CS-US trials appears to be the standard training phase for the studies assessing factors modulating or underlying the expression of ethanol-mediated CTA in infants (Pautassi et al., 2002a, 2005b; Hunt et al., 1990). Moreover, pups can acquire ethanol-mediated taste aversions without the need of explicit CSs. Molina and Chotro (1989) as well as Hunt et al. (1993) and Lee et al. (1998) found that pups given only high-dose ethanol (≥2.5 g/kg, i.g.) subsequently avoided the odor and taste of ethanol. These studies took advantage of the fact that about 12% of ethanol is excreted unchanged through respiration, salivation, perspiration, and urine (Winger et al., 1992), thus allowing perception of ethanol's odor and taste cues during the toxic process and acquisition of aversion to them. The level of ethanol intoxication leading to this chemosensory stimulation seems to be around 200 mg%: conditioned aversion following ethanol was found when BALs were greater than 250 mg% but not when BALs were lower than 150 mg% (Molina et al., 1989).
At high doses (≥2.0 g/kg, yielding BALs >150 mg%), the ability of ethanol to induce CTA in preweanling rats may be related to its capacity to activate the emetic centers of the brainstem. Arias et al. (2007) found similar one-trial CTA in pups receiving ethanol or lithium chloride (LiCl) (2.5 g/kg and 0.5% of body weight, respectively; CS: saccharin). When briefly stimulated with the taste CS, these rats exhibited increased wall climbing, head shaking, locomotion, and grooming, and less mouthing movements than controls. These conditioned disgust reactions, indicating a change in the palatability of the tastant from relatively neutral to aversive, were similar across ethanol- and LiCl-treated pups and were inhibited by systemic administration of domperidone, a peripheral dopamine receptor antagonist with potent anti-emetic effects (Arias et al., 2007). These results suggest that, in the preweanling rat, ethanol may share some postabsorptive effects with LiCl. Emetic-like effects are critical for the expression of LiCl-mediated disgust reactions in the infant rat (Pautassi et al., 2008a).
Even relatively low ethanol doses successfully establish CTA in preweanlings. Hunt et al. (1990) found CTA in rat pups given two pairings of sucrose and 1.2 g/kg ethanol. A striking finding was that preweanlings also expressed CTA when employing a much lower ethanol dose, 0.4 g/kg. Results by Hunt et al. (1990) pinpoint certain specific conditions in which ethanol-mediated taste learning is likely to occur. Specifically, low-dose ethanol (0.4 g/kg) elicited CTA only when (i) ethanol administration during conditioning preceded sucrose by 30 min, and (ii) rats also received 0.4 g/kg ethanol immediately before testing. Presumably, the 30-min interval allowed the CS to be experienced during a post-administration interval characterized by maximum BALs for that particular low dose. In contrast, the need for ethanol administration at testing implied that the expression of the aversive memory was state-dependent. For conditioned rejection of the sucrose CS to occur, testing had to be conducted under the same pharmacological state experienced during conditioning. Another study also analyzed the animal's ability to concurrently perceive the state of alcohol intoxication as a distinctive state and to develop conditioned responses to ethanol-paired flavors. Fernandez-Vidal et al. (2003) trained adolescent rats (PD30-33) to discriminate a moderate ethanol toxic state from a non-drug state by allowing rats to have access to sucrose after ethanol (0.5 g/kg) or sham intubation. The adolescents learned this discrimination after four training trials. Perhaps more interesting, sucrose-seeking and intake increased in those rats in which sucrose availability had been signaled by ethanol. A common denominator between the Hunt et al. (1990) and Fernandez-Vidal et al. (2003) studies is state-dependent learning when employing low-dose ethanol. Additionally, in both studies a few pairings of sucrose and the postabsorptive consequences of ethanol altered later responsiveness to the sweet taste. However, although infants exhibited ethanol-mediated conditioned aversion, conditioned sucrose preference was found in adolescent rats. These results suggest that age-related differences may exist in terms of ethanol's ability to induce conditioned flavor learning.
Age-related differences in ethanol-mediated CTA were also found within the period of infancy. Hunt et al. (1991) reported CTA in 16-day-old, but not in 10 or 20 day-old, rats that received pairings of sucrose and ethanol (1.6 g/kg). The younger animals only expressed CTA when employing a higher dose (3.0 g/kg). Ethanol-mediated aversion was also related to ambient temperature, with low temperatures promoting acquisition of the CTA. Very few studies have analyzed ethanol's ability to induce conditioned flavor responses before PD10. In an intriguing study (Arias and Chotro, 2006a), 7- to 8-day-old rats receiving ethanol (3.0 g/kg) showed heightened ethanol intake and more appetitive responses to ethanol's flavor than rats treated with vehicle when tested 3 days later. At the same dose, ethanol intoxication induced taste and flavor aversion in older animals (10- to 11-day-old). A follow up study (Chotro and Arias, 2007) found that pretreatment with an opioid antagonist (naloxone, 1 or 10 mg/kg) inhibited the heightened ethanol consumption in the younger pups but had no effect on the aversion observed in the older rats. These studies (Arias and Chotro, 2006a; Chotro and Arias, 2007) suggest that sensitivity to the motivational effects of ethanol may differ across early ontogeny and also underscore the involvement of the opioid system in ethanol's hedonics.
Infants also appear to be affected differently by ethanol pre-exposure than adults. As stated above, prior ethanol exposure disrupts later ethanol-mediated CTA in adult subjects (Berman and Cannon, 1974). Pautassi et al. (2005b) gave infant rats 4 days of non-reinforced ethanol exposure (2.5 g/kg, PD10-13) followed by pairings of ethanol (0.5 or 2.5 g/kg, i.g., PD 14-15) and sucrose. Pups receiving the highest dose exhibited ethanol-mediated CTA after one conditioning trial. Interestingly, after two CS-US pairings, the lower dose also promoted CTA. No sign was evident of conditioned aversions being altered by ethanol pre-exposure. Conditioned taste aversion induced by ethanol was also assessed in rat pups that had experienced alcohol in uterus and then been reared by an alcohol intoxicated dam (Pueta et al., 2005). Ethanol-mediated CTA (1.0 – 2.0 g/kg) was expressed following a single conditioning trial and was unaffected by prenatal or nursing experiences with ethanol. These results indicate high sensitivity to ethanol's aversive effects in the infant rat. These animals exhibit drug-mediated CTA after ethanol pre-exposure and when conditioned with a relatively low dose of ethanol (0.5 g/kg; maximal BAL 42 mg%). One of these studies (Pautassi et al., 2005b) employed a discriminative conditioning procedure, a fact that may explain the strength of the ethanol-mediated CTA as well as its apparent insensitivity to the pre-exposure treatment. In Pautassi et al., (2005b) a tactile stimulus (CS−) predicted the initial phase of ethanol intoxication, while the gustatory stimulus (sucrose, CS+) was presented during a later period of the toxic processes, characterized by peak BALs. Employment of a CS−/CS+ Pavlovian strategy is known to facilitate, particularly in young rats, the acquisition of aversive memories otherwise not detected through simple excitatory conditioning procedures that only utilize a CS+ (Kucharski et al., 1985 Spear et al., 1989). It should also be noted that prenatal ethanol has been found to ameliorate ethanol-mediated CTA during infancy (Arias and Chotro, 2006b). This study employed a high ethanol dose (3.0 g/kg, one or three conditioning trials).
3. Beyond the aversive unconditioned effects of ethanol: Appetitive and negative reinforcement effects
3.1. Appetitive reinforcing effects of ethanol in preweanling rats
Associative learning has been suggested as one likely mechanism underlying the link between early ethanol exposure and heightened consumption of the drug. As proposed by Spear and Molina (2005) and later developed by Chotro et al. (2007) and Abate et al. (2008), the young organism may associate the positive reinforcing consequences of ethanol with stimuli present during drug administration. This process would later lead to enhanced preference for the drug or for stimuli predicting its positive consequences. In the preceding section, we accounted for studies involving ethanol as US and gustatory cues as CS. The results provide little indication that infant rats could perceive the positive reinforcing effects of ethanol when assessed in conditioning models. Apparently, we need to look elsewhere to find evidence of such an outcome.
3.1.1. Ethanol-mediated conditioned place preference in infant rats
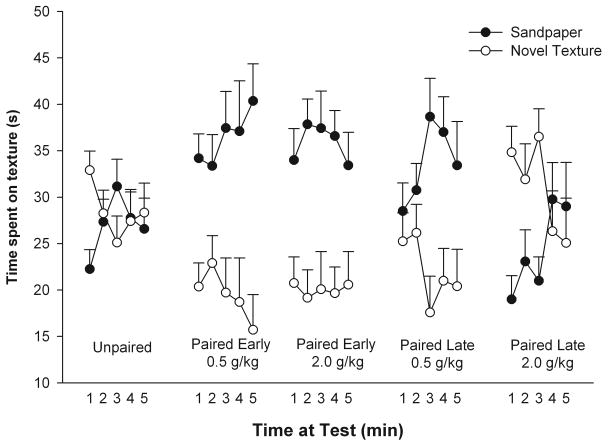
Ethanol-mediated appetitive effects are easily observed in mice when employing the CPP procedure (Cunningham et al., 2006a; Green and Grahame, 2008). However, under similar testing conditions, rats usually display a conditioned place aversion (CPA). Similar conclusions were derived from a study that sought to pair tactile stimuli with the postabsorptive effects of ethanol in infant rats (Molina et al., 1996). In this work, 10-day-old pups were subjected to paired or unpaired presentations of a soft texture and ethanol intoxication (2.0 g/kg). Importantly, for reasons that will be explained later in this review, the CS presentation occurred 30 min following drug administration, when alcohol reached peak concentrations in brain and blood. This procedure resulted in the expression of conditioned aversion. That is, those subjects that received paired CS-ethanol presentations avoided the soft texture during the test session. Lee et al. (1998) similarly reported ethanol-mediated CPA in infant rats that drank ethanol from the floor of a distinctive chamber.
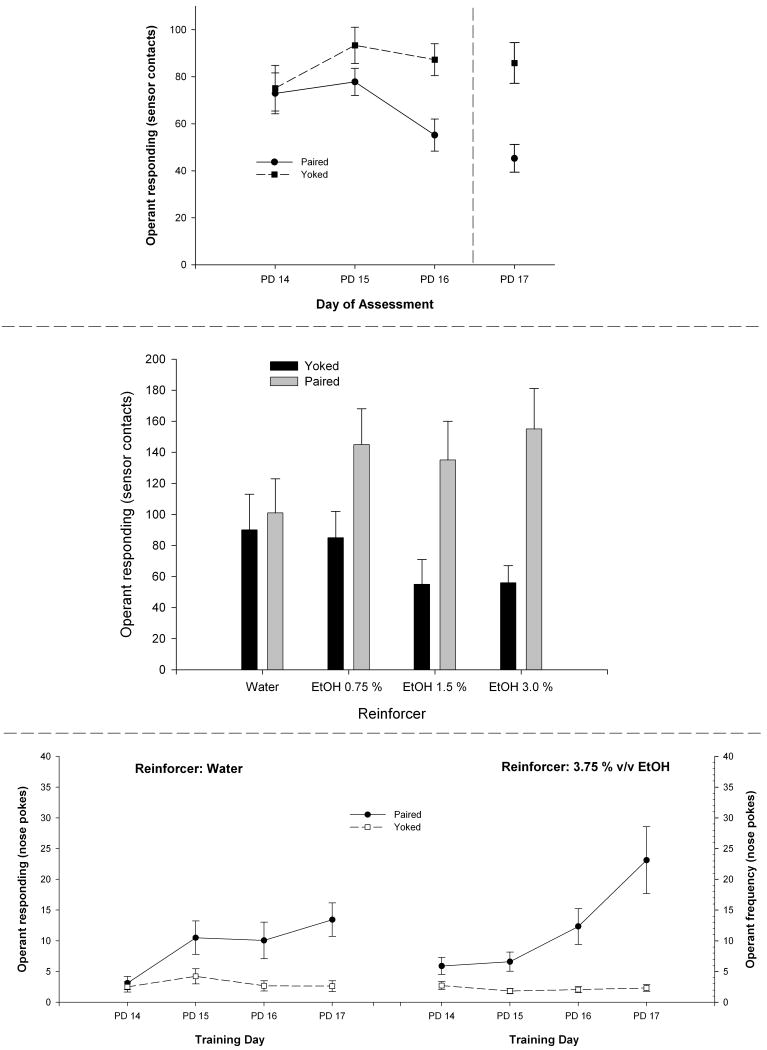
Pautassi et al. (2002a) sought to assess responsiveness to tactile cues signaling ethanol intoxication in 2-week-old rats. A main procedural difference from the Molina et al. (1996) study was that the tactile CS (sandpaper) was presented relatively soon after induction of ethanol intoxication (2.5 g/kg). Our objective was to pair the tactile CS with the initial, early ethanol postabsorptive interval, when BALs are still rising. By doing this, we were guided by data derived from animal and human preclinical studies. Investigations in mice (Cunningham and Prather, 1992; Risinger and Cunningham, 1992) have suggested that during the onset of the state of intoxication, ethanol may exert appetitive unconditioned effects, and aversive consequences are likely to emerge at later stages. Cunningham et al. (2002) had also found that minor variations between the timing of ethanol administration and CS exposure exerts dramatic effects on the outcome of an ethanol-mediated CPP. Additionally, human studies have indicated that subjects at risk for developing alcohol-related problems show heightened heart rate, psychomotor stimulation, and positive hedonic ratings during the ascending but not descending limb of the blood alcohol curve (Conrod et al., 2001). In summary, the duration of tactile conditioning in Pautassi et al (2002a) was restricted to a temporal window (i.e., post-administration interval of 5-15 min) that overlapped with the initial, ascending limb of the blood alcohol curve. Following sandpaper presentation, pups were exposed to an alternative, gustatory CS (saccharin). Intraoral stimulation with the gustatory CS occurred 25-35 min after ethanol administration. Pups were exposed to a differential conditioning procedure in which a tactile cue signaled the onset of ethanol's effects and a taste cue was associated with the later peak of the blood alcohol curve. Would this procedure result in differential motivational learning (i.e., appetitive and aversive conditioned responses involving the tactile and gustatory cues, respectively), or would ethanol's aversive effects prevail, with both cues being aversively conditioned? Not surprisingly, saccharin intake assessments revealed the expression of CTA. However, no evidence of appetitive or aversive learning was found when examining responsiveness toward the texture CS.
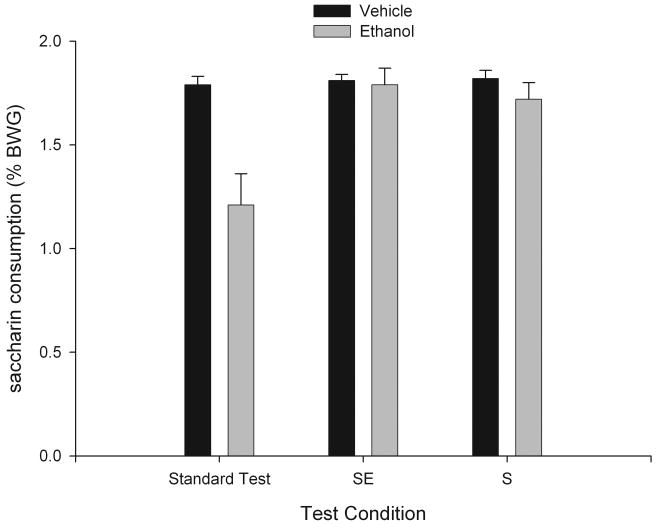
The data also revealed, however, that infants indeed processed information about the contingency between the texture CS and the onset of the ethanol toxic process. As shown in Fig. 1, a dramatic and complete inhibition of ethanol-mediated CTA was found when infants were exposed to this tactile cue during testing or immediately before assessment of saccharin intake (Pautassi et al., 2002a). This intriguing phenomenon suggested that pups were able to encode differential hedonic effects during the course of ethanol's toxic process. That is, the tactile cue encoded information about ethanol's postabsorptive effects that were perhaps appetitive and counteracted the expression of ethanol-mediated aversive conditioned responses. Subsequent replications confirmed the reliability of this empirical finding and also dispelled alternative explanations such as the possibility of the tactile cue exerting nonspecific interference effects on the expression of CTA (Pautassi et al., 2002b). In addition to the possibility of ethanol exerting appetitive consequences, these results can also be explained in terms of inhibitory and excitatory conditioning associated with ethanol's support of aversive conditioning. Under this framework, the tactile cue and the saccharin taste predicted the absence and presence, respectively, of ethanol's aversive effects. Therefore, contingent presentation of both CSs canceled the expression of the otherwise strong aversive excitatory response. Regardless, the most fruitful outcome of this experiment was the finding of a non-aversive motivational effect of ethanol early in development. This observation led us to further scrutinize the pups' perceptions of ethanol's hedonic effects, particularly during the onset of postabsorptive processing of ethanol.
Figure 1.
Saccharin consumption scores expressed through percentage body weight gain (% BWG) as a function of conditioning treatment (postnatal days 14-15, 2.5 g/kg ethanol or saline as unconditioned stimulus paired with saccharin, conditioned stimulus) and testing conditions on postnatal day 16 [standard intake test, sandpaper exposure immediately before test (SE), or sandpaper concurrently presented with saccharin during the intake test (S)]. During conditioning, sandpaper was presented during the rising limb of the blood ethanol curve (5-15 min postadministration). Vertical lines represent SEM. Adapted from: Pautassi, R.M., Godoy, J.C., Spear, N.E., Molina, J.C., 2002. Early responsiveness to stimuli paired with different stages within the state of alcohol intoxication. Alcohol. Clin. Exp. Res. 26, 644-654, Blackwell Publishing. Used with permission from publisher.
3.1.2. Further analysis of ethanol-mediated tactile conditioning in infants
Often overlooked, method of drug delivery exerts significant effects on expression of drug-mediated motivational learning. The rate of glucose utilization and neurotransmitter turnover differs when using forced or self-administration procedures with drugs such as cocaine or morphine (Porrino et al., 1998; Smith et al., 1984). Furthermore, different routes of administration result in differential peak drug levels and rates of drug accumulation (Kuczenski and Segal, 2005). With regard to ethanol, Ciccocioppo et al. (1999a) found that ethanol (1.0 g/kg) induced a CTA in selectively bred Marchigian adult rats when employing the i.g. but not intraperitoneal (i.p.) route. Mode of ethanol administration also affected ethanol-mediated CPP (Ciccocioppo et al., 1999b). The oral route of administration has greater face validity than the i.g. or i.p. routes of ethanol administration when the objective is to model the typical pattern of alcohol self-administration observed in humans. The intraoral (i.o.) route also allows researchers to analyze the specific role of the orosensory properties of ethanol (e.g., taste, smell) in the acceptance and modulation of the drug's effects. In adult rats, the orosensory effects of ethanol appear to constitute a “taste barrier” that precludes substantial consumption of the drug (Kiefer et al., 2005; Samson et al., 1988).
These observations were considered when continuing to pursue the analysis of ethanol's positive reinforcing effects in infants (Pautassi et al., 2008b; Nizhnikov et al., 2009). The general strategy of these experiments involved exposing 14- to 15-day-old pups to distinctive tactile cues while sober or while experiencing a given effect of ethanol administered i.o. (Pautassi et al., 2008b), i.p., i.g. (Nizhnikov et al., 2008; Nizhnikov, Pautassi, Truxell, Spear, unpublished) or via vapor inhalation (Nizhnikov et al., 2009). These conditioning sessions were followed by a drug-free test in which the pup had the opportunity to choose between the ethanol-related cue and an alternative stimulus. Ethanol dose was kept relatively low compared with the preceding studies (e.g., Pautassi et al., 2002a; b). We were guided by an increasing amount of literature indicating that ethanol appetitive consequences might be more easily observed during early development when dosage is kept below 1.0 g/kg. These data encompassed our own work involving ethanol's interaction with aversive conditioning (Pautassi et al., 2002a; b, 2006, 2007) and evidence gathered in neonate rats by means of a surrogate nipple technique (Petrov et al., 2001, 2003; Cheslock et al., 2001). This literature is discussed in detail in sections 3.2 and 3.3.
A first experiment was intended to assess, primarily, the hedonic value of ethanol's flavor (Pautassi et al., 2008b). During daily conditioning sessions (PD14-15), infant rats were exposed to a tactile CS for 20 min in contiguity with i.o. delivery of ethanol (5% v/v) or water. Twenty-four hours later, a tactile preference test indicated that pups avoided the CS that had been paired with i.o. ethanol stimulation. The results revealed that pups perceive the orosensory features of i.o.-delivered ethanol as an aversive US, reflected in the rejection (i.e., CPA) of a stimulus signaling the taste and smell of ethanol. However, this assertion did not preclude the possibility that the delayed, postingestive consequences of ethanol may exert positive reinforcing effects. We tested this possibility in a follow-up experiment. The study comprised an ethanol pre-exposure phase (PD13) defined by passive i.o. stimulation with water or 5% ethanol. During conditioning (PD14-15), i.o. ethanol administration (0.33-0.66 g/kg, BALs <70 mg%) was immediately followed by CS exposure (sandpaper; trial duration, 7.5 min). The goal was to capture the onset of the ethanol postabsorptive process when BALs are rising. Control groups experienced both the CS and i.o. ethanol but in an explicit unrelated fashion (unpaired groups) or experienced only the CS (CS-only group). When tested on P16, ethanol-preexposed rats given pairings of sandpaper and postabsorptive ethanol exhibited greater preference for sandpaper than unpaired counterparts. This increased predilection was observed even compared with the CS-only group.
Strikingly corresponding results were found in a recent study that used conditioning procedures similar to those in Pautassi et al. (2008b), but delivered ethanol via vapor inhalation (Nizhnikov et al., 2009). Vapor ethanol exposure has the advantage of minimizing handling and injection-related stress associated with i.p. or i.g. administration (Pal and Alkana, 1997), and seems to be one of the instances in which ethanol exposure occurs in human infancy (Choonara et al., 1994; Puschel, 1981). Pups were preexposed to vapor ethanol on PD13, before experiencing pairings of a tactile cue (sandpaper, CS+) and ethanol's effects on PD 14-15. Aversive learning (CPA) emerged when employing relatively long conditioning trials (12 min, maximal BALs: 55 mg%). Aversive reinforcement was seemingly driven by aversive sensory stimulation associated with a high level of ethanol exposure (particularly that related to potential irritation of the respiratory system). Yet, ethanol-mediated appetitive learning (i.e., CPP) was found in rat pups given vapor ethanol in distributed conditioning trials and pre-exposed to the drug prior to training. In these animals, BALs at conditioning were low (15 mg%) and the CS+ signaled the onset of the ethanol postabsorptive process.
These results (Pautassi et al., 2008; Nizhnikov et al., 2009) helped clarify how sensory and postabsorptive effects of ethanol interact to determine ethanol-seeking and acceptance in infant rats. As previously observed in older rats (Kiefer et al., 2005), the infant appears to perceive the chemosensory features of ethanol as an aversive stimulus, a factor that may limit oral consumption of the drug. This assertion does not discount that oral ethanol can be accepted by the infant and exert positive reinforcing effects in alternative, self-administration models, particularly when high levels of intake are achieved in short periods of time (Sanders and Spear, 2007; see sections 1.4 and 4). These results also show that when the “taste barrier” is overcome, the postingestive consequences of the drug support first-order appetitive conditioning. The conclusions derived from this rat model fit well with those found with certain distinct inbred strains of mice, such as DBA/2J (D2). This strain drinks very little ethanol in the home cage, particularly when contrasted with other strains, such as C57BL/6J (Belknap et al., 1993; Camarini and Hodge, 2004). Differences in taste and olfactory reaction to the preabsorptive characteristics of ethanol have been suggested to underlie this phenomenon (Camarini and Hodge, 2004). Interestingly, when ethanol chemosensory factors are bypassed by means of systemic administration, D2 mice express robust and reliable ethanol-induced CPP (Cunningham et al., 2006a).
In both studies (Pautassi et al., 2008; Nizhnikov et al., 2009), pre-exposure to alcohol was required for the conditioned preference to emerge. Indeed, pre-exposure to ethanol has been one of the few manipulations that allows expression of conditioned place preference by ethanol in naïve, adult heterogeneous rats (Bienkowsky et al., 1995: Reid et al., 1985). As already mentioned (see section 1.3), adult heterogeneous rats generally do not exhibit appetitive learning when ethanol's postabsorptive effects are paired with distinctive tactile cues (e.g., Cunningham et al., 1993). The studies assessing the effects of ethanol pre-exposure in rats have produced conflicting results. Despite data indicating that ethanol pre-exposure promotes ethanol-induced appetitive learning (Bienkowsky et al., 1995), other data indicates a lack of effect of ethanol pre-exposure on subsequent ethanol-mediated learning (Pautassi et al., 2005). Based on mouse data, Cunningham and co-workers (2002) proposed that preexposure to ethanol may diminish the aversive effects of ethanol while leaving the appetitive effects unchanged (Cunningham et al., 2002b). Generally, however, the role of ethanol pre-exposure in the expression of ethanol reinforcement is uncertain and more work is needed to clarify the conditions in which this treatment alters ethanol reinforcement.
A recent set of studies conducted in our lab indicated that pre-exposure to alcohol is not always required for the emergence of ethanol-mediated conditioned preference in the preweanling rat (Nizhnikov et al., 2008; Nizhnikov, Pautassi, Truxell, Spear, unpublished). In most of these experiments pups were trained in a differential conditioning procedure in which a distinctive tactile CS (sandpaper) was paired with the onset of the intoxication whereas an alternative texture signaled the absence of ethanol's effects. Conditioned preference was found following 1.0 but not 2.0 g/kg ethanol and only when the drug was delivered i.g. (but not if i.p., Nizhnikov et al., 2008). Subsequent experiments (Nizhnikov, Pautassi, Truxell, Spear, unpublished) replicated this result and found that ethanol-mediated preference was inhibited by pre-treatment with either a general opioid antagonist (naloxone, 0.75 -- 1.5 mg/kg) or with antagonists specific for mu- and delta receptors (CTOP and naltrindole, respectively). These results not only underscore the importance of method of drug delivery in ethanol's hedonics but also fit well with human (Peterson et al., 2006) and animal experiments (neonate and older infant rats: Nizhnikov et al., 2006a; 2007; Arias et al; 2008; 2009; mice: Bechtholt & Cunningham 2005) suggesting that activation of the endogenous opioid system is critical for appetitive effects of ethanol. Some of these rat studies (Arias et al; 2008; 2009) will be described in the next section.
3.1.3. Motor activation as a measure of ethanol's hedonics
Motor activation induced by psychoactive drugs has been long considered an index, albeit indirect, of positive, rewarding central effects (Orsini et al., 2004: Wise and Bozarth, 1987). The theoretical postulate is that a common neurobiological factor underlies forward locomotion (also known as “psychomotor activation”) and these reinforcing effects, namely, the activation of the medial forebrain bundle. Ethanol induces robust biphasic motor effects in mice (Cunningham et al., 1993), with activation at low doses and sedation at high doses (Masur and Boerngen, 1980). These effects seem to be mediated by several neurotransmitter systems, notably the opioid, dopaminergic and glutamatergic systems (for review and references, see Pastor & Aragon, 2006). For instance, Camarini et al. (2000) found acute motor activation by ethanol (2.0 g/kg) in mice, which was counteracted in a dose-response manner by naloxone (1.0 – 3.0 mg/kg). It has been difficult to observe these results in rats, although central administration to specific brain regions have been show to exert some motor stimulating effects (Arizzi-LaFrance et al., 2006; Pastor & Aragon 2008). Generally, the most common outcome of ethanol administration in genetically heterogeneous rats is a dose–response sedative effect (Chuck et al.2006). Low ethanol doses, however, exert motor activation in rats selectively bred for high ethanol intake [i.e., alcohol-preferring (P) or Sardinian alcohol preferring rats (Waller, 1986; Agabio et al., 2001)]. Perhaps more important for the purposes of this review is a series of studies recently conducted by Arias and co-workers (2008; 2009; in press). These studies assessed acute motor effects of ethanol in preweanling heterogeneous rats (8-12 day-olds) and found results strikingly similar to those found in adult mice. Specifically, moderate and high (1.25 and 2.5 g/kg, respectively), but not low (0.5 g/kg), ethanol doses exerted motor activating effects when assessed during the onset of the intoxication (5-10 min post-administration). The highest dose, in turn, exerted sedation at 30–35 min. There were also signs of acute tolerance, with preweanlings quickly recovering from sedation within the state of intoxication, despite the fact that blood ethanol levels remained high and stable (Arias et al., 2008). A subsequent study (Arias et al., 2009) assessed the role of opioid and GABAB receptors in ethanol-mediated motor activation in infant rats. Naloxone (1.0 – 2.0 mg/kg) and baclofen (0.5 – 2.5 mg/kg) significantly attenuated the stimulating effects of ethanol, hence suggesting a modulatory role for these transmitter systems.
It is interesting to note that there are striking parallels between the time course of ethanol's motor (Arias et al., 2008) and motivational effects (Molina et al., 2006; 2007; Nizhnikov et al., 2008). Both effects seem to share a common neurobiological mechanism (i.e., the opiodergic transmitter system). This phenomenon will be further commented in the discussion section.
3.2. Can ethanol exert negative reinforcement consequences early in life?
The motivational consequences of ethanol are not restricted to its appetitive and aversive effects. Ethanol ameliorates dysphoria and reduces anxiety (Wilson et al., 2004). These anxiolytic effects modulate, through negative reinforcement mechanisms, the initiation and maintenance of drug-seeking and consumption (Koob and Britton, 1996). Assessment of these anti-anxiety-like effects in infant rats has been hindered by several factors. Techniques commonly employed in tests of adults (e.g., elevated plus maze, light-dark transition test) are not well suited for infants. The length of the training protocol usually outlasts the stage of infancy, and obvious sensory limitations of the infant hinder assessment of early anti-anxiety-like effects of ethanol (but see Iijima and Chaki, 2005, for a model based on the assessment of ultrasonic vocalizations). We attempted to address these problems by taking advantage of the highly developed associative learning abilities of the infant. Infant rats can learn to avoid a distinctive odor CS predicting aversive stimulation (e.g., Miller et al., 1989, 1991). In a first experiment (Pautassi et al., 2005a), we paired a lemon scent (CS) with high-rate i.o. infusion of a flavored tastant (US). Conditioning took place after the administration of various doses of ethanol or its vehicle. During the test session, vehicle-treated pups showed a lower preference for the lemon CS than did unpaired controls, hence exhibiting conditioned aversion. Ethanol dose-dependently affected learning acquisition. Low doses (0.25-0.50 g/kg) inhibited aversive learning; whereas infant rats given a higher dose (1.25 g/kg) showed levels of odor aversion similar to controls given vehicle. These results suggested that the affective, perhaps anxiolytic, properties of low-dose ethanol counteracted the acquisition of aversive learning. In other words, the postabsorptive effects of ethanol weakened the ability of the US to mediate conditioned aversion (Pautassi et al., 2005a). Although useful, confounding variables with this strategy precluded clear interpretation. The unconditioned effects of ethanol acted on the hedonic representation of the US, the hedonic representation of the CS, the associative link between the latter two elements, and the processing of the context where the learning experience took place. These considerations were considered when designing an alternate approach that is similar to a US revaluation procedure (Delamater & Lolordo, 1991; Holland & Rescorla, 1975).
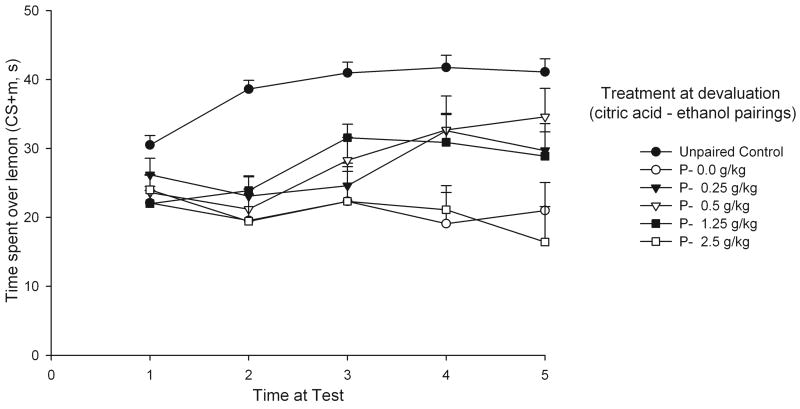
The magnitude of an aversive memory, derived from a CS-US association, is by no means fixed after initial training (Kraemer et al., 1992). Post-training pairings of the original US and alternative USs of similar or opposite hedonic value result in a heightened or ameliorated conditioned response (inflation and devaluation, respectively). This phenomenon inspired an experiment (Pautassi et al., 2006) in which 14-day-old pups were exposed first to a distinct lemon scent (CS) while stimulated with an aversive taste (citric acid, i.o., US). Twenty-four hours later, during the devaluation phase, the rats were briefly given the citric acid solution while experiencing the initial postabsorptive effects of ethanol (post-administration interval 5-10 min). A two-way odor preference test revealed that CS-US pairings resulted in strong avoidance of lemon odor (i.e., less time spent close to the lemon scent) in control pups given vehicle (no ethanol) during the devaluation phase, whereas this conditioned aversion was significantly ameliorated in subjects given ethanol (0.25, 0.5, and 1.25 g/kg, BALs 17-70 mg%) paired with citric acid flavor during this phase (Figure 2). These results indicate that ethanol reduces a conditioned fear response by acting on the rat's representation of the US, an effect presumably related to its anxiolytic effects. The revaluation paradigm also detected biphasic motivational effects of ethanol. At high doses, ethanol's ability to revalue the aversive memory changed during the course of the toxic process. Specifically, infants exhibited as much conditioned odor aversion as controls if re-exposed to citric acid 5-15 min after 2.5 g/kg ethanol, but the conditioned aversion was enhanced (i.e., inflated) if the pairing was delayed until a later post-administration interval (25-35 min). Thus, at high doses, the initial and late stages of the ethanol post-administration process differed with respect to hedonic effects, with aversive effects predominating at about 30 min post-administration.
Figure 2.
Time spent on conditioned stimulus (lemon odor), as a function of paired (P) or unpaired (control conditions) presentations of lemon odor and citric acid stimulation (aversive conditioning) and time at test (min 1, 2, 3, 4, and 5). Animals subsequently experienced citric acid after being administered with ethanol (0.25, 0.5, 1.25, or 2.5 g/kg) or with vehicle (0.0 g/kg, devaluation phase). Vertical bars represent the standard error of the means. Adapted from: Pautassi, R.M., Sanders, S., Miller, S., Spear, N., Molina, J.C., 2006. Early ethanol's anxiolytic effects assessed through an unconditional stimulus revaluation procedure. Alcohol. Clin. Exp. Res. 30, 448-459, Blackwell Publishing. Used with permission from publisher.
One could wonder if there is any evidence of ethanol's devaluating effects in ontogenetic stages other than infancy. To our knowledge, there has been no such an attempt. Becker and Flaherty (1982), however, found in adult rats results strikingly analogous to those of Pautassi et al (2006; 2007). Specifically, ethanol (0.5 g/kg, i.g.) reduced the negative contrast that occurred when rats were shifted from 32% to 4% sucrose. Negative contrast is thought to be associated with frustration and anxiety-like behavior (Becker et al., 1984).
3.2.1. Neural mechanisms underlying the early negative reinforcement effects of ethanol
Ethanol interacts with several neurotransmitters, including facilitative effects on GABAergic transmission that are frequently linked to ethanol's anti-anxiety effects (Wilson et al., 2004). Could ethanol's effects in a devaluation paradigm, demonstrated by Pautassi et al. (2006), be GABA-related? We compared the effects of ethanol and the fast-acting GABAA agonist midazolam on the expression of citric acid-induced ultrasonic vocalizations (Pautassi et al., 2007). Rat pups emit ultrasonic vocalizations when confronted with stressors, such as maternal separation (Iijima and Chaki, 2005). These vocalizations are hypothesized to reflect anxiety and can be inhibited by several compounds, including midazolam (Engel and Hard, 1987). We found similar calming effects of 0.5 g/kg ethanol and 0.09 mg/kg midazolam. The effects of these drugs were then assessed in the devaluation paradigm employed by Pautassi et al. (2006). Training was defined by pairings of lemon odor and citric acid i.o. infusions. One day later, pups were briefly stimulated with citric acid following administration of ethanol (0.5 g/kg), midazolam (0.09 or 0.18 mg/kg) or vehicle. Both vehicle- and midazolam-treated animals spent significantly less time near the lemon CS, thus expressing conditioned odor aversion. This response was completely inhibited in pups that received 0.5 g/kg ethanol. We concluded that ethanol's devaluation effects are not shared by midazolam, suggesting that these effects of ethanol may not be GABAA-mediated. Notably, ethanol at the dose tested in this study exerted appetitive effects when assessed in other behavioral preparations (Molina et al., 2006; Pautassi et al., 2008b, also see section 3.3). Therefore, a potential role for the appetitive effects of ethanol in the devaluation of the avoidance response should not be discounted.
3.3. Alternative learning preparations, more robust results: Ethanol exerts positive reinforcing effects shortly after birth and when assessed by second-order conditioning
3.3.1. Tests of ethanol reinforcement in neonatal rats
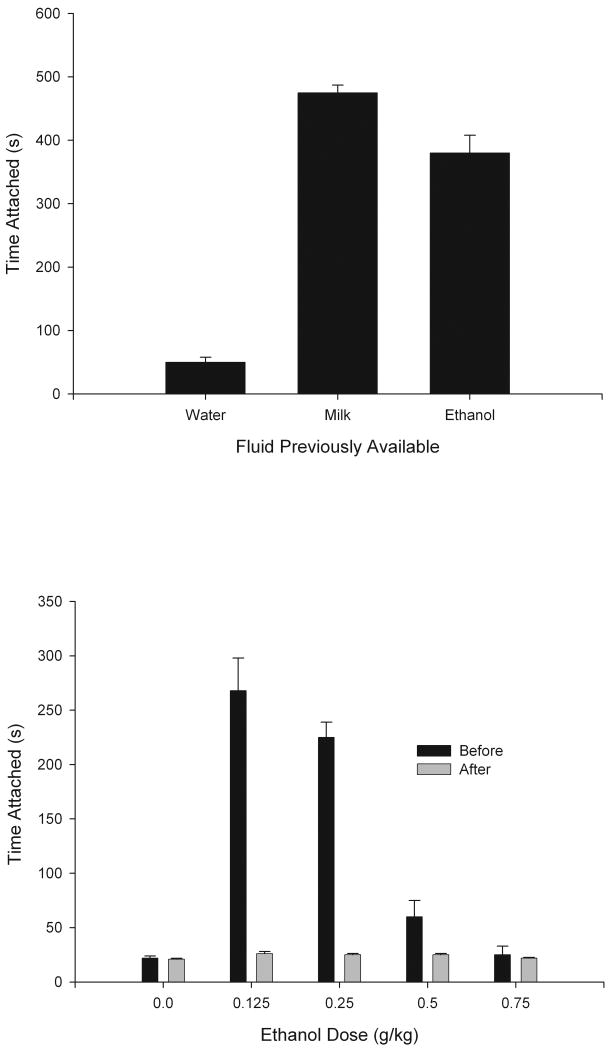
Shortly after birth, as early as 3 hours postnatally, neonatal rats readily self-administer ethanol through a latex nipple made to resemble that of a rat dam (termed a “surrogate” nipple). Ingestion from a surrogate nipple dispensing 5% or 10% ethanol over a 10-minute period results in detectable BALs of 15-25 mg% and absolute ethanol intake of about 0.5 g/kg (Cheslock et al. 2001; Varlinskaya et al., 1999). If a similar nipple that does not deliver any fluid (“empty” nipple) is given an hour later, neonates readily attach if they had previously experienced ethanol, milk or saccharin but not if they had previously experienced only an empty nipple or a nipple delivering water. It seems that ethanol endowed the empty nipple with conditioned reinforcing properties. Subsequent experiments (described below) identified the pharmacological effects of ethanol (as opposed to its taste or caloric content) as the source of reinforcement. The data have further indicated no difference in the reinforcement effects of ethanol, milk and saccharin in this circumstance (Cheslock et al., 2001; Petrov et al., 2001). To achieve more complete experimental control over the nature and contingency of the CS-US pairing, an early step was to apply a paradigm previously found effective for assessing milk reinforcement at this age (Cheslock et al., 2000). In this paradigm, pups learn that a brief (60-sec) exposure to a novel odorant predicts intraoral infusion of ethanol. Although one pairing of this kind is sufficient to increase responsiveness to an empty nipple in the presence of the odorant, significantly more conditioning is evident with 2-5 pairings. Control groups given only the CS (odorant exposure) or only the US (ethanol infusion), or both in unpaired fashion, have yielded little or no responding to an empty nipple in the presence of the odorant (Cheslock et al., 2001).
Ethanol reinforcement was found to depend on concentration of the ethanol in these neonates, but in an unexpected fashion. Although responsiveness to an empty nipple was equivalent an hour after consuming either saccharin, 5% or 10% ethanol from a similar nipple, after an interval of 24 hours elapsed there was greater responsiveness by pups previously given 10% ethanol than for those previously given 5% or saccharin. This result is notable because in these experiments, the pups ingested less 10% ethanol solution than 5% ethanol solution (although absolute intake of ethanol was equivalent in these two conditions). This finding adds to other evidence that ingestion does not always have a 1:1 correspondence to strength of reinforcement (e.g., Czachowski et al., 2001, 2005; Files et al., 1997, 1998; Ritz et al., 1994).
As mentioned, although it was clear that ethanol exerts appetitive reinforcing effects in the neonate, it was unclear whether the source of such reinforcement was the gustatory or pharmacological effects of ethanol. To minimize the role of ethanol's chemosensory components, Cheslock et al., (2001, Exp. 8) injected (i.p.) neonates with an arbitrary, small amount of ethanol (10 μl of 5% ethanol) followed 5 min later by exposure to a nipple delivering water (thus pairing the nipple with the postingestive effect of ethanol). In the control condition the order of nipple and i.p. ethanol was reversed. The results indicated reinforcement from i.p. ethanol, but required verification in an extensive subsequent study (Petrov et al., 2003) that included several control experiments in addition to fine-grain variation in ethanol dose (0.0, 0.125, 0.25, 0.5, or 0.75 g/kg). The lower two doses (yielding BALs of 15 to 35 mg%) induced robust appetitive conditioning, indicated by greater nipple attachment at test in paired (“before”) compared with unpaired (“after”) animals (Fig. 3, bottom panel). The basis of ethanol reinforcement in neonatal rats appeared to be primarily pharmacological. To test this further by eliminating hematogenic detection of gustatory or olfactory cues from i.p. ethanol, Nizhnikov et al. (2006c, 2007) determined significant ethanol reinforcement in neonates from central ethanol delivered intracisternally. It therefore seems clear that the pharmacological effects of ethanol are sufficient for appetitive reinforcement in neonatal rats, at central doses as low as 25 mg% (Nizhnikov et al., 2006c).
Figure 3.
Upper panel: Neonatal responsiveness to an empty surrogate nipple (total time attached, expressed in seconds) following exposure to the surrogate nipple providing water, milk, or 5% ethanol. Vertical bars represent the standard error of the means (SEM). Adapted from: Cheslock, S.J., Varlinskaya, E.I., Petrov, E.S., Silveri, M.M., Spear, L.P., Spear, N.E., 2001. Ethanol as a reinforcer in the newborn's first suckling experience. Alcohol. Clin. Exp. Res. 25, 391-402, Blackwell Publishing. Used with permission from publisher. Lower panel: Time attached (seconds) to an empty surrogate nipple in pups pre-exposed to the surrogate nipple while experiencing the postaborptive effects of ethanol (0.125, 0.25, 0.5, or 0.75 g/kg, i.p). Control pups (“After” condition) were given ethanol administration 5 min after nipple exposure. Vertical bars represent the standard error of the means (SEM). Adapted from: Petrov, E.S., Varlinskaya, E.I., Spear, N.E., 2003. Reinforcement from pharmacological effects of ethanol in newborn rats. Alcohol. Clin. Exp. Res. 27, 1583-1591. Blackwell Publishing. Used with permission from publisher.
Toward determining the neural mechanisms of this phenomenon, Nizhnikov et al. (2006b) demonstrated that systemic administration of μ and κ antagonists (d-Pen-Cys-Tyr-d-Trp-Orn-Thr-Pen-Thr-NH2 [CTOP] and norbinaltorphimine, respectively) completely blocked ethanol's reinforcing effects, suggesting the involvement of the opioid neurotransmitter system. The central reinforcing effects of ethanol were similarly blocked by catalase inhibitors (i.e., (sodium azide), indicating that products derived from ethanol metabolism in the brain, such as acetaldehyde, may underlie expression of this phenomenon (Nizhnikov et al., 2007). Interestingly, ethanol's appetitive effects in the nipple technique can be increased by prenatal ethanol exposure. Nizhnikov et al. (2006a) found that neonates exposed to ethanol in uterus (1 g/kg, gestational days 17-20) showed robust reinforcement when employing 0.25, 0.5, or 0.75 g/kg ethanol, demonstrating a broadening of the effective reinforcing doses of ethanol. Only the intermediate dose provided effective ethanol reinforcement for control pups not prenatally exposed to ethanol.
3.3.2. Second-order conditioning procedure
One advantage of using an immature (infant) rat model is that infants possess robust higher-order stimuli processing capabilities. For example, 2-week-old rats encode stimuli of different sensory modalities by taking into account their so-called amodal properties, including their common affective and intensity properties (Kraebel and Spear, 2000; Spear and Molina, 1987; Spear and McKinzie, 1995). These learning and memory abilities are expressed early during development as demonstrated by sensory preconditioning (Chen et al., 1992) and second-order conditioning (Miller et al., 1990).
Recent experiments (Molina et al., 2006, 2007) have provided robust evidence that ethanol exerts appetitive effects during the second week of life, as indexed by a test of second-order conditioning. With reinforcers other than ethanol, this test had been known to reveal conditioned place preference in infant rats (Miller et al., 1990) and conditioned approach in adult pigeons (Rescorla, 1980) when inherent weakness in the circumstance of first-order conditioning otherwise precluded evidence of conditioning. Molina et al. (2006, 2007) suspected a similar weakness in tests of CPP after primary conditioning with ethanol in infant rats, hence their application of second-order conditioning. Among several possibilities was the likelihood that conventional circumstances of primary conditioning with ethanol as a US (paired with, for example, intraoral stimulation of water) yield conditioned responses that compete with selection of the correct alternative in the CPP test. Molina et al. (2006; 2007) identified one candidate --conditioned wall climbing -- that might conceivably preclude expression of CPP.
To test ethanol-mediated second-order conditioning, experiments used relatively low (0.25 and 0.5 g/kg) or relatively high (2.0 g/kg) ethanol doses (Molina et al., 2006). The main question was whether a gustatory CS paired with ethanol's postabsorptive effects would indicate reinforcement by ethanol. This question was answered not through direct responsiveness to the taste but rather by assessing whether the ethanol-paired taste would serve as an effective second-order reinforcer to transfer motivational information to a tactile cue.
In the first of these studies (Molina et al., 2006), 14-day-old rats received ethanol (0.0, 0.25, or 0.5 g/kg, i.g.) paired with i.o. sucrose (CS1, first-order conditioning phase). Sucrose was delivered during the onset of ethanol's effects (5-20 min after ethanol intubation) in an intermittent, discrete fashion. During the second-order conditioning phase 24 hours later, pups were briefly re-exposed to the taste while experiencing a rough texture (sandpaper). When tested in a tactile preference test, control pups that received pairings of only sucrose and 0.0 g/kg ethanol (water) spent roughly 50% of the test on either texture: sucrose-sandpaper pairings alone experienced during the second conditioning phase were not sufficient to alter sandpaper preference during the test session. Yet pups given the sucrose-ethanol pairing prior to sucrose-sandpaper had 60-70% preference for the sandpaper, significantly more than their control counterparts. This indicated CPP and ethanol reinforcement. A follow-up experiment confirmed these results, even when pairing, as a test of the generality of this CPP, a relatively neutral gustatory stimuli (water) with ethanol (0.5 and 2.0 g/kg) during the first conditioning phase. (Molina et al., 2006).
Recently, the second-order conditioning procedure was modified to examine the apparent biphasic nature of ethanol's motivational effects (Molina et al., 2007). In this case “biphasic” refers to initial appetitive effects followed by aversive consequences as BALs reach peak values (e.g., Pohorecky, 1977). This was accomplished by varying the post-administration time interval in which conditioning takes place. Fourteen-day-old rat pups received pairings of i.o. water (CS1) and ethanol (US, 0.5 or 2.0 g/kg) during an early (5-20 min) or late (30-45 min) post-administration interval. Phase 2 followed on PD15 and comprised pairings of sandpaper (CS2) and water. Sandpaper preference registered during the 5-min test is shown in Fig. 4. Animals given pairings of the CS1 and the early or late effects of low-dose ethanol (0.5 g/kg; BAL 40 mg%) exhibited greater conditioned preference for sandpaper than did the (unpaired) controls. A similar outcome was observed in animals receiving a higher dose of ethanol (2.0 g/kg; BAL 157 mg%) and conditioned during the onset of intoxication (5-20 min). In contrast, an aversion for sandpaper emerged when the CS1 was paired with the late stage of the toxic process induced by 2.0 g/kg ethanol (BAL 200 mg%). The experiment indicated that, at high doses, the initial and later stages of ethanol's effects seem to differ in their hedonic value, a result consistent with data gathered by means of alternative experimental procedures (Pautassi et al., 2002a; 2006).
Figure 4.
Time spent over sandpaper and a novel surface (seconds) as a function of intraoral water paired with the early (5-20 min postadministration) or late (30-45 min postadministration) postabsortive effects of ethanol (0.5 or 2.0 g/kg) and time at test (1-5 min). Unpaired control groups were exposed to intraoral water and ethanol's effects, but in an explicitly unrelated fashion. In this study, intraoral water was subsequently paired with sandpaper. Vertical bars represent the standard error of the means (SEM). Adapted from: Molina, J.C., Pautassi, R.M., Truxell, E., Spear, N., 2007. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol 41, 41-55, Elsevier. Used with permission from publisher.
Although beyond the scope of this review, the second-order conditioning procedure has been recently employed to assess appetitive reinforcement by ethanol in ontogenetic stages other than infancy. Ethanol (0.5-2.0 g/kg, i.g.) induced appetitive effects in adolescent, but not adult, rats (Pautassi et al., 2008c).
4. Ethanol-mediated operant responding in infant rats
The experiments thus far reviewed employed a type of training in which salient stimuli (CSs) are paired with the effects of experimenter-administered ethanol. Motivational effects of ethanol were then indexed by exposing the animal to the CS. These Pavlovian conditioning procedures are rightfully valued for their analytical power (Cunningham, 1993). They allow systematic variation of dose, post-administration time, and stimulus intensity (Rescorla, 1988). However, they lack face validity when contrasted with the typical human pattern of ethanol self-administration. Compelling evidence of ethanol's positive reinforcing effects in laboratory animals can also be obtained by having animals (e.g., rats, mice, primates) work or emit a particular behavior to gain access to oral ethanol. Assessment with operant procedures is important for understanding the seeking behaviors associated with ethanol availability and the actual self-administration of the drug (Tabakoff and Hoffmann, 2000).
In adult rats, implementation of operant procedures to assess the motivational effects of ethanol usually involves lever-pressing for small quantities of ethanol dispensed in a reservoir (Samson et al., 1988). Lever-pressing leading to physiologically relevant BALs can be achieved, but requires a rather lengthy protocol that includes rats having previous access to oral ethanol for several days, sometimes mixed with sweeteners (e.g., Samson et al., 2004) or the induction of dependence previous to the actual operant testing (e.g., Roberts et al., 2000). Naive (i.e., without acclimatation to the sensory properties of ethanol) heterogeneous rats drink little ethanol, particularly as the concentration increases. Not surprisingly, rate of lever-pressing for ethanol in these animals can be quite low. Moreover, because naive heterogeneous rats usually distribute their operant responses over the time span of training sessions (30-60 min), they may never reach BALs sufficient to induce central reinforcing effects. The interacting constraints of low palatability and irrelevant levels of intoxication have posed difficulties in the development of an animal model of ethanol self-administration (Stewart et al., 1988). Several procedures have been devised to address these problems. Some early work reported successful initiation of ethanol-self administration following stressor exposure. This approach was later dismissed due to contradictory results and concerns about the procedure's apparent inability to discriminate between the positive and negative reinforcing effects of ethanol (for review, see Pohorecky, 1981; Roske et al., 1994). In a newer and widely employed procedure (Samson, 1986), rats initially learn to respond for a sweet solution. As training progresses, the rats are offered this solution with progressively decreasing sweetness mixed with progressively increasing concentrations of ethanol. After several weeks, rats readily lever-press for ethanol, even at high concentrations (e.g., 40%). Prolonged pre-exposure to ethanol also serves as a means of increasing later ethanol self-administration (Camarini and Hodge, 2004). Fidler et al. (2006) subjected rats to passively infused ethanol or its vehicle for up to 6 days and then trained these animals in an operant procedure defined by the contingency between licking a flavored solution and i.g. infusion of ethanol (delivered by means of a gastric fistula). Ethanol-preexposed rats, but not their ethanol-naive counterparts, showed substantial self-administration of ethanol (4-7 g/kg/day).
Some similarities emerge among the operant ethanol self-administration paradigms successfully developed for adult rats, including prolonged training or passive pre-exposure, complex motor requirements often combined with high-rate responding criteria, concurrent access to an alternative positive reinforcer (e.g., a sweet solution), and testing under stressful conditions. For example, Fidler et al. (2006) showed that ethanol can support substantial operant responding when employing procedures that (i) likely induce tolerance to the drug's aversive postingestive effects or sensitization to its rewarding effects due to the procedure's length, and (ii) minimize the role of the aversive chemosensory properties of the drug.
The employment of adult operant models of ethanol self-administration in infant rats has been hindered by the animals' rapid development and limited sensory and motor abilities. Only a few successful procedures have been created to assess whether rat pups will self-administer ethanol in an operant paradigm. The first of such attempts was by Dominguez et al. (1993), who employed an operant procedure [originally devised by Johanson and Hall (1979) to assess the reinforcing effects of milk] in which 3-4, 9-10, and 15-16-day-old pups were placed for long periods in a container equipped with a small lever. Application of a small amount of pressure to this lever (achieved by head probing or forelimb movements) yielded i.o. infusion of milk or a mixture of milk and ethanol. Pups were maternally deprived for 12 h prior to training, and establishment of operant responding required between 3 and 6 h. Interpretation of these data was somewhat complicated because of the pups' considerable loss of weight and possibly deprivation-induced stress as training progressed. Despite these methodological constraints, Dominguez et al. (1993) replicated the basic findings of Johanson and Hall (1979), including heightened responding in pups reinforced with milk compared with yoked controls. Lever pressing was much less when the milk contained 6% ethanol, although still greater than yoked controls. This pattern of responding remained across all ages tested. Ponce et al. (2006) found similar results in two-week-old rats. These animals rapidly learned to respond for i.o. sucrose, but mixing the sweet tastant with ethanol, either from the very start of training or when using a progressive substitution schedule, resulted in a significant drop in operant responding.
Lever-pressing can be considered a complex motor skill and, in fact, pups in the Dominguez et al. (1993) study required several hours to establish stable levels of responding. In a more recent study (Pautassi et al., 2008d; see Fig. 5, upper panel), we employed a much simpler operant response, namely contacting a touch-sensitive disk placed on the floor of the operant cage. The rationale behind this procedure was that this high-frequency behavior might result in high levels of intake in shorter periods of time, thus allowing ethanol's postingestive effects to play a role in maintaining operant responding. This was not the case, however. No evidence of ethanol's positive reinforcing effects was found (highest mean level of drug intake: 0.27 g/kg/20 min). In fact, sensor-touching was significantly less in pups reinforced with ethanol (3 or 5%) than in yoked controls given non-contingent drug infusions. As shown in Fig. 5 (upper panel), decreased responding in paired compared with yoked pups was found even during extinction sessions (i.e., when the reinforcer was withheld). Operant responding appeared to be driven by the pre-absorptive properties of ethanol (i.e., odor or taste), which apparently were aversive (Pautassi et al., 2008d).
Figure 5.
Upper panel: Mean number of target operant responses (sensor contacts) during training (postnatal days 14–16, PD 14-16) and extinction (PD 17) sessions as a function of the contingency between behavior and delivery of the reinforcer [target behavior either paired or unpaired (yoked control) with reinforcer availability]. The vertical dashed line in the y-axis indicates that PD16 was the last day that ethanol was delivered. Adapted from: Pautassi, R.M., Truxell, E., Molina, J.C., Spear, N.E., 2008. Motivational effects of intraorally-infused ethanol in rat pups in an operant self-administration task. Physiol. Behav. 93, 118-129, Elsevier. Middle panel: Mean number of target operant responses (sensor contacts) in neonates rats during a 15-minute session as a function of the contingency between behavior and delivery of the reinforcer [target behavior either paired or unpaired (yoked control) with availability of 0.75, 1.5, or 3.0% v/v ethanol]. Adapted from: Bordner, K.A., Molina, J.C., Spear, N.E., 2008. Analysis of ethanol reinforcement in 1-day-old rats: assessment through a brief and novel operant procedure. Alcohol. Clin. Exp. Res. 32, 580-592, Blackwell Publishing. Lower panel: Mean number of target operant responses (nose-pokes) during daily training sessions (PD 14–17), as a function of the contingency between behavior and delivery of the reinforcer [target behavior either paired or unpaired (yoked control) with availability of water or 3.75% v/v ethanol]. Adapted from: Ponce, L.F., Pautassi, R.M., Spear, N.E., Molina J.C., 2008. Ethanol-mediated operant learning in the infant rat leads to increased ethanol intake during adolescence. Pharmacol. Biochem. Behav. 90, 640-650, Elsevier. In all figures vertical lines represent standard errors of the mean. All figures used with permission from publisher.
These studies (Dominguez et al., 1993; Ponce et al., 2006; Pautassi et al., 2008d) provide further evidence that pups reject the orosensory attributes of i.o. ethanol. Any attempt to establish i.o. ethanol as a successful reinforcer in an operant setting will need to overcome this aversion. Two recent studies, however, have succeeded in inducing substantial ethanol-mediated responding in rat pups. A close examination of these studies should provide clues to the conditions that facilitate the emergence of ethanol's reinforcing properties. The first study (Bordner et al., 2008) employed a derivative of the surrogate nipple technique (described in section 3.3.1). These researchers noted that suckling-related behaviors (i.e., forelimb or head movements) occurred during both attachment to a surrogate nipple providing milk and when exposed to a milk-related CS. They hypothesized that neonates may associate the emission of suckling-related behaviors with the availability of milk. To test this hypothesis, one-day old rats were tested while resting in a supine position, secured by a vest. The vest kept the animal in a fixed positioned while allowing head or forelimb movements. During 15-min operant sessions, execution of these behaviors activated a touch-sensitive sensor located just above the head of the animal and resulted in i.o. infusion of a given solution. Pups readily learned to gain access to milk or low concentrations of ethanol (1.5 or 3%; Fig. 5, middle panel). Responding in animals provided with access to 5, 7.5, or 10% ethanol was higher than in unpaired controls but still much lower than responding for milk or lower ethanol concentrations. Operant responding for the low concentrations of ethanol was associated with BALs in the 15-30 mg% range. Increased responding in paired pups provided with low-concentrations of ethanol may be attributable to nonspecific activating effects of the drug. That is, operant responding while ingesting ethanol might conceivably exacerbate the normal repertoire of body movements in these animals. This possibility appears to be unlikely, however, given the substantial responding that continued during extinction. Sensor-touching in paired animals not only persisted but also showed some characteristics commonly associated with the expression of operant learning, such as an initial increase in overall responding compared with levels found during acquisition (i.e., a “spike” or “rebound effect”, also found in a similar study with milk reinforcement, Arias et al., 2007).
The results by Bordner et al. (2008) are consistent with those obtained by means of the surrogate nipple technique (Cheslock et al., 2001; Petrov et al., 2001). Both techniques indicate great sensitivity to ethanol's reinforcing effects in the neonatal rat. The ease with which these effects are observed at this early age suggests that sensitivity to ethanol's reinforcing effects might be greater shortly after birth before declining during subsequent development. A similar pattern has been suggested for alcohol intake when measured by a seemingly voluntary intake preparation (consumption-off-the-floor; Lee et al., 1998). Unselected infant rats exhibit a high initial acceptance of ethanol that increased during the second postnatal week, then decreases slowly thereafter to the low levels of acceptance observed in non-initiated adults (Sanders and Spear, 2007; Truxell et al., 2007). However, the nipple technique and the operant derivative of Bordner et al. (2008) may favor the expression of appetitive effects of ethanol by using more appropriate, age-specific and ecologically compatible procedures than those employed for older rats. Some support for the latter possibility derives from a recent study by Ponce et al. (2008). Infant rats were given a brief non-reinforced exposure to ethanol or its vehicle prior to operant conditioning. Daily operant sessions (20 min) were conducted from PD14 to PD17. The target behavior was nose-poking, a very simple behavior present throughout most of the ontogeny of the rat. Nose-poke responding resulted in i.o. infusion of a sizable amount of ethanol (25 μl, 3.75 or 7.5%) or its vehicle. Initial responding was very low (five or fewer nose-pokes per session). However, by the end of training on PD17, pups reinforced with ethanol showed greater frequency of responding and shorter latencies to execute the target behavior than infants reinforced with water or yoked controls, a phenomenon that was particularly clear in ethanol-preexposed animals (Ponce et al., 2008; Fig. 5, bottom panel). A subsequent experiment replicated these results and indicated that infant operant responding for ethanol resulted in detectable BALs (25-48 mg% on PD17). Moreover, infant learning mediated by ethanol affected voluntary intake of the drug during adolescence. Adolescent rats (PD 33-36) that had been trained to nose-poke for 3.75% ethanol during infancy drank more ethanol, assessed in a two-bottle choice test, than counterparts reinforced during infancy with water or yoked controls.
A common denominator between Bordner et al. (2008) and Ponce et al. (2008) is reliable operant conditioning when utilizing low concentrations of ethanol, but decreasing operant responding as the concentration increases. Additionally, in both studies, responding for ethanol was associated with very similar BALs (15-50 mg%; see below). Both techniques eliminate the need for shaping procedures or long acquisition phases in favor of shorter training sessions and relatively simpler (compared with lever-pressing) and age-specific target behaviors.
5. Summary, conclusions, and closing comments
Alcohol use and abuse are complex phenomena resulting from the interplay of genetic (Ducci and Goldman, 2008) and environmental variables (Spear and Molina, 2005). Epidemiological and animal preclinical research indicates that early prenatal and postnatal exposure to ethanol increases the likelihood of later alcohol problems. Exposure to alcohol in the womb ranks among the strongest predictors of alcohol consumption during adolescence and early adulthood (Baer et al., 1998, 2003; Streissguth, 2007; Yates, 1998). Animal models (Lopez and Molina, 1999; Spear and Molina, 2005) also indicate heightened ethanol consumption following early pre- or postnatal ethanol exposure. Spear and Molina (2005) posited an explanation for this phenomenon based on learning mechanisms. Their simplest account invoked a passive pre-exposure effect. Under the “mere exposure” hypothesis (Spear and Molina, 2005), non-reinforced exposure to ethanol leads to increased acceptance of the drug, but they ultimately proposed that early exposure to ethanol results in associative conditioning (Chotro et al., 2007; Abate et al., 2008). The young organism learns that chemosensory properties of ethanol (e.g., taste, flavor) or other stimuli present in the context of ethanol administration predict reinforcing postabsorptive effects of the drug, a process leading to heightened ethanol seeking and consumption later in life.
The present review is not intended to assess the associative hypothesis linking early contact with ethanol and later affinity for the drug or to establish a model for alcohol abuse or dependence in developing rats. The objective of this review is much narrower—to describe ethanol's motivational effects in the rat during the developmental window between PD0 and PD21, a period considered infancy. However, the present review did provide support for an accessory hypothesis of the theoretical account of Spear and Molina (2005), namely that ethanol can exert reinforcing effects in neonates and infants when tested in contemporary animal models. These affective consequences go beyond the well-known ability to associate gustatory stimuli with ethanol-mediated malaise. Furthermore, the results of Ponce et al. (2008) are consistent with the directional hypothesis of greater affinity for ethanol following early motivational learning with the drug. These researchers found that operant ethanol self-administration in infancy increased drug consumption during adolescence, a significant result even when compared with subjects given equivalent drug exposure during infancy but in a passive manner that required no operant response.
A summary of the studies analyzing ethanol-mediated motivational learning in infants can be found in Table 1. We described the ease with which ethanol intoxication results in CTA. Table 1 briefly describes these studies. When ethanol's postabsorptive effects are made contingent upon a taste, the infant rat readily expresses ethanol-related memories characterized by an aversive component (Hunt et al., 1990, 1991). At high doses, emetic-like effects appear to underlie this associative process (Arias et al., 2007). Infants acquire ethanol-mediated CTA at lower doses and fewer CS-US trials (0.4-0.5 g/kg, one or two trials; Pautassi et al., 2005b; Hunt and Spear, 1989) than older rats. The younger animals also appear more resistant than mature animals to the ameliorating effects of ethanol pre-exposure on later CTA mediated by the drug (Pautassi et al., 2005b; Pueta et al, 2005; but see Arias and Chotro, 2006b).
Table 1. Studies assessing motivational effects of ethanol in heterogeneous infant rats (i.e., postnatal days 1 to 21).
| Reference | Test age (days) | Procedure | Ethanol Dose or Level of intoxication | Outcome | Additional Details |
|---|---|---|---|---|---|
| Molina and Chotro, 1989 | 12 | CTA (see details) | 3.0 g/kg., i.g. | Reduced ethanol consumption | CS: hematogenic detection of gustatory/olfactory ethanol cues |
| Molina et al., 1989 | 12 | CTA (see details) | 3.0 g/kg., i.g. | Odor aversion | CS: hematogenic detection of gustatory/olfactory ethanol cues |
| Hunt et al., 1990 | 16 | CTA | 0.4, 1.2 g/kg, i.g. | Taste aversion | US reinstatement at test |
| Hunt et al., 1991 | 10, 16 or 20 | CTA | 1.6, 2.4 and 3.0 g/kg, i.g. | Taste aversion | Ontogenetic differences in CTA expression |
| Dominguez et al., 1993 | 3-4, 9-10, and 15-16 | Operant conditioning with milk alone or milk plus ethanol | --------- | Responding for milk but less for milk plus ethanol | Reinforcers were delivered i.o. following lever-pressing |
| Hunt et al., 1993 | 22 | CTA | 2.5 g/kg i.g. | Odor aversion | CS: hematogenic detection of gustatory/olfactory ethanol cues |
| Molina et al., 1996 | 12 | Tactile Conditioning | 2.0 g/kg i.g. | Tactile aversion | CS at peak BALs |
| Lee et al., 1998 | 13-14 | Tactile/odor conditioning | 1.32 – 4.5 g/kg | Tactile and odor aversion | US (ethanol) was self-administered |
| Abate et al., 2001 | 16 | CTA | 1 g/kg i.g. | Taste aversion | CS: cineole (eucalyptus oil) |
| Cheslock et al., 2001 | 1 | Classical Conditioning with surrogate nipple | 20-30 mg% | Appetitive Conditioning | US (ethanol) was self-administered |
| Petrov et al., 2001 | 1-2 | Classical Conditioning with surrogate nipple | --------- | Appetitive Conditioning | US (ethanol) was self-administered |
| Petrov et al., 2003 | 1 | Classical Conditioning with surrogate nipple | 0.125, 0.25, 0.50, 0.75 g/kg, i.p | Appetitive Conditioning | Effect selective for low dose ethanol (0.125 – 0.25 g/kg, i.p.). |
| Pautassi et al., 2005a | 15 | Aversive Odor Conditioning | 0.0., 0.25., 0.5, 1.25 g/kg. | Ethanol inhibits learning acquisition | Effect selective for low dose ethanol (0.25 – 0.5 g/kg). |
| Pautassi et al. 2005b | 16 | CTA | 0.5, 2.5 g/kg i.g. | Taste aversion | No effect of drug pre-exposure |
| Pueta et al., 2005 | 16 | CTA | 0.5, 1.0, 2.0 g/kg i.g. | Taste aversion | CTA at 1.0 or 2.0 g/kg unaffected by in uterus or nursing experience with ethanol |
| Arias and Chotro, 2006a | 9-16 | CTA | 3.0 g/kg i.g. | Taste aversion and preferences | Ontogenetic differences in CTA expression |
| Arias and Chotro, 2006b | 14-17 | CTA | 3.0 g/kg i.g. | Taste aversion | Attenuated by in uterus ethanol |
| Molina et al., 2006 | 15 | Second-order conditioning | 0.25, 0.5, 2.0 g/kg, i.g. | Tactile preferences | Behavioral activation was detected on CS1-CS2 transfer phase. |
| Nizhnikov et al., 2006a | 1 | Classical Conditioning with surrogate nipple | 0.25, 0.5, 0.75 g/kg | Appetitive Conditioning | Greater conditioning after in uterus ethanol experience |
| Nizhnikov et al., 2006b | 1 | Classical Conditioning with surrogate nipple | --------- | Appetitive Conditioning | μ and κ antagonists blocked conditioned reinforcement |
| Nizhnikov et al., 2006c | 1 | Classical Conditioning with surrogate nipple | 25 – 400 mg%, central administration | Appetitive Conditioning | Effect selective for concentrations between 25 to 200 mg% |
| Pautassi et al., 2006 | 15 | US devaluation after aversive conditioning | 0.25, 0.25, 0.5, 1.25, 2.5 g/kg, i.g. | Reduced aversive conditioning | Effect selective for low dose ethanol (0.25 – 1.25 g/kg). |
| Ponce et al., 2006 | 14-17 | Operant conditioning with sucrose alone or sucrose + ethanol | --------- | Responding for sucrose, less for sucrose+ ethanol | Reinforcers were delivered i.o. following nose pokes. |
| Arias et al., 2007 | 14-15 | CTA | 2.5 g/kg i.g. | Taste aversion | Inhibited by D2 antagonist |
| Chotro and Arias, 2007 | 9-16 | CTA | 3.0 g/kg i.g. | Flavor aversion and preferences | Ethanol-flavor learning inhibited by opioid antagonism. |
| Molina et al., 2007 | 15 | Second-order conditioning | 0.5, 2.0 g/kg, i.g. | Tactile preferences and aversion | Outcome dependent on timing of conditioning (CS paired with early or late ethanol's effects) |
| Nizhnikov et al., 2007 | 1 | Classical Conditioning with surrogate nipple | 25 – 200 mg%, central administration | Appetitive Conditioning | Catalase inhibition blocked ethanol reinforcement |
| Pautassi et al., 2007 | 15 | US devaluation after aversive conditioning | 0.5 g/kg, i.g. | Reduced aversive conditioning | Effect not shared by midazolam (0.09 or 0.18 mg/kg) |
| Arias et al., 2008 | 13 | Test of motor activation | 0.5, 2.5 g/kg, i.g. | Stimulant effect at 5-10 min, sedation at 30-35 | Effects observed following 2.5 but not 0.5 g/kg. Acute tolerance was also found |
| Bordner et al., 2008 | 1 | Operant conditioning | 15-30 mg% | Responding for ethanol | Greater effect for low ethanol concentrations (1.5 - 3% v/v). |
| Nizhnikov et al., 2008 | 16 | Tactile Conditioning | 1.0 – 2.0 g/kg, i.g. or i.p. | Tactile preference | Preference found only after intragastric intubation of 1.0 g/kg |
| Pautassi et al., 2008b | 16 | Tactile Conditioning | 0.33 – 0.66 g/kg i.o. | Tactile preference and aversions | Outcome dependent on CS predicting chemosensory or postabsorptive effects of ethanol |
| Pautassi et al., 2008d | 14-17 | Operant Conditioning | 0.12- 0.20 g/kg | Reduced operant responding | Ethanol delivered i.o. following sensor-touching |
| Ponce et al., 2008 | 14-17 | Operant Conditioning | 25- 48 mg% | Responding for ethanol | Effect facilitated by ethanol preexposure (PD13) |
| Arias et al., 2009 | 12 | Test of motor activation | 2.5 g/kg, i.g. | Stimulant effect at 5-10 min | Naloxone and Baclofen blocked the stimulant effect |
| Nizhnikov et al., 2009 | 16 | Tactile Conditioning | 15 – 55 mg%, induced by vapor inhalation | Tactile preference and aversions | Outcome dependent on CS predicting chemosensory or postabsorptive effects of ethanol |
PD, Postnatal Day; CTA, Conditioned Taste Aversion; CS, Conditioned Stimulus; US, Unconditioned Stimulus; BALs, Blood alcohol levels; i.g. Intrastric; i.o. Intraoral; i.p. Intraperitoneal. Unpublished experiments mentioned in-text are not included in this table.
We then discussed studies of conditioned place preference mediated by tactile conditioning (see table 1). Initial reports (Molina et al., 1996; Lee et al., 1998) were consistent with adult studies in finding ethanol-mediated conditioned aversions. But subsequent work (Pautassi et al., 2002a; Pautassi et al., 2008b) raised the possibility of ethanol-mediated conditioned tactile preferences. These non-aversive effects of ethanol were found when conditioned stimuli signaled the onset of the toxic process (Pautassi et al., 2002a) or when drug dosage was kept low and the apparent aversive stimulation associated with the i.o. route was minimized (Pautassi et al., 2008b). Nonreinforced exposure to ethanol before conditioning also facilitated early expression of ethanol's appetitive effects (Pautassi et al., 2008b; Nizhnikov et al., 2009), possibly by inducing tolerance to the drug's aversive effects or by sensitizing the animal to ethanol's positive reinforcing effects. Further evidence of ethanol's appetitive properties was found by a second-order conditioning procedure. Pairings of ethanol and a taste stimulus allowed the latter cue to transfer appetitive information to a distinctive surface (Molina et al., 2006, 2007). Second-order conditioning appears to be highly sensitive to ethanol's hedonic effects and can be easily transferred to ontogenetic stages other than infancy. Implementation of techniques with these features has long been claimed as a priority in the field of drug abuse research (Witt, 1994). Recent studies (Pautassi et al., 2008c) have employed this procedure in adolescent and adult rats to assess conditioned reinforcement mediated by ethanol.
This review sheds light on an interesting mechanistic issue, namely the apparent biphasic nature of ethanol's motivational effects. Biphasic effects of ethanol on neuroendocrine, autonomic, and motor parameters have been extensively described in mature rats (Pohorecky, 1977). However, not until recently has similar evidence been reported with regard to ethanol's motivational effects, and these studies were mainly conducted in mouse models (Cunningham and Prather, 1992). A common denominator across the experiments presented in the present review is that ethanol's hedonic effects vary across and between drug doses as a function of the course of ethanol intoxication. Ethanol-mediated aversion is more common at doses greater than 1.0 g/kg and when CS exposure is delayed until later post-administration intervals characterized by peak BALs (Pautassi et al., 2002a, b, 2005b; Hunt et al., 1990, 1991). In contrast, expression of alternative motivational consequences is associated with procedures that use relatively low ethanol doses or capture the onset of intoxication when BALs are still rising (Pautassi et al., 2002a, 2008b; Molina et al., 2006, 2007). These results are consistent with previous studies with mice indicating that a short trial duration (5 min) is more effective for inducing CPP than longer trial durations (i.e., 60 min; Cunningham & Prather, 1992; Risinger & Cunningham, 1992).
The results obtained through second-order conditioning (Molina et al., 2006, 2007) and ethanol-induced motor activation (Arias et al., 2008) clearly illustrate the biphasic nature of ethanol during infancy. Second-order appetitive conditioning resulted when using several combinations of dose and CS-US timing. However, conditioned aversion was also found in the same circumstances when the target CS was presented 25 min following administration of a relatively high dose (2.0 g/kg). Assessment of negative reinforcement by ethanol (Pautassi et al., 2005a, 2006, 2007) provides similar results. Low-dose (0.25-1.25 g/kg) but not high-dose (2.5 g/kg) ethanol weakened the expression of aversive conditioning. In fact, the delayed pharmacological effects of the latter dose exerted aversive effects, reflected in an augmented avoidance response at test (Pautassi et al., 2006). Furthermore, conditioned aversion mediated by high-dose ethanol was found following CS presentation at the peak of the blood ethanol curve (dose: 2.5 g/kg; Pautassi et al., 2002a, b). This conditioned aversion was dramatically suppressed when tested under the simultaneous presence of a tactile CS that had signaled the onset of intoxication. Employing a similar dose and infant rats of about the same age, Arias et al. (2008) found ethanol-induced psychomotor activation during the onset of the intoxication (5-10 min) but sedation if the test was delayed until postadministration time 30-35 min. In summary, these results support the hypothesis that the ascending and descending limbs of the blood ethanol curve are associated with different hedonic effects. Under this framework, the appetitive properties of ethanol may be present during the ascending limb of the blood ethanol curve but disappear or are counteracted by aversive effects as BALs peak and then begin to fall. This theory fits well with human research indicating that differential sensitivity to ethanol's activating and depressing effects might constitute a biological marker for predisposition to alcohol abuse (e.g., King et al., 2002). Subjects who are at risk for alcohol problems are hypothesized to be more sensitive to the rewarding effects of ethanol observed during the ascending limb of the blood ethanol curve, but less sensitive to the negative effects of ethanol that occur during the descending limb (Conrod et al., 2001; Newlin and Thompson, 1999).
The review also underscores the intrinsic value of ontogenetic analysis in the search for mechanisms underlying the affinity for ethanol and ethanol reinforcement. Specifically, future studies could benefit from the use of genetically heterozygous, developing rats as an experimental model for uncovering determinants of alcohol affinity and reinforcement. The rationale is that features of brain and behavior qualitatively and quantitatively fluctuate during the normal course of development. This provides an opportunity to identify mechanisms of ethanol's effects by identifying changes in brain corresponding to ontogenetic changes in the affinity for ethanol and conditioned reinforcement. An obvious example is the dramatic change in neurotransmitter function that occurs between birth and the second to third weeks of life. Some systems show ontogenetic increases in the density or number of, for example, μ and δ opioid receptors and NMDA glutamate receptors, while other systems exhibit a switch in function, notably GABAA receptors, κ opioid receptors, and 5-hydroxytryptamine (5-HT, serotonin) receptors (Herlenius and Lagercrantz, 2004; Spain et al., 1985). Among the numerous neurotransmitter systems, the opioid and dopaminergic systems have been repeatedly related to ethanol abuse and dependence. Shortly after birth μ receptors are more common than κ or δ receptors. The difference decreases during the first week of life, only to reappear by PD14-15 (Spain et al., 1985). In regard to the dopamine system, the expression of messenger RNA (mRNA) for D1A and D2 receptors in the striatum of newborn rats is similar to that seen in adults (Schambra et al, 1994). This is not the case for D4 mRNA, which is barely appreciable at PD1, achieves a maximum at PD3, only to decline by day 28 (Nair and Mishra, 1995). Another important site of action of ethanol is the GABAB receptor. GABA function exhibits a change from an excitatory role early in development to its more traditional role as an inhibitory system, approximately by PD9 (Rivera et al., 1999). As mentioned, there is a striking correlation between this developmental change in GABA function and acceptance of ethanol (Sanders and Spear, 2007).
Although the methods for analyzing early brain development at morphological, cellular, and molecular levels have been available for years (Sturrock et al., 1983; Sturrock, 1987; Spain et al., 1985), behavioral assays for assessing the reinforcing effects of ethanol in infants have been scarce and hampered by technical constraints. However, the present review indicates that sensitive models for assessing the aversive, appetitive, and negative reinforcing effects of ethanol in developing rats are now available and can be used to analyze potential neurophysiologic determinants of ethanol-related problems. An unfortunate weakness of the present review is that the information regarding the neurotransmitters that seem most relevant for ethanol reinforcement is quite limited. We consider this review a first step in the search for these underlying mechanisms. Still, the available information seems to indicate that the appetitive effects of ethanol in preweanlings is critically mediated by the opioid system, as shown in different learning preparations and age groups. Ethanol reinforcement in neonates, as indexed by the surrogate nipple technique, was suppressed by systemic administration of μ and κ opioid antagonists (Nizhnikov et al., 2006b). Moreover, pretreatment with a more general antagonist (naloxone) suppressed the heightened ethanol consumption in rat pups given non-reinforced ethanol exposure at PD7 (Arias and Chotro, 2006a). Analogous effects were found later in ontogeny, with a general opioid antagonist blocking the expression of ethanol-induced activation (Arias et al., 2009) and conditioned place preference by ethanol in 14-15 day old rats (Nizhnikov, Pautassi, Truxell, Spear, unpublished). CPP by ethanol in preweanlings was also reduced or eliminated by drugs tailored to block μ and κ receptors (CTOP and naltrindole, respectively; Nizhnikov, Pautassi, Truxell, Spear, unpublished). As mentioned, these appetitive properties of ethanol may be associated with the ascending limb of the blood ethanol curve. Interestingly, Froehlich et al. (1991; 1994) linked the appetitive properties of the drug during the onset of the intoxication to an ethanol-induced activation of the endogenous opioid system. Hence, it is possible that opioid antagonists inhibit early ethanol reinforcement through a disruption of ethanol's early stimulant effects. This hypothesis fits well with previous human data indicating that naltrexone blocks the acute psychomotor stimulant effects of alcohol during the rising limb of the intoxication (King et al., 1997; Peterson et al., 1996).
Behavioral reactions to pre-absorptive characteristics of ethanol (e.g., taste, odor) have been consistent across Pavlovian and operant conditions. Infants avoid tactile stimuli signaling i.o. delivery of ethanol (Pautassi et al., 2008b). Operant responding for sucrose or milk is quite robust in neonates and older infants, but contamination of these solutions with ethanol (even at low concentrations; Ponce et al., 2006) sharply decreased responding (Dominguez et al., 1993; Ponce et al., 2006). A negative association between ethanol concentration and amount of operant responding was found even in procedures in which overall responding for ethanol was robust (i.e., Bordner et al., 2008). Consistent with studies conducted in adults (Kiefer et al., 2005), these findings suggest that i.o. ethanol administration has aversive chemosensory consequences in infants. The latter statement does not contradict that infants may consume substantial oral ethanol if presented under more appropriate, age-related testing circumstances, which allow sharp increases in BAL over short periods. This observation is important considering findings of substantial intake of ethanol between PD6 and PD12 when tested through an independent feeding procedure (i.e., consumption-off-the-floor; Sanders and Spear, 2007).
Still unclear, however, are the critical factors promoting operant responding for oral ethanol in infant rats (Bordner et al., 2008; Ponce et al., 2008). Some similarities between studies include higher responding at lower ethanol concentrations and relatively simple response requirements (e.g., FR1, absence of shaping procedures, no timeout between reinforcers). Interestingly, in both studies, reliable ethanol-mediated responding was associated with similarly low BALs (20-50 mg %). This result does not appear to be simply a spurious coincidence. These BALs are in the same range as those that mediate appetitive pharmacological effects in neonates and infants when employing Pavlovian techniques. For example, ethanol administration yielding BALs as low as 25 mg % promoted appetitive learning with the surrogate nipple technique (Nizhnikov et al., 2006b). Ethanol doses resulting in BALs of 17-60 mg % devalued aversive conditioning mediated by an innate aversive tastant (citric acid; Pautassi et al., 2006, 2007). Pharmacological consequences of low-dose ethanol (0.5 g/kg, i.g., resulting in BALs of ∼50 mg%) have also been reported in adolescent rats (PD33). These animals exhibited conditioned preference for an ethanol-paired flavor (Fernandez-Vidal et al., 2003). Therefore, a reasonable postulate is that during early development the expression of the non-aversive effects of ethanol may be associated with moderate to low levels of intoxication in the range of 15-70 mg %.
In summary, this review was inspired by the hypothesis that experimental methods and evidence gathered during the past decade may allow characterization of rat infancy as a developmental period of high sensitivity to not only the aversive motivational properties of ethanol but also to the drug's positive reinforcing and negative reinforcing (anti-anxiety) effects. The studies reviewed provide substantial support for this assertion and provide a theoretical and methodological basis for researchers interested in analyzing the neurobiology of conditioned reinforcement and ethanol intake.
Acknowledgments
This work was supported by grants from NIAAA (AA11960, AA013098, AA015992) to NES. The authors wish to express their gratitude to Teri Tanenhaus for her technical assistance in the edition and copy-editing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abate P, Pepino MY, Dominguez HD, Spear NE, Molina JC. Fetal associative learning mediated through maternal alcohol intoxication. Alcohol Clin Exp Res. 2000;24:39–47. [PubMed] [Google Scholar]
- Abate P, Pueta M, Spear NE, Molina JC. Ethanol-mediated fetal learning modulates neonatal responsiveness to an ethanol scented artificial nipple. Alcohol Clin Exp Res. 2004;28(5 suppl):94A. doi: 10.1097/01.alc.0000125354.15808.24. [DOI] [PubMed] [Google Scholar]
- Abate P, Pueta M, Spear NE, Molina JC. Fetal learning about ethanol and later ethanol responsiveness: evidence against “safe” amounts of prenatal exposure. Exp Biol Med. 2008;233:139–154. doi: 10.3181/0703-MR-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate P, Spear NE, Molina J. Fetal and infantile alcohol-mediated associative learning in the rat. Alcohol Clin Exp Res. 2001;25:989–998. [PubMed] [Google Scholar]
- Abate P, Varlinskaya EI, Cheslock SJ, Spear NE, Molina JC. Neonatal activation of alcohol-related prenatal memories: impact on the first suckling response. Alcohol Clin Exp Res. 2002;26:1512–1522. doi: 10.1097/01.ALC.0000034668.93601.8F. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Iranmanesh A, Veldhuis J, Fisher L. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health Res World. 1998;22:67–72. [PMC free article] [PubMed] [Google Scholar]
- Agabio R, Carai MA, Lobina C, Pani M, Reali R, Vacca G, Gessa GL, Colombo G. Alcohol stimulates motor activity in selectively bred Sardinian alcohol-preferring (sP), but not in Sardinian alcohol-nonpreferring (sNP), rats. Alcohol. 2001;23:123–126. doi: 10.1016/s0741-8329(00)00144-0. [DOI] [PubMed] [Google Scholar]
- Aragon CM, Amit Z. Taurine and ethanol-induced conditioned taste aversion. Pharmacol Biochem Behav. 1993;44:263–266. doi: 10.1016/0091-3057(93)90460-b. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Increased palatability of ethanol after prenatal ethanol exposure is mediated by the opioid system. Pharmacol Biochem Behav. 2005;82:434–442. doi: 10.1016/j.pbb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Ethanol-induced preferences or aversions as a function of age in preweanling rats. Behav Neurosci. 2006a;120:710–718. doi: 10.1037/0735-7044.120.3.710. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Interactions between prenatal ethanol exposure and postnatal learning about ethanol in rat pups. Alcohol. 2006b;40:51–59. doi: 10.1016/j.alcohol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Naloxone and baclofen attenuate ethanol's locomotor-activating effects in preweanling Sprague-Dawley rats. Behav Neurosci. 2009;123:172–180. doi: 10.1037/a0014049. [DOI] [PubMed] [Google Scholar]
- Arias C, Molina JC, Mlewski EC, Pautassi RM, Spear N. Acute sensitivity and acute tolerance to ethanol in preweanling rats with or without prenatal experience with the drug, Pharmacol. Biochem Behav. 2008;89:608–22. doi: 10.1016/j.pbb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Ethanol induces locomotor activating effects in preweanling Sprague-Dawley rats. Alcohol. doi: 10.1016/j.alcohol.2008.09.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Pautassi RM, Murphy H, Molina JC, Spear NE. Similarity between conditioned aversion to ethanol and to lithium chloride in preweanling rats. Alcohol Clin Exp Res. 2007;31(5 supp):153A. [Google Scholar]
- Arias C, Spear NE, Molina JC, Molina A, Molina JC. Rapid acquisition of operant conditioning in 5-day-old rat pups: a new technique articulating suckling-related motor activity and milk reinforcement. Dev Psychobiol. 2007;49:576–588. doi: 10.1002/dev.20236. sic. [DOI] [PubMed] [Google Scholar]
- Arizzi-LaFrance MN, Correa M, Aragon CM, Salamone JD. Motor stimulant effects of ethanol injected into the substantia nigra pars reticulata: importance of catalase-mediated metabolism and the role of acetaldehyde. Neuropsychopharmacology. 2006;31:997–1008. doi: 10.1038/sj.npp.1300849. [DOI] [PubMed] [Google Scholar]
- Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. J Stud Alcohol. 1998;59:533–543. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry. 2003;60:377–385. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Cunningham CL. Ethanol-induced conditioned place preference is expressed through a ventral tegmental area dependent mechanism. Behav Neurosci. 2005;119:213–223. doi: 10.1037/0735-7044.119.1.213. [DOI] [PubMed] [Google Scholar]
- Becker HC, Flaherty CF. Influence of ethanol on contrast in consummatory behavior. Psychopharmacology. 1982;77:253–258. doi: 10.1007/BF00464576. [DOI] [PubMed] [Google Scholar]
- Becker HC, Jarvis MF, Wagner GC, Flaherty CF. Medial and lateral amygdalectomy differentially influences consummatory negative contrast. Physiol Behav. 1984;33:707–712. doi: 10.1016/0031-9384(84)90035-0. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Berman RF, Cannon DS. The effect of prior ethanol experience on ethanol-induced saccharin aversions. Physiol Behav. 1974;12:1041–1044. doi: 10.1016/0031-9384(74)90152-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Kuka P, Kowstowski W. Conditioned place preference after prolonged preexposure to ethanol. Pol J of Pharmachol. 1995;47:185–187. [PubMed] [Google Scholar]
- Bond NW, Di Giusto EL. Effects of prenatal alcohol consumption in open-field behaviour and alcohol preference in rats. Psychopharmacologia. 1976;46:163–165. doi: 10.1007/BF00421386. [DOI] [PubMed] [Google Scholar]
- Bordner KA, Molina JC, Spear NE. Analysis of ethanol reinforcement in 1-day-old rats: assessment through a brief and novel operant procedure. Alcohol Clin Exp Res. 2008;32:580–592. doi: 10.1111/j.1530-0277.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cunningham CL. The role of NMDA receptor binding sites in ethanol place conditioning. Behav Neurosci. 2004;118:822–834. doi: 10.1037/0735-7044.118.4.822. [DOI] [PubMed] [Google Scholar]
- Bozarth MA. Evidence for the rewarding effects of ethanol using the conditioned place preference method. Pharmacol Biochem Behav. 1990;35:485–487. doi: 10.1016/0091-3057(90)90191-j. [DOI] [PubMed] [Google Scholar]
- Bruins Slot LA, Koek W, Colpaert FC. Ethanol state dependence involving a lever press response requirement in rats. Behav Pharmacol. 1999;10:229–233. doi: 10.1097/00008877-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Camarini R, Hodge CW. Ethanol preexposure increases ethanol self-administration in C57BL/6J and DBA/2J mice. Pharmacol Biochem Behav. 2004;79:623–632. doi: 10.1016/j.pbb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Camarini R, Nogueira Pires ML, Calil HM. Involvement of the opioid system in the development and expression of sensitization to the locomotor-activating effect of ethanol. Int J Neuropsychopharmacol. 2000;3:303–309. doi: 10.1017/S146114570000211X. [DOI] [PubMed] [Google Scholar]
- Cannon DS, Berman RF, Baker TB, Atkins CA. Effect of preconditioning unconditioned stimulus experience on learned taste aversions. J Exp Psychol Anim Behav Process. 1975;1:270–284. doi: 10.1037//0097-7403.1.3.270. [DOI] [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE, Endrenyi L. Aversive conditioning by psychoactive drugs: effects of morphine, alcohol and chlordiazepoxide. Psychopharmacologia. 1973;29:239–246. doi: 10.1007/BF00414038. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Hughes S, Meltzer SB, Wahlgren D, Kassem N, Larson S, Riley EP, Hovell MF. Alcohol consumption among low-income pregnant Latinas. Alcohol Clin Exp Res. 2005;29:2022–2028. doi: 10.1097/01.alc.0000187160.18672.f9. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Spear LP, Spear NE. Enhancement of sensory preconditioning by a moderate dose of ethanol in infant and juvenile rats. Behav Neural Biol. 1992;57:44–57. doi: 10.1016/0163-1047(92)90746-q. [DOI] [PubMed] [Google Scholar]
- Cheslock SJ, Varlinskaya EI, Petrov ES, Silveri MM, Spear LP, Spear NE. Ethanol as a reinforcer in the newborn's first suckling experience. Alcohol Clin Exp Res. 2001;25:391–402. [PubMed] [Google Scholar]
- Cheslock SJ, Varlinskaya EI, Petrov ES, Spear NE. Rapid and robust olfactory conditioning with milk before suckling experience: promotion of nipple attachment in the newborn rat. Behav Neurosci. 2000;114:484–95. [PubMed] [Google Scholar]
- Chien YC, Huang YJ, Hsu CS, Chao JC, Liu JF. Maternal lactation characteristics after consumption of an alcoholic soup during the postpartum ‘doing-the-month’ ritual. Public Health Nutr. 2008;12:382–388. doi: 10.1017/S1368980008002152. [DOI] [PubMed] [Google Scholar]
- Chien YC, Liu JF, Huang YJ, Hsu CS, Chao JC. Alcohol levels in Chinese lactating mothers after consumption of alcoholic diet during postpartum “doing-the-month” ritual. Alcohol. 2005;37:143–150. doi: 10.1016/j.alcohol.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Chuck TL, McLaughlin PJ, Arizzi-LaFrance MN, Salamone JD, Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: sedation, ataxia, and bradykinesia. Life Sci. 2006;79:154–161. doi: 10.1016/j.lfs.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Choonara I. Percutaneous drug absorption and administration. Arch Dis Child. 1994;71:F73–74. doi: 10.1136/fn.71.2.f73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotro M, Arias C. Ontogenetic difference in ethanol reinforcing properties: the role of the opioid system. Behav Pharmacol. 2007;18:661–6. doi: 10.1097/FBP.0b013e3282f00754. [DOI] [PubMed] [Google Scholar]
- Chotro M, Arias C, Laviola G. Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neurosci Biobehav Rev. 2007;31:181–191. doi: 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C. Prenatal exposure to ethanol increases ethanol consumption: a conditioned response? Alcohol. 2003;30:19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Chhada M, Perfumi M, Froldi R, Massi M. Conditioned taste aversion induced by ethanol in alcohol-preferring rats: influence of the method of ethanol administration. Pharmacol Biochem Behav. 1999a;64:563–566. doi: 10.1016/s0091-3057(99)00104-5. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Froldi R, Quitadamo E, Massi M. Ethanol induces conditioned place preference in genetically selected alcohol-preferring rats. Psychopharmacology. 1999b;141:235–241. doi: 10.1007/s002130050830. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology (Berl) 2001;157:20–30. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO, Mankowski S. Biphasic effects of alcohol on heart rate are influenced by alcoholic family history and rate of alcohol ingestion. Alcohol Clin Exp Res. 1997;21:140–149. [PubMed] [Google Scholar]
- Conrod PJ, Pihl RO, Vassileva J. Differential sensitivity to alcohol reinforcement in groups of men at risk for distinct alcoholism subtypes. Alcohol Clin Exp Res. 1998;22:585–597. doi: 10.1111/j.1530-0277.1998.tb04297.x. [DOI] [PubMed] [Google Scholar]
- Croce P. El alcohol y ninos. In: Secretaria de Medicine Sanitaria, editor. Alcohol y Alcoholism. Sector Educacion para la Salud; Buenos Aires: 1987. [Google Scholar]
- Cunningham CL. Pavlovian drug conditioning. In: van Haaren F, editor. Methods in Behavioral Pharmacology. NY: Elsevier; 1993. pp. 349–381. [Google Scholar]
- Cunningham CL. Drug conditioning and seeking behavior. In: O'Donohue WT, editor. Learning and Behavior Therapy. Allyn and Bacon; Boston: 1998. pp. 518–540. [Google Scholar]
- Cunningham CL, Clemans JM, Fidler TL. Injection timing determines whether intragastric ethanol produces conditioned place preference or aversion in mice. Pharmacol Biochem Behav. 2002a;72:659–668. doi: 10.1016/s0091-3057(02)00734-7. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Fidler TL, Hill KG. Animal models of alcohol's motivational effects. Alcohol Res Health. 2000a;24:85–92. [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006a;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Hawks DM, Niehus DR. Role of hypothermia in ethanol-induced conditioned taste aversion. Psychopharmacology (Berl) 1988;95:318–322. doi: 10.1007/BF00181940. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Howard MA, Gill SJ, Rubinstein M, Low MJ, Grandy DK. Ethanol-conditioned place preference is reduced in dopamine D2 receptor-deficient mice. Pharmacol Biochem Behav. 2000b;67:693–699. doi: 10.1016/s0091-3057(00)00414-7. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Malott DH, Dickinson SD, Risinger FO. Haloperidol does not alter expression of ethanol-induced conditioned place preference. Behav Brain Res. 1992a;50:1–5. doi: 10.1016/s0166-4328(05)80282-7. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS. Drug-induced hypothermia and conditioned place aversion. Behav Neurosci. 1988;107:468–479. doi: 10.1037//0735-7044.107.3.468. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS, Bachtold JF. Ambient temperature effects on taste aversion conditioned by ethanol: contribution of ethanol-induced hypothermia. Alcohol Clin Exp Res. 1992b;16:1117–1124. doi: 10.1111/j.1530-0277.1992.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS, Noble D. Species difference in sensitivity to ethanol's hedonic effects. Alcohol. 1993;10:97–102. doi: 10.1016/0741-8329(93)90087-5. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Noble D. Conditioned activation induced by ethanol: role in sensitization and conditioned place preference. Pharmacol Biochem Behav. 1992;43:307–313. doi: 10.1016/0091-3057(92)90673-4. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Patel P, Milner L. Spatial location is critical for conditioning place preference with visual but not tactile stimuli. Behav Neurosci. 2006b;120:1115–1132. doi: 10.1037/0735-7044.120.5.1115. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Prather LK. Conditioning trial duration affects ethanol-induced conditioned place preference in mice. Anim Learn Behav. 1992;20:187–194. [Google Scholar]
- Cunningham C, Tull L, Rindal K, Meyer P. Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect. Psychopharmacology. 2002;160:414–424. doi: 10.1007/s00213-001-0990-1. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Philips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Czachowski C. Manipulations of serotonin function in the nucleus accumbens core produce differential effects on ethanol and sucrose seeking and intake Alcohol. Clin Exp Res. 2005;29:1146–1155. doi: 10.1097/01.alc.0000171944.50381.86. [DOI] [PubMed] [Google Scholar]
- Czachowski C, Legg B, Samson H. Effects of acamprosate on ethanol-seeking and self-administration in the rat. Alcohol Clin Exp Res. 2001;25:344–350. [PubMed] [Google Scholar]
- Davies BT, Parker LA. Novel versus familiar ethanol: a comparison of aversive and rewarding properties. Alcohol. 1990;7:523–529. doi: 10.1016/0741-8329(90)90043-c. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Harford TC, Grant BF. Family history as a predictor of alcohol dependence. Alcohol Clin Exp Res. 1992;16:572–575. doi: 10.1111/j.1530-0277.1992.tb01419.x. [DOI] [PubMed] [Google Scholar]
- Delameter AR, Lolordo VM. Event revaluation procedures and associative structures in Pavlovian conditioning. In: Dachowski L, Flaherty C, editors. Current Topics in Animal Learning: Brain, Emotion and Cognition. Hillsdale, NJ: Erlbaum; 1991. [Google Scholar]
- Dominguez HD, Bocco G, Chotro MG, Spear NE, Molina JC. Operant responding controlled by milk or milk contaminated with alcohol as positive reinforcers in infant rats. Pharmacol Biochem Behav. 1993;44:403–409. doi: 10.1016/0091-3057(93)90482-9. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Lopez MF, Molina JC. Neonatal responsiveness to alcohol odor and infant alcohol intake as a function of alcohol experience during late gestation. Alcohol. 1998;16:109–117. doi: 10.1016/s0741-8329(97)00169-9. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Ducci F, Goldman D. Genetic approaches to addiction: genes and alcohol. Addiction. 2008 doi: 10.1111/j.1360-0443.2008.02203.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranceaux NCE, Schuckit MA, Eng MY, Robinson SK, Carr LG, Wall TL. Associations of variations in alcohol dehydrogenase genes with the level of response to alcohol in non-Asians. Alcohol Clin Exp Res. 2006;30:1470–1478. doi: 10.1111/j.1530-0277.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- Engel J, Hard E. Effects of diazepam, ethanol and Ro 15-1788 on ultrasonic vocalization, locomotor activity and body righting in the neonatal rat. Alcohol Alcohol. 1987 1:709–712. [PubMed] [Google Scholar]
- Enoch M. Pharmacogenomics of alcohol response and addiction. Am J Pharmacogenomics. 2003;3:217–232. doi: 10.2165/00129785-200303040-00001. [DOI] [PubMed] [Google Scholar]
- Ericson M, Molander A, Lof E, Engel JA, Soderpalm B. Ethanol elevates accumbal dopamine levels via indirect activation of ventral tegmental nicotinic acetylcholine receptors. Eur J Pharmacol. 2003;467:85–93. doi: 10.1016/s0014-2999(03)01564-4. [DOI] [PubMed] [Google Scholar]
- Faden VB. Trends in initiation of alcohol use in the United States 1975 to 2003. Alcohol Clin Exp Res. 2006;30:1011–1022. doi: 10.1111/j.1530-0277.2006.00115.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Vidal JM, Spear NE, Molina JC. Adolescent rats discriminate a mild state of ethanol intoxication likely to act as an appetitive unconditioned stimulus. Alcohol. 2003;30:45–60. doi: 10.1016/s0741-8329(03)00093-4. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Bakner L, Cunningham CL. Conditioned place aversion induced by intragastric administration of ethanol in rats. Pharmacol Biochem Behav. 2004;77:731–743. doi: 10.1016/j.pbb.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Clews TW, Cunningham CL. Reestablishing an intragastric ethanol self-infusion model in rats. Alcohol Clin Exp Res. 2006;30:414–428. doi: 10.1111/j.1530-0277.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- Fildes VA. Breasts, Bottles and Babies: A History of Infant Feeding. Edinburgh University Press; Edinburgh: 1986. [Google Scholar]
- Files FJ, Denning CE, Hyytia P, Kiianmaa K, Samson HH. Ethanol-reinforced responding by AA and ANA rats following the sucrose-substitution initiation procedure. Alcohol Clin Exp Res. 1997;21:749–753. [PubMed] [Google Scholar]
- Files FJ, Denning CE, Samson HH. Effects of the atypical antipsychotic remoxipride on alcohol self-administration. Pharmacol Biochem Behav. 1998;59:281–285. doi: 10.1016/s0091-3057(97)00460-7. [DOI] [PubMed] [Google Scholar]
- Flores-Huerta S, Hernadez-Montes H, Argote RM, Villalpando S. Effects of ethanol consumption during pregnancy and lactation on the outcome and postnatal growth of the offspring. Ann Nutr Metab. 1992;36:121–128. doi: 10.1159/000177706. [DOI] [PubMed] [Google Scholar]
- Froehlich J, Li T. Opioid Involvement in Alcohol-Drinking. Models of Neuropeptide Action. 1994;739:156–167. doi: 10.1111/j.1749-6632.1994.tb19817.x. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Zweifel M, Harts J, Lumeng L, Li TK. Importance of delta opioid receptors in maintaining high alcohol drinking. Psychopharmacology. 1991;103:467–472. doi: 10.1007/BF02244246. [DOI] [PubMed] [Google Scholar]
- Garcia L, Garcia CHS, Calábria LK, da Cruz GCN, Puentes AS, Báo SN, Fontes W, Ricart CAO, Espindola FS, de Sousa MV. Proteomic Analysis of Honey Bee Brain upon Ontogenetic and Behavioral Development. J Proteome Res. 2009 Feb 9; doi: 10.1021/pr800823r. Downloaded from http://pubs.acs.org on February 24, 2009. [DOI] [PubMed]
- Gauvin DV, Holloway FA. Historical factors in the development of ETOH-conditioned place preference. Alcohol. 1992;9:1–7. doi: 10.1016/0741-8329(92)90002-r. [DOI] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and DSM-IV alcohol dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Alcohol Health Res World. 1998;22:144–147. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groblewski PA, Bax LS, Cunningham CL. Reference-dose place conditioning with ethanol in mice: empirical and theoretical analysis. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1251-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WG. The ontogeny of ingestive behavior. In: Stricker E, editor. Neurobiology of Food and Fluid Intake (series title: Handbook of Behavioral Neurobiology, vol 10) Plenum Press; New York: 1990. pp. 77–123. [Google Scholar]
- Hayashi T, Tadokoro S. Learning retardation and enhanced ethanol preference produced by postnatal pretreatments with ethanol in adult rats. Jpn J Pharmacol. 1985;37:269–276. doi: 10.1254/jjp.37.269. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp Neurol. 2004;190(suppl 1):S8–S21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Hitzemann R. Animal models of psychiatric disorders and their relevance to alcoholism. Alcohol Res Health. 2000;24:149–158. [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Rescorla RA. The effect of two ways of devaluing the unconditioned stimulus after first- and second-order appetitive conditioning. J of Exp Psychol: Anim Behav Proc. 1975;1:355–363. doi: 10.1037//0097-7403.1.4.355. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Molina JC, Rajachandran L, Spear LP, Spear NE. Chronic administration of alcohol in the developing rat: expression of functional tolerance and alcohol olfactory aversions. Behav Neural Biol. 1993;59:87–99. doi: 10.1016/0163-1047(93)90795-j. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Molina JC, Spear LP, Spear NE. Ethanol-mediated taste aversions and state-dependency in preweanling (16-day-old) rats. Behav Neural Biol. 1990;54:300–322. doi: 10.1016/0163-1047(90)90650-u. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Richardson R. Pharmacological dissociation of trace and long-delay fear conditioning in young rats. Neurobiol Learn Mem. 2007;87:86–92. doi: 10.1016/j.nlm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Spear LP, Spear NE. An ontogenetic comparison of ethanol-mediated taste aversion learning and ethanol-induced hypothermia in preweanling rats. Behav Neurosci. 1991;105:971–983. doi: 10.1037//0735-7044.105.6.971. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Spear NE. Hypothermia and ethanol-mediated taste aversion in infant rats. Paper presented at the Annual Meeting of the International Society for Developmental Psychobiology; San Francisco, CA. October, 1989.1989. [Google Scholar]
- Iijima M, Chaki S. Separation-induced ultrasonic vocalization in rat pups: further pharmacological characterization. Pharmacol Biochem Behav. 2005;82:652–627. doi: 10.1016/j.pbb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Ivkovich D, Paczkowski CM, Stanton ME. Ontogeny of delay versus trace eyeblink conditioning in the rat. Dev Psychobiol. 2000;36:148–160. doi: 10.1002/(sici)1098-2302(200003)36:2<148::aid-dev6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Johanson IB, Hall WG. Appetitive learning in 1-day-old rat pups. Science. 1979;205:419–421. doi: 10.1126/science.451612. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitorying the Future: National Survey Results on Drug Use, 1975-2006, vol 1, Secondary School Students [NIH Pub No 07-6205] National Institute on Drug Abuse; Bethedesa: 2007. [Google Scholar]
- Kiefer SW, Hill KG, Coonfield DL, Ferraro FM., 3rd Ethanol familiarity and naltrexone treatment affect ethanol responses in rats. Alcohol. 2005;37:167–172. doi: 10.1016/j.alcohol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Lawrence GJ, Metzler CW. Alcohol preference in rats lacking gustatory neocortex. Alcohol. 1987;4:37–43. doi: 10.1016/0741-8329(87)90058-9. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Britton KT. Neurobiological substrates for the anti-anxiety effects of ethanol. In: Begleiter H, Kissin B, editors. The Pharmacology of Alcohol and Alcohol Dependence. Oxford University Press; New York: 1996. pp. 477–506. [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kozlov AP, Varlinskaya EI, Spear NE. Ethanol, saccharin, and quinine: early ontogeny of taste responsiveness and intake. Alcohol Clin Exp Res. 2008;32:294–305. doi: 10.1111/j.1530-0277.2007.00581.x. [DOI] [PubMed] [Google Scholar]
- Kraebel KS, Spear NE. Infant rats are more likely than adolescents to orient differentially to amodal (intensity-based) features of single-element and compound stimuli. Dev Psychobiol. 2000;36:49–66. [PubMed] [Google Scholar]
- Kraemer PJ, Hoffmann H, Randall CK, Spear NE. Devaluation of Pavlovian conditioning in the 10-day-old rat. Anim Learn Behav. 1992;20:219–222. [Google Scholar]
- Kucharski D, Richter NG, Spear NE. Conditioned aversion is promoted by memory of CS- Anim Learn Behav. 1985;13:143–151. [Google Scholar]
- Kuczenski R, Segal DS. Stimulant actions in rodents: implications for attention-deficit/hyperactivity disorder treatment and potential substance abuse. Biol Psychiatry. 2005;57:1391–1396. doi: 10.1016/j.biopsych.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Lee JS, Crawford J, Spear NE. Characteristics and consequences of free-feeding ethanol ingestion during the first two postnatal weeks of the rat. Alcohol Clin Exp Res. 1998;22:1615–1622. [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of Drug Intake. Exp Clin Psychopharmacol. 2001;9:131–43. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Molina JC. Chronic alcohol administration in the rat pup: effects upon later consumption of alcohol and other palatable solutions. Addict Biol. 1999;4:173–183. doi: 10.1080/13556219971678. [DOI] [PubMed] [Google Scholar]
- Marglin SH, MacKechnie DK, Mattie M, Hui YH, Reid LD. Ethanol with small doses of morphine establishes a conditioned place preference. Alcohol. 1988;5:309–313. doi: 10.1016/0741-8329(88)90071-7. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Kiianmaa K, Gianoulakis C. Opioid propeptide mRNA content and receptor density in the brains of AA and ANA rats. Life Sci. 2000;66:1915–1927. doi: 10.1016/s0024-3205(00)00517-8. [DOI] [PubMed] [Google Scholar]
- Masur J, Boerngen R. The excitatory component of ethanol in mice: a chronic study. Pharmacol Biochem Behav. 1980;13:777–780. doi: 10.1016/0091-3057(80)90206-3. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Mennella J. Alcohol's effect on lactation. Alcohol Res Health. 2001;25:230–234. [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Beauchamp GK. Beer, breast feeding, and folklore. Dev Psychobiol. 1993;26:459–466. doi: 10.1002/dev.420260804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Jagielo JA, Spear NE. Age-related differences in short-term retention of separable elements of an odor aversion. J Exp Psychol Anim Behav Process. 1989;15:194–201. [PubMed] [Google Scholar]
- Miller JS, Jagielo JA, Spear NE. Differential effectiveness of various prior-cuing treatments in the reactivation and maintenance of memory. J Exp Psychol Anim Behav Process. 1991;17:249–258. doi: 10.1037//0097-7403.17.3.249. [DOI] [PubMed] [Google Scholar]
- Miller JS, Molina JC, Spear NE. Ontogenetic differences in the expression of odor- aversion learning in 4- and 8-day-old rats. Dev Psychobiol. 1990;23:319–330. doi: 10.1002/dev.420230404. [DOI] [PubMed] [Google Scholar]
- Molina JC, Bannoura MD, Chotro MG, McKinzie DL, Arnold HM, Spear NE. Alcohol-mediated tactile conditioned aversions in infant rats: devaluation of conditioning through alcohol-sucrose associations. Neurobiol Learn Mem. 1996;66:121–132. doi: 10.1006/nlme.1996.0053. [DOI] [PubMed] [Google Scholar]
- Molina JC, Chotro MG. Acute alcohol intoxication paired with appetitive reinforcement: effects upon ethanol intake in infant rats. Behav Neural Biol. 1989;51:326–345. doi: 10.1016/s0163-1047(89)90974-6. [DOI] [PubMed] [Google Scholar]
- Molina JC, Chotro MG, Dominguez HD. Fetal alcohol learning derived from ethanol contamination of the prenatal environment. In: Lecanuet JP, editor. Fetal Development: A Psychobiological Perspective. Lawrence Erlbaum; Hillsdale NJ: 1995. pp. 419–438. [Google Scholar]
- Molina JC, Chotro MG, Spear NE. Early (preweanling) recognition of alcohol's orosensory cues resulting from acute ethanol intoxication. Behav Neural Biol. 1989;51:307–325. doi: 10.1016/s0163-1047(89)90961-8. [DOI] [PubMed] [Google Scholar]
- Molina JC, Hoffmann H, Serwatka J, Spear NE. Conditioning of aversion to alcohol orosensory cues in 5- and 10-day rats: subsequent reduction in alcohol ingestion. Dev Psychobiol. 1986;19:175–183. doi: 10.1002/dev.420190304. [DOI] [PubMed] [Google Scholar]
- Molina JC, Hoffmann H, Serwatka J, Spear NE. Establishing intermodal equivalence in preweanling and adult rats. J Exp Psychol Anim Behav Process. 1991;17:433–447. doi: 10.1037//0097-7403.17.4.433. [DOI] [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41:41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JC, Ponce LF, Truxell E, Spear NE. Infantile sensitivity to ethanol's motivational effects: ethanol reinforcement during the third postnatal week. Alcohol Clin Exp Res. 2006;30:1506–1519. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- Montano Loza AJ, Ramirez Iglesias MT, Perez Diaz I, Cruz Castellanos S, Garcia Andrade C, Medina Mora ME, Robles Díaz G, Kershenobich D, Gutierrez Reyes G. Association of alcohol-metabolizing genes with alcoholism in a Mexican Indian (Otomi) population. Alcohol. 2006;39:73–79. doi: 10.1016/j.alcohol.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Morris DS, Tenkku LE, Salas J, Xaverius PK, Mengel MB. Exploring pregnancy-related changes in alcohol consumption between black and white women. Alcohol Clin Exp Res. 2008;32:505–512. doi: 10.1111/j.1530-0277.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- Mulligan CJ, Robin RW, Osier MV, Sambuughin N, Goldfarb LG, Kittles RA, Hesselbrock D, Goldman D, Long JC. Allelic variation at alcohol metabolism genes (ADH1B, ADH1C, ALDH2) and alcohol dependence in an American Indian population. Hum Genet. 2003;113:325–36. doi: 10.1007/s00439-003-0971-z. [DOI] [PubMed] [Google Scholar]
- Nair VD, Mishra RK. Ontogenic development of dopamine D4 receptor in rat brain. Develop Brain Res. 1995;90:180–183. doi: 10.1016/0165-3806(96)83499-7. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Eighth Special Report to the U.S. Congress on Alcohol and Health. National Institute on Alcohol Abuse and Alcoholism; Bethesda MD: 1993. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIH News. National Institute on Alcohol Abuse and Alcoholism; Bethesda MD: 2008. Li to Step Down as Director of the National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- Newlin DB, Thomson JB. Chronic tolerance and sensitization to alcohol in sons of alcoholics: II. Replication and reanalysis. Exp Clin Psychopharmacol. 1999;7:234–243. doi: 10.1037//1064-1297.7.3.234. [DOI] [PubMed] [Google Scholar]
- Nizhnikov ME, Molina JC, Spear NE. Central reinforcing effects of ethanol are blocked by catalase inhibition. Alcohol. 2007;41:525–534. doi: 10.1016/j.alcohol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizhnikov ME, Molina JC, Varlinskaya EI, Spear NE. Prenatal ethanol exposure increases ethanol reinforcement in neonatal rats. Alcohol Clin Exp Res. 2006a;30:34–45. doi: 10.1111/j.1530-0277.2006.00009.x. [DOI] [PubMed] [Google Scholar]
- Nizhnikov M, Pautassi RM, Molina JC, Spear NE. First-order tactile conditioning induced by ethanol in infant rats. Paper presented at the Society for Neuroscience (SFN) Meeting; November 15 - 19, 2008; Washington, DC, USA. 2008. [Google Scholar]
- Nizhnikov ME, Pautassi RM, Molina JC, Spear NE. Conditioned preferences and aversions in infant rats mediated through ethanol inhalation. Alcohol. 2009;43:1–12. doi: 10.1016/j.alcohol.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Nizhnikov ME, Varlinskaya EI, Petrov ES, Spear NE. Reinforcing properties of ethanol in neonatal rats: involvement of the opioid system. Behav Neurosci. 2006b;120:267–280. doi: 10.1037/0735-7044.120.2.267. [DOI] [PubMed] [Google Scholar]
- Nizhnikov ME, Varlinskaya EI, Spear NE. Reinforcing effects of central ethanol injections in newborn rat pups. Alcohol Clin Exp Res. 2006c;30:2089–2096. doi: 10.1111/j.1530-0277.2006.00253.x. [DOI] [PubMed] [Google Scholar]
- Orsini C, Buchini F, Piazza PV, Puglisi-Allegra S, Cabib S. Susceptibility to amphetamine-induced place preference is predicted by locomotor response to novelty and amphetamine in the mouse. Psychopharmacology. 2004;172:264–270. doi: 10.1007/s00213-003-1647-z. [DOI] [PubMed] [Google Scholar]
- Pal N, Alkana RL. Use of inhalation to study the effect of ethanol and ethanol dependence on neonatal mouse development without maternal separation: a preliminary study. Life Sci. 1997;61:1269–1281. doi: 10.1016/s0024-3205(97)00672-3. [DOI] [PubMed] [Google Scholar]
- Pastor R, Aragon CMG. The role of opioid receptor subtypes in the development of behavioral sensitization to ethanol. Neuropsychopharmacology. 2006;31:1489–99. doi: 10.1038/sj.npp.1300928. [DOI] [PubMed] [Google Scholar]
- Pastor R, Aragon CMG. Ethanol injected into the hypothalamic arcuate nucleus induces behavioral stimulation in rats: an effect prevented by catalase inhibition and naltrexone. Behavioural Pharmacology. 2008;19:698–705. doi: 10.1097/FBP.0b013e328315ecd7. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Arias C, Molina JC, Spear N. Domperidone interferes with conditioned disgust reactions but not taste avoidance evoked by a LiCl-paired taste in infant rats. Dev Psychobiol. 2008a;50:343–352. doi: 10.1002/dev.20288. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Godoy JC, Spear NE, Molina JC. Early responsiveness to stimuli paired with different stages within the state of alcohol intoxication. Alcohol Clin Exp Res. 2002a;26:644–654. [PubMed] [Google Scholar]
- Pautassi RM, Melloni C, Ponce LF, Molina JC. Acute ethanol counteracts the acquisition of aversive olfactory learning in infant rats. Alcohol. 2005a;36:99–105. doi: 10.1016/j.alcohol.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Molina JC, Spear N. Infant rats exhibit aversive learning mediated by ethanol's orosensory effects but are positively reinforced by ethanol's post-ingestive effects. Pharmacol Biochem Behav. 2008b;88:393–402. doi: 10.1016/j.pbb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent, but not adult, rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol Clin Exp Res. 2008c;32:2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov M, Molina JC, Boehm SL, 2nd, Spear N. Differential effects of ethanol and midazolam upon the devaluation of an aversive memory in infant rats. Alcohol. 2007;41:421–431. doi: 10.1016/j.alcohol.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Ponce LF, Molina JC. Effects of early exposure to ethanol on subsequent learning mediated by the unconditional attributes of the drug. Latin Am J Psychol. 2005b;37:131–149. [Google Scholar]
- Pautassi RM, Ponce LF, Spear NE, Molina JC. The pharmacological consequences of alcohol support inhibitory and excitatory aversive conditioning in infant rats. Alcohol Clin Exp Res. 2002b;26(5 suppl):151A. [Google Scholar]
- Pautassi RM, Sanders S, Miller S, Spear N, Molina JC. Early ethanol's anxiolytic effects assessed through an unconditional stimulus revaluation procedure. Alcohol Clin Exp Res. 2006;30:448–459. doi: 10.1111/j.1530-0277.2006.00049.x. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Truxell E, Molina JC, Spear NE. Motivational effects of intraorally-infused ethanol in rat pups in an operant self-administration task. Physiol Behav. 2008d;93:118–129. doi: 10.1016/j.physbeh.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peana AT, Enrico P, Assaretti AR, Pulighe E, Muggironi G, Nieddu M, et al. Key role of ethanol-derived acetaldehyde in the motivational properties induced by intragastric ethanol: a conditioned place preference study in the rat. 2008;32:249–258. doi: 10.1111/j.1530-0277.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Abate P, Spear NE, Molina JC. Heightened ethanol intake in infant and adolescent rats following nursing experiences with an ethanol-intoxicated dam. Alcohol Clin Exp Res. 2004;28:895–905. doi: 10.1097/01.alc.0000128223.95184.c9. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Pihl RO, Gianoulakis C, Conrod P, Finn PR, Stewart SH, LeMarquand DG, Bruce KR. Ethanol-induced change in cardiac and endogenous opiate function and risk for alcoholism. Alcohol Clin Exp Res. 1996;20:1542–52. doi: 10.1111/j.1530-0277.1996.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Bregman K, Smotherman WP. Sustained attachment to the nipple in the newborn rat depends on experience with the nipple, milk, and expression of oral grasping. Behav Neurosci. 1999;113:211–221. [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Spear NE. Self-administration of ethanol and saccharin in newborn rats: effects on suckling plasticity. Behav Neurosci. 2001;115:1318–1331. [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Spear NE. Reinforcement from pharmacological effects of ethanol in newborn rats. Alcohol Clin Exp Res. 2003;27:1583–1591. doi: 10.1097/01.ALC.0000089960.62640.58. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Biphasic action of ethanol. Biobehav Rev. 1977;1:231–240. [Google Scholar]
- Pohorecky LA. Interaction of alcohol and stress: a review. Neurosci Biobehav Rev. 1981;5:209–229. doi: 10.1016/0149-7634(81)90003-8. [DOI] [PubMed] [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Nursing from an ethanol-intoxicated dam induces short- and long-term disruptions in motor performance and enhances later self-administration of the drug. Alcohol Clin Exp Res. 2004;28:1039–1050. doi: 10.1097/01.alc.0000131298.32045.96. [DOI] [PubMed] [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Ethanol-mediated operant learning in the infant rat leads to increased ethanol intake during adolescence. Pharmacol Biochem Behav. 2008;90:640–650. doi: 10.1016/j.pbb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Nursing from an ethanol-intoxicated dam induces short- and long-term disruptions in motor performance and enhances later self-administration of the drug. Alcohol Clin Exp Res. 2004;28:1039–1050. doi: 10.1097/01.alc.0000131298.32045.96. [DOI] [PubMed] [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Ethanol reinforcing properties during infancy in the rat: operant self-administration of the drug. Alcohol Clin Exp Res. 2006;30(6 suppl):186A. [Google Scholar]
- Porrino LJ, Whitlow CT, Samson HH. Effects of the self-administration of ethanol and ethanol/sucrose on rates of local cerebral glucose utilization in rats. Brain Res. 1998;791:18–26. doi: 10.1016/s0006-8993(97)01519-9. [DOI] [PubMed] [Google Scholar]
- Pueta M, Abate P, Spear NE, Molina JC. Interactions Between Ethanol Experiences During Late Gestation and Nursing: Effects upon Infantile and Maternal Responsiveness to Ethanol. Int Jour Comp Psych. 2005;18:207–224. [Google Scholar]
- Puschel K. Percutaneous alcohol intoxication. Eur J Pediatr. 1981;136:317–318. doi: 10.1007/BF00443001. [DOI] [PubMed] [Google Scholar]
- Randall CL, Hughes SS, Williams CK, Anton RF. Effect of prenatal alcohol exposure on consumption of alcohol and alcohol-induced sleep time in mice. Pharmacol Biochem Behav. 1983;18(suppl 1):325–329. doi: 10.1016/0091-3057(83)90194-6. [DOI] [PubMed] [Google Scholar]
- Reid LD, Hunter GA, Beaman CM, Hubbell CL. Toward understanding ethanol's capacity to be reinforcing: a conditioned place preference following injections of ethanol. Pharmacol Biochem Behav. 1985;22:483–487. doi: 10.1016/0091-3057(85)90051-6. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian Second-Order Conditioning: Studies in Associative Learning. Lawrence Erlbaum; Hillsdale NJ: 1980. [Google Scholar]
- Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowyley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Boyce JM. Conditioning tastant and the acquisition of conditioned taste avoidance to drugs of abuse in DBA/2J mice. Psychopharmacology (Berl) 2002;160:225–232. doi: 10.1007/s00213-001-0973-2. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Ethanol produces rapid biphasic hedonic effects. Ann N Y Acad Sci. 1992;654:506–508. doi: 10.1111/j.1749-6632.1992.tb26014.x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Genetic differences in ethanol-induced conditioned taste aversion after ethanol preexposure. Alcohol. 1995;12:535–539. doi: 10.1016/0741-8329(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Garcia JM, Protz D, Rael AM, George FR. Ethanol-reinforced behavior in P, NP, HAD and LAD rats: differential genetic regulation of reinforcement and motivation. Behav Pharmacol. 1994;5:521–531. doi: 10.1097/00008877-199408000-00013. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsak K, Pirvola U, Saarma M, Kaila K. The K+/C− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Roberts A, Heyser C, Cole M, Griffin P, Koob G. Excessive ethanol drinking following a history of dependence: Animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and drug ‘wanting’. Psychopharmacology (Berl) 2004;171:352–353. [Google Scholar]
- Roma PG, Rinker JA, Serafine KM, Chen SA, Barr CS, Cheng K, et al. Genetic and early environmental contributions to alcohol's aversive and physiological effects. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.06.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roske I, Baeger I, Frenzel R, Oehme P. Does a relationship exist between the quality of stress and the motivation to ingest alcohol? Alcohol. 1994;11:113–124. doi: 10.1016/0741-8329(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson H, Cunningham C, Czachowski C, Chappell A, Legg B, Shannon E. Devaluation of ethanol reinforcement. Alcohol. 2004;32:203–212. doi: 10.1016/j.alcohol.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Samson HH, Pfeffer AO, Tolliver GA. Oral ethanol self-administration in rats: models of alcohol-seeking behavior. Alcohol Clin Exp Res. 1988;12:591–598. doi: 10.1111/j.1530-0277.1988.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Sanders S, Spear NE. Ethanol acceptance is high during early infancy and becomes still higher after previous ethanol ingestion. Alcohol Clin Exp Res. 2007;31:1148–1158. doi: 10.1111/j.1530-0277.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- Schambra U, Duncan G, Breese G, Fornaretto M, Caron M, Fremeau J. Ontogeny of D1a and D2 dopamine receptor subtypes in rat brain using in situ hybridization and receptor binding. Neuroscience. 1994;62:65–85. doi: 10.1016/0306-4522(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Calcagnetti DJ. Continued trends in the conditioned place preference literature from 1992 to 1996, inclusive, with a cross-indexed bibliography. Neurosci Biobehav Rev. 1998;22:827–846. doi: 10.1016/s0149-7634(98)00012-8. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Krimmer EC. Difference in response to the aversive properties and activity effects of low dose ethanol in LAS and HAS selectively bred rats. Psychopharmacology (Berl) 1992;107:564–568. doi: 10.1007/BF02245271. [DOI] [PubMed] [Google Scholar]
- Serwatka J, Molina JC, Spear NE. Weanlings' transfer of conditioned ethanol aversion from olfaction to ingestion depends on the unconditioned stimulus. Behav Neural Biol. 1986;45:57–70. doi: 10.1016/s0163-1047(86)80006-1. [DOI] [PubMed] [Google Scholar]
- Sherman JE, Hickis CF, Rice AG, Rusiniak KW, Garcia J. Preferences and aversions for stimuli paired with ethanol in hungry rats. Anim Learn Behav. 1983;11:101–106. [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JE, Co C, Lane JD. Limbic acetylcholine turnover rates correlated with rat morphine-seeking behaviors. Pharmacol Biochem Behav. 1984;20:429–442. doi: 10.1016/0091-3057(84)90282-x. [DOI] [PubMed] [Google Scholar]
- Spain JW, Roth BL, Coscia CJ. Differential ontogeny of multiple opioid receptors (μ, δ, and κ) J Neurosci. 1985;5:584–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence: alcohol sensitivity, tolerance, and intake. In: Galanter M, editor. Alcohol Problems in Adolescents and Young Adults: Epidemiology, Neurobiology, Prevention, Treatment (series title: Recent Developments in Alcoholism, vol 17) Kluwer Academic; New York: 2005. pp. 143–159. [PubMed] [Google Scholar]
- Spear NE, Kucharski D, Miller JS. The CS- effect in simple conditioning and stimulus selection during development. Animal Lear Behav. 1989;17:70–82. [Google Scholar]
- Spear NE, McKinzie DL. Intersensory integration in the infant rat. In: Lewkowicz DL, Lickliter R, editors. The Development of Intersensory Perception: Comparative Perspectives. Hillsdale, NJ: Erlbaum; 1995. pp. 133–161. [Google Scholar]
- Spear NE, Molina JC. The role of sensory modality in the ontogeny of stimulus selection. In: Krasnegor NA, editor. Perinatal Development: A Psychobiological Perspective. Academic Press; Orlando: 1987. pp. 83–110. [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Stanton ME. Multiple memory systems, development and conditioning. Behav Brain Res. 2000;110:25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- Stewart RB, Grupp LA. Some determinants of the motivational properties of ethanol in the rat: concurrent administration of food or social stimuli. Psychopharmacology (Berl) 1985;87:43–50. doi: 10.1007/BF00431776. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Perlanski E, Grupp LA. Ethanol as a reinforcer for rats: factors of facilitation and constraint. Alcohol Clin Exp Res. 1988;12:599–608. doi: 10.1111/j.1530-0277.1988.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Streissguth A. Offspring effects of prenatal alcohol exposure from birth to 25 years: the Seattle Prospective Longitudinal Study. J Clin Psychol Med Settings. 2007;14:81–101. [Google Scholar]
- Sturrock RR. Age-related changes in the number of myelinated axons and glial cells in the anterior and posterior limbs of the mouse anterior commissure. J Anat. 1987;150:111–127. [PMC free article] [PubMed] [Google Scholar]
- Sturrock RR, Smart JL, Tricklebank MD. A quantitative neurohistological study of the long term effects in the rat brain of stimulation in infancy. J Anat. 1983;136:129–144. [PMC free article] [PubMed] [Google Scholar]
- Szeto HH. Maternal-fetal pharmacokinetics and fetal dose-response relationships. Ann N Y Acad Sci. 1989;562:42–55. doi: 10.1111/j.1749-6632.1989.tb21006.x. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. Animal models in alcohol research. Alcohol Res Health. 2000;24:77–84. [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, van Dijk G, Yagaloff KA, Fisher SL, Schwartz M, Burn P, et al. Central infusion of melanocortin agonist MTII in rats: assessment of c-Fos expression and taste aversion. Am J Physiol Regul Integr Comp Physiol. 1998;274:R248–R254. doi: 10.1152/ajpregu.1998.274.1.R248. [DOI] [PubMed] [Google Scholar]
- Truxell E, Spear NE. Immediate acceptance of ethanol in infant rats: ontogenetic differences with moderate but not high ethanol concentration. Alcohol Clin Exp Res. 2004;28:1200–1211. doi: 10.1097/01.alc.0000134220.34842.18. [DOI] [PubMed] [Google Scholar]
- Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31:755–765. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Tupala E, Tiihonen J. Cortical dopamine D1 receptors in type 1 and type 2 alcoholics measured with human whole hemisphere autoradiography. Psychiatry Res. 2008;162:1–9. doi: 10.1016/j.pscychresns.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Tyndale RF. Genetics of alcohol and tobacco use in humans. Ann Med. 2003;35:94–121. doi: 10.1080/07853890310010014. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, O'Shaughnessy M, Mucha RF, Kalant H. Motivational properties of ethanol in naive rats as studied by place conditioning. Pharmacol Biochem Behav. 1983;19:441–445. doi: 10.1016/0091-3057(83)90117-x. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Petrov ES, Cheslock SJ, Spear NE. A new model of ethanol self-administration in newborn rats: gender effects on ethanol ingestion through a surrogate nipple. Alcohol Clin Exp Res. 1999;23:1368–1376. [PubMed] [Google Scholar]
- Waller MB, Murphy JM, McBride WJ, Lumeng L, Li TK. Effect of low dose ethanol on spontaneous motor activity in alcohol-preferring and -nonpreferring lines of rats. Pharm Biochem Behav. 1986;24:617–623. doi: 10.1016/0091-3057(86)90567-8. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Burghardt PR, Ford KA, Wilkinson MB, Primeaux SD. Anxiolytic effects of diazepam and ethanol in two behavioral models: comparison of males and females. Pharmacol Biochem Behav. 2004;78:445–458. doi: 10.1016/j.pbb.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Windle M. Alcohol use among adolescents and young adults. Alcohol Res Health. 2003;27:79–85. [PMC free article] [PubMed] [Google Scholar]
- Winger G, Hofmann FG, Woods JH. A Handbook on Drug and Alcohol Abuse: The Biomedical Aspects. 3rd. Oxford University Press; New York: 1992. [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Witt ED. Mechanisms of alcohol abuse and alcoholism in adolescents: a case for developing animal models. Behav Neural Biol. 1994;62:168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]
- Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta TS. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol, and drug dependence. Alcohol Clin Exp Res. 1998;22:914–920. [PubMed] [Google Scholar]