Abstract
Histone deacetylase (HDAC) inhibitors are promising new epi-drugs, but the presence of both class I and class II enzymes in HDAC complexes precludes a detailed elucidation of the individual HDAC functions. By using the class II-specific HDAC inhibitor MC1568, we separated class I- and class II-dependent effects and defined the roles of class II enzymes in muscle differentiation in cultured cells and in vivo. MC1568 arrests myogenesis by (i) decreasing myocyte enhancer factor 2D (MEF2D) expression, (ii) by stabilizing the HDAC4–HDAC3–MEF2D complex, and (iii) paradoxically, by inhibiting differentiation-induced MEF2D acetylation. In vivo MC1568 shows an apparent tissue-selective HDAC inhibition. In skeletal muscle and heart, MC1568 inhibits the activity of HDAC4 and HDAC5 without affecting HDAC3 activity, thereby leaving MEF2–HDAC complexes in a repressed state. Our results suggest that HDAC class II-selective inhibitors might have a therapeutic potential for the treatment of muscle and heart diseases.
Keywords: differentiation, epigenetic drugs, HDAC inhibitor, signal transduction
Introduction
Growing evidence supports a therapeutic potential for histone deacetylases (HDACs) against diseases such as cancer (Minucci & Pelicci, 2006), neurodegenerative disorders or cardiac hypertrophy (Zhang et al, 2002; Chang et al, 2004; Vega et al, 2004; Mejat et al, 2005; Yang & Gregoire, 2005; Trivedi et al, 2007). The 18 human HDACs are divided into four subsets: class I (1–3,8), class II (4, 5, 7, 9 form the class IIa, whereas 6, 10 belong to class IIb), class III that are referred to as sirtuins (SIRT1–7) and class IV (HDAC 11). Class I, II and IV HDACs share common features such as the dependence on zinc for their enzymatic activity, whereas class III HDACs are NAD+-dependent. Although class I HDACs are nuclear and believed to act predominantly at the chromatin level, class II HDACs shuttle between the cytoplasm and nucleus and target selected physiological programmes. Class IIa HDACs compete, in a signal-responsive manner, with p300 for direct binding to myocyte enhancer factor 2 (MEF2), inhibiting the expression of MEF2-responsive genes. HDAC4 and HDAC5 are predominantly expressed in the heart, skeletal muscle and brain, the same tissues that express the highest levels of MEF2. MEF2 activates or represses myogenesis, depending on its interactions with HDACs, through the MADS/MEF2 domains, thus revealing a regulating role for chromatin modifiers in muscular gene activation (Lu et al, 2000a). Gene ablation experiments support the organ-specific function of class II HDACs; HDAC5 or HDAC9 knockout leads to cardiac hypertrophy (Chang et al, 2004; McKinsey & Olson, 2004, 2005) and HDAC4 null mice show skeletal defects possibly linked to altered RUNX2 (runt-related transcription factor 2) action (Vega et al, 2004). HDAC6 inhibition stimulates tubulin acetylation and influences cell motility (Palazzo et al, 2003; Zhang et al, 2003), but the role of HDAC6 in cellular management of misfolded proteins (Kawaguchi et al, 2003) has also been reported. Class II HDACs recruit corepressors and/or protein-modifying enzymes that, in turn, inactivate transcription factors. The diverse HDAC actions provide a strong rationale for analysing their functions by using selective inhibitors. Here we provide a comparative cell-biological analysis of pan (suberoyl anilide hydroxamic acid; SAHA) and class II selective (MC1568) HDAC inhibitors (Mai et al, 2005). We report that MC1568 blocks muscle cell differentiation by a new repressive mechanism. We also show that MC1568 acts in vivo repressing MEF2 function through a non-enzymatic mechanism. These findings are discussed in view of the therapeutic implications of selective HDAC inhibitors.
Results
Cancer cells are not affected by HDAC class II inhibition
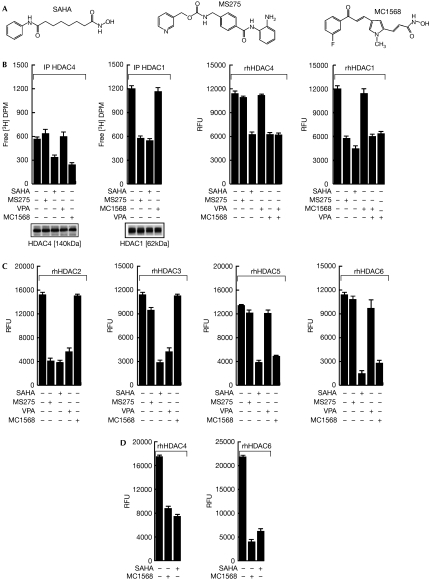
Although MC1568 (Fig 1A; Mai et al, 2005) blocks the cellular activity of HDAC4 in vitro, no inhibition is seen with MS275, which inhibits class I (1, 2 and, to a lesser extent, 3), with the exception of HDAC8, or with valproic acid (VPA; Fig 1B left). MC1568 was tested using in vitro HDAC assays with HDAC1, 2, 3, 4, 5 and 6 recombinant proteins on histone (Fig 1B right, C) and non-histone substrates (Lahm et al, 2007; Fig 1D right). The results show that MC1568 is a specific class II inhibitor. Note that recombinant human HDACs, the purity of which was verified (supplementary Fig 1A online), might still be missing further crucial components or post-translational modifications relevant for their function. Furthermore, the HDAC inhibition was proportional to the incubation time (supplementary Fig 1B online; data not shown). At the cellular level, MC1568 inhibited HDAC6 in both breast (ZR75.1) and haematological (U937) cancer cells, as revealed by hyper-acetylation of α-tubulin (supplementary Fig 2D,1G online). Although class I inhibitors block cell-cycle progression by inducing p21CIP1/WAF1 and tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis (Nebbioso et al, 2005), MC1568 failed to inhibit cell proliferation to any significant extent (supplementary Fig 2A–C,E,F online and supplementary Fig 3A–C online). Thus, the anticancer effects of pan-HDAC inhibitors might be related to their class I inhibition. However, synergistic effects owing to simultaneous class I and II inhibition or antiproliferative effects of class II HDAC inhibitors when used at higher concentrations cannot be excluded.
Figure 1.
Enzymatic inhibition of class specific histone deacetylase inhibitors. (A) The chemical structures of suberoyl anilide hydroxamic acid (SAHA), MS275 and MC1568. (B) Left: cell-based histone deacetylase (HDAC)4 and HDAC1 assay with HDAC inhibitors (all used at 5 μM, except valproic acid (VPA), which was used at 1 mM); right: in vitro HDAC4 and HDAC1 assay with HDAC inhibitors on histone substrate (all used at 5 μM, except VPA, which was used at 1 mM). (C) In vitro HDAC2, 3, 5 and 6 assay with HDAC inhibitors (all used at 5 μM, except VPA, which was used at 1 mM on histone substrate). (D) HDAC4 and HDAC6 assay on the substrate trifluoroacetyl-lysine and the specific HDAC6 substrate. DPM, distintegrations per minute; IP, immunoprecipitation; RFU, rate fluorescence unit; rhHDAC, recombinant human histone deacetylase.
MC1568 induces MEF2 repressory complex stabilization
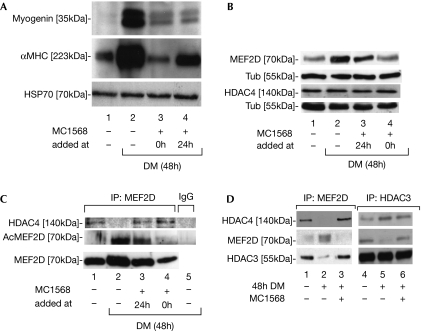
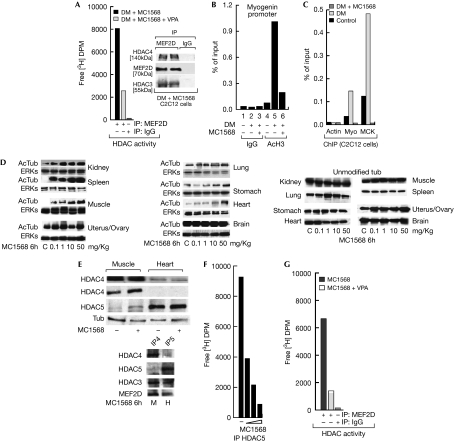
The observation that class IIa HDACs are active in a ternary complex with HDAC3–SMRT (silencing mediator for retinoid and thyroid hormone receptor)–NCOR (nuclear receptor co-repressor 1; Fischle et al, 2002) has previously precluded analysis of the individual roles of HDAC3 and class II HDACs; however, MC1568 allowed us to distinguish between the class I and class II effects. MC1568 blocked the induction of myogenin and α-myosin heavy chain (αMHC) gene expression 48 h after the addition of differentiation medium (Fig 2A lanes 1–3); even when added 24 h after the induction of differentiation, the inhibitor still blocked myogenin (Fig 2A lane 4). MEF2D induction (Lu et al, 2000a) was also repressed. HDAC4 levels were not affected (Fig 2B lane 2, Fig 2C lane 4). Interestingly, analogues of MC1568 (supplementary Fig 4A online and refs therein), reported to be inactive on class II HDACs and clearly inactive on recombinant human HDAC4 (supplementary Fig 4 online), were unable to repress myogenesis as shown by αMHC gene expression (supplementary Fig 4B online), thus confirming the specificity of the MC1568 effect and the relevance of enzymatic inhibition. Furthermore, MEF2D immunoprecipitation revealed that its acetylation (Fig 2C lanes 1 and 2) was inhibited by MC1568 (Fig 2C lane 4) and the concomitant release of co-precipitated HDAC4 was blocked (Fig 2C lanes 2–4; see also Fig 2D). Given that class II HDACs are inhibited in the presence of MC1568 (Fig 1), this deacetylation must be due to the action of HDAC class I. Indeed, co-immunoprecipitation experiments corroborated that HDAC3 forms a complex with MEF2D, which is disrupted on differentiation yet stabilized by MC1568 (Fig 2D). Neither HDAC1 nor HDAC2 were found to be associated with MEF2D (data not shown), indicating that MEF2D deacetylation is HDAC3-mediated. Complexes immunoprecipitated with MEF2D retained HDAC activity in the presence of MC1568, whereas co-exposure to VPA abolished this activity (Fig 3A). Thus, HDAC3, present in the MEF2D complex, is responsible for the maintenance of HDAC activity in the presence of MC1568. Furthermore, quantitative PCR chromatin immunoprecipitation (qPCR ChIP) revealed that histone H3 acetylation at the MEF2D target gene myogenin promoter is also inhibited by MC1568 (Fig 3B), consistent with a model in which MEF2 deacetylation and/or stabilization of the HDAC3–HDAC4–MEF2D complex are a consequence of the treatment with MC1568. Accordingly, MEF2D qPCR ChIP confirmed its presence on myogenin and muscle creatine kinase (MCK) promoters during differentiation and its absence during MC1568 co-incubation, whereas HDAC3 and HDAC4 were absent in both conditions (Fig 3C and supplementary Fig 5A online). In addition, MyoD recruitment to responsive promoters was altered by MC1568, as well as by its activity on 4REluc and 3xMEF2luc reports (supplementary Fig 5B,C online). Our results reveal that the pharmacological block of class II HDACs unexpectedly results in the deacetylation of MEF2 by HDAC3 and stabilization of the inhibitory HDAC3–HDAC4–MEF2D complex, thus blocking MEF2 target gene activation.
Figure 2.
MC1568 stabilize the MEF2–HDAC4–HDAC3 complex and blocks myogenesis of C2C12 cells. (A) Western blot of myogenin and αMHC in the absence and presence of differentiation medium (DM) with or without MC1568 added to the DM at the start of differentiation (lane 3) or after 24 h (lane 4); HSP70 indicates equal loading. (B) Western blot of MEF2D and HDAC4 expression in C2C12 cells; α-tubulin (Tub) indicates equal loading. (C) Immunoprecipitation (IP) assays using MEF2D antibodies for MEF2D IPs and HDAC4 co-IPs in C2C12 cells. Acetyl-MEF2D (AcMEF2D) levels were revealed with antibodies against acetylated lysines. (D) IPs using MEF2D and HDAC3 antibodies to show MEF2D, HDAC3 and HDAC4 complexes in differentiating C2C12 cells with or without treatment with MC1568; note that IgG-negative control IP did not show detectable bands. HDAC, histone deacetylase; HSP70, heat-shock protein 70; MEF2, myocyte enhancer factor 2; αMHC, α-myosin heavy chain.
Figure 3.
MC1568 blocks MEF2D transcriptional activity in C2C12 cells and shows inhibitory activities in mice. (A) Histone deacetylase (HDAC) assay on myocyte enhancer factor 2D (MEF2D) immunoprecipitation (IP) from C2C12 cells in the presence of MC1568 (5 μM) with or without valproic acid (VPA; 1 mM); the inset shows the quantities of co-immunoprecipitated MEF2D, HDAC4 and HDAC3. (B) Chromatin immunoprecipitation (ChIP) assay of acetyl-H3 (AcH3) levels on the myogenin promoter in C2C12 cells. (C) ChIP assay of MEF2D on the myogenin (Myo) and muscle creatine kinase (MCK) promoters in C2C12 cells. (D) MC1568 increases acetylation of tubulin (AcTub) in selected organs of mice in a dose-dependent manner. (E) HDAC4 and HDAC5 expression levels in skeletal muscle and heart after MC1568 treatment (50 mg/kg); note that although differentially expressed both HDACs are present. Bottom: IPs using HDAC4 and HDAC5 antibodies to reveal MEF2D and HDAC3 complexes in skeletal muscle and heart. (F) Heart-extract-HDAC5 immunoprecipitation assay on treatment with 1, 10 and 50 mg/kg MC1568. (G) HDAC assay from heart extracts on MEF2D IP in the presence of MC1568 with or without 1 mM VPA. DM, differentiation medium; DPM, disintegrations per minute; ERK, extracellular signal regulated kinase.
Tissue-selective activity of MC1568 in mice
We tested the HDAC inhibitory potential of MC1568 in CD1 (Crl:CD-1(ICR)) outbred mice. Administration of the 50 mg/kg dose every 2 days for a 10-day period did not result in detectable liver toxicity, weight loss or behavioural abnormalities. Even after a 6-h acute administration of MC1568, tubulin acetylation increased in organs such as the kidney, spleen, muscle and heart. No effects were observed in the stomach, ovary/uterus, lung or brain where tubulin was differently but constantly acetylated (Fig 3D). The observations that the muscle and heart showed different and reciprocal expression of HDAC4 and HDAC5 (Fig 3E), and that in both cases these HDACs formed complexes with HDAC3 and MEF2D (Fig 3E bottom part) suggest that in vivo the MC1568 might act in a similar manner to that in C2C12 cells (Fig 3C). Although immunoprecipitation experiments carried out using HDAC5 reveal a clear dose-dependent HDAC5 inhibition in heart extracts from MC1568-treated mice (Fig 3F), MEF2D immunoprecipitations from identical heart extracts show residual HDAC activity that can be inhibited only by class I HDAC inhibitors, thus revealing that in vivo the MEF2D complex also retains class I HDAC-dependent deacetylase activity in the presence of MC1568. Note that in this respect the myogenin expression levels are altered by MC1568 administration in a dose-dependent manner in vivo (supplementary Fig 4C online), thus strongly suggesting a mechanism for (de)regulation of myogenesis for MC1568.
Discussion
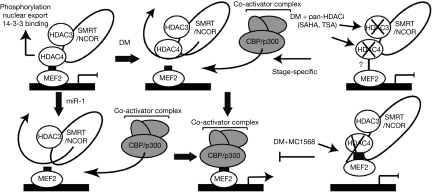
We have shown that MC1568 (Mai et al, 2005; Butler & Kozikowski, 2008; Itoh et al, 2008) acts at three distinct levels to interfere with myogenic signalling (Fig 4). First, it blocks the class II HDAC enzymatic activity, concomitantly stabilizing the interaction of MEF2 with the HDAC4–NCOR–HDAC3 complex (Fig 2C,D), without altering HDAC4 acetylation levels (supplementary Fig 6 online). This sustains muscle-specific gene suppression. Second, the stabilization of the HDAC4–MEF2D complex indirectly stabilizes the association of HDAC3 with MEF2 (Fig 2D lanes 3 and 9), thereby providing an enzymatically active HDAC (Fig 3A). Our finding is fully compatible with the observation that the HDAC catalytic domain mediates the repression activity of the carboxy-terminal regions of class II HDACs (Lu et al, 2000b), whereas the amino-terminal extensions seem to repress transcription by recruiting class I HDACs and the corepressor C-terminal binding protein (Zhang et al, 2001). In support of this idea, the N-terminal region of class II HDACs (in particular HDAC4) has been reported to be responsible for MEF2 repression in a deacetylase-independent manner (Lomonte et al, 2004). That inhibition of class II HDAC enzymatic activity leads to MEF2D repression reveals that HDAC4 absence (Chen et al, 2006; Potthoff et al, 2007) can have the opposite effect of blocking its enzymatic activity. Third, inhibition of HDAC4 during differentiation does not block MEF2D deacetylation (Fig 2C). Indeed, HDAC4 might not function as a MEF2 deacetylase (Zhao et al, 2005). HDAC3, present in the complex, is responsible for MEF2D deacetylation, as its activity is not blocked by MC1568. That HDAC3 is necessary for cardiac energy metabolism has recently been confirmed in a study on a conditional HDAC3-null allele (Montgomery et al, 2008). The inability to recruit co-activators and the sustained activity of class I HDACs are likely to account for the low histone H3 acetylation on MEF2 target chromatin (Fig 3B). Finally, MC1568 stabilization of HDAC4–MEF2D binding might account for the absence of MEF2D (Fig 3C) and MyoD (supplementary Fig 5B online) on responsive promoters. The fact that the activatory role of MyoD on myogenesis measured in transfection on two responsive reporters is impaired by MC1568 (supplementary Fig 5C online) strongly supports our hypothesis, not excluding the possibility that additional mechanisms might still occur. In vivo MC1568 showed organ-selective effects. The fact that MC1568 showed inhibitory action on HDAC5 in vitro and in vivo (Fig 1, 3), and the data from the mice heart and skeletal muscles confirm the mechanism described in C2C12 cells (Fig 3; supplementary Fig 4C,5 online). Recent studies have pointed to the possible use of HDAC inhibitors in the treatment of cardiac hypertrophy (Antos et al, 2003; McKinsey & Olson, 2005; Trivedi et al, 2007). Interestingly, class II HDACs function as signal-responsive repressors of cardiac hypertrophy and heart failure, presumably through their ability to block MEF2 target genes (Zhang et al, 2002). Therefore, class II-specific HDAC inhibitors, which inhibit HDAC activity and block MEF2-mediated transactivation, might represent new tools. The challenge will be to ensure that sufficient MEF2 activity is maintained for homoeostatic control in the heart, as MEF2 ablation leads to cardiomyopathy and an increased risk of myocardial infarction in humans (Naya et al, 2002; Wang et al, 2003). Our results show new mechanisms by which HDAC class II inhibition blocks myogenesis and demonstrate that HDAC class II inhibition is fundamentally different from the inhibition of HDAC expression. Future work will reveal the potential of class II inhibitors in the treatment of cardiac diseases.
Figure 4.
Regulatory complexes formed with MEF2 at responsive genes. Myocyte enhancer factor 2 (MEF2) recruits class II histone deacetylases (HDACs) such as HDAC4 to responsive genes, resulting in repression. HDAC4 binds to HDAC3 on a co-repressor (nuclear receptor corepressor (NCOR)/silencing mediator of retinoic acid and thyroid hormone receptor (SMRT)) platform. Differentiation medium (DM) results in the dissociation of the MEF2–HDAC4 interaction, allowing association of a co-activator complex and target gene expression, as CREB (cyclic AMP response element binding)-binding protein (CBP)/p300 and HDAC4 bindings to MEF2 are mutually exclusive. The miR-1 achieves the same effect by down regulating HDAC4. HDAC inhibition does not result in the same events as the removal of HDAC4. Pan-histone decetylase inhibitors (HDACi) block both HDAC3 and HDAC4 and, depending on the stage of myogenesis, might block or stimulate differentiation. By contrast, MC1568 blocks HDAC4 activity but enhances the HDAC4–MEF2 interaction, thus resulting in enforced repression. SAHA, suberoyl anilide hydroxamic acid; TSA, tricostatin A.
Methods
Drugs. SAHA (MERCK, Readington, NJ, USA), MS275 (Schering AG, Berlin-Wedding, Germany), MC1568 (Mai et al, 2005), MC1617 and MC1757 were dissolved in dimethylsulphoxide and used as indicated; VPA was purchased from SIGMA (St Louis, MO, USA).
Cell lines. U937 and ZR75.1 were cultured using standard procedures. C2C12 cells were cultured in DMEM supplemented with 20% FCS (‘C2C12 growth medium') or with 2% horse serum (‘C2C12 differentiation medium') and 50 μg/ml penicillin-streptomycin and 2 mM glutamine.
Cell-based human HDAC1 and HDAC4 assay. Assays were carried out as reported previously (Mai et al, 2005). Also see supplementary information online.
Fluorimetric human recombinant HDAC1, 2, 3, 4, 5 and 6 assays. The HDAC assay was carried out according to the supplier's instructions (BIOMOL, Plymouth Meeting, PA, USA), using the specific purified recombinant proteins. Also see supplementary information online.
HDAC4 and HDAC6 assay on non-histone substrates. The assay has been carried out as described in Lahm et al (2007) on the trifluoroacetyl lysine substrate or on the HDAC6 selective substrate (Heltweg et al, 2004). Cell-cycle differentiation and apoptosis studies were carried out as described in Mai et al (2005, 2006) and Nebbioso et al (2005).
Antibodies. Antibodies against HDAC4, acetyl-tubuline and tubulin were obtained from SIGMA and Abcam (Cambridge, UK); HDAC1 and HDAC5 from Abcam; p21 and MEF2D from Becton Dickinson (Franklin Lakes, NJ, USA); acetyl lysines from Upstate (Bedford, MA, USA); myogenin, MHC, HSP70 and HDAC3 from Santa Cruz (Santa Cruz, CA, USA) and Abcam.
Immunoprecipitation assay and coupling. See supplementary information online.
C2C12 transfection experiments. See supplementary information online.
Chromatin Immunoprecipitation assay. Acetyl H3 (Upstate), MEF2D (Becton Dickinson), MyoD and purified IgG (Santa Cruz) were used. ChIP assays were carried out as described in Nebbioso et al (2005) and Denissov et al (2007). For details see supplementary information online.
Muscle differentiation assays. C2C12 cells were incubated with growth (C) or differentiation medium (D) for 48 h. C2C12 cells were incubated with (D) and treated with MC1568 at both 0 and 24 h after the starting of differentiation. Cells were collected at 48 h and Western blots, IP or ChIP were carried out.
Mice treatment and tissue homogenization. See supplementary information online. Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
This paper has been written in memory of Gianni Bollino, an unforgettable friend. We thank Schering AG for MS275, MERCK for suberoyl anilide hydroxamic acid, P. Gallinari for the trifluoroacetyl-lysine substrate, L. Bagella for the 4REluc and E. Olson for the 3xMEF2luc. This work was supported by EU LSHC-CT2005-518417 (L.A., H.G.); PRIN2006052835_003 (L.A.); Regione Campania L.5 2005 (L.A.); PRIN2006 (A.M.); AIRC (A.M., L.A.); FUTURA onlus (A.B.); Fondazione onlus Luigi Califano (L.A.).
Footnotes
The authors declare that they have no conflict of interest.
References
- Antos CL, McKinsey TA, Dreitz M, Hollingsworth LM, Zhang CL, Schreiber K, Rindt H, Gorczynski RJ, Olson EN (2003) Dose-dependent blockade to cardiomyocyte hypertrophy by histone deacetylase inhibitors. J Biol Chem 278: 28930–28937 [DOI] [PubMed] [Google Scholar]
- Butler KV, Kozikowski AP (2008) Chemical origins of isoform selectivity in histone deacetylase inhibitors. Curr Pharm Des 14: 505–528 [DOI] [PubMed] [Google Scholar]
- Chang S, McKinsey TA, Zhang CL, Richardson JA, Hill JA, Olson EN (2004) Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol Cell Biol 24: 8467–8476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ (2006) The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38: 228–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denissov S, van Driel M, Voit R, Hekkelman M, Hulsen T, Hernandez N, Grummt I, Wehrens R, Stunnenberg H (2007) Identification of novel functional TBP-binding sites and general factor repertoires. EMBO J 26: 944–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, Voelter W, Verdin E (2002) Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell 9: 45–57 [DOI] [PubMed] [Google Scholar]
- Heltweg B, Dequiedt F, Marshall BL, Brauch C, Yoshida M, Nishino N, Verdin E, Jung M (2004) Subtype selective substrates for histone deacetylases. J Med Chem 47: 5235–5243 [DOI] [PubMed] [Google Scholar]
- Itoh Y, Suzuki T, Miyata N (2008) Isoform-selective histone deacetylase inhibitors. Curr Pharm Des 14: 529–544 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP (2003) The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115: 727–738 [DOI] [PubMed] [Google Scholar]
- Lahm A (2007) Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc Natl Acad Sci USA 104: 17335–17340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonte P, Thomas J, Texier P, Caron C, Khochbin S, Epstein AL (2004) Functional interaction between class II histone deacetylases and ICP0 of herpes simplex virus type 1. J Virol 78: 6744–6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, McKinsey TA, Zhang CL, Olson EN (2000a) Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol Cell 6: 233–244 [DOI] [PubMed] [Google Scholar]
- Lu J, McKinsey TA, Nicol RL, Olson EN (2000b) Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc Natl Acad Sci USA 97: 4070–4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai A, Massa S, Pezzi R, Simeoni S, Rotili D, Nebbioso A, Scognamiglio A, Altucci L, Loidl P, Brosch G (2005) Class II (IIa)-selective histone deacetylase inhibitors. 1. Synthesis and biological evaluation of novel (aryloxopropenyl)pyrrolyl hydroxyamides. J Med Chem 48: 3344–3353 [DOI] [PubMed] [Google Scholar]
- Mai A et al. (2006) Small-molecule inhibitors of histone acetyltransferase activity: identification and biological properties. J Med Chem 49: 6897–6907 [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Olson EN (2004) Cardiac histone acetylation--therapeutic opportunities abound. Trends Genet 20: 206–213 [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Olson EN (2005) Toward transcriptional therapies for the failing heart: chemical screens to modulate genes. J Clin Invest 115: 538–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejat A, Ramond F, Bassel-Duby R, Khochbin S, Olson EN, Schaeffer L (2005) Histone deacetylase 9 couples neuronal activity to muscle chromatin acetylation and gene expression. Nat Neurosci 8: 313–321 [DOI] [PubMed] [Google Scholar]
- Minucci S, Pelicci PG (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6: 38–51 [DOI] [PubMed] [Google Scholar]
- Montgomery RL, Potthoff MJ, Haberland M, Qi X, Matsuzaki S, Humphries KM, Richardson JA, Bassel-Duby R, Olson EN (2008) Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J Clin Invest 118: 3588–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya FJ, Black BL, Wu H, Bassel-Duby R, Richardson JA, Hill JA, Olson EN (2002) Mitochondrial deficiency and cardiac sudden death in mice lacking the MEF2A transcription factor. Nat Med 8: 1303–1309 [DOI] [PubMed] [Google Scholar]
- Nebbioso A et al. (2005) Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat Med 11: 77–84 [DOI] [PubMed] [Google Scholar]
- Palazzo A, Ackerman B, Gundersen GG (2003) Cell biology: tubulin acetylation and cell motility. Nature 421: 230. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Wu H, Arnold MA, Shelton JM, Backs J, McAnally J, Richardson JA, Bassel-Duby R, Olson EN (2007) Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Invest 117: 2459–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi CM et al. (2007) Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3 beta activity. Nat Med 13: 324–331 [DOI] [PubMed] [Google Scholar]
- Vega RB et al. (2004) Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 119: 555–566 [DOI] [PubMed] [Google Scholar]
- Wang L, Fan C, Topol SE, Topol EJ, Wang Q (2003) Mutation of MEF2A in an inherited disorder with features of coronary artery disease. Science 302: 1578–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Gregoire S (2005) Class II histone deacetylases: from sequence to function, regulation, and clinical implication. Mol Cell Biol 25: 2873–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN (2002) Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110: 479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, McKinsey TA, Lu JR, Olson EN (2001) Association of COOH-terminal-binding protein (CtBP) and MEF2-interacting transcription repressor (MITR) contributes to transcriptional repression of the MEF2 transcription factor. J Biol Chem 276: 35–39 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P (2003) HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J 22: 1168–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sternsdorf T, Bolger TA, Evans RM, Yao TP (2005) Regulation of MEF2 by histone deacetylase 4- and SIRT1 deacetylase-mediated lysine modifications. Mol Cell Biol 25: 8456–8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information