Abstract
Slit proteins are secreted ligands that interact with the Roundabout (Robo) receptors to provide important guidance cues in neuronal and vascular development. Slit–Robo signalling is mediated by an interaction between the second Slit domain and the first Robo domain, as well as being dependent on heparan sulphate. In an effort to understand the role of the other Slit domains in signalling, we determined the crystal structure of the fourth Slit2 domain (D4) and examined the effects of various Slit2 constructs on chick retinal ganglion cell axons. Slit2 D4 forms a homodimer using the conserved residues on its concave face, and can also bind to heparan sulphate. We observed that Slit2 D4 frequently results in growth cones with collapsed lamellipodia and that this effect can be inhibited by exogenously added heparan sulphate. Our results show that Slit2 D4–heparan sulphate binding contributes to a Slit–Robo signalling mechanism more intricate than previously thought.
Keywords: neurons, guidance cues, signalling
Introduction
Slit ligand proteins secreted by the midline are important in neuronal development as they mediate a change in axonal response from attraction to repulsion. This change occurs when Slit binds to the transmembrane Roundabout (Robo) receptors of commissural neurons that have crossed the spinal cord floorplate (reviewed in Dickson & Gilestro, 2006). The Slits are large multi-domain proteins characterized by four consecutive leucine-rich repeat (LRR) domains (D1–D4) at their amino terminus. The minimal component required for Slit–Robo signalling is the N-terminal LRR region of Slit2 (Chen et al, 2001; Nguyen Ba-Charvet et al, 2001), specifically Slit D2 (Howitt et al, 2004) and a fragment spanning Robo domains Ig1 and Ig2 (Ig1–2; Liu et al, 2004). The structure of Slit2 D2 bound to Robo1 Ig1 showed that these domains interact through highly conserved electrostatic and hydrophobic regions (Morlot et al, 2007b). Heparan sulphate (HS), a highly charged polysaccharide, is essential in the repulsive guidance activities of Slit2 (Hu, 2001; Inatani et al, 2003), and Syndecan, a transmembrane protein containing HS, was later identified as a necessary component for Slit–Robo signalling (Johnson et al, 2004; Steigemann et al, 2004). Heparin, a chemically related analogue of HS, binds to both Slit2 D2 and Robo Ig1–2 (Hussain et al, 2006). In fact, the recent structure of Drosophila Robo Ig1–2 in complex with heparin shows that the Robo and Slit D2 heparin-binding sites are in close proximity, resulting in a continuous binding patch (Fukuhara et al, 2008). These results suggest that HS forms an integral part of the Slit–Robo signalling complex.
Slit dimerization is mediated by D4 and might be important for Slit–Robo signalling (Howitt et al, 2004). Here, we present the structure of the Slit2 D4 domain and show that it forms a stable non-symmetrical dimer through highly conserved residues on its concave face. Functional studies on the effect of Slit2 constructs on chick retinal–ganglion-cell (RGC) axons show that a Slit2 fragment spanning domains D2–D4, as well as the individual domains D2 and D4, elicits a response in vitro. Furthermore, we show that Slit2 D4 can bind to HS in vitro and that exogenously added HS can inhibit the collapse response of chick RGC growth cones to Slit2 D4. Taken together, these results provide compelling evidence that Slit2 dimerization is functionally relevant and that heparin binding to Slit2 is more complex than understood earlier.
Results And Discussion
Overall structure
The Slit2 D4 structure was solved by molecular replacement using a modified model of human Slit2 D3 (Morlot et al, 2007a) and was refined at 1.8 Å resolution. The crystallographic asymmetrical unit contains two molecules in an anti-parallel dimeric arrangement. Each monomer adopts a slightly curved right-handed LRR fold similar to that of other Slit2 LRR domains (Morlot et al, 2007a, 2007b). Slit2 D4 has five LLRs and two flanking cysteine-rich capping domains (Fig 1A). The concave face is composed of a continuous seven-stranded β-sheet. The first two β-strands are anti-parallel and form the N-terminal cap, whereas each of the five LRRs contributes a parallel β-strand. The convex surface is composed of variable loops and short helices. As seen for Slit2 D2 and D3, the N-terminal cap is a double hairpin stabilized by two disulphide bridges, whereas the carboxy-terminal cap adopts an irregular fold containing a 12-residue α-helix, two 310 helices and two disulphide bridges.
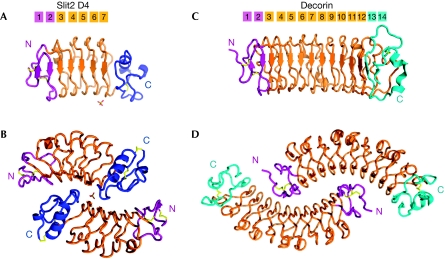
Figure 1.
Structure of Slit2 D4 and the Decorin dimer in similar orientations. The Slit2 D4 and decorin amino-terminal caps are coloured purple, whereas their carboxy-terminal caps are coloured blue and cyan. The LRRs are coloured orange and the disulphide bridges are in yellow (Slit2 D4 N-terminal cap: Cys727–Cys733 and Cys731–Cys740; Slit2 D4 C-terminal cap: Cys863–Cys886 and Cys865–Cys907). The sulphate ion is indicated by sticks. (A) Human Slit2 D4 monomer. (B) Human Slit2 D4 dimer. (C) Bovine decorin monomer. (D) Bovine decorin dimer.
Slit2 D4 dimer
Multi-angle laser light scattering (MALLS) confirmed that Slit2 D4 exists as a homodimer in solution (data not shown). The dimer has a compact globular shape (30Å × 60Å × 70 Å) (Fig 1B) and shows a quaternary structure resembling that of the extracellular-matrix protein decorin (Fig 1C,D; Scott et al, 2004). Both decorin and Slit2 D4 dimerize through their concave face, but in distinct ways. The decorin dimer is mainly formed by interactions involving the N-terminal capping domain, LRR1 and the central LRRs, but not the C-terminal LRRs. In Slit2 D4, the entire domain, including the C-terminal cap, is involved. In addition, unlike decorin, the Slit2 D4 dimer is not perfectly symmetrical, as the two monomers differ in conformation (r.m.s.d.181Cα=0.45 Å). The most significant difference occurs between residues 875 and 881, where the Cα positions deviate by as much as 2.4 Å. This region corresponds to the ‘β-switch' loop, which adopts different conformations in the free and ligand-bound structures of glycoprotein Ib-α (Huizinga et al, 2002) and which characterizes this class of LRR domains.
The dimer interface
Dimerization buries 1,240 Å2 of the solvent-accessible surface for each monomer of Slit2 D4. This represents 13.6% of the total surface, which is significantly larger than the buried Slit2 D2 surface on binding to Robo1 Ig1 (657 Å2). Dimerization involves mainly electrostatic interactions, but several hydrophobic residues from LRR 2–5 and the C-terminal capping domain (Leu781, Ile807, Leu829, Ala855, Ile883, Leu896 and Leu898) also participate. An important feature of the dimer interface is the β-switch loop, which reaches deep into the concave surface of the opposite monomer, forming extensive intermolecular contacts (Fig 2A–C). The atomic B-factors of the loop are comparatively higher in one of the two monomers, suggesting that the dimer tolerates flexibility within its interface, a property linked to high affinity (Seiradake et al, 2006).
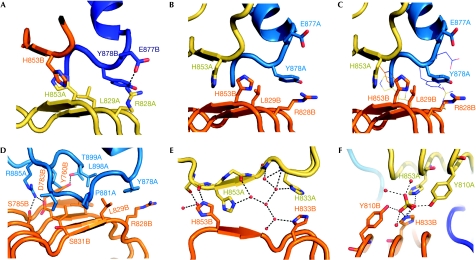
Figure 2.
Molecular interactions at the dimer interface. (A) Central region of the Slit2 D4 dimerization interface. Residues that differ in chains (A) and (B) are shown as sticks. The β-switch loop of chain (B) is coloured purple. (B) The asymmetric dimer region with the chain (A) β-switch loop is coloured purple. (C) Chain (A) and (B) superpositions. The orientation of the ribbon and sticks model is as in (B) (chain (A) on top, chain (B) at the bottom). Lines represent the alternative residue conformation in the opposite chains, as seen in (A). (D) The β-switch loop of chain (A) and interacting residues. (E) The central four-histidine cluster and water network. (F) The sulphate binding site.
The dimer interface contains a total of 11 direct intermolecular hydrogen bonds. Arg885 contributes six of these by forming a salt bridge with Asp762 and Asp783 and a hydrogen bond with Ser785 (Fig 2D). Arg828 and Glu877 form an additional salt bridge, which is non-symmetrical because of differences in the two β-switch loops (Fig 2A,B). Similarly, there are non-symmetrical hydrogen bonds involving His853: to avoid steric clashes with the adjacent β-switch loop, the two His853 side chains hydrogen bond to either the Ser852 or the Tyr878 carbonyl group (Fig 2C). This results in the His853 imidazole ring of monomer A becoming sandwiched between Pro881 and the His853 imidazole ring of monomer B. Two additional hydrogen bonds are observed between Tyr760 and Thr899, with the Tyr side chain also maintaining hydrophobic interactions. Last, 18 well-ordered water molecules mediate an extensive network of hydrogen bonds, which further stabilize the dimer interface (Fig 2E).
Heparan sulphate binding
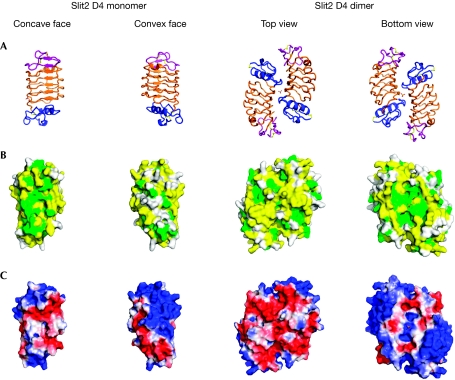
The surface of the Slit2 D4 homodimer shows a striking electrostatic potential distribution, with the top surface being highly negatively charged and the bottom mainly positively charged (Fig 3A,C). More specifically, the bottom face shows three basic patches, one from each monomer and one from the dimer interface. Contributing to these patches are residues Lys746, Arg788, Arg812 and Arg814. The distribution of basic residues extends around the sides to cover the N-terminal half of each molecule in the dimer (Fig 3C). Here, the contributing residues include Lys750, Arg754, Lys771 and Lys777. In the basic patch at the dimer interface, we observe a well-ordered sulphate ion (Fig 3A,C). This ion is held tightly in place, forming hydrogen bonds with Tyr810 and His833 from each monomer and four water-mediated hydrogen bonds with the protein backbone (Fig 2F).
Figure 3.
Sequence conservation and electrostatic surface representation of Slit2 D4 with the sulphate ion in sticks. (A) Structural representation, in four orientations and coloured as in Fig 1. (B) Sequence surface conservation as viewed in (A) and coloured in green: identical; yellow: partly conserved; white: poorly conserved. (C) Electrostatic surface potential calculated with Adaptive Poisson–Boltzmann Solver (APBS; Baker et al, 2001; from −2 coloured in red: negatively charged, to +2 coloured in blue: positively charged).
Heparin is an integral part of Slit–Robo signalling and has been shown to bind to Slit2 D2, the C-terminal cysteine-knot domain of Slit2 and Robo1 Ig1–2. Although heparin has not been implicated in binding to Slit2 D4, the large basic regions and the conspicuous sulphate-binding site suggest a possible interaction. To test this hypothesis, we carried out surface plasmon resonance experiments in which either Slit2 D2 or Slit2 D4 was flowed over a HS-conjugated sensor chip. We observed a strong interaction between Slit2 D4 and HS, characterized by a dissociation constant (Kd) of 50 nM (supplementary Fig S5B online). Although this domain binds to HS more weakly than Slit2 D2 (Kd=10 nM; supplementary Fig S5C online), the results nevertheless support our structure-based hypothesis and we were able to reduce HS binding by reversing the charge of the large basic region on the bottom face (data not shown).
Biological activity of Slit2 constructs
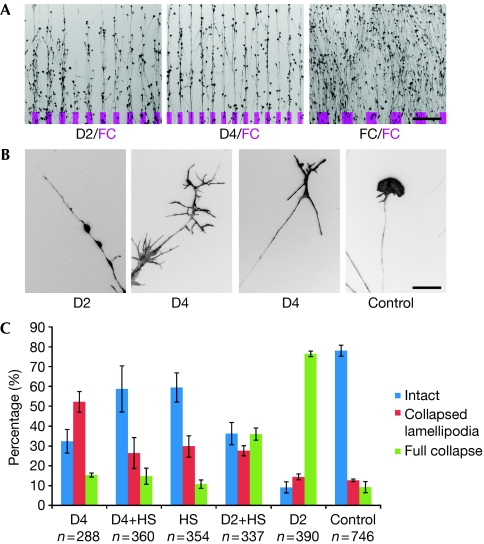
Full-length Slit2 and Slit2 D2 have previously been shown to cause the collapse of chick and Xenopus RGC axon growth cones (Hussain et al, 2006; Piper et al, 2006). We carried out similar in vitro assays with our Slit2 constructs on chick RGCs and tested them in a ‘stripe and collapse' assay. When presented in substrate-bound form, the Slit2 D2–D4 fragment (encompassing D2, D3 and D4), Slit2 D2 and Slit2 D4 are all equally avoided by growing axons that can choose between the respective Slit2 construct and laminin (data not shown) or FC (fragment constant, Fig 4A), a neutral protein substrate. In the collapse assay, both soluble Slit2 D2–4 (data not shown) and Slit2 D2 resulted in typical growth cone collapse with fully retracted lamellipodia and filopodia (Fig 4B). Here, Slit2 D2–4 induced a collapse of more than 75% of the growth cones at protein concentrations 5–10 times lower than those used with the Slit2 D2 domain (data not shown). Slit2 D4 also affected the growth of chick RGCs at concentrations similar to those used with Slit2 D2. In this case, however, the majority of the growth cones showed a ‘bare branch morphology', characterized by elongated filopodia and collapsed lamellipodia (Fig 4B). Quantifications of growth-cone responses to the different Slit2 constructs are given in Fig 4C.
Figure 4.
Biological activity of Slit2 contructs. (A) Stripe assay with axons given the choice between substrate-bound lanes covered with Slit2 D2, Slit2 D4 and FC compared with lanes covered with FC (indicated in magenta). Scale bar, 200 μm. (B) Representative single growth cones after treatment with soluble Slit2 D2 (full collapse), Slit2 D4 (bare branch morphology) and control treatment (intact morphology). Scale bar, 20 μm. (C) Heparan sulphate (HS) oligomers (12-mers) alter the effect of Slit2 D2 and D4 on growth cones incubated for 15 min with 100 μg/ml HS oligomers, followed by incubation for 30 min with 100 μg/ml HS oligomers and 50 μg/ml Slit2 D2 or D4, respectively. Blue bars indicate the percentage of intact growth cones, red bars indicate growth cones with bare branch morphology and green bars indicate fully collapsed growth cones. Bars indicate the s.e.m.; n indicates the number of growth cones scored.
Soluble heparin inhibits the collapse of Xenopus RGCs induced by full-length Slit2 but not by Slit2 D2 (Hussain et al, 2006). This led to the conclusion that heparin might have a dual function in Slit–Robo signalling, reflecting the heparin-binding properties of the Slit2 D2 and Slit2 CT domains. In line with this, we find that soluble HS oligomers (12-mers) inhibit the collapse of chick RGC growth cones induced by Slit2 D2–4 (data not shown). We also found a partial reduction of Slit2 D2-induced collapse by HS oligomers (P<0.005; χ2-test). More interesting is the fact that the addition of HS oligomers to the cell-culture medium abolished the bare branch morphology of growth cones induced by Slit2 D4 and led to a morphology indistinguishable from controls using HS oligomers alone (P<0.005; χ2-test) (Fig 4C).
Slit D4 sequence comparison
Slit D4 domains are highly conserved among bilateral organisms (for example, human Slit2 D4 has 98% and 53% sequence identity to its murine and Drosophila orthologues; supplementary Fig S6 online). The most highly conserved residues map to the concave dimerization surface of the molecule, whereas the convex face is much less conserved (Fig 3B). The conservation of residues in the D4 dimer interface and the large, accessible surface area buried on dimerization (∼2,500 Å2) strongly suggest that all Slit family members dimerize in a similar manner through the fourth LRR domain. Out of the 21 residue positions involved in dimerization, 11 are invariant across the sequence alignment, including a continuous motif comprising residues 882–886 within the β-switch loop. Seven residue positions are either identical or show highly conservative substitutions across homologues. Only three positions show chemically significant variations: two (Leu781 and Glu877) are located at the periphery of the dimer interface, whereas the third (Lys879) participates in dimer formation exclusively through main-chain atoms. Hence, variations at these positions are not expected to significantly compromise dimerization. In addition, our attempts to disrupt the dimerization interface by multiple mutageneses have so far failed, indicating a high stability of the dimer (supplementary information online).
The sulphate-binding residues, Tyr810 and His833 (which are integral to the dimerization interface), are strictly conserved across the Slit D4 domains. By contrast, the potential heparin-binding residues on the bottom face (Lys746, Arg788, Arg812 and Arg814) and those on the N-terminal convex face (Lys750, Arg754, Lys771 and Lys777) are less well conserved in other Slit family members (supplementary Fig S6 online). In fact only three of these seven residues are conserved in Drosophila. This might explain why no heparin interaction with Drosophila Slit D4 was observed (Hussain et al, 2006). The heparin-binding potential of the fourth LRR domain is therefore likely to vary among Slit homologues, which might be important for modulating the functional properties of different Slits.
A functional role for Slit2 D4
Our in vitro assays show that chick RGCs were responsive to similar concentrations of Slit2 D2 and D4. In contrast to Slit2 D2, which leads to a full collapse and retraction of the growth cone, Slit2 D4 has a less pronounced effect, collapsing only lamellipodia and leaving filopodia mainly intact. We also observed that Slit2 D2–D4 fragment elicited growth cone collapse at much lower concentrations than Slit2 D2. Thus, although dimerization might not be essential for an axonal response, it clearly has a potentiating effect. How Slit2 D4 elicits a response is unclear, as Slit2 D4 is not known to bind to a Robo receptor and does not bind to Robo1 (data not shown). One possibility is that the interaction of Slit2 D4 with cell-surface HS elicits a response. Such an interaction might feasibly be mediated by Syndecan, which is known to be important for Slit–Robo signalling in Drosophila (Johnson et al, 2004; Steigemann et al, 2004). These studies suggested that Syndecan acts as a co-receptor by presenting Slit to Robo and stabilizing the complex, but the possibility that the highly conserved cytoplasmic domains of Syndecan might have a more subtle modulatory function was not ruled out.
More recent studies support the Slit–Robo HS stabilizing effect, where the loss in response of Xenopus RGC axons towards Slit2 D2 on treatment with heparinase 1 can be reversed by the addition of soluble heparin (Hussain et al, 2006). However, it is not understood why chick and Xenopus RGCs are unresponsive to full-length Slit2 constructs incubated with heparin but remain responsive to heparin-incubated Slit2 D2. This has been suggested to be because heparin has a dual role in signaling: modulating the Slit2 distribution through its CT region; and participating in a ternary Slit2–Robo1–heparin complex that is necessary for signalling (Hussain et al, 2006). In our studies, Slit2 D2–4 complex, which lacks the CT region, Slit2 D4 and, at least partly, Slit2 D2 remain sensitive to incubation with HS. These results are indicative of a more complex signalling mechanism that involves HS.
Syndecans are better known as co-receptors but they have been shown to signal independently (Couchman, 2003). In Caenorhabditis elegans it has been reported that SAX-3/Robo has a SLT-1/cSlit1 independent function, which is dependent on certain heparin modifications (Bulow & Hobert, 2004), and that Syndecan can function autonomously in axon guidance (Rhiner et al, 2005). Syndecan can also regulate neuronal growth-cone signalling by interacting with other HS receptors, such as the leukocyte-common antigen-related (LAR)-receptor tyrosine phosphatase in Drosophila (Fox & Zinn, 2005). In addition, others have shown that Robo can bind to heparin alone (Hussain et al, 2006; Fukuhara et al, 2008). Our observation that Slit2 D4 is active on growing RGC axons in a HS-dependent manner raises the possibility that Slit2 D4 might interact with Syndecan to induce a partial cytoskeletal rearrangement, resulting in the bare branch morphology. Indeed, other HS-binding receptors at the cell surface, such as LAR or Robo, could also be implicated in modulating such a signal. In conclusion, our results show an important role for Slit2 dimerization through Slit2 D4. Furthermore, that purified Slit2 D4 has a HS-dependent effect suggests that D4 has a more complex part in Slit2 signalling than as a simple dimerization module. Clearly, further studies are required to identify the HS-dependent interaction partners of Slit2 D4 on the cell surface.
Methods
Preparation of Slit2 domains. Human Slit2 constructs (Slit2 D2 (residues 271–479), Slit2 D4 (residues 726–907) and Slit2 D2–4 (residues 271–907)) were essentially produced and purified as described earlier (Morlot et al, 2007a). Further detail can be found in the supplementary information online.
Crystallization and X-ray data collection of Slit2 D4. Purified and deglycosylated Slit2 D4 was crystallized from solutions containing 0.2 M LiSO4, 0.1 M Tris pH 8.5 and 30% polyethyleneglycol 4000. The crystals were frozen in a cryoprotectant solution containing all solution components and 10–15% glycerol, and diffraction data were collected at the European Synchrotron Radiation Facility (Grenoble, France; beamline ID14-EH4). Further details can be found in the supplementary information online.
Growth cone stripe and collapse assays. RGCs were dissected and cultivated overnight in F12 medium on a laminin-coated coverslip. To test the effect of a protein, the protein solution was mixed with culture medium. The culture medium of the explants was exchanged with the protein-containing medium and the cultures were incubated for 30 min at 37°C. To test the inhibitory effect of HS the RGCs were first incubated with fresh medium containing HS, followed by incubation with medium containing HS and protein solution. The cultures were then fixed, stained with fluorescently labelled phalloidin and photographed for growth-cone collapse evaluation. For the stripe assay, the protein was not added to the culture medium after outgrowth, but rather bound in a striped pattern onto the cover slip on which the axons were cultivated. To produce a striped pattern, a silicone matrix with small channels was placed on a coverslip and filled with protein solution. The pattern was then coated with laminin. To make the patterns visible, the protein of interest was mixed with a fluorescently labelled inactive protein. Further details on the in vitro stripe and collapse assays that were used to analyse the effect of Slit2 constructs on chick RGCs are described in the supplementary information online.
Surface plasmon resonance spectroscopy binding assay. HS was biotinylated at the reducing end and immobilized on a BiAcore (Uppsala, Sweden) sensorchip. One flow cell was left untreated and used as a negative control. For binding assays, the proteins were simultaneously injected over the control and the HS. Further details can be found in the supplementary information online. Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Information
Acknowledgments
We thank C. Morlot for her assistance in the initial stage of this study, A. Schuller for help with the functional assays, R. Aricescu for providing technical advice and C. Petosa for critical reading of the paper. Partial funding for this project was provided by the European Union FP6 Integrated Project ‘SPINE2-Complexes' and the Deutsche Forschungsgemeinschaft (BA 1034/14-2 to M.B.). E.S. was supported by a European Union FP6 Marie Curie Early Stage Research Training Fellowship under Contract MEST-CT-2004-504640 (E-STAR). The atomic coordinates and structure factors have been deposited in the Protein Data Bank (www.pdb.org) with the accession code: 2wfh.
Footnotes
The authors declare that they have no conflict of interest.
References
- Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA 98: 10037–10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulow HE, Hobert O (2004) Differential sulfations and epimerization define heparan sulfate specificity in nervous system development. Neuron 41: 723–736 [DOI] [PubMed] [Google Scholar]
- Chen JH, Wen L, Dupuis S, Wu JY, Rao Y (2001) The N-terminal leucine-rich regions in Slit are sufficient to repel olfactory bulb axons and subventricular zone neurons. J Neurosci 21: 1548–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman JR (2003) Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mol Cell Biol 4: 926–937 [DOI] [PubMed] [Google Scholar]
- Dickson BJ, Gilestro GF (2006) Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol 22: 651–675 [DOI] [PubMed] [Google Scholar]
- Fox AN, Zinn K (2005) The heparan sulfate proteoglycan syndecan is an in vivo ligand for the Drosophila LAR receptor tyrosine phosphatase. Curr Biol 15: 1701–1711 [DOI] [PubMed] [Google Scholar]
- Fukuhara N, Howitt JA, Hussain SA, Hohenester E (2008) Structural and functional analysis of Slit and heparin binding to immunoglobulin-like domains 1 and 2 of Drosophila Robo. J Biol Chem 283: 16226–16234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitt JA, Clout NJ, Hohenester E (2004) Binding site for Robo receptors revealed by dissection of the leucine-rich repeat region of Slit. EMBO J 23: 4406–4412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H (2001) Cell-surface heparan sulfate is involved in the repulsive guidance activities of Slit2 protein. Nat Neurosci 4: 695–701 [DOI] [PubMed] [Google Scholar]
- Huizinga EG, Tsuji S, Romijn RA, Schiphorst ME, de Groot PG, Sixma JJ, Gros P (2002) Structures of glycoprotein Ibalpha and its complex with von Willebrand factor A1 domain. Science 297: 1176–1179 [DOI] [PubMed] [Google Scholar]
- Hussain SA et al. (2006) A molecular mechanism for the heparan sulfate dependence of slit–robo signaling. J Biol Chem 281: 39693–39698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatani M, Irie F, Plump AS, Tessier-Lavigne M, Yamaguchi Y (2003) Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science 302: 1044–1046 [DOI] [PubMed] [Google Scholar]
- Johnson KG, Ghose A, Epstein E, Lincecum J, O'Connor MB, Van Vactor D (2004) Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr Biol 14: 499–504 [DOI] [PubMed] [Google Scholar]
- Liu Z, Patel K, Schmidt H, Andrews W, Pini A, Sundaresan V (2004) Extracellular Ig domains 1 and 2 of Robo are important for ligand (Slit) binding. Mol Cell Neurosci 26: 232–240 [DOI] [PubMed] [Google Scholar]
- Morlot C, Hemrika W, Romijn RA, Gros P, Cusack S, McCarthy AA (2007a) Production of Slit2 LRR domains in mammalian cells for structural studies and the structure of human Slit2 domain 3. Acta Crystallogr D Biol Crystallogr 63: 961–968 [DOI] [PubMed] [Google Scholar]
- Morlot C, Thielens NM, Ravelli RB, Hemrika W, Romijn RA, Gros P, Cusack S, McCarthy AA (2007b) Structural insights into the Slit–Robo complex. Proc Natl Acad Sci USA 104: 14923–14928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Ma L, Wang KH, Marillat V, Sotelo C, Tessier-Lavigne M, Chedotal A (2001) Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J Neurosci 21: 4281–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Anderson R, Dwivedy A, Weinl C, van Horck F, Leung KM, Cogill E, Holt C (2006) Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron 49: 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhiner C, Gysi S, Frohli E, Hengartner MO, Hajnal A (2005) Syndecan regulates cell migration and axon guidance in C. elegans. Development 132: 4621–4633 [DOI] [PubMed] [Google Scholar]
- Scott PG, McEwan PA, Dodd CM, Bergmann EM, Bishop PN, Bella J (2004) Crystal structure of the dimeric protein core of decorin, the archetypal small leucine-rich repeat proteoglycan. Proc Natl Acad Sci USA 101: 15633–15638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiradake E, Lortat-Jacob H, Billet O, Kremer EJ, Cusack S (2006) Structural and mutational analysis of human Ad37 and canine adenovirus 2 fiber heads in complex with the D1 domain of coxsackie and adenovirus receptor. J Biol Chem 281: 33704–33716 [DOI] [PubMed] [Google Scholar]
- Steigemann P, Molitor A, Fellert S, Jackle H, Vorbruggen G (2004) Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr Biol 14: 225–230 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Information