Abstract
Purpose
In prostate cancer bearing host, regulatory T cells restrain activity of tumor antigen specific T cells. Since B7:CD28 interactions are needed for both function of CD4+CD25+ Treg cells and the CD8+ effective T cells, targeting this pathway may help to overcome the immunotherapy barriers.
Experimental design
The anti-B7−1/B7−2 mAbs were administrated to a transgenic mouse model of prostate cancer (TRAMP) ectopically expressing SV40 large T antigen (TAg) in different tumor development stages for prevention and therapy of prostate cancer. The treatment was also tested in treating transplanted MC38 colon adenocarcinoma in mice.
Results
Here we showed that short-term administration of anti-B7−1/B7−2 mAbs in TRAMP mice leads to significant inhibited primary tumor growth and the size of metastatic lesions. The treatment is effective to inhibit MC38 colon cancer growth. Correspondingly, this treatment results in a transient reduction of Treg in both thymus and the periphery. In vivo cytotoxicity assay revealed TAg-specific CTL effectors in anti-B7 treated, but not control IgG-treated TRAMP mice.
Conclusions
Transient blockade of B7−1/2 alters the balance between Treg and cancer-reactive T cells to enhance cancer immunotherapy.
Keywords: regulatory T cells, costimulatory molecule, prostate cancer
Introduction
Many of tumor antigens identified so far are self-antigens (1-4) and may therefore trigger immune tolerance. Logically, mechanisms that mediate self-tolerance may contribute to inadequacy of tumor immunity. The best characterized mechanism of self-tolerance is clonal deletion (5, 6). In this context, we have demonstrated that tumor antigen controlled by tissue-specific promoter is also expressed in the thymus to trigger clonal deletion (7).
In addition to clonal deletion, CD4+CD25+ regulatory T (Treg) cells play a pivotal role in the maintenance of peripheral self-tolerance (8-12). Accumulating evidence also support a role for Treg in restrained cancer immunity. Thus, cancer patients have elevated numbers of Treg cells in the blood of malignant effusions (13-15). Treg cells are also recruited and accumulated at tumor sites in animal models and in cancer patients (16-18). Correlation between the number of CD4+CD25+ Treg cells and clinical outcomes in some, although not all, cancer patients supported the hypothesis that Treg may suppress the effector function of tumor antigen-specific T cells, allowing tumor growth in the presence of tumor antigen-specific T cells (19, 20). Consistent with this concept, the removal of CD4+CD25+ Treg cells by an anti-CD25 antibody promoted rejection of transplanted tumor cells (21). However, this approach has showed little efficacy in animals with spontaneous tumors, which better reflect the challenge of cancer immunotherapy. In a recent study using a transgenic model of prostate dysplasia, anti-CD25 mAb treatment at 12 weeks of age caused only 25% reduction in the prostate mass at 20 weeks, although extended observation has not been carried out to document long term effect (22).
Alternatively, it is worth considering conditions that are selectively required for the generation and maintenance of Treg. CD28−/− and B7−1/B7−2−/− mice have markedly decreased numbers of CD4+CD25+ Treg cells in the thymus as well as in the periphery (23-25). Meanwhile, we and others have reported a significant role for B7:CD28 interaction in clonal deletion of some, although not necessarily all self antigens (26, 27). As such, transient blockade of B7−1/2 may reduce Treg while increase the frequency of cancer-reactive T cells, thus overcoming the two major barriers to effective cancer immunity.
TRAMP is a well established mouse model for prostate cancer with clearly defined progression of prostate cancer that resembles the human disease (28). Metastasis to periaortic lymph nodes and lungs can be detected frequently (29). By the time the mice are 24−30 weeks old, the prostate cancer become palpable in the abdomen. We have adopted the TRAMP mouse model to test our hypothesis because the challenge of treating established spontaneous tumors. We report here that transient blockade of B7−1/2 with monoclonal antibodies resulted in temporal deletion of Treg and rescue of cancer-reactive T cells from clonal deletion. These effects associated with increased effector function of cytotoxic T lymphocytes. Remarkably, the relatively simple treatment confers prevention and therapy of the spontaneous prostate cancer and transplantable colon cancer. Since recombinant protein that blocks B7−1 and B7−2 has already been approved for human use, the path for translating our observation into patient care is considerably shorter than most therapeutic approach.
Methods
Experimental animals
C57BL/6 mice and TRAMP mice expressing the SV40 Tag controlled by rat probasin regulatory elements in the C57BL/6 background were purchased from the Jackson Laboratory (Bar Harbor, ME). The mice were bred at the animal facilities of the Ohio State University (Columbus, OH) and the University of Michigan (Ann Arbor, MI). All animal experimental procedures were reviewed and approved by The Ohio State University and University of Michigan Institutional Animal Care and Use Committees. Mice were typed for SV40 Tag by isolation of mouse tail genomic DNA. The PCR-based screening assay was described previously(7). Transgenic mice expressing TCR specific for SV40 large T antigen (TGB) have been described (30). Generation of TRAMP mice expressing TGB TCR (TGB-TRAMP) was also described(7).
Antibody treatment of the TRAMP mice
TRAMP mice were treated with anti-B7−1 and anti-B7−2 antibodies at two different stages. In the first regiment, 4−6 week old TRAMP mice were injected intraperitoneally with 5 injections of anti-B7−1 (Rat anti-mouse CD80, clone 3A12, (31)) and anti-B7−2 (Hamster anti-mouse CD86, clone GL1, ATCC, (32)) antibodies or control hamster/rat IgG (Sigma) at 100 μg/antibody/injection every other day. Long term prostate cancer incidence was recorded by physical examination. In the second regiment, 25 week old TRAMP male mice without palpable prostate cancer were treated intraperitoneally with the anti-B7 or control IgG at 100 μg/antibody/injection for 5 injections every other days. The MRI examination was carried out before treatment and 8 weeks later at age of 33 weeks. In a separate experiment, 25 week old mice were treated with one intraperitoneal injection of 1 mg anti-CD25 (PC61) or control rat IgG (33). The efficiency of anti-CD25 depletion was examine by flow cytometry with staining PBL using conjugated anti-CD4, anti-CD25 (clone 7D4, ATCC) and anti-Foxp3. The MRI examination was carried out before treatment and 5 weeks later at age of 30 weeks. For long term prostate cancer incidence study, anti-B7 and control IgG treated mice were examined at least weekly for palpable tumor at lower abdomen, and were euthanasized when they either become moribund or with tumor size exceeding 5% of body weight.
6−8 wks old TRAMP or TRAMP/TG-B mice were sub-lethally irradiated (500 Rad) on day 0 and the treatment started on day 1 with either anti-B7−1/2 mAbs (100 μg/each) or control rat/hamster IgG (100 μg/each) intraperitoneally. The mice were treated 6 times every other days. One week after the last treatment, the mice were sacrificed and the total thymocytes and splenocytes were harvested and stained with fluorochrome-conjugated antibodies anti-CD4 (RM4.5), anti-CD8 (53−6.7), anti-Vβ8.1+8.2 (MR5−2) (BD, San Diego).
For transplantable tumor model, MC38 murine colon carcinome cells were grow in RPMI medium with 5% FBS and subcutaneously injected to male C57BL/6 mice (5×105/mouse). 10 day after injection, mice were divided evenly into two groups based on the tumor sizes, and administrated i.p. with either anti B7 or control IgG 3 times every other day. Peripheral blood was collected at 0, 6 days (0 day is the day before the administration of antibodies) and the splenocytes were collected at 14 days and stained with anti-CD4, CD8, CD25 and Foxp3 antibodies (BD),
Proliferation of T cells to antigenic peptides
Total spleen cells (3 × 105/well) from control Ig or anti-B7-treated TRAMP × TG-B (H-2bxk) F1 mice were cultured with the given concentrations of SV40 Tag K560−568 peptide or control HSV gB peptide in Click's Eagle-Hank's amino acid medium for 72 h. The proliferation of T cells was determined by incorporation of [3H]thymidine (TdR) pulsed (1 μCi/well) during the last 6 h of culture. The data presented are means of triplicates with variation from the means <15%.
Peptide synthesis
All peptides used were synthesized by Research Genetics, Inc. (Huntsville, AL). The peptides were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mg/ml and diluted in PBS or culture medium before use. Peptides used were SV40 Tag 560−568 SEFLLEKRI (7) and HSV gB peptide gB498−505: SSIEFARL (34).
Immunohistochemistry
Mouse organs were fixed with 10% buffered formalin. Tissue sections were stained with hematoxylin and eosin (H&E), and examined under a microscope. Frozen sections were prepared and stained with 2 μg/ml of antibodies specific for CD3 (2C11, hamster IgG). CD3 positive foci were counted using 20× microscope visual fields.
In vivo cytotoxity assay
Spleen cells from C57BL/6 mice were pulsed with 10 μg/ml of either SV40 Tag 560−568 SEFLLEKRI or a control peptide HSV gB 498−505 SSIEFARL in the presence of either 0.5 mM or 5 mM of CFSE, respectively. After mixing at a 1:1 ratio, the labeled cells were injected intravenously into recipients and spleen cells were harvested 20 hours later and analyzed by flow cytometry for the relative abundance of CFSElow (SV40 Tag peptide) and CFSEhi (HSV peptide) populations.
Detection of anti-dsDNA
Anti-DNA antibodies were measured by ELISA according to the published procedure (35).
Magnetic resonance imaging (MRI) of prostate
The progression of prostate cancer in the TRAMP model was measured by MRI as described (36). Briefly, MRI experiments was performed on a Varian system equipped with a 7.0-Tesla, 18.3-cm horizontal bore magnet (300-MHz proton frequency). For MRI examination, the mice will be anesthetized with sodium pentobarbital (70 mg/kg intraperitoneally) and maintained at 37°C inside the magnet using a heated circulation water blanket, with pelvis motion (due to respiration) minimized by a small plastic support placed before insertion into a 3-cm diameter quadrature birdcage coil (USA Instruments). Multislice images was acquired using a T1-weighted spin echo sequence (TR/TE = 880/13, field of view = 30 × 30 mm using a 128 × 128 matrix, slice thickness = 1.5 mm, and slice separation = 1.0 to 1.6 mm.). Each set contained 9 to 25 slices and enough sets was obtained to provide contiguous image data of the prostate tumor. Prostate volume will be measured using the formula V=4/3[(D1+D2/4]3 π, where D1 and D2 correspond to the longest and shortest (transverse and sagittal) diameter measured from the MRI image. The accuracy of this measurement was confirmed by comparing prenecropsy MRI volumes to postnecropsy actual prostate volumes in select cases.
Results
Anti B7−1/B7−2 antibody treatment of young TRAMP mice reduced Treg cells in both the thymus and periphery and delay development of prostate cancer
We and others have reported that targeted mutation of CD28 and B7−1/2 abrogated generation of regulatory T cells (23). To test whether this pathway can be targeted for transient reduction of Treg, we treated C57BL/6 mice with either anti B7−1/B7−2 mAbs or control IgG, 5 times every other day. Thymi and spleens were harvested 8 days after the last injection. Cells were stained for flow cytometry analysis. This treatment did not affect either the total cellularity or the numbers of CD4 and CD8 T cells (Fig. 1a). However, the numbers of CD4+FoxP3+CD25+ cells were reduced by 50% in thymus and by 4 folds in the spleen (Fig. 1b). When gated on lymphocyte gate, all CD4+ T cells are CD3+ (supplemental Fig. 1). Therefore, all FoxP3+CD25+ cells analyzed in this study are Treg. These data indicate that Treg cells can be significantly reduced in both the thymus and spleen by anti B7−1/B7−2 antibodies.
Fig. 1. Anti B7−1/B7−2 antibody treatment reduced Treg both in the thymus and periphery of normal mice and delayed the development of palpable tumors.

C57BL/6 mice were injected i.p. with either anti B7−1/B7−2 mAbs (1:1 mixture of 100μg 3A12 and 100μg GL-1), or control IgG (1:1 mixture of 100μg hamster and 100μg rat IgG) at 6 weeks old, 5 times every other day. The mice were sacrificed 8 days after the last injection. Thymocytes and splenocytes were harvested and analyzed by flow cytometry. Plots shown are from CD4+ cells among the lymphocyte gates. a. Anti B7−1/B7−2 antibody treatment did not alter the number of thymocytes, spleen cells and CD4 and CD8 subsets. b. Anti B7−1/B7−2 antibody treatment reduced CD25+Foxp3+ cells both in thymus and spleen. Statistical analysis was done using the Student T test. *, P<0.05; **, P<0.01. c. Kaplan Meier analysis of tumor incidence. The experimental endpoint is 2 cm in tumor diameter as determined by palpation. Data shown have been repeated 3 times.
In order to investigate whether anti B7−1/B7−2 antibody treatment delay the development of prostate cancer, 4-week old male TRAMP mice were treated with either control IgG or anti-B7−1/B7−2 antibodies and the incidence of cancer development were followed by physical examination. Using 50% of mice with palpable prostate cancer as a reference point, we observed that anti-B7 delayed the tumor development by more than 14 weeks (Fig. 1c). Therefore, anti-B7 treatment may be valuable for prevention of prostate cancer development.
Enhanced tumor specific cytotoxity after anti-B7−1/B7−2 antibody treatment
To test tumor antigen-specific immunity following anti-B7−1/B7−2 treatment, we further investigated the tumor specific cytotoxity by an in vivo killing assay. Six-week old male TRAMP mice were injected i.p. with anti B7−1/B7−2 mAbs or control IgG 5 times every other day. Two weeks after the first injection, they received an i.v. injection of a 1:1 mixture of SV40 Tag peptide-pulsed (CFSElo) and control HSV gB peptide-pulsed (CFSEhi) spleen cells. The spleens were harvested 20 hours later and analyzed by flow cytometry. As shown in Fig. 2a, in mice treated with anti-B7 antibodies, the SV40 TAg pulsed targets were preferentially eliminated while the CFSElo and CFSEhi cells remained the 1:1 ratio in control Ig treated mice. These data demonstrated anti-B7 treatment enhanced CTL response against the SV40 large T antigen without intentional immunization.
Fig. 2. Anti B7−1/B7−2 antibody treatment enhanced TAg-specific CTL and partially rescured clonal deleted TAg-specific T cells in the TRAMP mice.
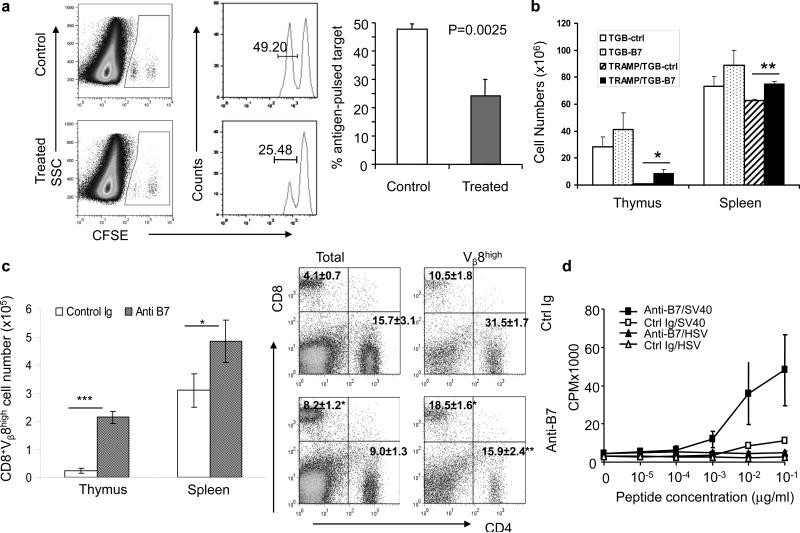
a. Six-week old male TRAMP mice were treated i.p. with either anti B7−1/B7−2 mAbs (1:1 mixture of 100μg 3A12 and 100μg GL-1), or control IgG (1:1 mixture of 100μg hamster and 100μg rat IgG) 5 times every other day. 2 weeks after the first injection, mice received an i.v. injection of a 1:1 mixture of TAg peptide-pulsed (CFSElo) and control HSV peptide-pulsed (CFSEhi) spleen cells (5×106 each). Twenty hours later, the spleens were harvested and analyzed by flow cytometry. The left panel shows representative profiles; while right panel shows summary of two experiments involving a total of 6 mice per group.. CFSEhi and CFSElo cells are gated as indicated. The number shown in the gates are the % of gated cells. b-d. Anti-B7 treatment rescued tumor-reactive T cells from the force of clonal deletion. TRAMP/TGB double transgenic or TGB single transgenic mice that received sublethal irradiation (500 Rad) were treated with either control Ig or anti-B7 mAbs five times every other days. The thymocytes and splenocytes were harvested on day 7 after final treatment and analyzed by flow cytometry. b. Thymocyte and splenocyte cellularities in TGB single transgenic and TRAMP/TGB double transgenic mice treated with control Ig or anti-B7 antibodies. c. Increase of CD8+Vβ8high T cells in thymus and spleen. Left panel: Summary of CD8+Vβ8high cell number change in thymus and spleen from TRAMP/TGB double transgenic mice. Right panels: Representative profiles of CD4 and CD8 T cells in the spleens of control Ig or anti-B7 treated mice. The left flow cytometry panels show those for total spleen cells, while the right flow cytometry panels show those for gated Vβ8hi cells. d. Antigen-reactivity of T cells rescued by anti-B7. The splenocytes from TRAMP/TGB double transgenic mice were stimulated with either SV40 T antigenic peptide or control HSV peptide for 72 hours and pulsed with 3HTdR to determine the rate of T cell proliferation. Data shown in b, c and d are means+/-SEM (n=3) and the conclusions have been confirmed with another independent experiment.
Anti-B7 antibodies rescued SV40 large T-specific T cells from clonal deletion in the TRAMP mice
Our previous studies have demonstrated that SV40 large T antigen is expressed in the thymic peripheral antigen-expressing cells in the TRAMP mice and that such expression caused nearly complete deletion of transgenic T cells expressing an TCR specific for a SV40 large T specific peptide presented by H-2Kk (7). Moreover, we reported that perinatal blockade of B7−1 and B7−2 reduced clonal deletion of autoreactive T cells (26). To test whether the anti-B7- treatment rescue SV40 T antigen-specific T cells from clonal deletion in the TRAMP mice, we produced TRAMP mice expressing the SV40 Tag specific TGB TCR and divided the double transgenic mice with either anti-B7 mAbs or control Ig G treatment groups.
As the mice recovered from irradiation, a new wave of bone marrow derived cells will differentiate into mature T cells in the thymus. This de novo process increases sensitivity of blocking studies (37) (and our unpublished observations). In order to study the effect of anti-B7 treatment on newly formed T cells undergone thymic development and clonal deletion, we gave sublethal irradiation (500 Rad) to TGB single transgenic and TRAMP/TGB double transgenic mice. At one week after 6 treatments, the thymic cellularity and mature CD8 T cells were measured by flow cytometry. As shown in Fig. 2b, due to clonal deletion, the numbers of reconstituted thymocytes were extremely low in the double transgenic TGB-TRAMP mice compared with single transgenic TGB. Importantly, anti-B7 treatment increased thymic cellularity by approximately 10-fold (Fig. 2b). A corresponding increase in the CD8 T cells expressing high levels of Vβ8+ transgenic TCR was observed in both spleen and thymus (Fig. 2c, left panel). When the spleen cells were analyzed for CD4/CD8 T cell ratios, it was clear that, perhaps due to clonal deletion, T cells in the control Ig-treated mice have lost the predominance of CD8 subset due to expression of MHC class I-restricted TCR. This is corrected to a large extent by anti-B7 treatment (Fig. 2c, right panel). Thus, anti-B7 treatment greatly reduced efficiency of clonal deletion. However, the numbers of transgenic T cells in the anti-B7 treated TGB-TRAMP mice were still much reduced in comparison to TGB mice, which demonstrated that the rescue is only partial.
To test the whether the T cells rescued by anti-B7 treatment were responsive to tumor antigen, we stimulated spleen cells from control Ig or anti-B7 treated mice with different concentration of the SV40 T antigenic peptide or control peptide from HSV peptide. As shown in Fig. 2d, anti-B7-treated spleen cells underwent a significant proliferation to SV40 T antigenic peptide. Based on the dose response, the anti-B7-treated spleen cells were at least 100-fold more responsive than the control Ig-treated spleen cells, which corresponded to increased number of antigen-specific T cells. Therefore the anti-B7 rescued T cells are functional. However, after in vitro stimulation, the rescued T cells showed poor cytotoxicity (data not shown), which suggests that the rescued T cells may be functionally impaired to some extent.
Anti B7−1/B7−2 antibody treatment cause significant albeit transient reduction of Treg in mice with established prostate cancer
One of the most difficult challenges in cancer immunotherapy is the treatment of established solid tumors. It has been shown that microscopic lesion of prostate cancer can be observed in the TRAMP mice between 18 and 24 weeks of age (29). To confirm the development of tumor in the 25 weeks TRAMP mice in our colony, we used the magnet resonance imaging (MRI) to compare the size of the prostate at 25 weeks. As shown in Fig. 3a, all of the 12 TRAMP mice tested had considerably larger prostate organ sizes comparing to non-TRAMP littermate. Thus, essentially all of the 25 weeks old TRAMP mice developed cancer in the prostate.
Fig. 3. Anti B7−1/B7−2 mAbs treatments of mice with established prostate cancer inhibited cancer progression.


a. MRI measurement of prostate volumes of 25 weeks old normal and TRAMP mice. Left panels: Representative local images of male B6 and TRAMP mice. The prostate were identified with thick white outlines. Right panel: Prostates sizes of three B6 and 12 TRAMP mice, all at 25 weeks of age. b and c. Anti-B7 treatment initiated at 25-week old TRAMP mice transiently depleted Treg. Male TRAMP mice were administrated i.p. with either anti B7−1/B7−2 mAbs (1:1 mixture of 100μg 3A12 and 100μg GL-1), or control IgG (1:1 mixture of 100μg hamster and 100μg rat IgG) 5 times every other day. Peripheral blood was taken at 0 week, 1 week, 2 week and 6 week. 0 week is the day before injection. Cells were stained for flow cytometry. Plots are gated on CD4+ cells. b. CD25+FoxP3+ cell number started to reduce following the first week of treatment, and almost recovered to normal levels one month after the treatment was stopped. Data shown have been repeated 2 times, involving a total of 12 mice per group. d. MRI image of TRAMP mice at 25 weeks and 33 weeks (8 weeks after starting treatments with either control Ig or anti-B7 mAbs). Summary data shown are ratio of prostate volumes at 33 weeks vs 25 weeks when the treatments started. d. Kaplan Meier analysis for incidence of palpable tumors in TRAMP mice treated with either control Ig or anti-B7 antibodies at age of 25 weeks.
To determine the impact of anti-B7 antibodies for B7−1 and B7−2, we injected either control or anti-B7 mAbs every other day for 5 times. The blood samples were collected at 0, 1, 2 or 6 weeks after antibody treatment and stained for either anti-CD25 or anti-Foxp3 in conjunction with anti-CD4. As shown in Fig. 3b, in comparison to control Ig-treated mice, significant reduction of Treg can be observed in the peripheral blood at one and two weeks after completion of the treatment. Interestingly, the number of Treg is restored to normal levels at 6 weeks after completion of the treatments. Thus, in mice bearing established prostate cancer, anti-B7−1 and anti-B7−2 antibodies caused a significant, albeit transient reduction of Treg in tumor bearing mice.
Anti-B7 antibodies delayed growth of established prostate cancer without autoimmune side effects
In order to determine whether anti-B7 antibodies can confer therapeutic effect in mice with established prostate cancer, we randomly divided 25 week TRAMP mice into two groups, and measured their tumor size prior to the treatment with either control Ig or anti-B7 antibodies, starting at 25 weeks. After 5 injections, the mice were followed for the tumor tumor progression by either palpation or MRI. As shown in Fig. 3c, at age 33 weeks (8 weeks after first treatment), in the control IgG-treated group, the volume of prostate expanded by 2.5−9 fold with an average of more than 4.5 fold. In contrast, all but one anti-B7 treated mice show less than two fold expansion of the prostate volume. Mann-Whitney test indicate that the difference was statistically significant (P=0.04). Since the tumors are not palpable at the beginning of the treatment, we also used the time when the mice developed palpable tumors as a second endpoint with larger sample size (12 mice for each group). As shown in Fig. 3d, even treated as late as 25 weeks of age, the anti-B7 antibodies delayed tumor development by approximately 7 weeks.
In the TRAMP model, lymph node metastasis has occurred at 25 weeks (29), we therefore tested the impact of anti-B7 treatment on metastatic lesions in other organs, including lung, kidney, and liver. As shown in Fig. 4a, 3/6 mice in the control Ig-treated group have substantially higher number of metastatic lesions in lung (2/6). In addition, massive metastatic lesions were found in kidney (1/6) and liver (2/6) (data not shown). Only one case of metastasis was observed in the anti-B7 treated group, and the metastasis is limited to the lung. In addition, the metastic lesions in the anti-B7 treated group was substantially smaller than those found in the control Ig treated group (Fig. 4a).
Fig. 4. Anti-B7 blockade in tumor-bearing mice reduces the number and size of metastatic lesions in the TRAMP mice and increases infiltration of T cells into tumors but does not cause autoimmunity.


a. Internal organs from mice from Fig. 3 were analyzed for metastatic lesions. Three sections of liver, lung, kidney, intestine and heart, 30 microns apart, were examined double blind by a pathologist. A representative field of lung sections of control Ig-treated mice (3 of 6 mice analyzed have metastasis) and the only metastatic lesion in anti-B7 treated group are shown. Metastatic lesion are marked with yellow arrows. In the control Ig treated group, massive metastases were also observed in the liver (2/6) and kidney (1/6). b-d. Mice from Fig. 3 were analyzed for infiltrating lymphocytes and autoimmune reactions. b. Representative tumor sections stained with anti-CD3 mAb. c. FACS profiles showing representation of CD4, CD8 T cells and the CD4+CD25+Foxp3+ T cells. Left top panels shows profiles of mononuclear cells isolated from the tumor, while the left lower panels are profiles from the gated CD4 T cells. Data shown are from pooled cells from 6 mice per group. The top right panel shows frequencies of CD4 and CD8 T cells among mononuclear cells isolated from the prostate cancer, while the lower right panel shows the ratio of Treg over CD4 or CD8 T cells. Data shown are means+/-SEM (n=6). d. Serum anti-double stranded DNA antibodies. Data shown are O.D. 490 from an ELISA, using 1:50 dilution of sera. Data shown are means and SEM, involving 6 mice per group.
Corresponding to reduced tumor growth, we have observed increased T-cell infiltrating into tumors. Immunohistochemistry staining revealed an increased numbers of T cell infiltration (Fig. 4b). Quantitative analysis by flow cytometry indicated that the frequency of T cells among the mononuclear cells from the collagenase-treated prostate cancer tissue increased by 4−5 fold, with the majority of the T cells are of CD8 subsets (Fig. 4b). In both groups, higher pencentage of CD4+ T cells expressed Foxp3 than what was found in the lymphoid organ (Fig. 4c, left lower panel), similar to observations made by others (33). Nevertheless, the % of Treg is significantly lower in the anti-B7 treated group. Moreover, the ratio of Treg over effector CD4 and CD8 T cells significantly decreased in anti-B7-treated group (Fig. 4c, lower righ). Therefore, anti-B7 treatment alters the ratio of Treg over effector T cells in the tumor, presumbly in favor of local immune response.
A general concern for immunotherapy of cancer is autoimmune side effect. In order to determine whether autoantibodies were induced in tumor-bearing TRAMP mice, the sera were collected at 1 week, 2 weeks and 6 weeks after the start of anti B7−1/B7−2 antibody treatments. The anti-dsDNA antibodies were detected by ELISA. As shown in Fig. 4d, while an increase in anti-DNA antibodies were detected at 6 weeks after control Ig treatment, presumably due to tumor growth, such increase was not observed in the anti-B7 treated mice. Histological analysis showed no inflammation of internal organs in either group (data not shown). Therefore, anti-B7 antibodies can induce significant protection against established tumor without eliciting autoimmune side effect.
Anti-B7 antibodies inhibit MC38 colon carcinoma cell growth
To confirm the general anti-tumor effect of anti-B7 treatment, we tested it with MC38 colon carcinoma tumor model. Male C57BL/6 mice were injected 5×106 MC38 tumor cells subcutaneously. Ten days after injection, mice developed palpable tumors and were divided evenly into two groups based on the tumor sizes. MC38 tumor bearing mice were administrated i.p. with either anti B7−1/B7−2 mAbs (1:1 mixture of 100μg 3A12 and 100μg GL-1), or control IgG 3 times every other day. Peripheral blood samples were taken at 0 day, 6 day, and spleens were collected at 14 day after completion of antibody treatments. As shown in Fig. 5a and 5b, at 6 days and 14 days after anti-B7 antibody treatment, the CD4+CD25+ and CD4+Foxp3+ Treg cells were significantly reduced. Correspondingly, anti-B7 treatment conferred a significant reduction in the growth rate of MC38 colon carcinoma (Fig. 5c, p=0.035).
Fig. 5. Anti B7−1/B7−2 mAbs treatments of mice bearing MC38 colon carcinoma.

Eight weeks old male C57BL/6 mice were injected s.c. with 5×105 MC38 tumor cells. Ten days after injection, mice were divided evenly into two groups based on the tumor sizes. The mice were administrated i.p. with either anti B7−1/B7−2 mAbs (1:1 mixture of 100μg 3A12 and 100μg GL-1), or control IgG (1:1 mixture of 100μg hamster and 100μg rat IgG) 3 times every other day. Peripheral blood was taken at 0 day, 6 day, and spleen was collected at 14 day. 0 day is the day before the administration of antibodies. Cells were stained for flow cytometry. Plots of gated CD4+ cells are presented. a. CD4+FoxP3+CD25+ cell number started to reduce following the first week of treatment. Representative profiles are shown in the left and the summary data are shown in the right panel. b. Anti-B7 treatment delayed growth of MC38 tumor (six mice per group). Data shown are means and SEM of tumor diameters at different time points. The day 1 is defined as the day for first injection of antibody. The statistical significance is determined by Plos Fisher's test.
Transient depletion of Treg by anti-CD25 antibody delays established prostate cancer growth in TRAMP mice
To test whether transient depletion of Treg alone inhibits tumor growth, we treated 25 week old TRAMP mice with anti-CD25 antibody to deplete CD4+CD25+ cells. Male 25 week old TRAMP mice were examined by MRI to measure prostate size and divided into two groups. The mice were treated i.p. with either 1mg anti-CD25 mAbs (PC61) or 1mg control rat Ig. Peripheral blood samples were taken at 0 day, 3 day, and spleen were collected on day 35. 0 day is the day before injection. As shown in Fig. 6a, 99% of Foxp3+CD25+ cells were depleted in three days after anti-CD25 treatment, however, the Foxp3+CD25+ cells were fully recovered to normal levels in 5 weeks after the treatment. Five weeks after anti-CD25 treatment when mice reached 30 weeks old, two groups of TRAMP mice were re-examined by MRI. As shown in Fig. 6b, the prostate sizes were enlarged by 3−5 folds during 5 weeks period due to the aggressive prostate cancer growth. Compared with the control group, the prostate sizes were increased by 2−3 folds in anti-CD25 treated group (Fig. 6c). The significant difference revealed an effect of Treg depletion on tumor growth. However, this treatment is substantially less effective than transient B7 blockade (the average after/before treatment prostate size ratio in anti-CD25 treatment group is 2.55 after 5 weeks, compared with anti-B7 treatment average ratio is 1.72 after 8 weeks) (Fig. 3).
Fig. 6. Anti-CD25 treatments of mice with established prostate cancer inhibited cancer progression.

a. Anti-CD25 (clone PC61) treatment initiated at 25-week old TRAMP mice transiently depleted Treg. Male TRAMP mice were administrated i.p. with one dose of anti-CD25 (1 mg/mouse) or control rat IgG (1 mg). Peripheral blood was taken at 0 week, 1 week, 2 week and 6 week. 0 week is the day before injection. Cells were stained for flow cytometry. Plots of gated CD4+ cells are shown. The conjugated anti-CD25 from a different clone 7D4 was used to avoid blocking by the depleting antibody. CD4+CD25+Foxp3+ cells were reduced at 6 days after anti-CD25 treatment but fully recovered at 35 days. b. MRI image of TRAMP mice at 25 weeks and 30 weeks (5 weeks after the treatments with either control Ig or anti-CD25 antibody, 5 mice per group). Summary data shown are ratio of prostate volumes at 30 weeks vs 25 weeks when the treatments started. The difference was compared by a student t-test.
Discussion
Traditionally, blockade of costimulatory molecules B7−1 and B7−2 has been explored for treatment of autoimmune diseases and transplant rejection (38). Recent studies that reveal a critical role for B7−1/2 in the production and maintenance of Treg (23-25) and in clonal deletion of self-reactive (26) as well as cancer-reactive T cells (7) suggest that this pathway may be targeted for overcoming the barrier of immune tolerance in cancer setting. The data described herein demonstrated unexpected efficacy of this new approach.
We have chosen the TRAMP mice, which developed malignant transformation of prostate epithelial cells as early as 12 weeks to test this notion. Our data demonstrated that a short-term anti-B7 blockade prior to the development of pathological lesions delays the development of palpable tumor for approximately 14 weeks. These data demonstrate that a short-term anti-B7 treatment may prevent the development of prostate cancer among individuals with predisposition of prostate cancer.
It is generally agreed that immunotherapy is very inefficient for treatment of established tumors (39). This can be more challenging in transgenic tumor models where malignant tumor cells continue to arise due to transgenic expression of oncogenes. Our data demonstrated that even when administrated at a time when the TRAMP mice show more than three fold enlargement of prostate size, transient blockade of B7−1 and B7−2 dramatically reduced the rate of tumor growth. Thus, at eight weeks after initiation of the treatment, the prostate of the control Ig-treated expanded by five fold in volume. In contrast, those from anti-B7-treated mice expanded by less than two fold during the same period. When the palpable tumors were used as endpoint, the anti-B7 treatment at 25 weeks reduced tumor development by 7 weeks. Nevertheless, perhaps because of the continuous production of new cancer cells from the germline insertion of SV40 large T antigen and waning of antibodies, short term treatment did not completely eradicated the tumors. Since the majority of tumors that developed in human have clonal origin, the malignant transformation is likely less frequent that what is observed in transgenic model of spontaneous tumors. Therefore, the relatively simple treatment may show greater efficacy. Given the broad function of B7−1 and B7−2 in host immune system, including T cell costimulation at both priming and effector phases, Treg generation and maintenance, and clonal deletion, it is unlikely that a single mechanism is responsible for the therapeutic efficacy reported herein.
First, we have demonstrated significant, albeit transient reduction of Treg in both thymus and in the peripheral blood. Since the treatment with anti-CD25 antibody also showed some efficacy in slowing prostate tumor growth in TRAMP mice, Treg depletion alone is sufficient to convey significant, although less marked, protection. It is worth noting that anti-CD25 antibody depletes almost 95% of CD4+CD25+ cells in 6 days, however, 60% of CD4+ Foxp3+ cells were still remained in peripheral blood at the same time. Since the treated mice had more CD25−Foxp3+ cells than the untreated, anti-CD25 ablated part of CD25+Foxp3+ cells and down-regulation of CD25 on others. On the other hand, anti-B7 treatment caused similar extent of reduction in the CD4+Foxp3+ cells regardless of their CD25 phenotype. It is unclear whether the different depletion profile contributed to different efficacy.
Interestingly, the number of Treg returns to normal levels at 6 weeks after reconstitution. It is therefore of interest why the anti-tumor effect appears to have lasted long after the frequency of Treg is restored. In this regards, it should be emphasized that in vivo Treg reconstitution is almost universal for all methods of Treg depletion, including antibody elimination and treatment of toxin targeting Treg that express the specific receptor for the toxin (40-42). In all cases, however, restoration of Treg did not prevent the immune response against antigen or pathogen. These studies suggested that numerical restoration of Treg is usually not accompanied by immune suppression of ongoing immune response and therefore made it plausible that temporary reduction of Treg can promote cancer immunity.
Second, in line of the function of B7 in clonal deletion of autoreactive T cells, including some tumor-reactive T cells, it is possible that anti-B7 treatment also rescues some tumor-reactive T cells that are otherwise deleted. In this regards, we showed that transient blockade of B7−1 and B7−2 reduced the clonal deletion of SV40 T-reactive CTL. Therefore, it is likely that anti-B7 blockade may also increase the frequency of tumor-reactive T cells. Taken together, by reducing the burden of Treg and increasing the frequency of cancer-reactive T cells, B7 blockade resets the balance between regulatory burden and effector function. These two factors provide plausible explanation for the prevention described herein. Since the TGB mice do not survive long enough for us to study clonal deletion at 25 weeks, due to insertional mutation by TCR transgene (43), the impact of rescue of tumor-reactive T cells in the therapy setting remains to be demonstrated.
It is possible to argue that since the majority of cancer patients developed cancer late in their life when the thymic function has deteriorated, the rescue of TCR repertoire may be less relevant for cancer immunotherapy in human. Nevertheless, we would like to point out that continuous production of T cells has been demonstrated throughout the life-span (44). Moreover, it is worth pointing out that hormone ablation is part of the standard therapy for prostate cancer. An unexpected benefit of this therapy is reinvigoration of thymic function (45). Therefore, it may be valuable to combine anti-B7 blockade with hormone ablation in human prostate cancer treatment.
Finally, it is worth pointing out that blockade of B7−1 and B7−2 with their soluble receptor CTLA4Ig has been approved for therapy of autoimmune disease with little side effect (38). In this study, we showed that despite the modulation of Treg and rescue of potentially self-reactive T cells, anti-B7 blockade does not trigger autoimmune side effect. The availability of a safe drug makes blockade of B7−1 and B7−2 an attractive approach for the cancer immunotherapy.
Supplementary Material
Supplemental Fig. 1. CD4+ cells from lymphocyte gate express CD3. Profiles shown from left to right are those from sequential gating.
Acknowledgement
We thank the University of Michigan Cancer Center Imaging Core for the MRI analysis of prostate cancer. Part of the study was done when the laboratories were at the Ohio State University. This study was supported by grants from the Department of Defense and American Cancer Society to PZ, National Institutes of Health (AI064350, CA12001) to YL.
Footnotes
Statement of Clinical Relevance
Despite the conceptual advances in cancer immunotherapy, clinical development has been slow. Immunotherapy has so far failed to show clear-cut effect once cancers are established in advance stage. Here in this paper, we demonstrated that temporary blockade of B7−1 and B7−2 reduced the number of regulatory T cells and conveyed considerable therapeutic effects in TRAMP mice with spontaneous prostate cancer. To our knowledge, this is the first time that the prostate cancer in the TRAMP mice can be effectively treated when the large tumors can be demonstrated. Mechanistically we showed that transient blockade of B7−1/2 resets the balance of Treg and cancer-reactive T cells to confer prevention and therapy of prostate cancer. A second major advantage is that the data can be easily translated into human use as the drug that blocks B7−1 and B7−2 (FDA approved CTLA4Ig) has already been approved for the treatment of autoimmune diseases. It is possible to dramatically shorten the path of clinical development for the novel immunotherapy.
References
- 1.Boel P, Wildmann C, Sensi ML, et al. BAGE: a new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity. 1995;2:167–75. doi: 10.1016/s1074-7613(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 2.Brichard V, Van Pel A, Wolfel T, et al. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489–95. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaugler B, Van den Eynde B, van der Bruggen P, et al. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994;179:921–30. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 5.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–6. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 6.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988;336:73–6. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 7.Zheng X, Gao JX, Zhang H, Geiger TL, Liu Y, Zheng P. Clonal deletion of simian virus 40 large T antigen-specific T cells in the transgenic adenocarcinoma of mouse prostate mice: an important role for clonal deletion in shaping the repertoire of T cells specific for antigens overexpressed in solid tumors. J Immunol. 2002;169:4761–9. doi: 10.4049/jimmunol.169.9.4761. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in postthymectomy autoimmune oophoritis in mice. I. Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J Exp Med. 1982;156:1565–76. doi: 10.1084/jem.156.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunological reviews. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 11.Randolph DA, Fathman CG. Cd4+Cd25+ regulatory T cells and their therapeutic potential. Annual review of medicine. 2006;57:381–402. doi: 10.1146/annurev.med.57.121304.131337. [DOI] [PubMed] [Google Scholar]
- 12.Moller G. Do suppressor T cells exist? Scandinavian journal of immunology. 1988;27:247–50. doi: 10.1111/j.1365-3083.1988.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 13.Woo EY, Yeh H, Chu CS, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–6. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 14.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72. [PubMed] [Google Scholar]
- 15.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 16.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. The Journal of experimental medicine. 2004;200:771–82. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. The Journal of experimental medicine. 1982;155:1063–74. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett B, Kryczek I, Cheng P, Zou W, Curiel TJ. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol. 2005;54:369–77. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 19.Wei S, Kryczek I, Zou L, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer research. 2005;65:5020–6. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 20.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 21.Steitz J, Bruck J, Lenz J, Knop J, Tuting T. Depletion of CD25(+) CD4(+) T cells and treatment with tyrosinase-related protein 2-transduced dendritic cells enhance the interferon alpha-induced, CD8(+) T-cell-dependent immune defense of B16 melanoma. Cancer research. 2001;61:8643–6. [PubMed] [Google Scholar]
- 22.Tien AH, Xu L, Helgason CD. Altered immunity accompanies disease progression in a mouse model of prostate dysplasia. Cancer research. 2005;65:2947–55. doi: 10.1158/0008-5472.CAN-04-3271. [DOI] [PubMed] [Google Scholar]
- 23.May KF, Chang X, zhang H, et al. B7-deficient autoreactive T cells are highly susceptible to supression by CD4+CD25+ regulatory T cells. J Immunol. 2007;178 doi: 10.4049/jimmunol.178.3.1542. In Press. [DOI] [PubMed] [Google Scholar]
- 24.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 25.Tang Q, Henriksen KJ, Boden EK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–52. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 26.Gao J-X, Zhang H, Bai XF, et al. Perinatal blockade of B7−1 and B7−2 inhibits clonal deletion of highly pathogenic autoreactive T cells. J Exp Med. 2002;195:959–71. doi: 10.1084/jem.20011948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noel PJ, Alegre ML, Reiner SL, Thompson CB. Impaired negative selection in CD28-deficient mice. Cell Immunol. 1998;187:131–8. doi: 10.1006/cimm.1998.1332. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gingrich JR, Barrios RJ, Morton RA, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–102. [PubMed] [Google Scholar]
- 30.Geiger T, Gooding LR, Flavell RA. T-cell responsiveness to an oncogenic peripheral protein and spontaneous autoimmunity in transgenic mice. Proc Natl Acad Sci U S A. 1992;89:2985–9. doi: 10.1073/pnas.89.7.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Guo Y, Liu Y. A major costimulatory molecule on antigen-presenting cells, CTLA4 ligand A, is distinct from B7. J Exp Med. 1993;178:1789–93. doi: 10.1084/jem.178.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman GJ, Borriello F, Hodes RJ, et al. Murine B7−2, an alternative CTLA4 counter-receptor that costimulates T cell proliferation and interleukin 2 production. J Exp Med. 1993;178:2185–92. doi: 10.1084/jem.178.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degl'Innocenti E, Grioni M, Capuano G, et al. Peripheral T-cell tolerance associated with prostate cancer is independent from CD4+CD25+ regulatory T cells. Cancer research. 2008;68:292–300. doi: 10.1158/0008-5472.CAN-07-2429. [DOI] [PubMed] [Google Scholar]
- 34.Bonneau RH, Salvucci LA, Johnson DC, Tevethia SS. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross- reactive cytotoxic T lymphocyte clones. Virology. 1993;195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Chen HM, Subudhi SK, et al. Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nat Med. 2002;8:1405–13. doi: 10.1038/nm1202-796. [DOI] [PubMed] [Google Scholar]
- 36.Eng MH, Charles LG, Ross BD, et al. Early castration reduces prostatic carcinogenesis in transgenic mice. Urology. 1999;54:1112–9. doi: 10.1016/s0090-4295(99)00297-6. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Fu YX. LIGHT (a cellular ligand for herpes virus entry mediator and lymphotoxin receptor)-mediated thymocyte deletion is dependent on the interaction between TCR and MHC/self-peptide. J Immunol. 2003;170:3986–93. doi: 10.4049/jimmunol.170.8.3986. [DOI] [PubMed] [Google Scholar]
- 38.Bluestone JA, St Clair EW, Turka LA. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24:233–8. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Yu P, Rowley DA, Fu YX, Schreiber H. The role of stroma in immune recognition and destruction of well-established solid tumors. Curr Opin Immunol. 2006;18:226–31. doi: 10.1016/j.coi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 41.Lahl K, Loddenkemper C, Drouin C, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. Journal of Experimental Medicine. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott-Browne JP, Shafiani S, Tucker-Heard G, et al. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. Journal of Experimental Medicine. 2007;204:2159–69. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Liu Y, Wu C, et al. Epm2a suppresses tumor growth in an immunocompromised host by inhibiting Wnt signaling. Cancer Cell. 2006;10:179–90. doi: 10.1016/j.ccr.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 45.Sutherland JS, Goldberg GL, Hammett MV, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–53. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. CD4+ cells from lymphocyte gate express CD3. Profiles shown from left to right are those from sequential gating.


