Abstract
We sought to determine the influence of sildenafil on the diffusing capacity of the lungs for carbon monoxide (DLCO) and the components of DLCO (pulmonary capillary blood volume Vc, and alveolar–capillary membrane conductance DM) at rest and following exercise with normoxia and hypoxia. This double-blind placebo-controlled, cross-over study included 14 healthy subjects (age = 33 ± 11 years, ht = 181 ± 8 cm, weight = 85 ± 14 kg, BMI = 26 ± 3 kg/m2, peak normoxic VO2 = 36 ± 6 ml/kg, mean ± SD). Subjects were randomized to placebo or 100 mg sildenafil 1 h prior to entering a hypoxic tent with an FiO2 of 12.5% for 90 min. DLCO, Vc, and DM were assessed at rest, every 3 min during exercise, at peak exercise, and 10 and 30 min post exercise. Sildenafil attenuated the elevation in PAP at rest and during recovery with exposure to hypoxia, but pulmonary arterial pressure immediately post exercise was not different between sildenafil and placebo. Systemic O2 saturation and VO2peak did not differ between the two conditions. DLCO was not different between groups at any time point. VC was higher with exercise in the placebo group, and the difference in DM between sildenafil and placebo was significant only when corrected for changes in Vc (DM/Vc = 0.57 ± 0.29 vs. 0.41 ± 0.16, P = 0.04). These results suggest no effect of sildenafil on DLCO, but an improvement in DM when corrected for changes in Vc during short-term hypoxic exposure with exercise.
Keywords: Viagra, Hypoxia, Pulmonary blood volume, Membrane diffusion
Introduction
Exposure to normobaric hypoxia and high altitude both result in hypoxic pulmonary vasoconstriction. This vasoconstriction causes an increase in pulmonary arterial pressures (PAP) and may increase an individual's susceptibility to the development of pulmonary edema (Bartsch et al. 2005; Grover 1965). However, previous work has demonstrated that elevations in PAP may not be the sole determinant for increased lung water in subjects who are susceptible to high altitude pulmonary edema (HAPE) (Sartori et al. 2000). A more likely explanation for the development of HAPE is that elevations in pulmonary arterial pressures contribute to HAPE but lung fluid accumulation requires alterations in other factors that regulate lung water, such as a concurrent inability to clear fluid from the lungs.
Nitric oxide (NO) is a potent vasodilator that reduces pulmonary arterial pressure via activation of cyclic guanosine monophosphate (cGMP). However, cGMP is rapidly degraded by phosphodiesterases. Selective inhibition of phosphodiesterase-5 (PDE5) results in sustained pulmonary arterial vasodilation, with less effect on the systemic vasculature (Cohen et al. 1996; Jackson et al. 1999; Zusman et al. 1999). Sildenafil is FDA approved to treat patients with pulmonary arterial hypertension and is being studied to determine whether it improves performance and prevents or reduces the symptoms of high altitude illness in sojourners to high altitude (Aldashev et al. 2005; Galie et al. 2005; Ghofrani et al. 2004; Sebkhi et al. 2003; Zhao et al. 2001).
Previous work has demonstrated that sildenafil attenuates the increase in pulmonary arterial pressures with resting exposure to normobaric hypoxia, and may improve exercise time with normobaric hypoxia and during exposure to high altitude under some experimental conditions (Faoro et al. 2007; Ghofrani et al. 2004; Ricart et al. 2005). In addition, one study has shown sildenafil to improve oxygen saturation during several days of altitude exposure (Richalet et al. 2005). However, the exact mechanisms for these effects remain unclear. In addition, few studies have examined the possible benefits of sildenafil supplementation on gas transfer in the lungs. No study has examined the influence of sildenafil with hypoxia on the diffusing capacity of the lungs for carbon monoxide (DLCO) and whether or not alterations in DLCO and the components of DLCO (alveolar–capillary membrane conductance DM, and pulmonary capillary blood volume Vc) influence systemic oxygen saturation with sildenafil during hypoxic exposure. In addition, most studies exploring the impact of sildenafil with high altitude focus on elite athletes and climbers, rather than normally trained individuals, despite the great numbers of non-elite athletes that travel to altitude each year. Therefore, the purpose of this study was to determine the influence of sildenafil on DLCO, Vc, DM, exercise performance, and cardiovascular function, with exposure to hypoxia in normal subjects. We hypothesize that sildenafil will attenuate the increase in pulmonary arterial pressure with exposure to hypoxia at rest and during exercise and that this will influence DLCO, DM, and Vc.
Methods
Protocol
The protocol was reviewed and approved by the Mayo Clinic Institutional Review Board, all participants provided written informed consent prior to study, and all aspects of the study were performed according to the declaration of Helsinki. Fourteen non-smoking healthy subjects of below average fitness agreed to participate in the study. Exclusion criteria included cardiovascular abnormalities, pregnancy, inability to exercise, history of high altitude illness, and time spent at altitude within six months prior to participating in the study. Before performing the main protocols, subjects underwent screening tests which included baseline blood sampling, an incremental cycle ergometry test to exhaustion (to rule out cardiovascular abnormalities), a complete blood count (CBC, to rule out anemia) and a pregnancy test in women.
In addition to the screening visit, the study involved three separate visits to our laboratory for the assessment of pulmonary arterial pressures, cardiovascular function, pulmonary capillary blood volume (Vc), alveolar–capillary membrane conductance (DM), exercise performance and blood markers (cGMP, brain natriuretic peptide, BNP, and endothelin-1, ET-1) with exposure to: (1) normoxia, (2) hypoxia with placebo, and (3) hypoxia with sildenafil. Prior to exercise under all three-study conditions, the subjects were monitored during 60 min of quiet rest.
Hypoxic exposure
The subjects were exposed to acute resting hypoxia and hypoxic exercise on two separate occasions which were identical, apart from the administration of either sildenafil or placebo. A pill containing a therapeutic level of sildenafil citrate (100 mg) or placebo was administered orally during normoxia 60 min prior to exposure to hypoxia, which allowed for adequate time for the drug to take maximal effect (Michelakis et al. 2002). Following resting normoxia, the subjects were exposed to hypoxia (12.5% O2) for 60 min in a low-oxygen tent (Colorado Altitude Training, Boulder, CO), also while at rest. Following this rest period the subjects were transported to the exercise laboratory for hypoxic exercise. During transport, the subjects were fitted with a portable mask connected to a gas reservoir attached to hypoxic gas (also 12.5% O2) which allowed for transport to the physiology laboratory without exposure to room air. The total resting hypoxic exposure averaged 90 min.
Drug administration
We used a randomized double blind placebo-controlled crossover study design. The randomization of the drug administration for the two hypoxic exposure visits was performed by the pharmacy of the Mayo Clinic Clinical Research Unit, while Pfizer supplied the study drug. None of the investigators, study technicians, or subjects had any impression as to which drug was given on either study day. The investigators were blinded until the completion of the study by all of the subjects.
Data collection
Gas exchange and exercise performance
All exercise tests were performed on the same cycle ergometer (Corival Lode B.V., The Netherlands) during normoxia and hypoxia. The wattage used for the ergometry test was the same for both hypoxia visits, was determined from the subject's maximal exercise test, and the intensity was increased every 3 min during exercise. The subjects were allowed to exercise until exhaustion under all three conditions (a rating of perceived exertion of 18 out of 20 was used to determine the end of exercise) (Borg 1982). Oxygen uptake (VO2) and the elimination of carbon dioxide (VCO2) were measured continuously during all stages of the exercise tests using a Medical Graphics CPX/D (St Paul, MN) metabolic cart interfaced with a Perkin Elmer MGA-1100 mass spectrometer (Pamona, CA). This system has been validated against classic “bag” collection techniques, and stability is verified by regular testing at standard exercise intensities by laboratory personnel (Proctor and Beck 1996). During exercise the subject's heart rate was monitored with a 12-lead electrocardiogram (Marquette Electronics, Milwaukee, WI) and oxygen saturation (SaO2) was assessed with a finger pulse-oximeter (Nellcor N-595, Pleasanton, CA).
Pulmonary capillary blood volume and alveolar–capillary conductance
We measured the disappearance of nitric oxide (NO) in concert with carbon monoxide (CO) for the assessment of Vc and DM using one maneuver as described by Tamhane et al. (2001). As described by Tamhane et al. DLNO is essentially DMNO; therefore DM and Vc were determined according to the Roughton Forster equation and according to differences in molecular weight and solubility of CO and NO using the equations: 1/DLCO = 1/DMCO + 1/ΘCO × Vc, where 1/θ = (0.73 + 0058 PAO2) × 14.6/Hb. DMCO is determined from the following ratio DLNO/DMCO = DMNO/DMCO = αNO/αCO × √(MWco/MWNO) which yields a ratio of 1.97 although others have calculated this to be as high as 2.42. We used a correction factor of 1.97 for hypoxic conditions, as a ratio of 2.42 yields unreasonable values for Vc (as low as 20 ml). Triplicate maneuvers of the diffusing capacity of the lungs for carbon monoxide (DLCO) and nitric oxide (DLNO) were performed at rest and at 10 and 30 min following exercise under all three conditions. Duplicate maneuvers were performed every 3 min during exercise. The inspired O2 in the rebreathe bag was 21% for the room air measures and 12.5% for the hypoxia and hypoxic exercise measures. In the present study there were no differences in PAO2 during the rebreathe maneuver during the hypoxia and sildenafil and hypoxia and placebo conditions (PAO2, normoxia= 137.2, hypoxia with placebo = 63.5, hypoxia with sildenafil = 63.1 mmHg). The sample gasses used for hypoxic exposure with sildenafil and hypoxic exposure with placebo were identical. Alveolar PO2 was measured using a mass spectrometer and a blood sample for hemoglobin was obtained before each test.
Diffusing capacities of the lungs for carbon monoxide and nitric oxide were measured using the rebreathing technique with gases sampled on the same mass spectrometer that was used for the assessment of cardiac output as well as a NO analyzer (Sievers Instruments, Boulder, CO.) using custom analysis software as described in detail previously (Snyder et al. 2006). Briefly, a 5-1 rebreathe bag was filled with 0.3% carbon monoxide (C18O), 40 PPM NO (diluted immediately before the test in the bag from an 800 PPM gas mixture), and O2. The volume of gas used to fill the rebreathe bag was determined by the tidal volume of the subject and 500 ml was added to the subject's tidal volume to insure the bag did not collapse. The bag volume was adjusted during exercise, accordingly. Consistent bag volumes were assured using a timed switching circuit which, given a consistent flow rate from the tank, resulted in the desired volume (Johnson et al. 1992). The switching circuit and tank were checked prior to each test for accurate volumes. At the end of a normal expiration (end-expiratory lung volume, EELV) the subjects were switched into the rebreathe bag and instructed to nearly empty the bag with each breath for ten consecutive breaths. The respiratory rate during the rebreathe maneuver was controlled at 32 breaths/min with a metronome at rest and for each stage of exercise that the subjects were breathing at a rate less than 32 breaths/min. Following each diffusing capacity maneuver, the rebreathe bag was emptied with a suction device and refilled immediately prior to the next maneuver. At the start of each maneuver, there was no residual gas in the dead space of the apparatus nor from the exhaled air from the subjects as determined through gas sampling with the mass spectrometer. For our lab, the coefficients of variation are 4.1% for the DLCO measure, 8.3% for the DLNO measure, 7.2% for DM, and 6.4% for Vc.
Cardiac output, stroke volume, and heart rate
Cardiac output (Q) was assessed at rest, every 3 min during exercise and at 10 and 30 min of recovery. Cardiac output was determined using a previously-validated 8–10 breath acetylene rebreathe technique using the same 5-1 bag containing the diffusion mixture with the addition of 0.7% C2H2 and 9%. He as described previously (Bell et al. 2003; Johnson et al. 2000). Briefly, a pneumotachograph was connected to a non-rebreathing Y valve (Hans Rudolph, KC, MO) with the inspiratory port connected to a pneumatic switching valve (Hans Rudolph, KC, MO) which allowed for rapid switching from room air to the test gas mixture. Gases were sampled using the same mass spectrometer which was integrated with custom analysis software for the assessment of Q.
Blood pressure was obtained using the auscultation technique with the same technician performing all measures. Mean arterial pressure (MAP) was calculated using the equation for rest: MAP = DBP + 1/3(SBP − DBP), and MAP = DBP + 1/2(SBP − DBP) for calculation during exercise, where DBP is diastolic blood pressure and SBP is systolic blood pressure. Stroke volume was calculated from Q and HR.
Assessment of cGMP, BNP, and ET-1
Cyclic guanosine monophosphate was assessed using radioimmunoassay in the Mayo Clinic immunochemical core laboratory. Plasma BNP was determined immunoenzymatically using Unicel DXI 800 (Beckman Coulter, Fullerton, CA). Endothelin-1 levels were determined from a plasma sample using immunoassay (Quantiglo, R and D systems, Minneapolis, MN).
Pulmonary arterial pressure
Pulmonary arterial pressure was calculated from the tricuspid regurgitation (TR) velocity as described previously (Yock and Popp 1984) using the equation ΔP = Δ4V2, where P is the pressure and V is the tricuspid regurgitant velocity. The same sonographer performed all echocardiographic measures. Venous injections of agitated saline were used to enhance the signal for TR velocity, as needed. Color Doppler echocardiography was used to locate the tricuspid regurgitation jet. The maximal velocity was determined by careful application of the continuous wave sampler within and parallel to the regurgitation jet. The right atrial pressure was added to the trans-tricuspid pressure gradient as a measure of peak right ventricular systolic pressure, which is equivalent to pulmonary artery systolic pressure in the absence of pulmonary stenosis. Right atrial pressure was estimated based on an algorithm which utilizes the caliber of the inferior vena cava (IVC), the magnitude of IVC collapse with inspiration, the ratio of systolic (S) to diastolic (D) hepatic vein velocity measured by pulsed wave Doppler and the peak velocity (A) of retrograde hepatic vein flow (Nagueh et al. 1996; Ommen et al. 2000).
Statistical analysis
All statistical analyses were performed using the SPSS statistical software package (v.12, SPSS Inc., Chicago, IL). To examine the group differences between normoxia, hypoxia with placebo and hypoxia with sildenafil at rest and during exercise and recovery we performed a repeated measures ANOVA with a Tukey HSD test to determine differences between the three conditions. All data were found to have homogeneity of variance prior to the ANOVA using the Levene's test for equality of variance. The alpha level for the ANOVAs and post hoc analyses was set at 0.05 for determining statistical significance.
Results
Subject characteristics
Fourteen subjects were consented and completed all aspects of the study. Of the 14, one subject was a female, the average age was 33 years, the average BMI was 26 kg/m2, and the average maximal oxygen uptake from the cycle ergometer test was 97% of predicted (predicted based on gender, age, height and weight using the equation by Hansen et. al 1984, Table 1).
Table 1.
Subject characteristics
| n | 14 (13 males, 1 female) |
|---|---|
| Age (years) | 33 ± 10 |
| Height (cm) | 182 ± 8 |
| Weight (kg) | 87 ± 15 |
| BMI (kg/m2) | 26 ± 3 |
| VO2peak (ml kg min) | 36 ± 6 |
| VO2peak (% predicted) | 97 ± 18 |
BMI body mass index, VO2peak maximal oxygen uptake, determined from cycle ergometry. Percent predicted values were calculated using the predicted equation provided by Hansen et al. (Hansen et al. 1984). Values are mean ± SD
Resting cardiovascular and metabolic parameters
Sildenafil resulted in higher cGMP levels when compared to placebo at rest (Fig. 1). The estimated pulmonary arterial systolic pressure was higher at rest with placebo compared to sildenafil (Tables 2, 3, 4). Oxygen saturation was lower under both hypoxia conditions with no differences according to medication. Hypoxia with placebo resulted in an increase in diastolic blood pressure when compared to normoxia; this effect was not present with sildenafil. Mean arterial blood pressure was lowest with hypoxia and sildenafil when compared to both of the other conditions. Endothelin-1 was higher under both hypoxic conditions, with no differences between sildenafil or placebo.
Fig. 1.
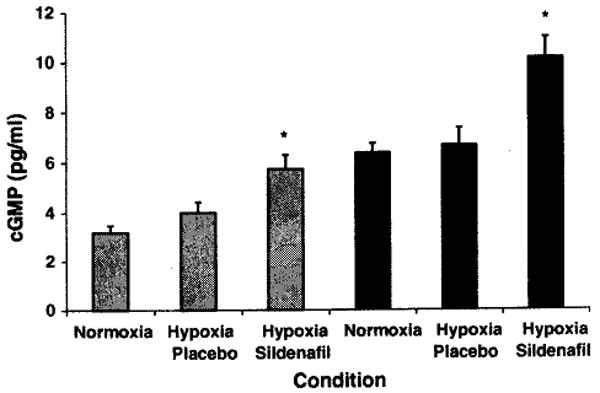
cGMP at rest and at peak exercise with normoxia, hypoxia with placebo, and hypoxia with sildenafil. Grey bars represent resting data while black bars represent peak exercise data. *P < 0.05 sildenafil versus placebo. Bars represent SEM
Table 2.
Cardiovascular response to hypoxia at rest with placebo and sildenafil
| Rest | |||
|---|---|---|---|
| Minute | |||
| Condition | Norm | HypPla | HypSil |
| n | 14 | 14 | 14 |
| Watts | 0 | 0 | 0 |
| VO2 (ml kg min) | 4.9 ± 1.0 | 5.0 ± 0.9 | 5.2 ± 0.9 |
| RVSP (mmHg) | 27 ± 6 | 34 ± 8† | 27 ± 4* |
| SaO2 (%) | 98.4 ± 0.9 | 89.7 ± 2.5† | 88.9 ± 3.5† |
| Q (1/min) | 4.5 ± 0.9 | 4.2 ± 0.8 | 4.6 ± 0.8 |
| HR (beats/min) | 68 ± 12 | 67 ± 10 | 71 ± 10 |
| SV (ml/beat) | 69 ± 21 | 65 ± 18 | 67 ± 18 |
| SBP (mmHg) | 107 ± 11 | 106 ± 12 | 103 ± 11 |
| DBP (mmHg) | 72 ± 5 | 76 ± 9† | 69 ± 5* |
| MAP (mmHg) | 84 ± 6 | 86 ± 8 | 81 ± 6* |
| BNP (pg/ml) | 5.9 ± 5.3 | 8.3 ± 7.1 | 8.8 ± 4.9 |
| ET-1 (pg/ml) | 1.0 ± 0.5 | 1.5 ± 0.4† | 1.4 ± 0.4† |
VO2 oxygen uptake, RVSP right ventricular systolic pressure, SaO2 oxygen saturation, Q cardiac output, HR heart rate, SV stroke volume, SBP systolic blood pressure, DBP diastolic blood pressure, MAP mean arterial blood pressure, BNP brain-type natriuretic peptide, ET-1 endothelin-l.
P < 0.05 compared to normoxia
P < 0.05 placebo versus sildenafil. Values are mean ± SD
Table 3.
Cardiovascular response during exercise with placebo and sildenafil
| Exercise | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minutes | 3 | 6 | 9 | 12 | ||||||||
| Condition | Norm | HypPla | HypSild | Norm | HypPla | HypSild | Norm | HypPla | HypSild | Norm | HypPla | HypSild |
| n | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 10 | 10 | 10 |
| Watts | 38 ± 7 | 38 ± 7 | 38 ± 7 | 75 ± 15 | 75 ± 15 | 75 ± 15 | 113 ± 22 | 113 ± 22 | 113 ± 22 | 150 ± 29 | 150 ± 29 | 150 ± 29 |
| VO2 (ml/kg/min) | 17 ± 3 | 16 ± 4 | 19 ± 5 | 21 ± 5 | 20 ± 4 | 21 ± 5 | 25 ± 4 | 23 ± 5 | 24 ± 3 | 30 ± 5 | 24 ± 3† | 27 ± 3†* |
| RVSP (mmHg) | 53 ± 14 | 55 ± 20 | 57 ± 16 | |||||||||
| SaO2 (%) | 98.5 ± 0.7 | 83.5 ± 5.8† | 83.2 ± 3.7† | 97.6 ± 1.6 | 82.4 ± 4.6† | 83.4 ± 3.6† | 97.9 ± 1.7 | 80.8 ± 4.0† | 82.4 ± 2.4†* | 98.2 ± 0.8 | 81.1 ± 3.9† | 82.2 ± 3.0† |
| Q (1/min) | 9.0 ± 1.7 | 9.1 ± 2.1 | 8.8 ± 1.8 | 11.0 ± 1.9 | 10.7 ± 2.4 | 10.4 ± 1.9 | 13.1 ± 2.6 | 12.4 ± 3.8 | 11.9 ± 2.4 | 14.5 ± 5.0 | 14.4 ± 5.0 | 13.1 ± 2.9 |
| HR (beats/min) | 93 ± 17 | 101 ± 17 | 103 ± 17 | 105 ± 18 | 116 ± 16† | 118 ± 22† | 124 ± 18 | 137 ± 18† | 138 ± 21† | 141 ± 21 | 158 ± 17† | 157 ± 21† |
| SV (ml/beat) | 100 ± 23 | 91 ± 26 | 88 ± 30 | 109 ± 29 | 89 ± 40 | 92 ± 23 | 109 ± 33 | 89 ± 39 | 83 ± 36 | 97 ± 45 | 92 ± 34 | 72 ± 40 |
| SBP (mmHg) | 121 ± 12 | 117 ± 14 | 119 ± 13 | 132 ± 12 | 130 ± 16 | 131 ± 17 | 143 ± 11 | 149 ± 21 | 141 ± 20 | 157 ± 16 | 161 ± 23 | 151 ± 21 |
| DBP (mmHg) | 75 ± 11 | 70 ± 10 | 59 ± 19† | 73 ± 9 | 62 ± 8† | 55 ± 18† | 71 ± 8 | 56 ± 20† | 45 ± 26 | 66 ± 9 | 37 ± 30† | 32 ± 30† |
| MAP (mmHg) | 98 ± 9 | 94 ± 10 | 89 ± 14 | 103 ± 8 | 96 ± 10† | 93 ± 12 | 107 ± 8 | 103 ± 17 | 93 ± 19†* | 112 ± 9 | 92 ± 33† | 86 ± 32† |
| BNP (pg/ml) | 9.3 ± 8.4 | 9.2 ± 8.2 | 9.2 ± 6.8 | |||||||||
| ET-1 (pg/ml) | 0.7 ± 0.2 | 1.2 ± 0.2† | 1.3 ± 0.2† | |||||||||
VO2 oxygen uptake, RVSP right ventricular systolic pressure, SaO2 oxygen saturation, Q cardiac output, HR heart rate, SV stroke volume, SBP systolic blood pressure, DBP diastolic blood pressure, MAP mean arterial blood pressure, BNP brain-type natriuretic peptide, ET-1 endothelin-1.
P < 0.05 compared to normoxia
P < 0.05 placebo versus sildenafil. Values are mean ± SD
Table 4.
Cardiovascular response during recovery with placebo and sildenafil
| Recovery | ||||||
|---|---|---|---|---|---|---|
| Minutes | 10 | 30 | ||||
| Condition | Norm | HypPla | HypSil | Norm | HypPla | HypSil |
| n | 14 | 14 | 14 | 14 | 14 | 14 |
| RVSP (mmHg) | 29 ± 7 | 37 ± 8† | 34 ± 11 | 26 ± 5 | 37 ± 9† | 30 ± 6* |
| SaO2 (%) | 97.7 ± 1.0 | 88.2 ± 4.0† | 88.5 ± 2.1† | |||
| Q (1/min) | 4.7 ± 0.6 | 4.5 ± 1.0 | 4.9 ± 1.0 | |||
| HR (beats/min) | 74 ± 11 | 79 ± 13 | 83 ± 14† | |||
| SV (ml/beat) | 47 ± 8 | 48 ± 14 | 49 ± 13 | |||
| SBP (mmHg) | 101 ± 8 | 105 ± 8 | 103 ± 9 | |||
| DBP (mmHg) | 74 ± 9 | 73 ± 8 | 75 ± 9 | |||
| MAP (mmHg) | 73 ± 30 | 80 ± 24 | 70 ± 30 | |||
VO2 oxygen uptake, RVSP right ventricular systolic pressure, SaO2 oxygen saturation, Q cardiac output, HR heart rate, SV stroke volume, SBP systolic blood pressure, DBP diastolic blood pressure, MAP mean arterial blood pressure, BNP brain-type natriuretic peptide, ET-1 endothelin-1.
P < 0.05 compared to normoxia
P < 0.05 placebo versus sildenafil. Values are mean ± SD
Cardiovascular and metabolic response to exercise
During most of exercise there were no differences in any of the cardiovascular or metabolic measurements between hypoxia with placebo and hypoxia with sildenafil. However, at 12 min of exercise VO2 was higher with sildenafil when compared to placebo. The SaO2, DBP and MAP were lower under both hypoxic conditions while HR and ET-1 were higher, when compared to normoxia. At peak exercise there was no difference in any of the cardiovascular parameters, time to exhaustion, or VO2 between placebo and sildenafil (VO2peak averaged 36 ± 6 ml kg min with normoxia, 30 ± 6 with hypoxia and placebo and 29 ± 5 with hypoxia and sildenafil, values are mean ± SD). At peak exercise cGMP was higher with sildenafil when compared to placebo.
Recovery cardiovascular and metabolic parameters
During recovery the pulmonary arterial pressure was lower with sildenafil than with placebo but no differences were observed in any of the measured or calculated cardiovascular parameters according to drug.
DLCO, Vc, and DM at rest, during exercise, and during recovery
The diffusing capacity of the lungs for carbon monoxide was higher under both hypoxic conditions (at rest, during exercise, and recovery) when compared to normoxia but there was no difference in DLCO between hypoxia with placebo and hypoxia with sildenafil (Fig. 2). Pulmonary capillary blood volume was higher with hypoxia regardless of the drug condition, but there were no significant differences between placebo or sildenafil (figure). Alveolar–capillary membrane conductance (DM) was higher with hypoxia when compared to normoxia. As would be expected, DLNO considered alone follows the pattern of change represented by the DM values. Alveolar–capillary membrane conductance corrected for pulmonary capillary blood volume (DM/Vc) was significantly lower with hypoxic exposure and placebo when compared to both normoxia and hypoxia with sildenafil at rest, during exercise, and during recovery (Fig. 3).
Fig. 2.
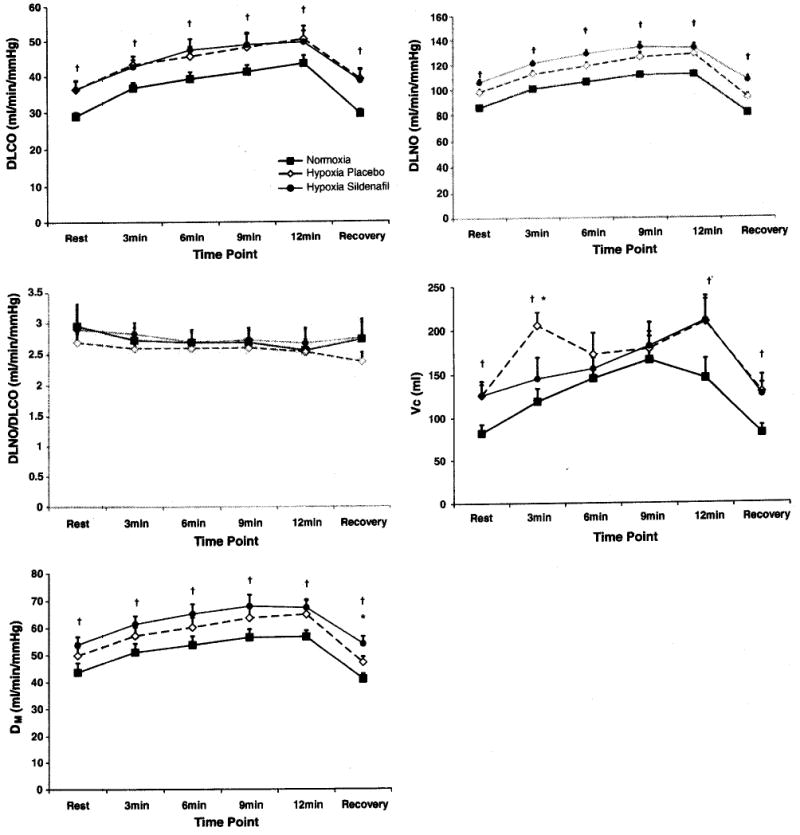
Diffusing capacity of the lung for carbon monoxide and components at rest, during exercise, and during recovery with normoxia, hypoxia and placebo, hypoxia and sildenafil. The top panel represents DLCO, followed by a panel representing DLNO, and a panel representing the DLNO to DLCO ratio with the final two panels representing pulmonary Capillary blood volume (Vc), and alveolar–capillary membrane conductance (DM). Filled black squares represent the normoxic condition, open diamonds represent the hypoxia with placebo condition, and filled circles represent the hypoxia with sildenafil condition. †P < 0.05 compared to normoxia. *P < 0.05 sildenafil versus placebo. Bars represent SEM
Fig. 3.
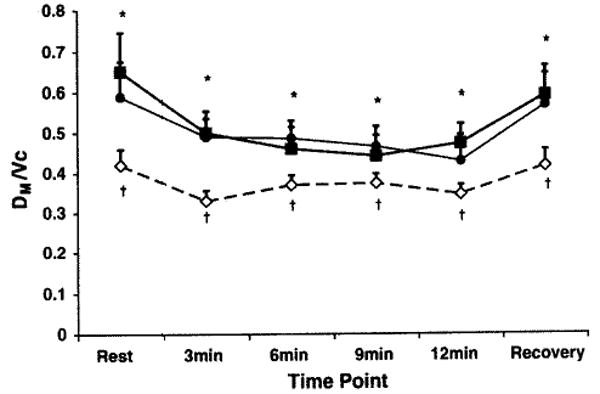
Alveolar–capillary membrane conductance relative to pulmonary capillary blood volume at rest, during exercise and during recovery with normoxia, hypoxia with placebo and hypoxia with sildenafil. Filled black squares represents normoxic condition, open diamonds represents hypoxia with placebo condition, and filled circles represents hypoxia with sildenafil condition. †P < 0.05 compared to normoxia. *P < 0.05 sildenafil versus placebo. Bars represent SEM
Discussion
We have found that sildenafil does not influence DLCO, DM, or Vc, but results in an improvement in alveolar–capillary membrane conductance relative to pulmonary capillary blood volume (DM/Vc) when compared to placebo. Correcting DM for Vc is a useful marker of efficiency of gas transfer, so this improvement suggests that sildenafil is preserving the integrity of the alveolar–capillary membrane during hypoxic exposure, although the exact mechanism for this is not known. Despite the improvement in the DM to Vc ratio, we found no differences in maximal oxygen uptake or in oxygen saturation. Further study with a longer duration of hypoxic exposure is necessary to assess the functional importance of what we interpret to be preservation of alveolar–capillary membrane efficiency with sildenafil. Other investigators have had variable findings in this regard. For example, studies at simulated altitude in 14 subjects found that oxygen saturation at maximum exercise capacity decreased to 61% in placebo treated subjects but only 67% in sildenafil treated subjects, a finding that could not be replicated by the same investigators at Everest base camp (Ghofrani et al. 2004). Failure in our study for saturation to be higher with sildenafil may reflect the relatively short duration of the hypoxic exposure used in the present study. Ricart et al. found sildenafil improved exertional systemic saturation from 63.5 to 65% with 90 min of hypoxic exposure (Ricart et al. 2005). Richalet and colleagues found that 24 h at 4350 m altitude was required before a difference in systemic saturation between sildenafil and placebo was observed (Richalet et al. 2005).
Although sildenafil blunted the increase in hypoxic resting pulmonary arterial pressure as well as the pulmonary arterial pressure observed during recovery, we did not observe an effect on the echocardiographically estimated pulmonary arterial pressure immediately following hypoxic exercise. We speculate that this may reflect the technical difficulty in estimating such pressures after peak exercise, particularly when using echocardiography to estimate PAP, when heart rate and respiratory excursion is near maximal. Although we observed a small difference in VO2 after 12-min of exercise, we did not demonstrate differences in peak wattage or time to exhaustion. Failure to see improvement in exercise time with sildenafil differs from findings of other investigators (Ghofrani et al. 2004). This may reflect differing experimental conditions and subjects. Our subjects were of average conditioning, not trained mountaineers or athletes, and peak exercise capacity under hypoxia may not be limited by right ventricular hemodynamics in such subjects. The use of untrained volunteers is an interesting aspect of the present study, however, due to the large numbers of non-climbers that travel to high altitude each year. It is possible that more sustained hypoxic exposure, where sildenafil may be expected to improve oxygen saturation, would be more likely to demonstrate an effect of sildenafil on maximal exercise capacity in subjects of average conditioning.
As expected, sildenafil resulted in an increase in cGMP when compared to placebo which suggests that the drug was still active during all of the testing. Also of interest, the cGMP assessed following exercise was higher under all three condition (normoxia, hypoxia with placebo, hypoxia with sildenafil) when compared to resting values. The increase in cGMP with exercise has been shown following acute exercise bouts and with exercise training and has been implicated as a possible mechanism for the improvement in vascular function with exercise training (Chang et al. 2003; Maeda et al. 2004).
Exercise is thought to challenge the ability of the lungs to regulate fluid and may lead to lung fluid accumulation in horses, elite athletes, and humans at high altitude. Interestingly, in the present study, alveolar–capillary membrane conductance, when considered alone or when corrected for differences in Vc, returned to baseline values during recovery under all three exercise conditions. These findings are in agreement with previous studies by us and others which have recently demonstrated that normobaric hypoxic exercise may not be an adequate stimulus to increase lung water in normally-trained healthy humans (Gallagher et al. 1988; Guenette et al. 2007; Hanel et al. 1994; Hodges et al. 2007; MacNutt et al. 2007; Snyder et al. 2006). Therefore, as with previous studies, the present study provides no evidence that short-duration hypoxic exercise results in lung fluid accumulation in healthy but untrained subjects.
Although, we demonstrated an improvement in DM in the present study, it was a small effect and only became significant when correcting for differences in Vc. In addition, we did not demonstrate differences in VO2peak, time to exhaustion with exercise, or in SaO2 which have been demonstrated by some other investigators, perhaps reflecting differences in experimental conditions and/or study subjects.
Limitations
Our study utilized normobaric hypoxia, rather than hypobaric hypoxia. Previous work has demonstrated that there may be important differences between normobaric hypoxia and high altitude that deserves further study (Bland et al. 1977; Levine et al. 1988). Thus, future work should focus on the influence of sildenafil on DLCO, Vc, and DM with exposure to high altitude.
We assessed pulmonary arterial pressure using echocardiography. While this has proven an accurate estimate of pulmonary arterial pressure, the gold standard remains right heart catheterization. Due to the invasive nature of right heart catheterization we chose to estimate pulmonary arterial pressure based on echocardiography.
Conclusion
The results of the present study suggest that sildenafil helps to sustain alveolar–capillary membrane conductance with hypoxic exposure at rest, during exercise and following recovery. However, this improvement in conductance was only present when corrected for pulmonary capillary blood volume, and was not coupled with an augmentation in peak wattage, maximal exercise time, or SaO2. Future studies should focus on the influence of sildenafil on DLCO, DM, and Vc during more sustained hypoxia and high-altitude exposure.
Acknowledgments
This work was supported by a grant to Dr. Frantz from Pfizer, Inc., NIH Grant HL71478, and AHA Grant 56051Z. At the time the study was performed Dr. Snyder was supported by the Mayo Clinic Nephrology and Hypertension Training Grant (DK007013-31). We would like to thank Minelle Hulsebus and Kathy O'Malley for their help with date collection, as well as the efforts of the study participants. We would also like to thank the staff of the General Clinical Research left (GCRC) for their assistance throughout this study. The Mayo Clinic GCRC is supported by US Public Health Service grant M01-RR00585.
Contributor Information
Eric M. Snyder, Email: snyder@pharmacy.arizona.edu, Department of Pharmacy Practice and Science, University of Arizona, 1703 E. Mabel, Tucson, AZ 85721, USA
Thomas P. Olson, Cardiovascular Diseases, College of Medicine, Mayo Clinic, Rochester, MN, USA
Bruce D. Johnson, Cardiovascular Diseases, College of Medicine, Mayo Clinic, Rochester, MN, USA
Robert P. Frantz, Cardiovascular Diseases, College of Medicine, Mayo Clinic, Rochester, MN, USA
References
- Aldashev AA, Kojonazarov BK, Amatov TA, Sooronbaev TM, Mirrakhimov MM, Morrell NW, Wharton J, Wilkins MR. Phosphodiesterase type 5 and high altitude pulmonary hypertension. Thorax. 2005;60:683–687. doi: 10.1136/thx.2005.041954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch P, Mairbaurl H, Maggiorini M, Swenson ER. Physiological aspects of high-altitude pulmonary edema. J Appl Physiol. 2005;98:1101–1110. doi: 10.1152/japplphysiol.01167.2004. [DOI] [PubMed] [Google Scholar]
- Bell C, Monahan KD, Donato AJ, Hunt BE, Seals DR, Beck KC. Use of acetylene breathing to determine cardiac output in young and older adults. Med Sci Sports Exerc. 2003;35:58–64. doi: 10.1097/00005768-200301000-00010. [DOI] [PubMed] [Google Scholar]
- Bland RD, Demling RH, Selinger SL, Staub NC. Effects of alveolar hypoxia on lung fluid and protein transport in unanesthetized sheep. Circ Res. 1977;40:269–274. doi: 10.1161/01.res.40.3.269. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Chang HJ, Chung JH, Choi BJ, Choi TY, Choi SY, Yoon MH, Hwang GS, Shin JH, Tahk SJ, Choi BI. Endothelial dysfunction and alteration of nitric oxide/cyclic GMP pathway in patients with exercise-induced hypertension. Yonsei Med J. 2003;44:1014–1020. doi: 10.3349/ymj.2003.44.6.1014. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Hanson K, Morris K, Fouty B, McMurty IF, Clarke W, Rodman DM. Inhibition of cyclic 3′-5′-guanosine monophosphate-specific phosphodiesterase selectively vasodilates the pulmonary circulation in chronically hypoxic rats. J Clin Invest. 1996;97:172–179. doi: 10.1172/JCI118386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faoro V, Lamotte M, Deboeck G, Pavelescu A, Huez S, Guenard H, Martinot JB, Naeije R. Effects of sildenafil on exercise capacity in hypoxic normal subjects. High Alt Med Biol. 2007;8:155–163. doi: 10.1089/ham.2007.1058. [DOI] [PubMed] [Google Scholar]
- Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- Gallagher CG, Huda W, Rigby M, Greenberg D, Younes M. Lack of radiographic evidence of interstitial pulmonary edema after maximal exercise in normal subjects. Am Rev Respir Dis. 1988;137:474–476. doi: 10.1164/ajrccm/137.2.474. [DOI] [PubMed] [Google Scholar]
- Ghofrani HA, Reichenberger F, Kohstall MG, Mrosek EH, Seeger T, Olschewski H, Seeger W, Grimminger F. Sildenafil increased exercise capacity during hypoxia at low altitudes and at Mount Everest base camp: a randomized, double-blind, placebo-controlled crossover trial. Ann Intern Med. 2004;141:169–177. doi: 10.7326/0003-4819-141-3-200408030-00005. [DOI] [PubMed] [Google Scholar]
- Grover RF. Pulmonary circulation in animals and man at high altitude. Ann N Y Acad Sci. 1965;127:632–639. doi: 10.1111/j.1749-6632.1965.tb49429.x. [DOI] [PubMed] [Google Scholar]
- Guenette JA, Sporer BC, Macnutt MJ, Coxson HO, Sheel AW, Mayo JR, McKenzie DC. Lung density is not altered following intense normobaric hypoxic interval training in competitive female cyclists. J Appl Physiol. 2007;103(3):875–882. doi: 10.1152/japplphysiol.00247.2007. [DOI] [PubMed] [Google Scholar]
- Hanel B, Clifford PS, Secher NH. Restricted postexercise pulmonary diffusion capacity does not impair maximal transport for O2. J Appl Physiol. 1994;77:2408–2412. doi: 10.1152/jappl.1994.77.5.2408. [DOI] [PubMed] [Google Scholar]
- Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49–S55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- Hodges AN, Sheel AW, Mayo JR, McKenzie DC. Human lung density is not altered following normoxic and hypoxic moderate-intensity exercise: implications for transient edema. J Appl Physiol. 2007;103:111–118. doi: 10.1152/japplphysiol.01087.2006. [DOI] [PubMed] [Google Scholar]
- Jackson G, Benjamin N, Jackson N, Allen MJ. Effects of sildenafil citrate on human hemodynamics. Am J Cardiol. 1999;83:13C–20C. doi: 10.1016/s0002-9149(99)00043-0. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol. 1992;73:874–886. doi: 10.1152/jappl.1992.73.3.874. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol. 2000;88:1650–1658. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- Levine BD, Kubo K, Kobayashi T, Fukushima M, Shibamoto T, Ueda G. Role of barometric pressure in pulmonary fluid balance and oxygen transport. J Appl Physiol. 1988;64:419–428. doi: 10.1152/jappl.1988.64.1.419. [DOI] [PubMed] [Google Scholar]
- MacNutt MJ, Guenette JA, Witt JD, Yuan R, Mayo JR, McKenzie DC. Intense hypoxic cycle exercise does not alter lung density in competitive male cyclists. Eur J Appl Physiol. 2007;99:623–631. doi: 10.1007/s00421-006-0388-1. [DOI] [PubMed] [Google Scholar]
- Maeda S, Tanabe T, Otsuki T, Sugawara J, Iemitsu M, Miyauchi T, Kuno S, Ajisaka R, Matsuda M. Moderate regular exercise increases basal production of nitric oxide in elderly women. Hypertens Res. 2004;27:947–953. doi: 10.1291/hypres.27.947. [DOI] [PubMed] [Google Scholar]
- Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation. 2002;105:2398–2403. doi: 10.1161/01.cir.0000016641.12984.dc. [DOI] [PubMed] [Google Scholar]
- Nagueh SF, Kopelen HA, Zoghbi WA. Relation of mean right atrial pressure to echocardiographic and Doppler parameters of right atrial and right ventricular function. Circulation. 1996;93:1160–1169. doi: 10.1161/01.cir.93.6.1160. [DOI] [PubMed] [Google Scholar]
- Ommen SR, Nishimura RA, Hurrell DG, Klarich KW. Assessment of right atrial pressure with 2-dimensional and Doppler echocardiography: a simultaneous catheterization and echocardiographic study. Mayo Clin Proc. 2000;75:24–29. doi: 10.4065/75.1.24. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Beck KC. Delay time adjustments to minimize errors in breath-by-breath measurement of VO2 during exercise. J Appl Physiol. 1996;81:2495–2499. doi: 10.1152/jappl.1996.81.6.2495. [DOI] [PubMed] [Google Scholar]
- Ricart A, Maristany J, Fort N, Leal C, Pages T, Viscor G. Effects of sildenafil on the human response to acute hypoxia and exercise. High Alt Med Biol. 2005;6:43–49. doi: 10.1089/ham.2005.6.43. [DOI] [PubMed] [Google Scholar]
- Richalet JP, Gratadour P, Robach P, Pham I, Dechaux M, Joncquiert-Latarjet A, Mollard P, Brugniaux J, Cornolo J. Sildenafil inhibits altitude-induced hypoxemia and pulmonary hypertension. Am J Respir Crit Care Med. 2005;171:275–281. doi: 10.1164/rccm.200406-804OC. [DOI] [PubMed] [Google Scholar]
- Sartori C, Allemann Y, Trueb L, Lepori M, Maggiorini M, Nicod P, Scherrer U. Exaggerated pulmonary hypertension is not sufficient to trigger high-altitude pulmonary oedema in humans. Schweiz Med Wochenschr. 2000;130:385–389. [PubMed] [Google Scholar]
- Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR. Phosphodiesterase Type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation. 2003;107:3230–3235. doi: 10.1161/01.CIR.0000074226.20466.B1. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Beck KC, Hulsebus ML, Breen JF, Hoffman EA, Johnson BD. Short-term hypoxic exposure at rest and during exercise reduces lung water in healthy humans. J Appl Physiol. 2006;101:1623–1632. doi: 10.1152/japplphysiol.00481.2006. [DOI] [PubMed] [Google Scholar]
- Tamhane RM, Johnson RL, Jr, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest. 2001;120:1850–1856. doi: 10.1378/chest.120.6.1850. [DOI] [PubMed] [Google Scholar]
- Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- Zhao L, Mason NA, Morrell NW, Kojonazarov B, Sadykov A, Maripov A, Mirrakhimov MM, Aldashev A, Wilkins MR. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation. 2001;104:424–428. doi: 10.1161/hc2901.093117. [DOI] [PubMed] [Google Scholar]
- Zusman RM, Morales A, Glasser DB, Osterloh IH. Overall cardiovascular profile of sildenafil citrate. Am J Cardiol. 1999;83:35C–44C. doi: 10.1016/s0002-9149(99)00046-6. [DOI] [PubMed] [Google Scholar]


