Abstract
Background
The role of obesity as a risk factor for cardiovascular disease in patients with chronic kidney disease (CKD) is poorly understood. Waist to hip ratio (WHR) is less influenced by muscle and bone mass than body mass index (BMI). We compared WHR and BMI as risk factors for cardiac events (myocardial infarction, fatal coronary disease) in persons with CKD.
Study Design
Cohort Study.
Setting and Participants
Persons with CKD, defined as a baseline estimated glomerular filtration rate between 15 and 60 mL/min/1.73m2, drawn from two community studies: the Atherosclerosis Risk in Communities Study and the Cardiovascular Health Study.
Predictor
Waist to Hip Ratio, Waist Circumference and Body Mass Index.
Outcomes and Measurements
Myocardial infarction and fatal coronary heart disease.
Results
Among 1,669 participants with CKD, mean age was 70.3 years and 56% were women. Mean WHR was 0.97 ± 0.08 in men and 0.90 ± 0.07 in women; mean BMI was 27.2 ± 4.6 kg/m2. Over a mean of 9.3 years of follow-up, there were 334 cardiac events. In multivariable adjusted Cox models the highest WHR group (n=386) was associated with an increased risk of cardiac events compared with the lowest WHR group [HR (95% CI) = 1.36 (1.01–1.83]. Obesity defined by BMI >30 kg/m2 (n= 381) was not associated with cardiac events [HR (95% CI) = 0.86 (0.62–1.20)] in comparison to participants with normal BMI. The results with waist circumference were similar to those with BMI.
Limitations
Absence of a gold standard for measurement of visceral fat.
Conclusions
WHR, but not BMI, is associated with cardiac events in persons with CKD. Relying exclusively on BMI may underestimate the importance of obesity as a cardiovascular disease risk factor in persons with CKD.
INTRODUCTION
Chronic kidney disease (CKD) is increasing in incidence and prevalence in the US with approximately 13% of adults in the US affected.1 CKD is now recognized as an independent risk factor for myocardial infarction (MI) and cardiovascular mortality.2–5 With the increasing incidence of hypertension and diabetes in the aging population, the number of individuals with CKD is increasing. Similar to the general population, the primary cause of death in persons with CKD is cardiovascular disease (CVD).
Obesity also increases the risk of CVD-related mortality in the general population.6, 7 During the past 20 years, the prevalence of obesity among adults has risen dramatically in the United States, with the latest data from the National Center for Health Statistics showing that 30 percent of U.S. adults (>60 million people) are obese.8 However, the role of obesity as a risk factor for MI and mortality in patients with CKD is not well understood. Previous studies that have used body mass index (BMI) to evaluate obesity as a risk factor for adverse outcomes in CKD have conflicting results,9–13 potentially reflecting the role of muscle, fat and bone mass in determining BMI. In CKD, where muscle wasting is common, lower BMI may reflect either decreased visceral fat (and lower CVD risk) or decreased muscle mass (and higher CVD risk), with the net effect of BMI on outcomes dependent on the relative contributions of each.12
Waist to hip ratio (WHR), a measure of central obesity and visceral fat, may be a better indicator of obesity than other anthropometric measures, including BMI, as high WHR can reflect both an increase in visceral fat as well as a relative lack of gluteal muscle, both of which have been found to be independently associated with cardiovascular disease risk.14–17 Since WHR is a more sensitive marker for central obesity and potentially less influenced by muscle mass, WHR may be better indicate risk associated with obesity in a population with a high prevalence of muscle loss and malnutrition, such as individuals with CKD. Therefore we compared WHR and BMI as risk factors for MI and cardiovascular mortality in individuals with CKD.
METHODS
Study Population
Individual patient data were pooled from two limited-access, publicly-available, community-based longitudinal studies: Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health Study (CHS). ARIC recruited 15,792 subjects, between the age of 45 to 64 years, from four geographically diverse communities throughout the US between 1987 and 1989 with follow up visits in 3-year intervals.18 CHS included 5,201 subjects, 65 years and older, randomly selected from Medicare eligibility files during 1989 and 1990. As African-Americans were underrepresented in the initial cohort, a second cohort of 687 African-American participants was recruited between 1992 to 1993.19 A more detailed description of the methods utilized for the ARIC and CHS studies has been previously published.18, 19
Both studies have meticulous ascertainment of CVD events during follow-up period. Pooling these cohort studies provides a large CKD cohort that allows thorough examination of our hypothesis and enhances generalizability by drawing from multiple community studies with a broad age range.
Assessment of Kidney Function
In ARIC, baseline serum creatinine was assessed in 15,582 (99%) subjects, while in CHS it was assessed in 5,716 (97%) subjects. Because serum creatinine assays vary across laboratories, we indirectly calibrated mean individual study creatinine values from ARIC and CHS to National Health and Nutrition Examination Survey (NHANES) III values and used the 4-variable modification of diet in renal disease (MDRD) equation to estimate glomerular filtration rate (GFR).3, 20
Baseline Anthropomorphic Measures
In both ARIC and CHS, waist circumference (WC) was measured using the smallest circumference between the lower ribs and iliac crests. Hip circumference was measured using the greatest circumference between iliac crest and thighs (measured at the level of maximal protrusion of the gluteal muscles). Trained personnel performed all measurements. WHR was calculated as the ratio of waist circumference to hip circumference. BMI was calculated by dividing the weight (kg) by height2 (m2). The quality control scheme for anthropometry involved equipment calibration and monitoring as well as between-technician and within-technician assessments of reliability.
Baseline Covariates
Other baseline characteristics included demographics (age, sex, race, education status), lifestyle characteristics (smoking, alcohol intake), medication use, past medical history (diabetes, hypertension and CVD), physical examination findings (systolic blood pressure), and laboratory variables (Serum creatinine, total cholesterol, albumin, hemoglobin). Race was defined as white or African American. Education level was dichotomized by high school graduation status. Cigarette smoking and alcohol use were dichotomized as current users and non-users. Diabetes was defined by self-reported history, use of oral hypoglycemic medications or insulin, or a fasting glucose ≥126mg/dL (7.0 mmol/L). Hypertension was defined as systolic blood pressure ≥140mmHg, diastolic ≥;90mmHg or use of antihypertensive medication. Baseline CVD was defined by history of recognized or silent myocardial infarction, angina based on the Rose questionnaire, stroke, transient ischemic attack, intermittent claudication, and prior coronary angioplasty or bypass procedures. CKD was defined by baseline eGFR between 15 and 60 ml/min/1.73m2 (0.25 and 1 mL/s/1.73m2).
Study Sample
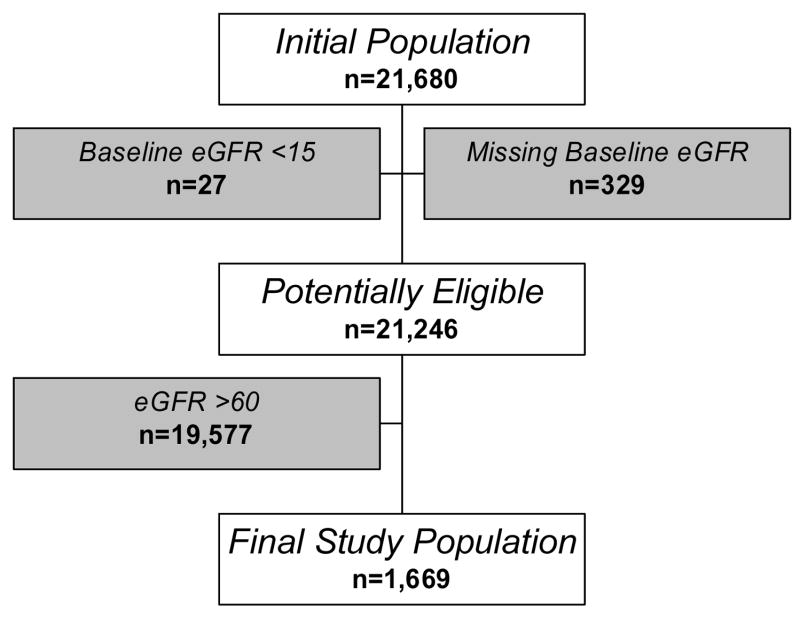
From a pooled sample of 21,680 individuals, 356 were excluded for missing baseline serum creatinine (n=329) or had a baseline eGFR <15 ml/min/1.73m2 (0.25 mL/s/1.73m2) (n=27). Among the remaining 21,246 individuals, 1,669 had CKD. An additional 42 had missing baseline covariate data, yielding a final study sample of 1,627 subjects (Figure 1).
Figure 1.
Derivation of the study cohort
Outcomes
The primary study outcome was a cardiac composite of MI and fatal coronary heart disease (CHD). The definition of MI in both ARIC and CHS included evolving Q-wave MI, chest pain plus abnormal enzymes and either an evolving ST-T pattern or a new left bundle branch block. Both studies ascertained CHD events and mortality from CHD after baseline by evaluating all hospitalizations and deaths. For patients hospitalized with potential MI, trained abstractors recorded the presenting signs and symptoms, including chest pain, cardiac enzymes, and related clinical information. Out-of-hospital fatal CHD events were investigated by an interview with one or more next of kin and a questionnaire completed by the patient’s physician. A committee of physicians using standardized criteria validated the CHD events. All fatal events were evaluated by consensus committees and classified by specific cause of death. Secondary outcomes included stroke alone and a composite outcome of MI/fatal CHD, stroke and all-cause mortality.
Statistical Analysis
BMI was examined as a categorical variable according to the World Health Organization classification: normal (BMI 18.5–24.9 kg/m2), overweight (BMI 25–29.9 9 kg/m2), and obese (BMI >30.0 kg/m2). Because there are no well-accepted cut-points for WHR and because our goal was to compare WHR with BMI, we used the same percentages stratified by sex to divide the participants into three WHR groups. Therefore, each group of WHR had similar number of persons to that of the BMI groups. WHR and BMI were also evaluated as continuous variables. Baseline characteristics were compared across WHR groups using analysis of variance for continuous variables and Chi-square tests for categorical variables. We examined the relationship of WHR and BMI using Pearson correlation and kappa statistics for agreement.
The functional forms of BMI and WHR and their associations with cardiac outcomes were examined using restricted cubic splines with four knots and are graphically presented using the Design library of S-Plus 6.1 (Insightful Inc. Seattle, WA).21 We then examined the association between BMI and WHR categories and cardiac outcomes using Kaplan Meier survival analyses. Multivariable Cox models were used to calculate the hazard ratio (HR) for cardiac outcomes in relation to either WHR or BMI, after adjusting for demographics (age, sex, race), lifestyle characteristics (smoking, alcohol intake), baseline CVD, CVD risk factors (history of diabetes, history of hypertension, baseline creatinine, total cholesterol level, albumin level) and study of origin. Patients were censored at the time of non-CHD deaths. The assumption for proportional hazards was examined using scaled Schoenfeld and Deviance residuals. The proportion hazards assumption was not violated.
Additional Analyses
To evaluate the utility of WC alone, it was studied as a continuous term in sex-specific models of the primary cardiac outcome. To evaluate if the effect of WHR might be modified by sex, an interaction between WHR (as a continuous variable) and sex was evaluated. We also examined whether the relationship between WHR and cardiac outcomes was modified by the presence of baseline CVD using an interaction term for WHR and CVD. As hypertension, high cholesterol level, and diabetes may result from central and total adiposity and may mediate the relationship between body habitus and mortality, sensitivity analyses were performed without adjusting for these factors. To examine if results differed by study, we evaluated stratified Cox models, which incorporate different baseline hazards for each study. Finally, we repeated the analyses using different cut-points as defined by Kwan et al. and Kovesdy et al., to define the BMI and WHR groups [group1 (<10th percentile), group 2 (10th to 50th percentile), group 3 (50th to 90th percentile), and group 4 (> 90th percentile)]. 10, 22 Analyses were performed using SAS version 9.1.
RESULTS
Baseline Characteristics
Among 1,669 participants with CKD, 1,225 (73.4%) were from CHS. The mean (± standard deviation) age was 70.3 ± 10.2 years, 56.4% were women and 14.3% African-Americans. Mean WHR was 0.93 ± 0.08 (0.97 ± 0.08 in men and 0.90 ± 0.07 in women) and mean BMI was 27.2 ± 4.6 kg/m2 for both men and women. Mean serum creatinine was 1.3 ± 0.4 mg/dL (115 ± 35 μmol/L), with mean eGFR of 51.1 ± 8.5 mL/min/1.73m2 (.85 ± .14 mL/s/1.73m2; Table 1). Participants with higher WHR were more likely African-American, had a higher prevalence of diabetes and CVD, and higher systolic blood pressure and total cholesterol levels. After excluding 4 participants with BMI <18.5, there were 590 (35.4%) participants in the ‘normal’ weight group, 693 (41.5%) in the ‘overweight’ group and 386 (23.1%) in the ‘obese’ group.
Table 1.
Baseline characteristics of the CKD cohort based on WHR groups
| Characteristic | WHR 1 (N = 590) | WHR 2 (N = 693) | WHR 3 (N = 386) | Total (N= 1669) | Trend P-value |
|---|---|---|---|---|---|
| WHR (range) Men | 0.78–0.95 | 0.95–1.02 | 1.02–1.28 | 0.78–1.28 | |
| Women | 0.65–0.87 | 0.87–0.96 | 0.96–1.20 | 0.65–1.20 | |
|
| |||||
| Age (years) | 71.2 ± 10.2 | 69.2 ± 9.9 | 70.3 ± 10.2 | 70.3 ± 10.2 | 0.003 |
| Female | 58.8 | 46.8 | 70.0 | 56.4 | 0.01 |
| Black | 10.2 | 14.6 | 20.2 | 14.3 | <0.001 |
| High School Graduate | 71.7 | 68.9 | 64.0 | 68.8 | 0.01 |
| Study (ARIC) | 20.2 | 28.1 | 33.7 | 26.6 | <0.001 |
| History of CVD | 33.3 | 33.3 | 34.6 | 33.6 | 0.7 |
| History of Diabetes | 11.4 | 20.2 | 27.2 | 18.7 | <0.001 |
| History of Hypertension | 69.5 | 76.9 | 76.6 | 74.2 | 0.006 |
| Smoking | 11.0 | 13.4 | 16.3 | 13.2 | 0.02 |
| WHR (mean) | 0.86 ± 0.06 | 0.95 ± 0.04 | 1.02 ± 0.05 | 0.93 ± 0.08 | <0.001 |
| BMI (kg/m2) | 25.1 ± 3.8 | 27.6± 4.1 | 29.6 ± 5.1 | 27.2 ± 4.6 | <0.001 |
| Systolic Blood Pressure (mmHg) | 134.0 ± 25.0 | 135.9 ± 23.2 | 136.3 ± 23.0 | 135.3 ± 23.8 | 0.1 |
| Hematocrit (%) | 41.1± 4.2 | 41.6 ± 4.6 | 40.6 ± 5.0 | 41.2 ± 4.6 | 0.2 |
| Creatinine (mg/dL) | 1.3 ± 0.4 | 1.4± 0.4 | 1.3 ± 0.3 | 1.3 ± 0.4 | 0.4 |
| Estimated GFR (ml/min/1.73m2) | 51.5 ± 8.5 | 51.0 ± 8.4 | 50.5± 8.9 | 51.1 ± 8.5 | 0.07 |
| Cholesterol (mg/dL) | 210.8 ± 42.8 | 215.1 ± 42.6 | 221.4 ± 47.6 | 215.0 ± 44.0 | <0.001 |
| Albumin (g/dL) | 4.0 ± 0.3 | 4.0 ± 0.3 | 3.9 ± 0.3 | 4.0 ± 0.3 | 0.004 |
Values are given as either percentages (%) or mean ± SD.
CVD, cardiovascular disease; WHR, waist to hip ratio; BMI, body mass index; GFR, glomerular filtration rate.
Concordance between WHR and BMI
The Pearson correlation between BMI and WHR was 0.31 and the weighted Kappa was 0.27. Approximately half of the participants were ranked in the same BMI and WHR group (Table 2).
Table 2.
Distribution of WHR and BMI
| BMI (range)** | BMI 1 20–24.9 kg/m2 | BMI 2 25–29.9 kg/m2 | BMI 3 >30 kg/m2 | Total |
|---|---|---|---|---|
| WHR (Range)** | ||||
| WHR 1 (0.65–0.95)* |
324
19.4% |
205
12.3% |
61
3.7% |
590 |
| WHR 2 (0.87–1.02)* | 197
11.8% |
333
19.9% |
163
9.8% |
693 |
| WHR 3 (0.96–1.28)* | 73
4.8% |
156
9.4% |
157
9.4% |
386 |
| Total | 594 | 694 | 381 | 1669 |
Of note there is overlap between the groups because of the sex stratification for WHR
The numbers in the WHR and BMI groups are very slightly different because of ties in values
The diagonal (bolded) region represents the concordance between groups of WHR and BMI
Univariate Analyses
Over mean follow-up of 9.3 years, there were 344 (20.6%) cardiac events, 198 (11.9%) stroke events and 775 (46.5%) composite events of MI/fatal CHD, stroke and all-cause mortality. Restricted cubic splines demonstrated that WHR was associated with cardiac events (p-value <0.001), while BMI was not associated with cardiac events (p-value 0.15). The test for overall effect of BMI on MI/fatal CHD was not significant (p-value=0.16). The test for overall effect of WHR on MI/fatal CHD showed a significant association (p-value < 0.001) and the test for departure from linearity was not significant for WHR (p-value=0.14) (Figure 2). Among participants in the lowest WHR group, 17.5% had cardiac events while 22.3% and 22.4% of the participants had cardiac events in the middle and highest WHR groups, respectively. Individuals in the middle and highest groups were at significantly increased risk of cardiac events compared to the lowest WHR group. In contrast, the individuals classified in the highest BMI group (considered obese) were at reduced risk of cardiac events (Table 3 and Figure 3).
Figure 2.
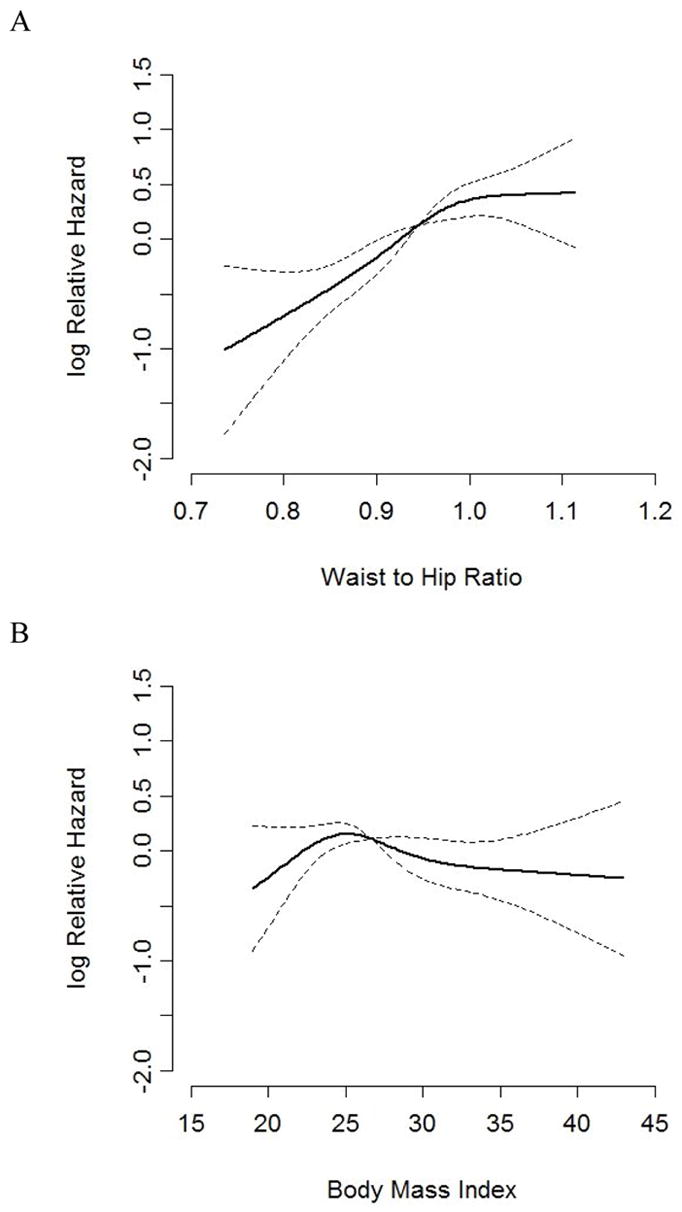
Graphical presentation of restricted cubic splines of BMI and WHR on the log hazard of cardiac events in unadjusted models. P <0.0001 for the association between WHR and cardiac events and p=0.15 for the association between BMI and cardiac events. Standard error of the predicted Xβ is zero at the reference value (the median)
Table 3.
Association of WHR and BMI with MI/Fatal CHD and a Composite Outcome
| Outcome | MI/Fatal CHD | Composite outcome*** | ||||||
|---|---|---|---|---|---|---|---|---|
| Statistics | Hazard Ratio | 95% CI | P-value | Hazard Ratio | 95% CI | P-value | ||
| Predictor | ||||||||
| WHR | Unadjusted | WHR 1 | 1.00 | - | - | 1.00 | - | - |
| WHR 2* | 1.33 | 1.03–1.70 | 0.03 | 1.13 | 0.96–1.33 | 0.1 | ||
| WHR 3* | 1.35 | 1.01–1.80 | 0.04 | 1.23 | 1.02–1.48 | 0.04 | ||
|
| ||||||||
| Adjusted | WHR 1 | 1.00 | - | 1.00 | - | - | ||
| WHR 2* | 1.18 | 0.91–1.52 | 0.20 | 1.03 | 0.87–1.22 | 0.7 | ||
| WHR 3* | 1.36 | 1.02–1.85 | 0.04 | 1.24 | 1.03–1.51 | 0.03 | ||
|
| ||||||||
| BMI | Unadjusted | BMI 1 | 1.00 | - | - | 1.00 | - | - |
| BMI 2** | 1.21 | 0.95–1.53 | 0.12 | 1.02 | 0.87–1.19 | 0.8 | ||
| BMI 3** | 0.80 | 0.59–1.09 | 0.16 | 0.69 | 0.57–0.85 | <0.001 | ||
|
| ||||||||
| Adjusted | BMI 1 | 1.00 | - | - | 1.00 | - | - | |
| BMI 2** | 1.20 | 0.93–1.53 | 0.16 | 1.04 | 0.89–1.23 | 0.6 | ||
| BMI 3** | 0.86 | 0.62–1.20 | 0.37 | 0.79 | 0.64–0.99 | 0.04 | ||
Model adjusted for significant variables: Demographics (age, sex, race), lifestyle characteristics (smoking, alcohol intake and education), baseline CVD, CVD risk factors (history of diabetes, history of hypertension, baseline kidney function, total cholesterol level, albumin level) and study of origin
In comparison with the reference of WHR
In comparison with the reference of BMI
Composite outcome includes MI/Fatal CHD, stroke and all-cause mortality
Figure 3.
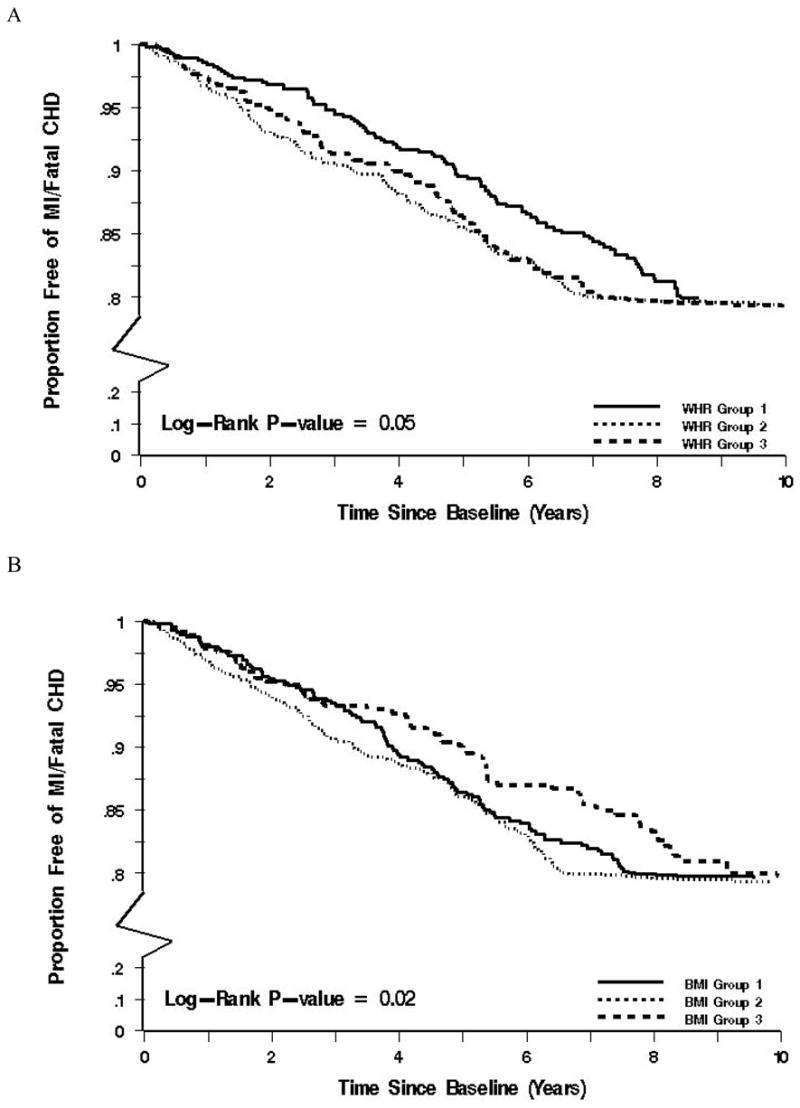
Kaplan Meier survival analysis by groups of WHR and BMI
Univariate Models
Primary outcome
In univariate analysis of the continuous terms for WHR and BMI, higher WHR was associated with cardiac events [HR= 1.53 (1.33–1.76), p<0.001) for each 0.1 unit increase], while BMI was not a significant risk factor for cardiac events [HR= 0.99 (0.97–1.01), p=0.30 for each 1 kg/m2 increase].
Secondary outcomes
WHR was not a significant risk for stroke in the unadjusted model [HR 1.12 (0.94–1.33), while higher BMI was protective [HR 0.89 (0.81–0.98)]. WHR was significantly associated with the composite outcomes in the unadjusted model [HR 1.32 (1.21–1.45)]. BMI was protective for development of the composite outcome in the unadjusted model [HR 0.92 (0.88–0.97).
Multivariable Models
Primary Outcome
In adjusted Cox models using continuous terms for WHR and BMI, higher WHR showed a trend towards increased risk of cardiac events [HR=1.16 (0.99–1.35), p-value =0.07) per 0.1 unit increase] while increasing BMI was not associated with cardiac events [HR= 1.00 (0.97–1.02), p =0.72)]. When evaluated by groups, the highest WHR levels were associated with a 36% increase in the risk of cardiac events [HR=1.36 (1.02–1.85), p=0.04], compared to the lowest group. Neither overweight nor obesity as defined by BMI classification was independently associated with cardiac events (Table 3).
Secondary Outcomes
In adjusted models, neither WHR nor BMI was significantly associated with stroke [HR 0.87 (0.71–1.08) and HR 0.91 (0.81–1.01), for each 0.1 unit increase in WHR and 1 kg/m2 unit increase in BMI, respectively].
Neither WHR nor BMI were significant risks for the composite outcome in the adjusted model [HR 1.06 (0.96–1.19) and HR 0.96 (0.91–1.01), respectively].
When evaluated by groups, the highest WHR levels were associated with a 23% increase in the risk of developing the composite outcome [HR=1.24 (1.03–1.51), p=0.03], compared to the lowest group. Overweight defined by BMI classification was not independently associated with the composite outcome, while obese was protective against the development of the composite outcome [HR=0.79 (0.64–0.99), p=0.04] (Table 3).
Additional Analyses
Using WC as a continuous term, in univariate analysis, higher WC was associated with cardiac events [HR= 1.11 (1.02–1.20), p=0.02) for each one cm increase]. In adjusted Cox models, increasing WC was not significantly associated with cardiac events [HR=1.03 (0.94–1.13), p-value =0.55) per one cm increase]. There was no interaction between WHR (as a continuous variable) and sex or WHR and CVD in the primary model (p=0.28 and p=0.77, respectively). If hypertension, cholesterol level, and diabetes were not included in multivariable model, the relationship between WHR and cardiac events was enhanced [HR=1.26 (95% CI: 1.08–1.47) per 0.1 unit increase, p=0.004], while BMI was still not associated with cardiac events [HR=1.01 (0.99–1.04) per 1 kg/m2 increase, p=0.27]. When, stratified by study, the results were unchanged (data not shown). Similarly the results did not change if different cut-offs for the BMI and WHR groups as proposed by Kwan et al. and Kovesdy et al were used (data not shown). 10, 22
DISCUSSION
In the current study, we demonstrated that the highest group of WHR is associated with MI/fatal CHD and a composite of MI/fatal CHD, stroke and all-cause mortality in subjects with CKD. Neither WC nor BMI, were significantly associated with higher risk for any of these outcomes. The highest BMI group (obese) was however protective for the composite outcome. To our knowledge, these findings have not been previously described.
These results are consistent with similar findings in the general population.23 For example, the INTERHEART study, a multi-center international prospective study with more than 27,000 participants, showed that the odds ratio of MI was significantly higher for each successive quintile of WHR, while those of BMI were not significant.7 Increased WHR may be a sign of higher waist circumference (reflecting increased visceral fat, a CVD risk factor), reduced hip circumference (reflecting low gluteal muscle mass and/or low peripheral fat mass), or a combination of these. High gluteal muscle mass and high peripheral fat may protect against CVD.17, 24
There are several mechanisms though which visceral fat may promote CVD. 1) Visceral fat leads to an increase in adipokines including leptin which may promote atherosclerosis by enhancing endothelial cell activation and migration, smooth muscle cell proliferation and vascular calcification.25, 26 Indeed, Wallace et al. noted that plasma leptin levels were independently associated with CVD events, after adjusting for traditional CVD risk factors.27 2) Proteins that are potentially protective against the development of diabetes and CVD (adiponectin, glycogen synthase, and peroxisome proliferator activated receptor-gamma) have lower levels of expression in visceral fat compared to the subcutaneous fat.28 3) Visceral fat has more beta-adrenergic receptors and a decreased function of antilipolytic receptors, which leads to higher rates of catecholamine-stimulated lipolysis and free fatty acids.29 4) Visceral fat produces more plasminogen activator inhibitor-1, an inhibitor of fibrinolysis compared with subcutaneous fat,30 while expression of angiotensinogen, a potential regulator of blood pressure, is also higher in visceral fat.31 5) Visceral fat is associated with other CVD risk factors such as metabolic syndrome (increased waist circumference, diabetes, hypertension, hypercholesterolemia and atherosclerosis), inflammation and oxidative stress.
In contrast to central fat, peripheral fat may confer a protective effect on cardiovascular health.17 Several studies, including the Hoorn study, have showed that fat mass in the lower body is inversely associated with the presence of diabetes.24, 32 In comparison to WHR, waist circumference alone is a more crude measurement for abdominal obesity, while WHR takes advantage of a reference body size - hip circumference. There are data from the general population that show a high correlation between WC, WHR and visceral fat. Seidell et al. demonstrated that WHR is highly correlated with visceral fat but not with subcutaneous fat. WC was highly correlated with both visceral and subcutaneous fat, while BMI was highly correlated with subcutaneous fat but not with visceral fat.33 The incorporation of a reference body size and the fact that WHR is not associated with subcutaneous fat (the latter which in fact may be protective) may explain why WHR is a better predictor of outcomes than WC.
The lack of agreement between WHR and BMI may reflect that these measures identify different features of obesity, thereby indicating different distributions of fat (central obesity in case of WHR vs. subcutaneous/total fat in case of BMI). Notably, BMI assesses the entire body mass without differentiating between its components, namely muscle, visceral fat, subcutaneous fat, bone and fluid. As CKD is associated with decreased muscle mass, BMI might not be the best clinical anthropometric measure of fat in this population. In our study, BMI demonstrated a trend toward a U shaped relationship with the primary outcome and the highest BMI group was in fact protective for the composite outcome. The protective effect of BMI has been seen particularly in patients with kidney failure (ESRD) and described as reverse epidemiology.9,10 Whether this represents a change in the causal relationship between classically understood risk factors and outcomes or a failure to adequately account for confounding factors such as inflammation and malnutrition is not known.
Strengths and Limitations
Pooling the ARIC and CHS cohorts provides a large diverse population with a broad age range within studies designed to evaluate CVD risk factors and events. However, there are several limitations. First, there was only one measurement of creatinine to identify participants with CKD. However, as subjects were not acutely ill at the time of study evaluation, these values likely are consistent with usual kidney function. Second, neither study assessed albuminuria at baseline. Third, results are limited by the absence of gold standard, such as DEXA, CT, or MRI for assessment of body fat composition. Previous studies have however compared WHR and BMI to these measurements and WHR was highly correlated with visceral fat measurement using DEXA, and was also shown to be comparable to single slice CT scan. 34 Finally, as these studies were conducted in the late 1980s and early 1990s, they did not measure several other non-traditional CVD risk factors such as homocysteine, oxidative stress markers and inflammatory markers; therefore unmeasured confounding remains a possibility.
Conclusion
WHR, but not BMI, was an independent risk factor for MI/fatal CHD in patients with CKD. BMI may not be an accurate measure of obesity in this population and the cardiovascular burden of obesity may be underestimated by relying on BMI. In conjunction with other clinical and laboratory data, WHR may be a helpful tool for risk stratification in patients with CKD. Future studies should confirm the consistency of these results in more advanced stages of CKD, as the results of this analysis are primarily generalizable to stage 3 CKD. Additional studies should also evaluate whether intentional reduction in WHR, whether through exercise, medication or surgical intervention, is associated with a decrease in CVD events in individuals with CKD.
Acknowledgments
The Atherosclerosis Risk in Communities (ARIC) Study and Cardiovascular Health Study (CHS) are conducted and supported by the National Heart, Lung and Blood Institute (NHLBI) in collaboration with the individual study investigators. This manuscript was not prepared in collaboration with the study investigators and does not necessarily reflect the opinions or views of the study investigators or the NHLBI.
Support: This research was supported by US National Institutes of Health grants K23 DK71636, R21 DK068310, and T32 DK007777 and Amgen Inc., Thousand Oaks, CA. Study sponsors were not involved in data analysis or interpretation of findings. Study sponsors were given the opportunity to review the manuscript prior to submission.
Footnotes
N section: An abstract based on this manuscript was presented as a poster presentation at the 2007 meeting of the American Society of Nephrology in San Francisco, CA.
Because an author of this manuscript is an editor for AJKD, the peer-review and decision-making processes were handled entirely by an Associate Editor (Bryan Becker, MD, University of Wisconsin) who served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
Financial Disclosure: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Weiner DE, Tighiouart H, Stark PC, et al. Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am J Kidney Dis. 2004;44:198–206. doi: 10.1053/j.ajkd.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13:745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 5.Shlipak MG, Heidenreich PA, Noguchi H, et al. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med. 2002;137:555–562. doi: 10.7326/0003-4819-137-7-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Yan LL, Daviglus ML, Liu K, et al. Midlife body mass index and hospitalization and mortality in older age. JAMA. 2006;295:190–198. doi: 10.1001/jama.295.2.190. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 10.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis. 2007;49:581–591. doi: 10.1053/j.ajkd.2007.02.277. [DOI] [PubMed] [Google Scholar]
- 11.Madero M, Sarnak MJ, Wang X, et al. Body mass index and mortality in CKD. Am J Kidney Dis. 2007;50:404–411. doi: 10.1053/j.ajkd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Beddhu S, Pappas LM, Ramkumar N, Samore M. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14:2366–2372. doi: 10.1097/01.asn.0000083905.72794.e6. [DOI] [PubMed] [Google Scholar]
- 13.Hsu C, Iribarren C, Go A. Body Mass Index and Risk for End-Stage Renal Disease. Ann Intern Med. 2006;144:701–702. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 14.Taylor RW, Keil D, Gold EJ, Williams SM, Goulding A. Body mass index, waist girth, and waist-to-hip ratio as indexes of total and regional adiposity in women: evaluation using receiver operating characteristic curves. Am J Clin Nutr. 1998;67:44–49. doi: 10.1093/ajcn/67.1.44. [DOI] [PubMed] [Google Scholar]
- 15.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 16.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 17.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 18.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 19.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 20.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 21.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Kwan BCH, Ramkumar N, Murtaugh MA, Bedddhu S. Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. J Am Soc Nephrol. 2006;17:99A. doi: 10.2215/CJN.04221206. [DOI] [PubMed] [Google Scholar]
- 23.Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Decreased muscle mass and increased central obesity are independently related to mortality in older men. Am J Clin Nutr. 2007;86:1339–1346. doi: 10.1093/ajcn/86.5.1339. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira I, Snijder MB, Twisk JW, et al. Central fat mass versus peripheral fat and lean mass: opposite (adverse versus favorable) associations with arterial stiffness? The Amsterdam Growth and Health Longitudinal Study. J Clin Endocrinol Metab. 2004;89:2632–2639. doi: 10.1210/jc.2003-031619. [DOI] [PubMed] [Google Scholar]
- 25.Yamagishi SI, Edelstein D, Du XL, et al. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–25100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 26.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88:954–960. doi: 10.1161/hh0901.090975. [DOI] [PubMed] [Google Scholar]
- 27.Wallace AM, McMahon AD, Packard CJ, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104:3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 28.Montague CT, Prins JB, Sanders L, et al. Depot-related gene expression in human subcutaneous and omental adipocytes. Diabetes. 1998;47:1384–1391. doi: 10.2337/diabetes.47.9.1384. [DOI] [PubMed] [Google Scholar]
- 29.Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann Med. 1995;27:435–438. doi: 10.3109/07853899709002451. [DOI] [PubMed] [Google Scholar]
- 30.Alessi MC, Peiretti F, Morange P, et al. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes. 1997;46:860–867. doi: 10.2337/diab.46.5.860. [DOI] [PubMed] [Google Scholar]
- 31.Dusserre E, Moulin P, Vidal H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim Biophys Acta. 2000;1500:88–96. doi: 10.1016/s0925-4439(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 32.Snijder MB, Dekker JM, Visser M, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–377. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 33.Seidell JC, Perusse L, Despres JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr. 2001;74:315–321. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 34.Schoen RE, Thaete FL, Sankey SS, Weissfeld JL, Kuller LH. Sagittal diameter in comparison with single slice CT as a predictor of total visceral adipose tissue volume. Int J Obes Relat Metab Disord. 1998;22:338–342. doi: 10.1038/sj.ijo.0800591. [DOI] [PubMed] [Google Scholar]