Summary
Background
IL-13 plays a key regulatory role in asthmatic responses and immunity to parasitic infection. In vivo, IL-13R-α2 is a critical modulator of IL-13 bioactivity. When inducibly expressed on the surface of fibroblasts and other cell types under inflammatory conditions, IL-13R-α2 contributes to resolution of IL-13 responses. A soluble form of IL-13R-α2 (sIL-13R-α2) can be detected in murine circulation, and functions as a regulator of IL-13 bioactivity. In humans, sIL-13R-α2 has been more difficult to detect. Recently, novel assay systems have been described to quantitate sIL-13R-α2 in human circulation, and revealed unexpectedly high levels of sIL-13R-α2 in healthy subjects.
Objective
To verify sIL-13R-α2 quantitation in human plasma samples under stringent conditions of signal verification and false-positive detection.
Methods
A standard ELISA protocol was evaluated for specificity using false-positive detection reagents. A more stringent ELISA protocol was developed by optimizing the composition of blocking and dilution buffers.
Results
Using the stringent assay protocol, endogenous sIL-13R-α2 was undetectable in plasma samples from a total of 120 asthmatics and 20 healthy subjects, and in bronchoalveolar lavage fluid from 10 asthmatics and eight healthy subjects undergoing allergen challenge.
Conclusion
These results underscore the necessity to perform rigorous assay controls in the biological matrix to be tested. Because the soluble form could not be demonstrated, our findings question a role for sIL-13R-α2 in the regulation of IL-13 bioactivity, and highlight the potentially important contribution of the membrane-bound form of IL-13R-α2 in humans.
Keywords: asthma, biomarkers, cytokines
Introduction
The T-helper type 2 (Th2) cytokine, IL-13, is a major regulatory cytokine in asthmatic responses. As a direct inducer of airway hyper-reactivity, lung inflammation, mucus hyper-secretion, and fibrosis, IL-13 represents a key target for the treatment of asthma [1]. Furthermore, IL-13 is an essential inducer of fibrosis in host responses to parasite infection [2]. IL-13 neutralization is an effective therapeutic strategy in animal models of asthma and reduces fibrosis in models of parasite infection [2, 3]. A recombinant soluble form of the natural IL-13 antagonist, IL-13R-α2, produced as a dimeric Fc construct, has been invaluable for validation of the role of IL-13 in these models [3–6].
In vivo, the IL-13 receptor system provides for rapid and sensitive control of cellular responses to this critical cytokine. Cellular responses to IL-13 are mediated through a receptor complex composed of the IL-13R-α1 and IL-4R-α chains, which also responds to the related cytokine, IL-4. IL-13 accesses the receptor through an initial, relatively low-affinity, interaction with IL-13R-α1 on the surface of B cells, monocytes, epithelial cells, and other cell types. This complex recruits IL-4R-α to form the high-affinity receptor that is competent for signal transduction through phosphorylation, dimerization, and nuclear translocation of the transcription factor, STAT6 [7–9]. IL-13 binding to IL-13R-α1 can be effectively competed by IL-13R-α2, which binds the cytokine with higher affinity, and at a site largely overlapping with that seen by IL-13R-α1 [10]. The IL-13R-α2 chain, which shares approximately 20% amino acid sequence homology with IL-13R-α1, is inducibly expressed on fibroblasts and other cell types [11, 12]. Although it may have some signalling capacity under certain activation conditions [13, 14], IL-13R-α2 is thought to act primarily as a ‘decoy’ receptor, sequestering IL-13 from the IL-13R-α1/IL-4R-α complex, and thus inhibiting its function [15–17].
In murine systems, both the soluble and cell surface forms of IL-13R-α2 may be induced during inflammatory conditions, resulting in ablation of IL-13 signalling responses [18–21]. Soluble IL-13R-α2 can be reliably quantitated in the sera of mice [18, 22, 23]. In human systems, surface IL-13R-α2 expression is readily detectable on activated fibroblasts, keratinocytes, and other cell types, but evidence of soluble IL-13R-α2 has been difficult to establish. Recently, novel assay formats have been developed to detect the soluble form of IL-13R-α2 (sIL-13R-α2) in human serum [23], and bronchoalveolar lavage (BAL) fluid [24], at unexpectedly high levels. Serum levels appear higher in subjects with active parasite infestation [23], and BAL fluid levels are lower in subjects with asthma [24], suggesting a functional role for sIL-13R-α2 in the regulation of IL-13 responses similar to that observed in mouse models.
Understanding the role and function of IL-13R-α2 in human systems is critical to the development of effective agents to modulate this key cytokine system. Newly developed assay formats must be rigorously evaluated to establish specificity of detection, which can be affected not only by the reagents but also by the assay configuration. In this report, we re-examine the quantitation of sIL-13R-α2 in human serum samples, and provide evidence that the high levels reported previously may have been detected erroneously.
Materials and methods
Healthy volunteer and asthma plasma samples
Samples used for assay development and standard curve generation were donated anonymously by healthy volunteers at Wyeth (Andover and Cambridge, MA, USA) under a physician reviewed and approved Wyeth Research Volunteer Blood Donor Program. Blood was drawn into Vacutainer® mononuclear cell preparation tubes (Becton-Dickinson, San Jose, CA, USA), and aliquots of plasma frozen at −80 °C.
Samples from subjects with asthma were collected from patients enrolled in the Wyeth-sponsored Asthma Observational Study 9998A1-900-WW (A Prospective, Non-interventional, Exploratory, Gene Expression Study in Adults with Mild, Moderate, and Severe Persistent Bronchial Asthma). Samples from healthy volunteers were collected from subjects enrolled in the Wyeth-sponsored Healthy Volunteer Observational Study 9999A1-900-WW (A Longitudinal Population-Based Study of Peripheral Blood Gene Expression Profiles in Healthy Volunteers). Informed consent was approved by local Institutional Review Boards and obtained from each patient.
Bronchoalveolar lavage samples
Thirty-six samples of BAL fluid were obtained from 18 patients undergoing segmental allergen challenge (SAC) in a Wyeth-sponsored study at the Cleveland Clinic. In this study, 10 mild asthmatic and eight non-asthmatic individuals underwent bronchoscopy and SAC with either timothy grass or ragweed allergen, based on season. The dose for the challenge was determined empirically for each asthmatic patient. Each non-asthmatic subject received a uniform allergen dose. BAL fluid was collected at time 0 (baseline) and 24 h post-challenge. For each collection, three 50 mL aliquots of normal saline solution warmed to 37 °C were instilled in each of two separate segments in the lingual and withdrawn by gentle hand aspiration. Processing of BAL was as described previously [25]. Studies have supported this timing for maximal BAL eosinophilia, cytokine release, and cell activation [26, 27].
Standard enzyme-linked immunosorbent assay protocol
The IL-13R-α2 capture ELISA from R&D Systems (Minneapolis, MN, USA) was used according to the manufacturer’s protocol, with minor modifications. The capture antibody was diluted to 4 µg/mL in PBS, and 100 µL/well was coated on assay plates (Immulon IV HBX; VWR) overnight at room temperature (RT). Plates were washed with PBS containing 0.05% Tween-20 (PBS-Tween), using a protocol of two wash cycles with 300 µL buffer per well, followed by 180° rotation of the plate, and then a repeat of the two wash cycles for a total of four wash cycles. A blocking solution of 5% non-fat dry milk (Bio-Rad, Hercules, CA, USA) diluted in PBS was added at 200 µL/well. Plates were incubated static for 1.5–2 h, RT, then washed. A seven-point standard curve ranging from 8000 to 125 pg/mL recombinant human IL-13 sR-α2/Fc chimera (R&D Systems), diluted in sample dilution buffer (R&D Systems), was added at 100 µL/well, along with samples of human serum, plasma, or BAL fluid diluted in sample dilution buffer. After static incubation for 2 h at 37 °C, plates were washed, and then incubated for 2 h, 37 °C, with biotinylated anti-human IL-13R-α2 (R&D Systems) diluted in PBS. The signal was detected using streptavidin–HRP conjugate (R&D Systems) for 20–30min, RT. Colour was developed using TMB reagents, and the reaction was stopped after 20 min by the addition of 50 µL/well of 2 N H2SO4. Plates were read at optical density 450 nm with wavelength correction set to 540 nm. Sample concentration was determined based on a four-parametric curve fit of the standard curve.
SpecificBlue enzyme-linked immunosorbent assay protocol
The SpecificBlue ELISA protocol was generated by empirical optimization of the R&D Systems ELISA kit, which used mouse IgG1 monoclonal anti-human IL-13R-α2 as capture antibody, and biotinylated affinity-purified polyclonal goat anti-human IL-13R-α2 serum as detector. Two biotinylated reagents were used for the detection of false-positive signal: goat anti-human TNF-α (R&D Systems) and goat anti-mouse IL-17A (R&D Systems). The methods used by R&D Systems to generate and purify these anti-sera were similar to those used for the goat anti-human IL-13R-α2 reagent. Plates were coated as described above, and then washed with a Dynex plate washer (Richfield, MN, USA) using PBS containing 0.05% Tween-20 (Pierce, Rockford, IL, USA) (PBS-Tween). After the first two cycles, plates were rotated 180° and the wash was repeated for a total of four cycles. All subsequent washes were performed in the same manner using the same buffer. Plates were blocked with 0.5 m NaCl SuperBlock® (Pierce) with NaCl added to a final concentration of 0.5 m. Buffer was made fresh for each use, added at 200 µL/well, and the plate was incubated shaking at 12 g for 3–5 min. A seven-point standard curve of recombinant human IL-13R-α2/Fc (R&D Systems) ranging from 8000 to 125 pg/mL with one blank was used for each plate. Dilution buffer for the standard curve consisted of control human healthy volunteer plasma diluted 1 : 4 in high salt (HS) PBS (500mm NaCl, 2.7mm KCl, 8.1mm Na2HPO4, and 1.47mm KH2PO4) plus 1% bovine serum albumin (BSA) (Sigma, St Louis, MO, USA). The dilution buffer was made fresh for each use.
Each sample was assayed in duplicate in three different assay formats: (1) IL-13R-α2 quantitation. Plasma (25 µL) was added to 75 µL sample dilution buffer (HS PBS+1% BSA), made fresh for each use. The plate was sealed and incubated static at 37 °C for 2 h±10 min, and then washed as described above. IL-13R-α2 detector antibody stock was diluted to a working concentration of 200 ng/mL in reagent diluent consisting of PBS+1% BSA. (2) False-positive detection. The false-positive control assay was run in parallel with the same protocol, using biotinylated antibody to TNF-α or IL-17A diluted to 300 ng/mL in reagent diluent. (3) Detection of exogenously added IL-13R-α2 standard (spike-in). To determine if matrix interference effects were present, binding to exogenously added IL-13R-α2 was determined in one of two ways: (i) IL-13R-α2 standard was added at a 1 : 4 dilution of plasma or (ii) IL-13R-α2 was added to undiluted plasma, incubated for 30min at room temperature, and the mixture was then diluted 1 : 4. No significant differences were observed between these procedures. The signal was detected using streptavidin–HRP conjugate (R&D Systems), followed by TMB substrate.
Results
Demonstration of false-positive signal in the interleukin-13R-α2 enzyme-linked immunosorbent assay
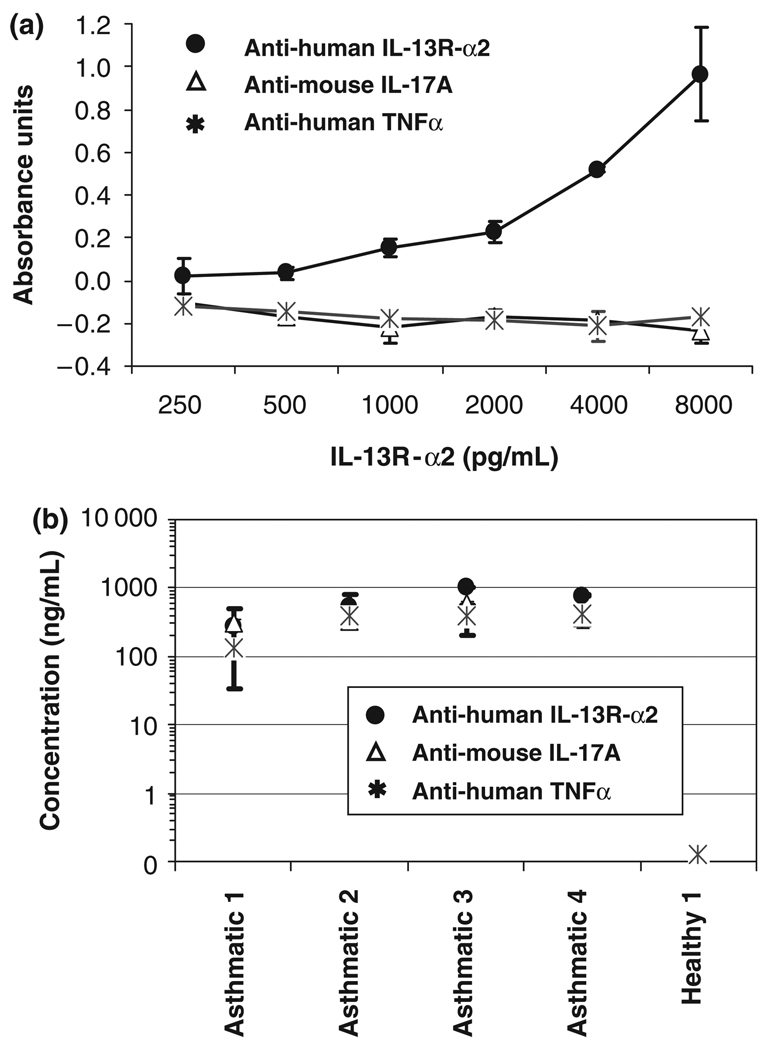
Using the standard protocol for the detection of human IL-13R-α2 with a commercially available ELISA kit, we found a high incidence of serum samples containing detectable levels of IL-13R-α2. Average values of 38 ng/mL were found for asthmatic subjects (n = 11) and 30 ng/mL for non-asthmatics (n = 12). Differences between the groups were not statistically significant (data not shown). When the IL-13R-α2 standard curve was run in the same protocol using false-positive control antibodies (biotinylated anti-TNF-α or anti-IL-17A), no false-positive signal was detected (Fig. 1a). However, when plasma samples were tested using false-positive control antibodies, positive signals were observed, which were not attributable to specific recognition of IL-13R-α2 (Fig. 1b).
Fig. 1.
Human IL-13R-α2 signal using the false-positive control detection antibodies. (a) Recombinant IL-13R-α2 standard curve in PBS+0.05% Tween dilution buffer was not detected with false-positive control antibodies (biotinylated anti-TNF-α or biotinylated anti-IL-17A). (b) Human plasma samples from asthmatics and healthy volunteers show an IL-13R-α2 false-positive signal using the false-positive control antibodies in the PBS-Tween buffer.
Abrogation of false-positive signals using the SpecificBlue enzyme-linked immunosorbent assay protocol
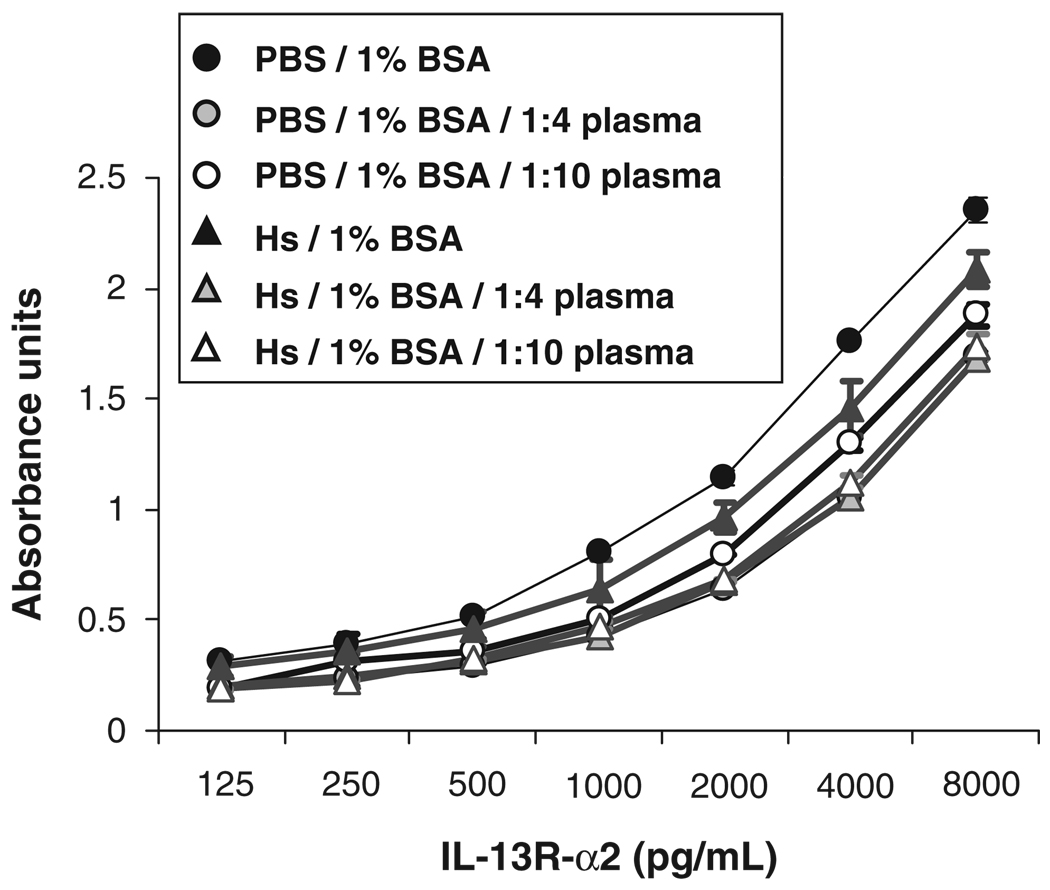
To reduce the false-positive signal for the detection of IL-13R-α2 standard, we tested the effects of increasing NaCl concentration in the blocking buffer, and addition of NaCl and 1% BSA to dilution buffers. Effects were tested both in dilution buffer and, to mimic the conditions of tested levels in plasma samples, in plasma diluted 1 : 4 in dilution buffer. We found that raising the NaCl concentration in both the blocking buffer (SuperBlock®) and the dilution buffer to 0.5 m, and adding 1% BSA to the dilution buffer abrogated the false-positive signal while preserving the specific anti-IL-13R-α2 signal. These conditions are referred to as the SpecificBlue ELISA protocol. Assay sensitivity was not compromised by the increase in NaCl concentration (Fig. 2). A standard curve of 8 ng/mL to 125 pg/mL was run in duplicate 10 times. At the 250 pg/mL concentrations, the %CV from these runs was 10.2±7.7%. At the 250 pg/mL concentrations, the %CV from these runs was 22.2±20%. Note that although the signals were highest with the protocol of PBS+0.05% Tween and no BSA, background was also highest under these conditions. Addition of 1% BSA decreased back-ground, and elevation of NaCl concentration to 0.5 m lowered it further.
Fig. 2.
Comparison of IL-13R-α2 standard curves in buffer and in diluted plasma. IL-13R-α2 standard was diluted in PBS or in high salt (HS) buffer containing 1% BSA and 0, 1 : 4 or 1 : 10 dilution of human control plasma. Assay sensitivity was comparable under all conditions, but signal was reduced in the presence of plasma. BSA, bovine serum albumin.
Levels of interleukin-13R-α2 in the plasma of patients with asthma and healthy volunteers
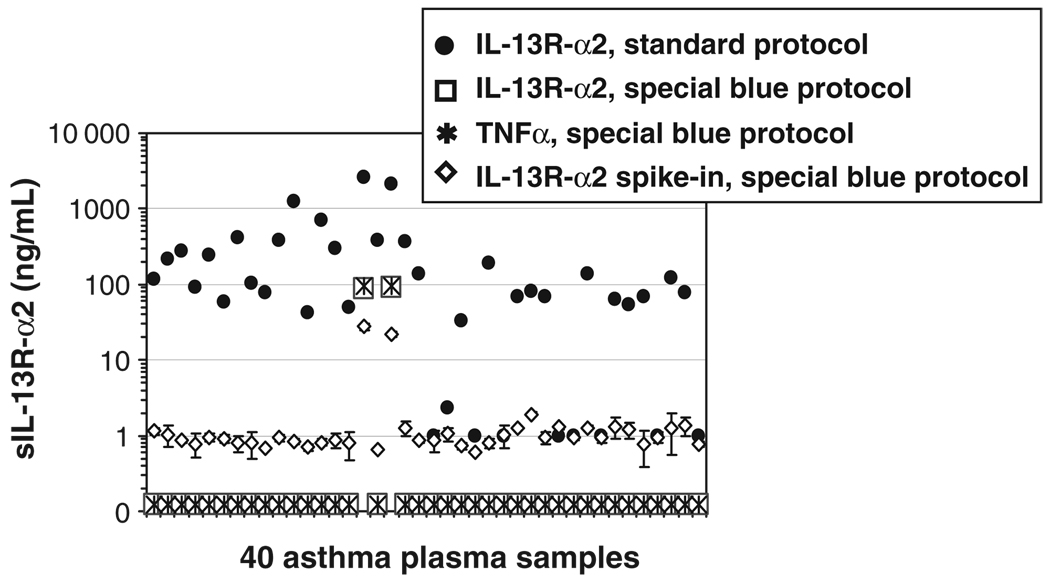
Using the SpecificBlue protocol modifications (0.5 m NaCl in blocking buffer and HS PBS+1% BSA in dilution buffer), levels of IL-13R-α2 were assayed in plasma samples from 120 asthmatics and 20 healthy volunteers (total N= 140). The signal obtained with the false-positive detection antibodies was also measured for each sample. In addition, signal from IL-13R-α2 standard exogenously added to each sample was measured as a control for false negatives. The signals were compared with those that had been obtained using the standard PBS+0.05% Tween protocol. Figure 3 shows the results obtained with 40 samples from asthmatic subjects. These results are representative of the results obtained with all 120 samples from asthmatic subjects, and with 20 samples from healthy volunteer controls. In all cases, a positive signal was obtained using the Standard ELISA protocol, and biotinylated anti-IL-13R-α2 detection antibody (Fig. 3). This signal was not specific, however, as all samples also gave a signal using the false-positive control detection antibodies, biotinylated anti-TNF-α (Fig. 3) or IL-17F (data not shown). The SpecificBlue ELISA protocol eliminated the false-positive detection in all but two cases (discussed below).
Fig. 3.
SpecificBlue ELISA abrogates false-positive IL-13R-α2 signals. Forty asthmatic plasma samples were run in the Standard (●) or the SpecificBlue (0.5m NaCl SuperBlock and HS PBS diluent plus 1% BSA;□) ELISA protocol. With the SpecificBlue ELISA, all but two of the false-positive signals (*) were below the detection limit. Recovery of spiked-in IL-13R-α2 (1ng/mL; ◇). Note that with the SpecificBlue protocol, the signals for specific IL-13R-α2 detection (□) and false-positive detection (*) overlap. BSA, bovine serum albumin; HS, high salt; sIL-13R-α2, soluble form of IL-13R-α2.
Detection of natural interleukin-13R-α2 exogenously added to plasma
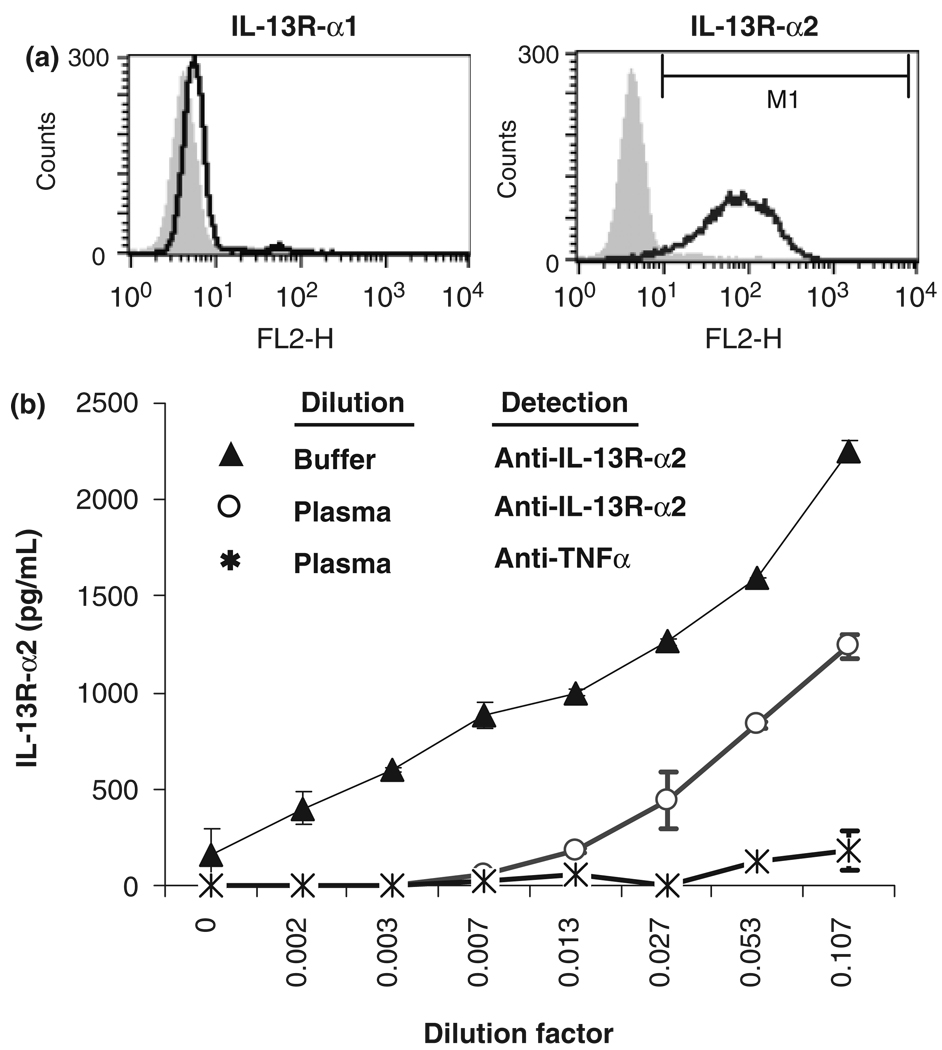
The standard used for this assay was a dimeric recombinant IL-13R-α2-Fc chimeric protein construct, while the soluble IL-13R-α2 in human plasma would be expected to be monomeric, non-polymorphic, and unconjugated to Fc. Therefore, we considered the possibility that IL-13R-α2 may have been present in the human plasma samples, but was undetectable in this assay format because the ELISA reagents were unable to recognize the natural form. Therefore, we prepared a Tween-20-solubilized membrane fraction of human A375 cells, which express high levels of IL-13R-α2 on the cell surface. The results confirmed that this preparation of natural IL-13R-α2 was detectable using the SpecificBlue protocol (Fig. 4).
Fig. 4.
Natural IL-13R-α2 is detectable using the SpecificBlue ELISA. (a) A375 human melanoma cells express high levels of cell surface IL-13R-α2, but not IL-13R-α1. (b) Natural IL-13R-α2 recovered from A375 cells solubilized with Tween-20 was detected using the SpecificBlue ELISA protocol in the absence (▲) or presence (◦) of control human plasma diluted 1 : 4. False-positive detection is shown (*).
Levels of interleukin-13R-α2 in bronchoalveolar lavage fluid samples
BAL fluid samples from 10 mild asthmatic and eight non-asthmatic subjects were assayed using the SpecificBlue protocol. For each subject, BAL fluid was collected at time 0 (pre-) and 24 h post-SAC. Representative data are shown in Table 1. All BAL fluid samples, both pre- and post-allergen challenge, gave signals with the false-positive detection using TNF-α-specific antibody in the SpecificBlue protocol. Only two BAL samples (#1480 and #1481) gave signals with specific detection reagents that were higher in magnitude than those seen with the false-positive detection reagents. No differences in magnitude or specificity of signal were noted related to the allergen challenge. These data demonstrate that the SpecificBlue protocol, successful in abrogating false-positive signals in plasma samples, did not completely abrogate false-positive signals in the BAL fluid matrix. Because the false-positive signal was equivalent to or higher than the signal seen with IL-13R-α2 detection in most cases (Table 1), our results do not convincingly show that IL-13R-α2 can be detected in BAL fluid. Because some IL-13R-α2 detection remained using the SpecificBlue protocol, however, we cannot definitively rule out this possibility.
Table 1.
sIL-13R-α2 detection in BAL fluid
| BAL donor number | Status | Allergen challenge* | IL-13R-α2 detection (ng/mL)† | False-positive detection (ng/mL)‡ |
|---|---|---|---|---|
| 1276 | HS | Post | 0.49 | 1.15 |
| 1314 | Asthmatic | Pre | 0.42 | 1.26 |
| 1427 | HS | Post | 0.13 | 1.13 |
| 1466 | HS | Pre | 0.20 | 1.27 |
| 1475 | Asthmatic | Pre | 0.18 | 1.08 |
| 1476 | Asthmatic | Post | 0.68 | 1.09 |
| 1480 | Asthmatic | Pre | 1.43 | 1.20 |
| 1481 | Asthmatic | Post | 1.66 | 1.06 |
| 1503 | Asthmatic | Pre | 0.88 | 0.98 |
| 1510 | HS | Pre | <0.125 | 1.09 |
| 1541 | Asthmatic | Pre | <0.125 | 0.94 |
| 1542 | Asthmatic | Post | 0.16 | 1.07 |
Samples were collected before (pre) or 24 h after (post) lung segmental challenge with Timothy grass or ragweed allergens.
Average reading (ng/mL) with IL-13R-α2 detection reagent, using the SpecificBlue protocol.
Average reading (ng/mL) with false-positive detection reagent in the SpecificBlue protocol, using TNF-α-specific antibody.
BAL, bronchoalveolar lavage; HS, high salt; sIL-13R-α2, soluble form of IL-13R-α2.
Discussion
sIL-13R-α2 is a naturally occurring regulator of IL-13 function in murine systems, and has been proposed to function similarly in humans. Nevertheless, sIL-13R-α2 has been difficult to detect reliably in samples of human serum or plasma. Recently, novel ELISA formats have been described by which sIL-13R-α2 was readily quantitated in the vast majority of human serum samples tested. Furthermore, levels appeared to be elevated in subjects undergoing IL-13- mediated immune responses, including those with active parasite infestation [23]. These findings raised the possibility that sIL-13R-α2 could function as a critical component of IL-13 regulation in humans, whose levels could be readily assayed for diagnostic or therapeutic use. The SpecificBlue ELISA, described in detail here, detects IL-13R-α2 at levels of 250 pg/mL or above. It has adequate sensitivity to detect the levels of 1–100 ng/mL sIL-13R-α2 that have been reported in human circulation [23], and reliably quantitated sIL-13R-α2 standard that was exogenously added to plasma. Using this assay, endogenous IL-13R-α2 was undetectable in plasma samples from a total of 120 asthmatics and 20 healthy subjects, and in BAL fluid from 10 asthmatics and eight healthy subjects.
Initial runs of the assay detected levels of sIL-13R-α2 exceeding 1 ng/mL in all human plasma samples tested, consistent with levels reported in the literature [23]. Further investigation revealed that these readings were false positives. When biotinylated detection reagents specific for TNF-α or IL-17A were substituted for the IL-13R-α2-specific detection reagent, readings of similar magnitude were obtained. Importantly, the false-positive readings were seen only in the biological samples, and not with standards diluted in media. Our findings underscore the necessity of performing numerous controls for the detection of false-positive signals when working in the plasma and serum matrixes. Using the SpecificBlue protocol, in which increased NaCl concentrations were used in blocking and dilution buffers, combined with 1% BSA in the dilution buffer, the false-positive signal was abrogated while the specific anti-IL-13R-α2 signal was preserved. In addition, we introduced the routine inclusion of false-positive control detection antibodies, to alert us if a signal detected from a plasma sample was attributable to something other than specific recognition of IL-13R-α2.
Even with the SpecificBlue protocol, two false-positive signals were detected among the 140 total plasma samples tested. This finding suggests that there are two sources of false-positive signal, only one of which can be abrogated using the SpecificBlue protocol described here. Preliminary evidence suggests that the second type of false-positive signal is attributable to rheumatoid factor (data not shown).
The lack of detectable sIL-13R-α2 in human samples has important implications for our understanding of IL-13 regulation in human immune responses. In the mouse, IL-13R-α2 acts as a carrier for IL-13 in the circulation, such that mice lacking IL-13R-α2 have accumulation of the cytokine in tissues, but depleted IL-13 levels in the blood [22]. In addition, IL-13R-α2 is thought to have a ‘decoy’ function. Whether on the cell surface or in soluble form, IL-13R-α2 binds to IL-13 and sequesters it from the lower affinity IL-13R-α1 chain, preventing biological responses to the cytokine [17, 28].
IL-13R-α2 can be expressed at high levels in synovial fibroblasts [28], dermal keratinocytes, epithelial cells, and macrophages, in addition to several brain tumours [29], suggesting potential roles in a number of immune responses. Cell surface IL-13R-α2 expression can be induced by high levels of IL-4 or IL-13, and regulated by TNF-α and IFNγ [11, 30, 31]. In murine models, induction of surface IL-13R-α2 results in ablation of IL-13 signalling responses [18–21]. Available evidence suggests a similar role for IL-13R-α2 in human cells. Reduced IL-13 responsiveness was seen with the human lung epithelial cell line, BEAS-2B [17], or the monocytic cell line, U937 [32], upon transfection of IL-13R-α2 and cell surface expression. In primary human fibroblasts and fibroblast lines, the STAT6 phosphorylation response to IL-13 was ablated following induction of surface IL-13R-α2 expression [11, 33]. Recently, IL-13R-α2 expression was shown to impact downstream responses. In primary human smooth muscle cells, acetylcholine-induced Ca2+ mobilization and proliferation, responses stimulated by IL-13 treatment, were reduced upon induction of cell surface IL-13R-α2 [34]. Levels of IL-13R-α2 expression on lung fibroblasts may be reduced in asthmatics [35], which could affect regulation of IL-13 function in the asthmatic lung. These observations strongly support the model of cell surface IL-13R-α2 acting as an antagonist of IL-13 bioactivity. Interestingly, IL-13R-α2 may abrogate responsiveness to IL-4 in addition to IL-13, by mechanisms that are still poorly defined [12, 34, 36].
The cell surface form of IL-13R-α2 has a short cytoplasmic region, which lacks known signalling motifs [37, 38], but is capable of internalizing bound IL-13 [39, 40]. Evidence suggests the existence of an intracellular pool of receptor, capable of rapidly populating the cell surface in response to inducing agents [41]. Even in murine systems, where the existence of sIL-13R-α2 is well validated, it is unclear how sIL-13R-α2 is generated. Available data indicate that very little, if any, IL-13R-α2 is spontaneously shed from the cell surface [32]. Cell surface IL-13R-α2 may be enzymatically cleaved from the cell surface to generate the soluble form. Intriguingly, the enzymatic activity of house dust mite allergens is capable of releasing cell surface-bound murine IL-13R-α2 [24], but this has not yet been shown for human IL-13R-α2. Another possibility is that sIL-13R-α2 may be encoded by an alternatively spliced transcript. Short alternative transcripts of IL-13R-α2 have been described in mice [37] and in humans [42]. Recently, a short transcript in the mouse was characterized as lacking the transmembrane region, thus encoding sIL-13R-α2, and its expression was preferentially induced in a mouse asthma model [18]. Although mouse and human IL-13R-α2 proteins share 59% amino acid homology [37], it is not yet clear whether any of the alternatively spliced transcripts observed in human cells encodes a form lacking the transmembrane region. The splice variants identified in the human IL-13R-α2 gene involved alternative usage of the first four (non-coding) exons, located in the 5' UTR [42]. Whether this could influence translation efficiency or further post-transcriptional processing to generate a soluble form is unknown.
The current findings suggest that levels of soluble IL-13R-α2 in human circulation are below 250 pg/mL, the detection limit of the SpecificBlue assay. This finding implies that a major component of the murine IL-13 regulatory system, sIL-13R-α2, is lacking in humans. IL-13-targeted therapeutics are currently being developed for asthma, glioblastoma, Hodgkin’s lymphoma, and other conditions. Initial reports of high circulating levels of IL-13R-α2 in humans had raised the possibility that sIL-13R-α2 could find utility as a biomarker for IL-13-mediated immune responses. Given the failure to detect sIL-13R-α2 using the currently available reagents in human plasma, even among severe asthmatics experiencing exacerbation attacks, we conclude that sIL-13R-α2 would not be an adequate biomarker for IL-13-mediated immune responses. Further studies are needed to evaluate the role and regulation of cell surface IL-13R-α2 as a natural regulatory system for IL-13 in humans, in order to develop effective IL-13 antagonists as therapeutics.
Acknowledgments
The authors thank Christopher Corcoran, Lisa Racie, and Dr Edward LaVallie for preliminary observations. We also thank Rob Thompson and Margaret Mentink-Kane for helpful discussions, and Pankaj Oberoi, Meso Scale Discovery, for discussion and valuable advice regarding false-positive signals.
References
- 1.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 2.Wynn TA. IL-13 effector functions. Ann Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 3.Wills-Karp M, Luyimbazi J, Xu X, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 4.Grunig G, Warnock M, Wakil AE, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasaian MT, Donaldson DD, Tchistiakova L, et al. Efficacy of IL-13 neutralization in a sheep model of experimental asthma. Am J Respir Cell Mol Biol. 2007;36:368–376. doi: 10.1165/rcmb.2006-0244OC. [DOI] [PubMed] [Google Scholar]
- 7.Corry DB, Kheradmand F. Biology and therapeutic potential of the interleukin-4/interleukin-13 signaling pathway in asthma. Am J Respir Med. 2002;1:185–193. doi: 10.1007/BF03256608. [DOI] [PubMed] [Google Scholar]
- 8.Andrews R, Rosa L, Daines M, Khurana HG. Reconstitution of a functional human type II IL-4/IL-13 receptor in mouse B cells: demonstration of species specificity. J Immunol. 2001;166:1716–1722. doi: 10.4049/jimmunol.166.3.1716. [DOI] [PubMed] [Google Scholar]
- 9.Andrews AL, Holloway JW, Puddicombe SM, Holgate ST, Davies DE. Kinetic analysis of the interleukin-13 receptor complex. J Biol Chem. 2002;277:46073–46078. doi: 10.1074/jbc.M209560200. [DOI] [PubMed] [Google Scholar]
- 10.Madhankumar AB, Mintz A, Debinski W. Alanine-scanning mutagenesis of alpha-helix D segment of interleukin-13 reveals new functionally important residues of the cytokine. J Biol Chem. 2002;277:43194–43205. doi: 10.1074/jbc.M205047200. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa M, Nakajima T, Tsukidate T, et al. TNF-alpha and IL-4 regulate expression of IL-13 receptor alpha2 on human fibro-blasts. Biochem Biophys Res Commun. 2003;312:1248–1255. doi: 10.1016/j.bbrc.2003.11.077. [DOI] [PubMed] [Google Scholar]
- 12.Andrews AL, Nasir T, Bucchieri F, Holloway JW, Holgate ST, Davies DE. IL-13 receptor alpha 2: a regulator of IL-13 and IL-4 signal transduction in primary human fibroblasts. J Allergy Clin Immunol. 2006;118:858–865. doi: 10.1016/j.jaci.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 13.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 14.Fichtner-Feigl S, Fuss IJ, Young CA, et al. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859–5870. doi: 10.4049/jimmunol.178.9.5859. [DOI] [PubMed] [Google Scholar]
- 15.Bernard J, Treton D, Vermot-Desroches C, et al. Expression of interleukin 13 receptor in glioma and renal cell carcinoma: iL13Ralpha2 as a decoy receptor for IL13. Lab Invest. 2001;81:1223–1231. doi: 10.1038/labinvest.3780336. [DOI] [PubMed] [Google Scholar]
- 16.Feng N, Schnyder B, Vonderschmitt DJ, Ryffel B, Lutz RA. Characterization of interleukin-13 receptor in carcinoma cell lines and human blood cells and comparison with the interleukin-4 receptor. J Recept Signal Transduct Res. 1995;15:931–949. doi: 10.3109/10799899509049865. [DOI] [PubMed] [Google Scholar]
- 17.Yasunaga S, Yuyama N, Arima K, et al. The negative-feedback regulation of the IL-13 signal by the IL-13 receptor alpha2 chain in bronchial epithelial cells. Cytokine. 2003;24:293–303. doi: 10.1016/j.cyto.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Tabata Y, Chen W, Warrier MR, Gibson AM, Daines MO, Hershey GK. Allergy-driven alternative splicing of IL-13 receptor alpha2 yields distinct membrane and soluble forms. J Immunol. 2006;177:7905–7912. doi: 10.4049/jimmunol.177.11.7905. [DOI] [PubMed] [Google Scholar]
- 19.Chiaramonte MG, Mentink-Kane M, Jacobson BA, et al. Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. J Exp Med. 2003;197:687–701. doi: 10.1084/jem.20020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsushita M, Yamamoto T, Nishioka K. Upregulation of interleukin-13 and its receptor in a murine model of bleomycin-induced scleroderma. Int Arch Allergy Immunol. 2004;135:348–356. doi: 10.1159/000082331. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto M, Morimoto M, Zhao A, et al. Functional importance of regional differences in localized gene expression of receptors for IL-13 in murine gut. J Immunol. 2006;176:491–495. doi: 10.4049/jimmunol.176.1.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood N, Whitters MJ, Jacobson BA, et al. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor alpha 2. J Exp Med. 2003;197:703–709. doi: 10.1084/jem.20020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mentink-Kane MM, Cheever AW, Thompson RW, et al. IL-13 receptor alpha 2 down-modulates granulomatous inflammation and prolongs host survival in schistosomiasis. Proc Natl Acad Sci USA. 2004;101:586–590. doi: 10.1073/pnas.0305064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daines MO, Chen W, Tabata Y, et al. Allergen-dependent solubilization of IL-13 receptor alpha2 reveals a novel mechanism to regulate allergy. J Allergy Clin Immunol. 2007;119:375–383. doi: 10.1016/j.jaci.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomassen MJ, Buhrow LT, Connors MJ, Kaneko FT, Erzurum SC, Kavuru MS. Nitric oxide inhibits inflammatory cytokine production by human alveolar macrophages. Am J Respir Cell Mol Biol. 1997;17:279–283. doi: 10.1165/ajrcmb.17.3.2998m. [DOI] [PubMed] [Google Scholar]
- 26.Kavuru MS, Dweik RA, Thomassen MJ. Role of bronchoscopy in asthma research. Clin Chest Med. 1999;20:153–189. doi: 10.1016/s0272-5231(05)70133-7. [DOI] [PubMed] [Google Scholar]
- 27.Follettie MT, Ellis DK, Donaldson DD, et al. Gene expression analysis in a murine model of allergic asthma reveals over-lapping disease and therapy dependent pathways in the lung. Pharmacogenom J. 2006;6:141–152. doi: 10.1038/sj.tpj.6500357. [DOI] [PubMed] [Google Scholar]
- 28.Feng N, Lugli SM, Schnyder B, et al. The interleukin-4/interleukin-13 receptor of human synovial fibroblasts: over-expression of the nonsignaling interleukin-13 receptor alpha2. Lab Invest. 1998;78:591–602. [PubMed] [Google Scholar]
- 29.Zheng T, Zhu Z, Liu W, et al. Cytokine regulation of IL-13Ralpha2 and IL-13Ralpha1 in vivo and in vitro. J Allergy Clin Immunol. 2003;111:720–728. doi: 10.1067/mai.2003.1383. [DOI] [PubMed] [Google Scholar]
- 30.David M, Ford D, Bertoglio J, Maizel AL, Pierre J. Induction of the IL-13 receptor alpha2-chain by IL-4 and IL-13 in human keratinocytes: involvement of STAT6, ERK and p38 MAPK path-ways. Oncogene. 2001;20:6660–6668. doi: 10.1038/sj.onc.1204629. [DOI] [PubMed] [Google Scholar]
- 31.David M, Bertoglio J, Pierre J. TNF-alpha potentiates IL-4/IL-13-induced IL-13Ralpha2 expression. Ann N Y Acad Sci. 2002;973:207–209. doi: 10.1111/j.1749-6632.2002.tb04633.x. [DOI] [PubMed] [Google Scholar]
- 32.Daines MO, Tabata Y, Walker BA, et al. Level of expression of IL-13R alpha 2 impacts receptor distribution and IL-13 signaling. J Immunol. 2006;176:7495–7501. doi: 10.4049/jimmunol.176.12.7495. [DOI] [PubMed] [Google Scholar]
- 33.Andrews AL, Bucchieri F, Arima K, et al. Effect of IL-13 receptor alpha2 levels on the biological activity of IL-13 variant R110Q. J Allergy Clin Immunol. 2007 doi: 10.1016/j.jaci.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 34.Kellner J, Gamarra F, Welsch U, Jorres RA, Huber RM, Bergner A. IL-13Ralpha2 reverses the effects of IL-13 and IL-4 on bronchial reactivity and acetylcholine-induced Ca+ signaling. Int Arch Allergy Immunol. 2007;142:199–210. doi: 10.1159/000097022. [DOI] [PubMed] [Google Scholar]
- 35.Plante S, Semlali A, Joubert P, et al. Mast cells regulate procollagen I (alpha 1) production by bronchial fibroblasts derived from subjects with asthma through IL-4/IL-4 delta 2 ratio. J Allergy Clin Immunol. 2006;117:1321–1327. doi: 10.1016/j.jaci.2005.12.1349. [DOI] [PubMed] [Google Scholar]
- 36.Rahaman SO, Sharma P, Harbor PC, Aman MJ, Vogelbaum MA, Haque SJ. IL-13R(alpha)2, a decoy receptor for IL-13 acts as an inhibitor of IL-4-dependent signal transduction in glioblastoma cells. Cancer Res. 2002;62:1103–1109. [PubMed] [Google Scholar]
- 37.Donaldson DD, Whitters MJ, Fitz LJ, et al. The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor alpha 1. J Immunol. 1998;161:2317–2324. [PubMed] [Google Scholar]
- 38.Caput D, Laurent P, Kaghad M, et al. Cloning and characterization of a specific interleukin (IL)-13 binding protein structurally related to the IL-5 receptor alpha chain. J Biol Chem. 1996;271:16921–16926. doi: 10.1074/jbc.271.28.16921. [DOI] [PubMed] [Google Scholar]
- 39.Kawakami K, Taguchi J, Murata T, Puri RK. The interleukin-13 receptor alpha2 chain: an essential component for binding and internalization but not for interleukin-13-induced signal transduction through the STAT6 pathway. Blood. 2001;97:2673–2679. doi: 10.1182/blood.v97.9.2673. [DOI] [PubMed] [Google Scholar]
- 40.Kawakami K, Takeshita F, Puri RK. Identification of distinct roles for a dileucine and a tyrosine internalization motif in the interleukin (IL)-13 binding component IL-13 receptor alpha 2 chain. J Biol Chem. 2001;276:25114–25120. doi: 10.1074/jbc.M100936200. [DOI] [PubMed] [Google Scholar]
- 41.Daines MO, Hershey GK. A novel mechanism by which interferon-gamma can regulate interleukin (IL)-13 responses. Evidence for intracellular stores of IL-13 receptor alpha -2 and their rapid mobilization by interferon-gamma. J Biol Chem. 2002;277:10387–10393. doi: 10.1074/jbc.M108109200. [DOI] [PubMed] [Google Scholar]
- 42.David MD, Bertoglio J, Pierre J. Functional characterization of IL-13 receptor alpha2 gene promoter: a critical role of the transcription factor STAT6 for regulated expression. Oncogene. 2003;22:3386–3394. doi: 10.1038/sj.onc.1206352. [DOI] [PubMed] [Google Scholar]