Abstract
Background
Directly administered antiretroviral therapy (DAART) is one approach to improve treatment adherence among human immunodeficiency virus (HIV)–infected drug users.
Methods
In this randomized, controlled trial (ClinicalTrials.gov identifier, NCT00367172), the biological outcomes of a 6-month community intervention of DAART were compared with those of self-administered therapy among HIV-infected drug users. Patients randomized to receive DAART received supervised therapy 5 days per week from workers in a mobile health care van. The primary outcome, using an intention-to-treat approach, was the proportion of patients achieving either a reduction in HIV-1 RNA level of ≥1.0 log10 copies/mL or an HIV-1 RNA level ≤400 copies/mL at 6 months. Secondary outcomes included the mean change from baseline in HIV-1 RNA level and CD4+ T lymphocyte count.
Results
Of the 141 patients who met the entry criteria, 88 were randomized to receive DAART, and 53 were randomized to receive self-administered therapy; 74 (84%) of 88 of the patients randomized to receive DAART accepted the intervention. Of the 74 patients who initiated DAART, 51 (69%) completed the full 6-month intervention. At the end of 6 months, a significantly greater proportion of the DAART group achieved the primary outcome (70.5% vs. 54.7; P = .02). Additionally, compared with patients receiving self-administered therapy, patients receiving DAART demonstrated a significantly greater mean reduction in HIV-1 RNA level (−1.16 log10 copies/mL vs. −0.29 log10 copies/mL; P = .03) and mean increase in CD4+ T lymphocyte count (+58.8 cells/µL vs. −24.0 cells/µL; P = .002)
Conclusions
This randomized, controlled trial was, to our knowledge, the first to demonstrate the effectiveness of DAART at improving 6-month virologic outcomes among drug users. These results suggest that DAART should be more widely available in HIV treatment programs that target drug users who have poor adherence to treatment.
HAART has dramatically reduced the morbidity and mortality associated with HIV disease [1, 2], but these benefits have not been conferred equally among all patient populations. Drug users have had particularly less-favorable outcomes, with HIV disease progression remaining at high levels [3, 4]. These poorer outcomes occur largely because this population is less likely to be prescribed HAART [5, 6], and when HAART is prescribed, drug users are less likely to demonstrate high levels of adherence to therapy [3, 7, 8]. Because adherence to HAART is the key determinant in the progression of HIV disease [9, 10], strategies that improve adherence should result in better clinical outcomes among drug users.
Directly observed therapy for tuberculosis has resulted in impressive improvements in adherence and clinical response, as well as marked reductions in the development of resistance [11–13]. The time-limited treatment of tuberculosis, the inherently different transmission patterns of tuberculosis and HIV infection, and the complexity of antiretroviral therapy have raised concerns about translating this model to use for HIV treatment [14–16]. Successful but noncomparative programs of directly administered antiretroviral therapy (DAART) have been implemented in methadone-maintenance programs [17, 18], community-based settings [19, 20], skilled nursing facilities [21, 22], and prisons [23]. One randomized, controlled trial of DAART failed to demonstrate an impact on virologic outcomes among a general population of low-income HIV-infected patients [24], but it is unclear how these results relate to drug users or other populations with problematic adherence [25].
Therefore, we conducted the first randomized, controlled trial to address this issue, consisting of 6 months of DAART versus self-administered therapy (SAT) among active drug users in a community setting. The objective was to determine the potential efficacy of a 6-month DAART program on HIV infection, using surrogate markers of HIV-1 RNA level and CD4+ T lymphocyte count.
METHODS
Study population
A randomized, controlled trial of 6 months of DAART versus SAT among drug users was conducted from 2001 through 2006. Patients were recruited from all 4 HIV clinics in New Haven, Connecticut. Entry criteria included (1) being HIV seropositive, (2) being eligible for and/or being prescribed HAART, (3) living in New Haven, (4) having used heroin and/or cocaine in the previous 6 months, and (5) receiving a treatment regimen of ≤2 doses per day.
Study design
After informed consent was received, eligible patients were randomized 2:1 to receive DAART or SAT, stratified on the basis of the following criteria: (1) antiretroviral experience, (2) problematic alcohol use, (3) baseline HIV-1 RNA level (dichotomized at ≤1000 copies/mL or >1000 copies/mL), and (4) baseline CD4+ T lymphocyte count (dichotomized at ≤350 cells/mL and >350 cells/mL). The 2:1 design was undertaken because of the possibility of refusals to participate in the DAART arm because of the perception that it would be too time-consuming.
During the 6-month intervention period, HIV-1 RNA levels (Amplicor 1.5; Roche) and CD4+ T lymphocyte counts (FACS; Quest) were obtained at the time of randomization and at 1, 3, and 6 months subsequent to randomization. Adherence was measured at baseline and at 1, 3, and 6 months using 3-day AIDS Clinical Trials Group self-reported adherence. Although self-report often overestimates true adherence [26], it has been demonstrated to be an effective way to compare adherence interventions [27]. For analysis purposes, high AIDS Clinical Trials Group adherence was defined as ≥80%, recognizing that clinical benefit has been associated with adherence levels ranging from 70% to 95% [28–30]. Among the patients receiving DAART, for both observed and nonobserved doses, all medications were placed in small plastic bags in a medication bottle with a Medication Electronic Monitoring System Version 6 Smart Cap (Aardex). Because these data were not available for patients receiving SAT, Medication Electronic Monitoring System data are not analyzed here. The study was approved by the Yale University Institutional Review Board and had a Certificate of Confidentiality.
Intervention groups
The components of the DAART intervention have been described elsewhere [31]. In brief, all medications were distributed at prearranged sites, in conjunction with a community health care van that travels on weekdays to 4 distinct inner-city neighborhoods in New Haven that have been negatively impacted by the substance abuse and HIV epidemics. All medications prescribed for chronic conditions were provided as DAART together with the antiretroviral medications. Receipt of 1 dose per day was observed by the outreach worker, and all other doses were provided for the patient to take later, with a reminder from a beeper. In instances in which a patient did not arrive for the daily supervised dose, the out-reach worker would use an array of contact information to find the patient. Each patient had 1–3 days of back-up medication available should they be too infirm or otherwise unable to appear for DAART visits. DAART was provided for 6 consecutive months, and patients were trained to package and self-administer their medications the month before transferring to complete self-administration of their therapy for an additional 6 months of monitoring.
The SAT arm continued to receive their HIV treatment and medications through community-based physicians, with follow-up visits at routinely scheduled intervals. Prior to randomization, all patients viewed a 30-min medication adherence video. The only additional interventions that the SAT arm received were the quarterly interview sessions with research interviewers. All patients, irrespective of randomization, received monetary compensation for interviews and phlebotomy. Patients who received DAART were provided a minimal non-monetary incentive for monthly Medication Electronic Monitoring System cap readings.
Outcomes
Outcomes were analyzed using an intention-to-treat approach and included all 141 patients, as randomized. The primary outcome was virologic success at 6 months, which, for this predominantly antiretroviral-experienced population, was defined a priori as having achieved an HIV-1 RNA level reduction of ≥1.0 log10 copies/mL or an HIV-1 RNA level <400 copies/mL [25, 32]. Missing values were imputed as failure. Secondary 6-month outcomes included (1) mean change in HIV-1 RNA level, (2) mean change in CD4+ T lymphocyte count, and (3) self-reported adherence. To explore these effects over the 6 months of the study, these approaches were confirmed using data at baseline and at 1, 3, and 6 months as inputs into generalized linear mixed effects models for virologic suppression and for viral load.
Statistical analysis
All statistical analyses were conducted using SAS, version 9.1.3 (SAS Institute). Analyses included all patients who were randomized. The primary outcome (the proportion of patients achieving virologic success) was analyzed using logistic regression, adjusting for baseline viral load and CD4+ T lymphocyte count. To achieve the primary and secondary aims of this study using a 2:1 randomization schema, 123 patients were needed for enrollment.
Changes in the HIV-1 RNA level were fitted to a linear regression with interval censoring using the SAS procedure Lifereg with the the dist = normal option. This robustly accounts for the large number of censored values because of viral loads at the lower limits of detection at baseline and at follow-up [25, 33, 34]. The fitted model included baseline HIV-1 RNA levels and CD4+ T lymphocyte counts. Missing values were imputed as zero change from baseline. Normal probability plots confirmed that data fit the parametric assumptions of the regression.
Mean change in the CD4+ T lymphocyte count from baseline to 6-month follow-up was assessed using a general linear model including baseline CD4+ T lymphocyte count and HIV-1 RNA level as covariates. CD4+ T lymphocyte counts were chosen to normalize the data, because they exhibited high dispersion [35–38]. Missing values were imputed as zero change from baseline.
For the longitudinal secondary outcomes, continuous viral load data were fitted as normally distributed data with an identity link using Proc Mixed in SAS, with undetectable values imputed at the lower limit of detection and missing values not analyzed. The binomial data of HIV-1 RNA levels ≤400 copies/mL were fit to a generalized linear model with a binomial distribution and a logit link using Proc NLmixed.
Reported P values were determined using Wald tests on the parameter estimate for the main effect of the treatment arm in the respective model. All analyses were adjusted for differences in baseline viral load and CD4+ T lymphocyte count. All inferences were made on the basis of a type I error rate equal to .05. The funding sources played no role in the design of the study, data collection, analysis or interpretation of results, or writing of the article.
RESULTS
The baseline characteristics of the study population are shown in table 1. The 2 treatment arms did not significantly differ with respect to any of the social and demographic characteristics assessed. Compared with the patients receiving SAT, the patients receiving DAART had a higher median HIV-1 RNA level (3.8 log10 copies/mL vs. 2.8 log10 copies/mL; P = .07) and a lower median CD4+ T lymphocyte count (283 cells/mL vs. 384 cells/µL; P = .04). These 2 laboratory measures were therefore controlled for in the final analyses.
Table 1.
Baseline characteristics of the 141 study patients.
| Characteristic | SAT arm (n = 53) | DAART arm (n = 88) | P |
|---|---|---|---|
| Age, median years (IQR) | 44.9 (40.7–49.7) | 42.7 (37.6–48.6) | .22 |
| Sex | .84 | ||
| Female | 16 (30.2) | 28 (31.8) | |
| Male | 37 (69.8) | 60 (68.2) | |
| Ethnicity | .54 | ||
| Black | 31 (58.5) | 51 (58.0) | |
| Hispanic | 11 (20.8) | 16 (18.2) | |
| White | 10 (18.9) | 21 (23.9) | |
| Other | 1 (1.9) | 0 (0.0) | |
| Education | .14 | ||
| Did not graduate high school | 14 (24.4) | 38 (43.2) | |
| High school graduate or GED | 25 (47.2) | 33 (37.5) | |
| Beyond high school | 14 (26.4) | 17 (19.3) | |
| Language | .82 | ||
| English | 45 (84.9) | 72 (81.8) | |
| Spanish | 8 (15.1) | 16 (18.2) | |
| Homeless | 31 (58.5) | 56 (63.6) | .59 |
| Monthly income | .21 | ||
| <$500 | 19 (35.9) | 45 (51.1) | |
| $500–$1000 | 29 (54.7) | 37 (42.1) | |
| >$1000 | 5 (9.4) | 6 (6.8) | |
| DAST-10 raw score a | .11 | ||
| Low | 27 (50.9) | 30 (34.1) | |
| Moderate | 10 (18.9) | 17 (19.3) | |
| High | 16 (30.2) | 41 (46.6) | |
| CESD depression score b | .45 | ||
| None | 23 (43.4) | 28 (32.6) | |
| Mild to moderate | 7 (13.2) | 14 (16.3) | |
| Severe | 23 (43.4) | 44 (51.2) | |
| Lifetime drug use | |||
| Heroin | 38 (71.7) | 61 (69.3) | .85 |
| Cocaine | 52 (98.1) | 85 (96.6) | >.99 |
| Injection drug use | 35 (66.0) | 57 (64.8) | >.99 |
| Antiretroviral therapy experience prior to the study | .79 | ||
| ≤3 years | 7 (13.2) | 10 (11.4) | |
| >3 years | 46 (86.8) | 78 (88.6) | |
| Dosing schedule | >.99 | ||
| Once daily | 8 (15.1) | 13 (14.8) | |
| Twice daily | 45 (84.9) | 75 (85.2) | |
| Baseline antiretroviral therapy regimens | .06 | ||
| NNTRI only | 16 (30.2) | 13 (14.8) | |
| PI only | 16 (30.2) | 41 (46.6) | |
| PI plus NNRTI | 6 (11.3) | 7 (8.0) | |
| Triple nucleoside | 10 (18.9) | 10 (11.4) | |
| Not receiving therapy | 5 (9.4) | 17 (19.3) | .15 |
| Future treatment option | |||
| <3 Susceptible ARV drug classes | 11 (20.8) | 23 (26.1) | .55 |
| 3 Susceptible ARV drug classes | 42 (79.2) | 65 (73.9) | |
| Major IAS DRM | |||
| None | 35 (66.0) | 59 (67.0) | .90 |
| 1–2 | 10 (18.9) | 14 (15.9) | |
| >2 | 8 (15.1) | 15 (17.0) | |
| HIV-1 load | .10 | ||
| >400 copies/mL | 29 (54.7) | 61 (69.3) | |
| ≤400 copies/mL | 24 (45.3) | 27 (30.7) | |
| HIV-1 RNA level, median log 10 copies/mL (IQR) | |||
| All patients | 2.8 (1.8–4.6) | 3.8 (2.2–5.1) | .07 |
| Patients with an HIV-1 RNA level ≤400 copies/mL | 4.4 (3.4–5.2) | 4.7 (3.8–5.4) | .38 |
| CD4+ T lymphocyte count | .22 | ||
| >350 cells/µL | 29 (54.7) | 38 (43.2) | |
| ≤350 cells/µL | 24 (45.3) | 50 (56.8) | |
| CD4+ T lymphocyte count, median cells/µL (IQR) | 384 (261–553) | 283 (103–538.5) | .04 |
NOTE. Data are no. (%) of patients, unless otherwise indicated. Reported P values were determined using Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for quantitative variables. ARV, antiretroviral; CESD, Center for Epidemiological Studies Depression Scale; DAART, directly administered antiretroviral therapy; DAST, Drug Abuse Screening Test; Major IAS DRMs, Major International AIDS Society Drug Resistance Mutations; SAT, self-administered therapy.
A low score is 0–2, a moderate score is 3–5, and a high score is ≥6.
None is a score of ≤14, mild to moderate is a score of 15–21, and severe is a score of ≥22.
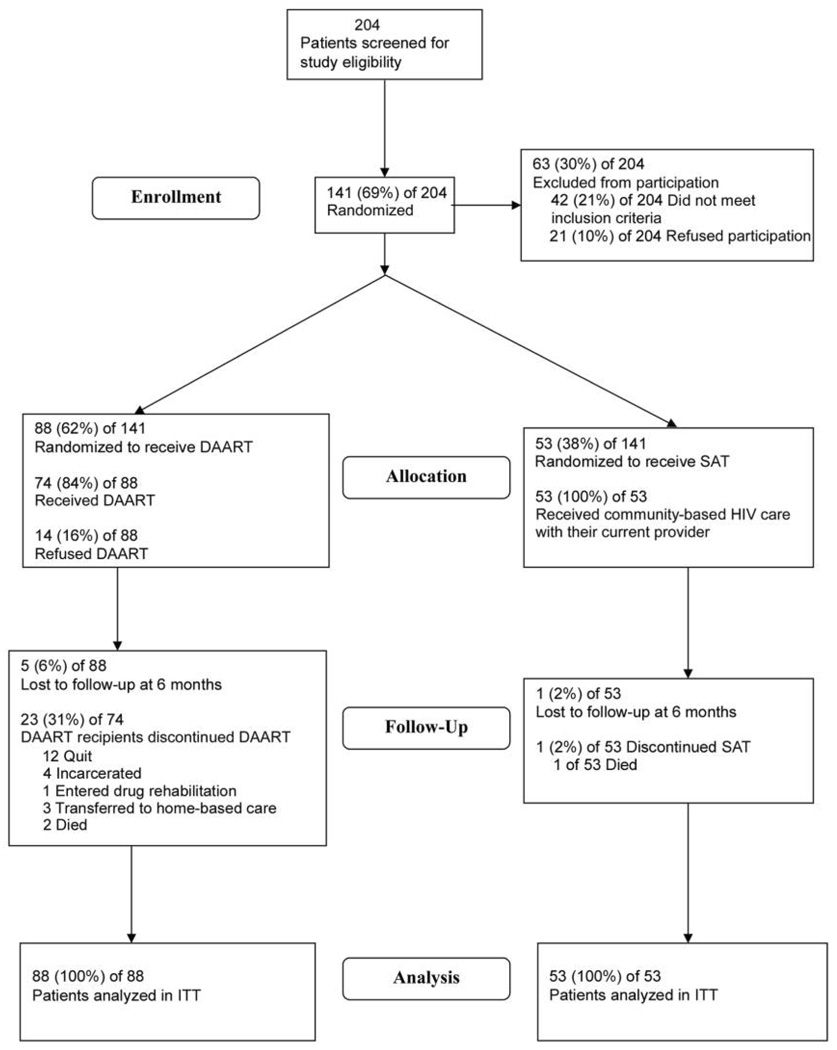
The disposition of patients screened and enrolled in the trial are shown in figure 1. Of the 88 patients randomized to receive DAART, 74 (84%) initiated DAART, and 51 (69%) of these patients completed the entire 6-month intervention. Among those patients who discontinued DAART (n = 23) the median time to discontinuation was 82 days (interquartile range, 67–118 days). Reasons for discontinuation of DAART included being lost to follow-up (n = 10), inpatient drug or medical treatment (n = 6), incarceration (n = 5), and death (n = 2)
Figure 1.
Disposition of study patients. DAART, directly administered antiretroviral therapy; ITT, intention-to-treat; SAT, self-administered therapy.
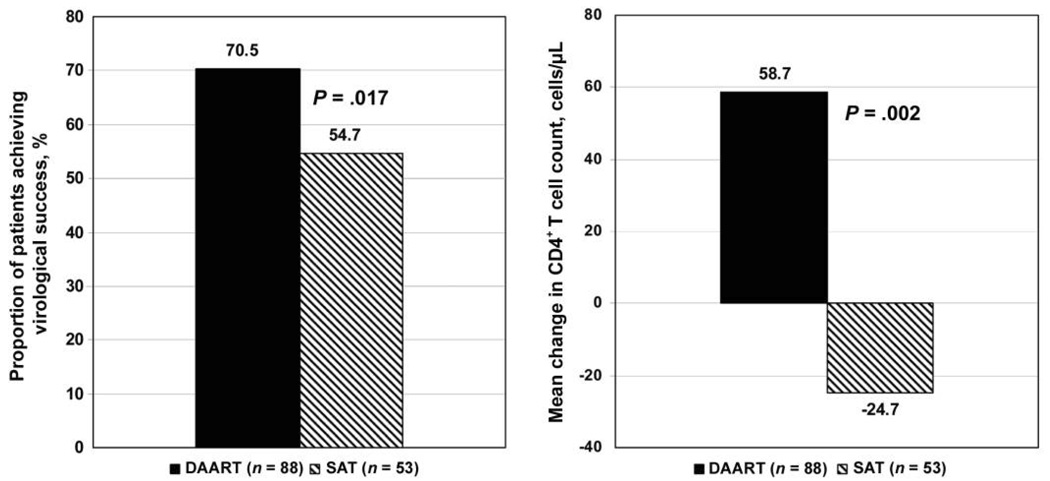
The proportion of patients achieving virologic success at 6 months (i.e., an HIV-1 RNA level reduction of ≥1.0 log10 copies/mL or achievement of an HIV-1 RNA level <400 copies/mL)—the primary outcome of interest (figure 2), adjusted for baseline viral load—was significantly higher in the DAART arm than in the SAT arm (70.5% vs. 54.7%; adjusted OR, 2.6; 95% CI, 1.2–5.5; P = .02).
Figure 2.
End-of-treatment virologic and immunologic outcomes. A, Proportion of patients achieving virologic success (i.e., an HIV-1 RNA level ≤400 copies/mL or a reduction in HIV-1 RNA level of ≥1.0 log10 copies/mL). B, Mean change in CD4+ T lymphocyte count. Reported P values were adjusted for baseline viral load and CD4+ T lymphocyte counts; the P value for CD4+ T lymphocyte counts represents that from the log-transformed model, which fit the normality assumption better. DAART, directly administered antiretroviral therapy; SAT, self-administered therapy.
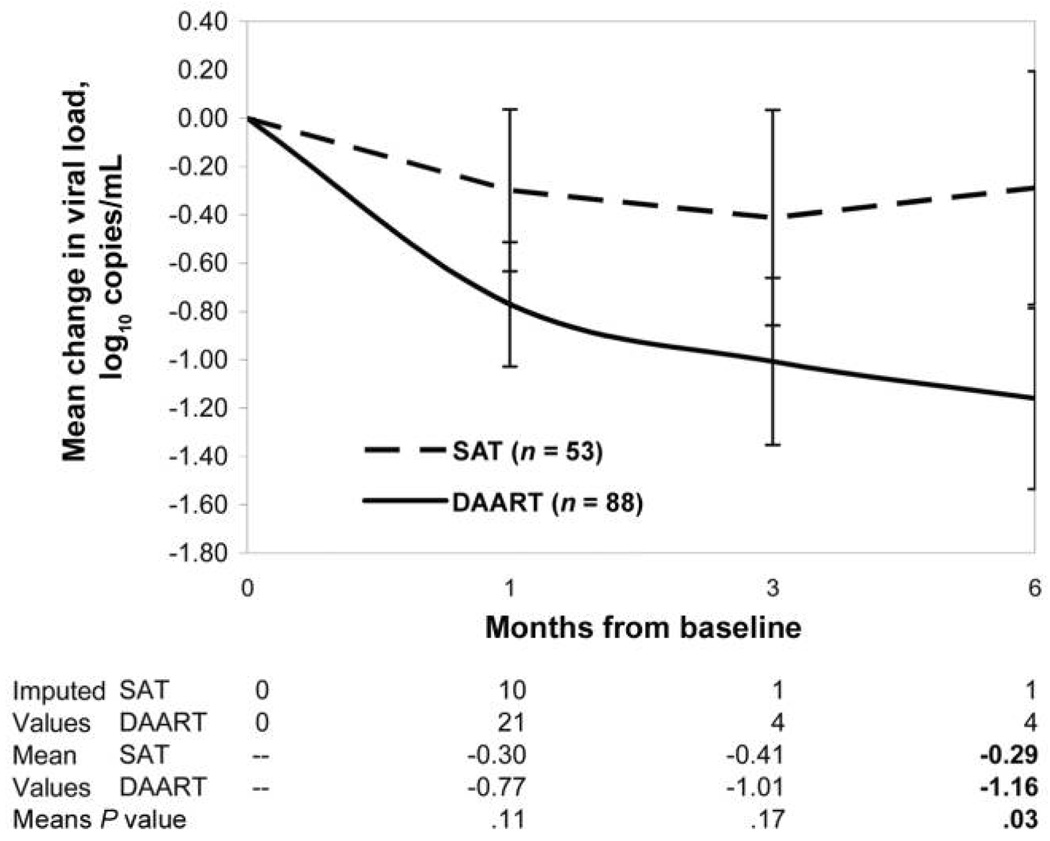
The mean reduction in HIV-1 RNA level, adjusted for censoring and baseline viral load, is presented in figure 3. At 6 months, the mean reduction in HIV-1 RNA level was significantly greater in the DAART arm than in the SAT arm (−1.16 log10 copies/mL vs. −0.29 log10 copies/mL; P = .03).
Figure 3.
Mean change in viral load during the intervention period. Raw values are shown, adjusted for censoring at the lower limits of detection. P values were adjusted for baseline HIV-1 RNA level and CD4+ T lymphocyte count. DAART, directly administered antiretroviral therapy; SAT, self-administered therapy.
Results from other measures are presented in table 2. At 6 months, the mean change in CD4+ T lymphocyte count after adjusting for the baseline CD4+ T lymphocyte count was significantly higher in the DAART arm than in the SAT arm (+58.8 cells/µL vs. −24.7 cells/µL; P = .002). Baseline-adjusted adherence outcomes from the 3-day AIDS Clinical Trials Group recall demonstrated greater adherence among patients receiving DAART, compared with patients receiving SAT, but this did not reach statistical significance (67.1% vs. 54.7%; P = .10). These results were robust to various missing data assumptions in which available case and last observation data points were carried forward. With each analytic approach, similar results were demonstrated (data not shown).
Table 2.
Summary of main outcome measures.
| Analysis | SAT (n = 53) | DAART (n = 88) | P |
|---|---|---|---|
| Proportion of patients achieving virologic success after the intervention | 54.7 | 70.5 | .02 |
| Virologic success after the intervention, adjusted OR (95% CI) | Referent | 2.5 (1.2–5.5) | .02 |
| Mean change in viral load from baseline to after the intervention, log10 copies/mL | −0.29 | −1.16 | .03 |
| Proportion of patients who achieved and HIV-1 RNA level ≤400 copies/mL after the intervention | 49.1 | 55.7 | .07 |
| Mean change in CD4+ T lymphocyte count from baseline to after the intervention | −24.7 | 58.8 | .002 |
| Proportion of patients with ≥80% ACTG adherence after the intervention | 54.7 | 67.1 | .10 |
| Mixed model difference in slope, SAT:DAART (95% CI) | Referent | −0.51 (−0.88 to −0.18) | .003 |
| Mixed model, OR of proportion of patients achieving virologic success over time (95% CI) | Referent | 2.3 (1.1–4.5) | .02 |
NOTE. All P values were adjusted for baseline HIV-1 RNA level and CD4+ T lymphocyte count. Reported means are the raw values. Missing values were imputed, except for mixed models, which were analyzed as available case. ACTG, AIDS Clinical Trial Group; DAART, directly administered antiretroviral therapy; SAT, self-administered therapy.
The longitudinal analyses also favored DAART. In a linear mixed model of viral load change from baseline, the fixed effect difference in slopes of viral load in the DAART arm versus the SAT arm was −0.53 (95% CI, −0.88 to −0.18; P = .003). In a generalized linear mixed model to determine the proportion of patients with an HIV-1 RNA level ≤400 copies/mL, the OR for the DAART group versus the SAT group was 2.3 (95% CI, 1.1–4.5; P = .02).
Similar results were seen in subgroup analyses stratifying the patients by virologic suppression at baseline. Among the 90 patients (64%) whose viral load was ≤400 copies/mL, 39 (76%) of 61 patients receiving DAART versus 12 (41%) of 29 patients receiving SAT achieved virologic success (adjusted OR, 2.7; 95% CI, 1.1–6.9; P = .04). The mean viral load reduction in the DAART group was also significantly greater than that in the SAT group (−1.6 log10 copies/mL vs. −0.83 log10 copies/mL; P = .04). Among the 51 patients (36%) for whom the baseline viral load was ≤400 copies/mL, 23 (86%) of 27 patients receiving DAART versus 17 (71%) of 24 patients receiving SAT maintained virologic suppression. Owing to the small sample size, this last comparison, however, did not reach statistical significance in the multiple regression analysis (adjusted OR, 2.5; 95% CI, 0.6–9.4; P = .20). Similarly, we were unable to make inferences when stratifying outcomes for antiretroviral-naive patients (12.1%) versus antiretroviral-experienced patients and for those receiving once-daily regimens (14.9%) versus twice-daily regimens because of the small number of patients in these subgroups.
During the course of the intervention period, 19 (36%) of 53 patients receiving SAT and 48 (55%) of 88 patients receiving DAART had a change in antiretroviral regimen (P = .04). This difference occurred predominantly because patients receiving DAART were more closely observed for adverse effects and problematic adherence by the DAART outreach workers and were referred to the clinician for antiretroviral medication change. Among those individuals who changed their initial antiretroviral regimen, a change to a once-daily protease inhibitor–based regimen occurred more often for patients receiving DAART than for patients receiving SAT (23 [47.9%] of 48 patients receiving DAART vs. 4 [21.1%] of 19 patients receiving SAT; P = .008).
DISCUSSION
To our knowledge, this is the first prospective, randomized, controlled trial of DAART among HIV-infected drug users—a group that, in prior studies, demonstrated poor adherence, compared with other populations of HIV-infected patients. Both acceptance (84% of those randomized to the intervention initiated DAART) and retention (69% of those who initiated DAART completed 6 months of therapy) were high. The intention-to-treat results from this trial demonstrate improved virologic and immunologic benefit in patients who receive DAART, compared with those who self-administer their HIV therapy, as prescribed by their clinician. Namely, at the end of treatment, 71% of patients receiving DAART versus 55% of patients receiving SAT achieved virologic success, and the mean reduction in viral load was ~1 log10 copies/mL greater among the patients receiving DAART. Patients who received DAART had an increase in CD4+ T lymphocyte count that was 180 cells/µL greater than that achieved by the patients who received SAT. This is particularly impressive because of the relatively high median CD4+ T lymphocyte count at baseline in a group of antiretroviral-experienced patients who had potentially achieved significant immunologic recovery prior to entry into this study. These results appear to be robust to inferences made through several different analytic approaches.
Because surrogate markers of virologic suppression and immunologic function are predictive of clinical outcomes [32], it is likely that improvements in these markers should provide clinical benefit to an HIV-infected population that has typically not fully benefited from antiretroviral therapy. In addition, reductions in HIV-1 RNA levels of the magnitude demonstrated among the patients receiving DAART (1.2 log10 copies/mL) have been significantly associated with reduced heterosexual transmission of HIV infection [39].
Despite these promising results, several questions about DAART remain if it is to be used more widely. This study, for example, does not describe the primary determinants of success of a DAART program. It is clear, both from this trial and from other observational studies [40], that an effective DAART program involves far more than simply directly observing treatment. Perhaps even more important to patient outcomes is the intensive social and medical support that DAART programs may provide. Indeed, in previously published reports involving the DAART arm of this study, training of the DAART workers [31] and provision of enhanced medical and social services were important determinants of outcome [41]. It is possible that these forms of support can be provided without the direct supervision component; DAART may simply be one logistically and programmatically effective way to do so. It is also unclear what role, if any, beepers played as a reminder for patients. Another study that used a “reminder” actually resulted in a reduction in adherence and no benefit from the device [42]. Additional prospective randomized, controlled trials and observational cohort studies of standard DAART, compared with “enhanced” DAART, in which additional services are provided, will be required to answer these important questions.
An additional component of the services provided as part of the DAART intervention is vigilance with respect to anti-retroviral treatment regimens. Patients receiving DAART were significantly more likely to be changed to an alternative regimen during the course of this study—in these instances, simplified regimens that involved a once-daily protease inhibitor combined with other antiretroviral medications. Although we were unable to assess this phenomenon because of the small number of patients, it may have been a result of the DAART outreach workers’ and DAART clinicians’ responsiveness to the adverse effects experienced by the patients, as well as the patients’ desires to simplify their regimens. These potential confounders will need to be addressed in future studies.
Other crucial issues include which patients are most likely to benefit from DAART, when DAART should be initiated, and for how long DAART should be continued. This study was designed to assess as broad a patient base as possible, including patients having, essentially, the entire range of HIV-1 RNA levels and CD4+ T lymphocyte counts. This was done to provide a rigorous, clear assessment of efficacy for DAART in a general, “real-world” population of drug users who are likely to be encountered by clinicians and public health practitioners. More research will be required to adequately describe patients who will benefit most from DAART interventions, because such interventions may not be cost-effective, unless patients with poor adherence to treatment are targeted [43].
The DAART intervention also provided a rather inflexible (and potentially undesirable or unrealistic) transition from DAART to SAT at 6 months, rather than at a point determined by a decision between the patient and provider or by some other predictor of long-term success. To determine the optimal duration of DAART, the durability of the intervention needs to be assessed. This will be partially informed by the 12-month outcomes of this study—a planned analysis that is forthcoming.
Of note, this trial does not provide evidence for the use of DAART as anything other than a voluntary program. All patients in this study had the option of refusing DAART without any repercussions in their HIV care, and 14 patients (16%) originally randomized to receive DAART withdrew participation without receiving a single directly administered dose, because they did not want to participate. It is unlikely that any use of DAART as a coercive strategy will ever be desirable on ethical, legal, public health, or clinical grounds [44].
In summary, this randomized, controlled trial of DAART among HIV-infected drug users achieved its primary outcome and several secondary outcomes, showing virologic and immunologic benefits at the end of the 6-month intervention. Among this difficult-to-engage population of drug users, we also exhibited high rates of both acceptance and retention of DAART. This trial provides, to our knowledge, the first support for DAART as an effective strategy to improve clinical outcomes among HIV-infected drug users, using a randomized, controlled design. Further investigation is warranted to delineate which patients should receive DAART, when DAART should be initiated, the optimal duration of DAART, and how services should be organized to reap the most benefit from DAART.
Acknowledgments
Financial support. National Institutes on Drug Abuse (R01 DA13805, K24 DA 0170720 to F.L.A., K23 DA 019381 to S.A.S., and K23 DA 022143 to R.D.B.) and National Institutes of Health Medical Science Training Program (GM07205 to D.S.R.).
Footnotes
Presented in part: 2nd International AIDS Society Conference on HIV Pathogenesis and Treatment, Paris, France, July 2003 (abstract 40).
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Yeni PG, Hammer SM, Hirsch MS, et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA panel. JAMA. 2004;292:251–265. doi: 10.1001/jama.292.2.251. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138:620–626. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- 3.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J Acquir Immune Defic Syndr. 2001;27:251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strathdee SA, Palepu A, Cornelisse PG, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- 6.Celentano DD, Galai N, Sethi AK, et al. Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS. 2001;15:1707–1715. doi: 10.1097/00002030-200109070-00015. [DOI] [PubMed] [Google Scholar]
- 7.Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 8.Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163:412–420. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- 9.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 10.Maher K, Klimas N, Fletcher MA, et al. Disease progression, adherence, and response to protease inhibitor therapy for HIV infection in an urban Veterans Affairs medical center. J Acquir Immune Defic Syndr. 1999;22:358–363. doi: 10.1097/00126334-199912010-00006. [DOI] [PubMed] [Google Scholar]
- 11.Chaulk CP, Kazandjian VA. Directly observed therapy for treatment completion of pulmonary tuberculosis: consensus statement of the Public Health Tuberculosis Guidelines panel. JAMA. 1998;279:943–948. doi: 10.1001/jama.279.12.943. [DOI] [PubMed] [Google Scholar]
- 12.Chaulk CP, Moore-Rice K, Rizzo R, Chaisson RE. Eleven years of community-based directly observed therapy for tuberculosis. JAMA. 1995;274:945–951. [PubMed] [Google Scholar]
- 13.Weis SE, Slocum PC, Blais FX, et al. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N Engl J Med. 1994;330:1179–1184. doi: 10.1056/NEJM199404283301702. [DOI] [PubMed] [Google Scholar]
- 14.Bangsberg DR, Mundy LM, Tulsky JP. Expanding directly observed therapy: tuberculosis to human immunodeficiency virus. Am J Med. 2001;110:664–666. doi: 10.1016/s0002-9343(01)00729-x. [DOI] [PubMed] [Google Scholar]
- 15.Mitty JA, Stone VE, Sands M, Macalino G, Flanigan T. Directly observed therapy for the treatment of people with human immunodeficiency virus infection: a work in progress. Clin Infect Dis. 2002;34:984–990. doi: 10.1086/339447. [DOI] [PubMed] [Google Scholar]
- 16.Lucas GM, Flexner CW, Moore RD. Directly administered antiretroviral therapy in the treatment of HIV infection: benefit or burden? AIDS Patient Care STDS. 2002;16:527–535. doi: 10.1089/108729102761041083. [DOI] [PubMed] [Google Scholar]
- 17.Conway B, Prasad J, Reynolds R, et al. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clin Infect Dis. 2004;38 Suppl 5:S402–S408. doi: 10.1086/421404. [DOI] [PubMed] [Google Scholar]
- 18.Lucas GM, Weidle PJ, Hader S, Moore RD. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a non-randomized comparative study. Clin Infect Dis. 2004;38 Suppl 5:S409–S413. doi: 10.1086/421405. [DOI] [PubMed] [Google Scholar]
- 19.Mitty JA, Macalino GE, Bazerman LB, et al. The use of community-based modified directly observed therapy for the treatment of HIV-infected persons. J Acquir Immune Defic Syndr. 2005;39:545–550. [PubMed] [Google Scholar]
- 20.Behforouz HL, Kalmus A, Scherz CS, Kahn JS, Kadakia MB, Farmer PE. Directly observed therapy for HIV antiretroviral therapy in an urban US setting. J Acquir Immune Defic Syndr. 2004;36:642–645. doi: 10.1097/00126334-200405010-00016. [DOI] [PubMed] [Google Scholar]
- 21.Tinoco I, Giron-Gonzalez JA, Gonzalez-Gonzalez MT, et al. Efficacy of directly observed treatment of HIV infection: experience in AIDS welfare homes. Eur J Clin Microbiol Infect Dis. 2004;23:331–335. doi: 10.1007/s10096-003-1099-8. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg B, Berkman A, Thomas R, et al. Evaluating supervised HAART in late-stage HIV among drug users: a preliminary report. J Urban Health. 1999;76:468–480. doi: 10.1007/BF02351504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wohl DA, Stephenson BL, Golin CE, et al. Adherence to directly observed antiretroviral therapy among human immunodeficiency virus–infected prison inmates. Clin Infect Dis. 2003;36:1572–1576. doi: 10.1086/375076. [DOI] [PubMed] [Google Scholar]
- 24.Wohl AR, Garland WH, Valencia R, et al. A randomized trial of directly administered antiretroviral therapy and adherence case management intervention. Clin Infect Dis. 2006;42:1619–1627. doi: 10.1086/503906. [DOI] [PubMed] [Google Scholar]
- 25.Smith-Rohrberg D, Altice FL. Randomized, controlled trials of directly administered antiretroviral therapy for HIV-infected patients: questions about study population and analytical approach. Clin Infect Dis. 2006;43:1221–1222. doi: 10.1086/508357. [DOI] [PubMed] [Google Scholar]
- 26.Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33:1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson CR, Simoni JM, Hoff P, Kurth AE, Martin DP. Assessing antiretroviral adherence via electronic drug monitoring and self-report: an examination of key methodological issues. AIDS Behav. 2007:161–173. doi: 10.1007/s10461-006-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross R, Yip B, Re VL, III, et al. A simple, dynamic measure of anti-retroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis. 2006;194:1108–1114. doi: 10.1086/507680. [DOI] [PubMed] [Google Scholar]
- 29.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43:939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 30.McQuay HJ, Bullingham RE, Paterson GM, Moore RA. Clinical effects of buprenorphine during and after operation. Br J Anaesth. 1980;52:1013–1019. doi: 10.1093/bja/52.10.1013. [DOI] [PubMed] [Google Scholar]
- 31.Altice FL, Mezger JA, Hodges J, et al. Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: implications for program replication. Clin Infect Dis. 2004;38 Suppl 5:S376–S387. doi: 10.1086/421400. [DOI] [PubMed] [Google Scholar]
- 32.Ledergerber B, Lundgren JD, Walker AS, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1–infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364:51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 33.Marschner IC, Betensky RA, DeGruttola V, Hammer SM, Kuritzkes DR. Clinical trials using HIV-1 RNA–based primary endpoints: statistical analysis and potential biases. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:220–227. doi: 10.1097/00042560-199903010-00002. [DOI] [PubMed] [Google Scholar]
- 34.Journot V, Chene G, Joly P, et al. Viral load as a primary outcome in human immunodeficiency virus trials: a review of statistical analysis methods. Control Clin Trials. 2001;22:639–658. doi: 10.1016/s0197-2456(01)00158-1. [DOI] [PubMed] [Google Scholar]
- 35.Weinfurt KP, Willke RJ, Glick HA, Freimuth WW, Schulman KA. Relationship between CD4 count, viral burden, and quality of life over time in HIV-1–infected patients. Med Care. 2000;38:404–410. doi: 10.1097/00005650-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Mustafa T, Sy FS, Macera CA, et al. Association between exercise and HIV disease progression in a cohort of homosexual men. Ann Epidemiol. 1999;9:127–131. doi: 10.1016/s1047-2797(98)00043-x. [DOI] [PubMed] [Google Scholar]
- 37.Yu LM, Easterbrook PJ, Marshall T. Relationship between CD4 count and CD4% in HIV-infected people. Int J Epidemiol. 1997;26:1367–1372. doi: 10.1093/ije/26.6.1367. [DOI] [PubMed] [Google Scholar]
- 38.Gries JM, Troconiz IF, Verotta D, Jacobson M, Sheiner LB. A pooled analysis of CD4 response to zidovudine and zalcitabine treatment in patients with AIDS and AIDS-related complex. Clin Pharmacol Ther. 1997;61:70–82. doi: 10.1016/S0009-9236(97)90183-1. [DOI] [PubMed] [Google Scholar]
- 39.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1–discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 40.Behforouz HL, Farmer PE, Mukherjee JS. From directly observed therapy to accompagnateurs: enhancing AIDS treatment outcomes in Haiti and in Boston. Clin Infect Dis. 2004;38 Suppl 5:S429–S436. doi: 10.1086/421408. [DOI] [PubMed] [Google Scholar]
- 41.Smith-Rohrberg D, Mezger J, Walton M, Bruce RD, Altice FL. Impact of enhanced services on virological outcomes in a directly administered antiretroviral therapy trial for HIV-infected drug users. J Acquir Immune Defic Syndr. 2006;43 Suppl 1:S48–S53. doi: 10.1097/01.qai.0000248338.74943.85. [DOI] [PubMed] [Google Scholar]
- 42.Mannheimer SB, Morse E, Matts JP, et al. Sustained benefit from a long-term antiretroviral adherence intervention: results of a large randomized clinical trial. J Acquir Immune Defic Syndr. 2006;43 Suppl 1:S41–S47. doi: 10.1097/01.qai.0000245887.58886.ac. [DOI] [PubMed] [Google Scholar]
- 43.Goldie SJ, Paltiel AD, Weinstein MC, et al. Projecting the cost-effectiveness of adherence interventions in persons with human immunodeficiency virus infection. Am J Med. 2003;115:632–641. doi: 10.1016/j.amjmed.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Liechty CA, Bangsberg DR. Doubts about DOT: antiretroviral therapy for resource-poor countries. AIDS. 2003;17:1383–1387. doi: 10.1097/00002030-200306130-00013. [DOI] [PubMed] [Google Scholar]