Abstract
Directly administered antiretroviral therapy (DAART) can improve health outcomes among HIV-infected drug users. An understanding of the utilization of DAART—initiation, adherence, and retention—is critical to successful program design. Here, we use the Behavioral Model to assess the enabling, predisposing, and need factors impacting adherence in our randomized, controlled trial of DAART versus self-administered therapy (SAT) among 141 HIV-infected drug users. Of 88 participants randomized to DAART, 74 (84%) initiated treatment, and 51 (69%) of those who initiated were retained in the program throughout the entire six-month period. Mean adherence to directly observed visits was 73%, and the mean overall composite adherence score was 77%. These results were seen despite the finding that 75% of participants indicated that they would prefer to take their own medications. Major causes of DAART discontinuation included hospitalization, incarceration, and entry into drug-treatment programs. The presence of depression and the lack of willingness to travel greater than four blocks to receive DAART predicted time-to-discontinuation.
Keywords: HIV, Acquired immunodeficiency syndrome, Substance abuse, Directly administered, antiretroviral therapy, Adherence, Directly observed therapy
Introduction
Since the introduction of highly active antiretroviral therapy, increasing adherence to antiretroviral medications has been critical to improving health outcomes and reducing health disparities among HIV-infected individuals (Altice and Friedland 1998). For active drug users in particular, poor adherence has been a central factor in their increased risk of morbidity, mortality, and antiretroviral drug resistance (Arnsten et al. 2002; Kozal et al. 2005; Lucas et al. 2001, 2002, 2006a). One strategy to improve adherence in this vulnerable population, adapted from directly observed therapy for tuberculosis control, is directly administered antiretroviral therapy (DAART). Recent data from feasibility pilot studies (Altice et al. 2003; Behforouz et al. 2004; Conway et al. 2004; Greenberg et al. 1999; Macalino et al. 2004; Mitchell et al. 2007; Mitty et al. 2005; Tinoco et al. 2004; Tyndall et al. 2007), case–control evaluations (Lucas et al. 2006b), and prospective randomized controlled trials (Altice et al. 2007; Arnsten et al. 2007; Macalino et al. 2007) support DAART implementation among patients with poor adherence. One randomized controlled trial among inner-city patients did not support DAART (Wohl et al. 2006); however, the population studied did not have a previous history of problematic adherence (Smith-Rohrberg and Altice 2006).
While these studies support the efficacy of DAART, an equally important issue is the real-world effectiveness of DAART, and the factors that impact patient outcomes. DAART fundamentally is about improving access to and uptake of HIV/AIDS treatment, particularly when the beneficiaries of DAART are among the highly vulnerable population of HIV-infected drug users. As such, to better understand the effectiveness of DAART and design more effective interventions, it is necessary to analyze the specific DAART utilization outcomes and the factors that may impact them. For evaluative purposes, the processes associated with the utilization of DAART can be divided into initiation, adherence, and retention.
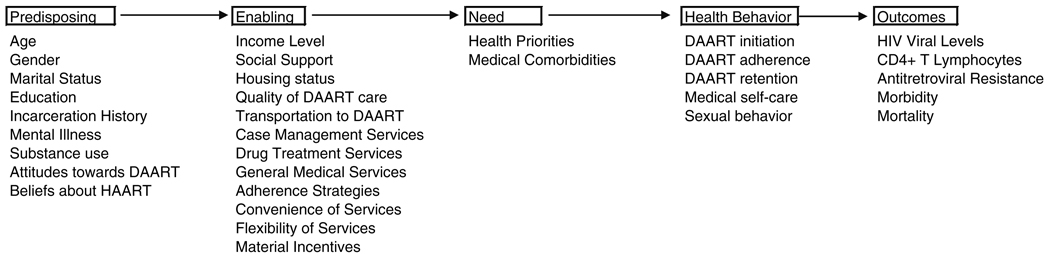
These utilization processes can be contextualized within the theoretical framework of the Behavioral Model (Andersen and Aday 1978; Andersen 1995) that has been adapted for vulnerable populations (Gelberg et al. 2000). This model of healthcare utilization posits that predisposing, enabling, and need factors impact the utilization of health services (Andersen and Aday 1978; Andersen 1995). Figure 1 presents this model, adapted from Gelberg et al. (2000), with the factors most relevant to DAART. Predisposing factors are demographic and social structure characteristics intrinsic to the individual that impact utilization. Relevant predisposing factors for the utilization of DAART among HIV-infected drug users may include age, race or ethnicity, mental illness, incarceration history, substance abuse, living situation, adherence strategies, and attitudes and beliefs about HIV care and about DAART. Enabling factors are personal and community resources that facilitate healthcare access. In the DAART context, these include the availability of general medical, case management, and social services, transportation, geographic proximity of DAART, adherence strategies, social networks, and income level. Finally, need factors are determined by individuals’ perceived health needs or priorities and their actual health status.
Fig. 1.
Predisposing, enabling, and need factors impacting initiation, adherence, and retention in DAART
Utilization processes are inherently linked to the setting of the DAART program. In our program on a community healthcare van, we previously reported that the predisposing factors of addiction severity and age and the enabling factors of convenient medical and case management services significantly predicted virological outcomes (Smith-Rohrberg et al. 2006). In a different program linked to a methadone clinic, of several demographic and social variables, positive urine toxicology screens were the only predictor of decreased retention. This was likely the result of participants being expelled from the methadone program if they were found to be using illicit drugs (Lucas et al. 2007). Although one might expect ongoing heroin and cocaine use to be important predisposing factors, these have not been strongly associated with outcomes (Conway et al. 2004; Smith-Rohrberg et al. 2006). On the other hand, the enabling factor of participation in a methadone program has improved DAART utilization in other studies (Arnsten et al. 2007; Lucas et al. 2004, 2006b). The enabling impact of providing ancillary social services was also demonstrated to be beneficial in a clinical trial involving low-income patients in Los Angeles. In that study, DAART was more acceptable to Latinos than to African Americans; the authors hypothesized that this was due to the greater value placed by Latinos on the social services provided, particularly because these services were delivered bilingually (Garland et al. 2007).
In this paper, we describe utilization outcomes (initiation, adherence, and retention) among participants in our community-based trial of DAART, as well as the enabling, predisposing, and need factors associated with this utilization. This extends our previous work describing some of the major predisposing (demographic and social characteristics) and enabling (income, availability of social, case management, and social services) factors associated with improved biological outcomes in this population (Smith-Rohrberg et al. 2006).
As such, the purpose of this paper is threefold. First, we will describe in greater detail the utilization outcomes measures: initiation, adherence, and retention. These descriptions will address the question: what utilization rates can be expected in community-based DAART programs? Next, we will describe those factors presumed to impact utilization—predisposing (demographic and social characteristics), enabling (income), need (general health status). These are all purely descriptive, addressing the question: which potential factors are present, and in what frequencies, in drug-using populations targeted by DAART programs? The final aim of the piece is more analytical. We will use survival analysis to ask the question: which utilization factors predict one particularly important utilization outcome—time-to-drop-out? Given the relatively small sample size, this analysis is hypothesis-generating, extending the descriptive data to provide one way of assessing how the predisposing, enabling, and need factors might impact utilization outcomes. Together, the data presented here will assist DAART researchers, clinicians, and program managers in framing implementation projects and research questions.
Methods
Participants and Study Design
The study design and DAART intervention have been described previously (Altice 2003; Altice 2004; Altice 2007; Smith-Rohrberg et al. 2006; Maru 2007). Briefly, a six-month, randomized controlled trial of DAART versus self-administered therapy (SAT) was conducted among 141 drug users. Participants were recruited from all of the HIV clinics in New Haven, Connecticut. Entry criteria included: (1) being HIV seropositive; (2) being eligible for and/or being prescribed antiretroviral medications; (3) residing within the city of New Haven; (4) lifetime history of heroin and/or cocaine use; and (5) receiving no more than a twice-daily regimen.
Following informed consent, eligible participants were randomized 2:1 to DAART or SAT and stratified on the following: (1) antiretroviral experienced versus naïve; (2) problematic alcohol use; (3) baseline HIV-1 RNA level, dichotomized as ≤ or > 1000 HIV-1 copies/ml; and (4) baseline CD4+ T lymphocyte count, dichotomized at ≤500 cells/ml and > 500 cells/ml. The 2:1 design was undertaken owing to the anticipated increase in refusals to participate in the DAART arm because subjects who underwent baseline interviews were not expected to agree to participate in DAART. Subjects randomized to DAART met the DAART Specialist after the baseline interview and upon learning of their randomization. For subjects already receiving antiretroviral therapy, medications were prepackaged and the first dose was observed that same day; most subjects received their first dose within a week, but some had up to a four-week delay while awaiting prescription information from the primary HIV provider.
For DAART, participants traveled to the Community Health Care Van (CHCV), a mobile medical program that provides an array of clinical services within four distinct areas of New Haven. There, they received DAART from a DAART Specialist. The DAART Specialist is a trained and experienced outreach worker who observes the participant take all prescribed chronic medications daily on weekdays and provides social support, medical and substance abuse referral, and case management services (Altice et al. 2004). Although most doses were observed on the CHCV, the DAART Specialist would occasionally travel to work-places, homes, drug-treatment settings and other prearranged settings depending upon their clients’ needs.
Weekend doses were dispensed on Fridays; participants were also provided an emergency supply of up to 3 days of antiretroviral medications. The exact number of emergency doses was determined at the discretion of the DAART Specialist based on safety and the likelihood that a participant would use an emergency dose as an excuse to skip a scheduled DAART visit. Subjects also carried a beeper to remind them when to take their evening dose. DAART participants were provided with a minimal non-monetary incentive ($5 dollars per week in food vouchers) for attending all DAART sessions in a given week. All participants, irrespective of randomization, received monetary compensation for interviews and for phlebotomy. DAART subjects were provided $10 for monthly MEMS cap readings. After 6 months of DAART, participants were transferred to SAT for the subsequent 6 months of observation.
Utilization Outcomes Measures
Participants were considered to have initiated treatment if they received at least one dose of DAART. The time-to-drop-out was considered to be the duration from the first observed dose of DAART to the last DAART visit the participant received. For 68 of the DAART participants (92% of those 74 participants who accepted DAART), electronic adherence monitoring was also available. Among these participants, for both observed and non-observed doses, all medications for a single dose were placed in small plastic bags in a medication bottle with a Medication Electronic Monitoring System (MEMS) Version 6 Smart Cap (Aardex, Union City, CA, USA). Thus, for a patient on a twice-daily regimen, two cap openings would be expected. Caps were collected monthly and data were downloaded to a computer database. Cap openings within 2 h of the scheduled dose were considered to be true medication events. In cases where more than one cap opening was observed during the expected interval, only a single event was recorded. Mean MEMS adherence was defined as the number of observed antiretroviral medication event openings divided by the expected event openings.
For descriptive purposes, a composite adherence score (CAS) was constructed in a manner adapted from Liu and colleagues (Liu et al. 2001; Paterson et al. 2002). The CAS is a strategy to make the adherence measurement more robust and less subject to bias, and to reduce missing values. This method makes use of the fact that MEMS and self-reported recall are highly correlated, especially when recall is assessed over the last 3 days, but that MEMS is somewhat more objective and sensitive at detecting poor adherence (Arnsten et al. 2001; Liu et al. 2006). The CAS was adapted for the context of the current trial, where we had available information from MEMS, observed doses, and self-report. For each expected dose, we recorded whether a MEMS event or a DAART observation occurred. Additionally, clients were asked during each DAART session about their adherence from the previous unobserved doses. If the patient reported that s/he had in fact taken the medication, but for some reason no MEMS event was recorded, these also were considered as adherent doses. Assessment of the correlates of CAS adherence was not performed; the CAS was used simply as a descriptive measure to depict the adherence of on-study DAART participants over the course of the intervention.
Measures of Predisposing, Enabling, and Need Factors
Baseline interviews assessed psychosocial and demographic characteristics, as well as adherence strategies, personal priorities, addiction severity using the DAST-10, depression using the Clinical Epidemiology Scale for Depressions (CES-D), the Medical Outcomes Study HIV Module (MOS-HIV), and attitudes towards DAART. All interviews were administered by independent research staff, at locations including our research offices, hospitals, prisons and drug-treatment settings. The demographic and social characteristics we used included previously described variables (Smith-Rohrberg et al. 2006; Altice et al. 2007): the predisposing factors of age, sex, education, drug abuse screening test and CES-D score, and the enabling factors of social support, homelessness status, and income level. The MOS-HIV (need factor) is a battery of 13 questions assessing participants’ current physical and mental health (Carretero et al. 1996; Murri et al. 1999; Wu 1999; Wu et al. 1997). The personal priorities (need factor) question involved a constrained choice approach by asking participants to indicate how they would spend $20, placing greater dollar amounts on those needs that are most important to them.
Statistical Analysis
All statistical analyses were implemented in SAS v9.1.3 (SAS Institute, Carey, NC). All inferences were made based on a type I error rate equal to 0.05. For comparisons between SAT and DAART participants or between DAART participants who dropped out and those who didn’t, Fisher’s exact test was used for categorical variables and the Wilcoxon rank-sum test for continuous variables.
For analysis of time-to-discontinuation among the DAART participants, drop-out defined as above, considering the last dose of therapy to be the time of discontinuation. Predisposing (demographic and social characteristics, beliefs about DAART), enabling (income, social support), and need (MOS-HIV score) factors were plotted Kaplan–Meier curves stratifying on responses to the baseline questionnaire. Variables were categorized as in Table 1. Both the mental health and physical health total scores were dichotomized at one standard deviation below the mean score (≥40 and <40). We used the Gehan (Wilcoxon) test statistic for testing significance, which is more sensitive when the proportional hazards assumption is partially violated. For those variables determined significant by Kaplan–Meier and for which the proportionality assumption was true, we additionally used Cox proportional hazards regression to estimate the hazard ratio. Owing to the small sample size and the exploratory aims of this study, multivariable analysis was not performed.
Table 1.
Baseline characteristics of the DAART arm (n = 88)
| Characteristic | Completed DAART (n = 51) Value |
Refused/did not complete (n = 37) Value |
P-value |
|---|---|---|---|
| Age, median years (IQR) | 43.7 (38–50) | 41.3 (37–47) | 0.41 |
| Gender | |||
| Female | 16 (31%) | 12 (32%) | >0.99 |
| Male | 35 (69%) | 25 (68%) | |
| Ethnicity | >0.99 | ||
| Black, not hispanic | 30 (59%) | 21 (57%) | |
| Hispanic | 12 (24%) | 9 (24%) | |
| White | 9 (18%) | 7 (19%) | |
| Primary language | 0.41 | ||
| English | 40 (78%) | 32 (86%) | |
| Spanish | 11 (22%) | 5 (14%) | |
| Education | 0.52 | ||
| Not high school graduate | 21 (41%) | 17 (46%) | |
| High school/GED | 18 (35%) | 15 (41%) | |
| Beyond HS | 12 (24%) | 5 (14%) | |
| Homeless | 21 (41%) | 11 (30%) | 0.37 |
| Monthly income | 0.25 | ||
| <$500 | 23 (45%) | 22 (59%) | |
| $500–$1000 | 23 (45%) | 14 (38%) | |
| >$1000 | 5 (10%) | 1 (3%) | |
| Lifetime drug use | |||
| Heroin | 39 (76%) | 22 (59%) | 0.10 |
| Cocaine | 50 (98%) | 35 (95%) | 0.57 |
| Injection drug use | 37 (73%) | 20 (54%) | 0.11 |
| DAST raw score | |||
| Low (0–2) | 18 (35%) | 12 (32%) | 0.16 |
| Moderate (3–5) | 13 (25%) | 4 (11%) | |
| High (6+) | 20 (39%) | 21 (57%) | |
| CESD depression score | 0.25 | ||
| None (<16) | 19 (38%) | 9 (25%) | |
| Present (≥16) | 31 (62%) | 27 (75%) | |
| Social support | 0.12 | ||
| Low (≤60 Huba scale) | 21 (41%) | 9 (24%) | |
| High (>60 Huba scale) | 30 (59%) | 28 (76%) | |
| HIV-1 viral load | |||
| ≤400 copies/ml | 17 (33%) | 10 (27%) | 0.64 |
| >400 copies/ml | 34 (67%) | 27 (73%) | |
| Median Log10 (IQR) | 3.8 (2.1–4.9) | 3.9 (2.4–5.2) | 0.52 |
| Mean Log10 (SE) | 3.6 (1.5) | 3.8 (1.6) | 0.58 |
| CD4+ T Lymphocytes | |||
| >350 cells/µl | 22 (43%) | 16 (43%) | >0.99 |
| ≤350 cells/µl | 29 (57%) | 21 (57%) | |
| Median CD4, cells/ml (IQR) | 246 (106–522) | 331 (81–541) | 0.78 |
| Mean CD4, cells/ml (SE) | 374 (46) | 231 (54) | 0.89 |
Note: For categorical variables, Fisher’s exact test was performed; as such, there is no associated test statistic. The test statistic for the others are as follows: median age, Wilcoxon test statistic = 536; median HIV viral load, Wilcoxon test statistic = 708; median CD4 count, Wilcoxon test statistic = 478; mean HIV viral load, t-test statistic = −0.55; mean CD4 count, t-test statistic = −0.14
Results
Utilization Outcomes
Of the 88 participants randomized to DAART, 14 (16%) refused participation in DAART; 51 (69%) of those participants who initiated the intervention remained in the intervention for the entire 6 months.
Adherence to observed doses was 54% (4172/7675) overall (intention-to-treat) and 67% (4172/6234) among those actively involved in the study prior to dropping out (on-treatment). Of the 1,786 observed doses missed due to identifiable causes (including those causes that led to complete drop-out from the study), 558 (35%) were due to entry into inpatient drug-treatment, 526 (33%) were due to incarceration, 4389 (25%) were because the patient started receiving home-based care from a visiting nurse, 207 (12%) because the treating physician had stopped medications for some reason, and 106 (7%) were due to hospitalization. Overall mean adherence among those actively involved and not having some identifiable reason for missing a dose (on-treatment and presently available) was 73% (4120/5584).
Among the 68 participants for whom detailed MEMS adherence data were available, total mean CAS adherence during the intervention prior to drop-out was 77% (12337/16020). Based on the CAS score, unsupervised dose adherence prior to drop-out was 69% (8354/12032).
Predisposing, Enabling, and Need Factors
Demographic and psychosocial characteristics as well as other predisposing and enabling factors are presented in Table 1. Among all participants, 101 (75%) indicated that they would prefer to take their own medications, but 118 (88%) responded that they would accept DAART if it were made compulsory to receiving HIV medications (predisposing factor; Table 2). More participants would prefer DAART to be provided in an anonymous setting (59%) rather than have someone come to them (41%). Only 56 (42%) participants indicated that they would be willing to travel up to one mile to receive DAART. Of potential sites for DAART, methadone maintenance clinics were the least acceptable to participants, acceptable to only 40% of participants, compared with 84–86% for the other venues.
Table 2.
Subject’s attitudes towards DAART at baseline (n = 141)
| Question | Response | Number of subjects | |
|---|---|---|---|
| You were given the choice, would you prefer to take your HIV medications on your own or have someone give you each of your doses of medication? | On own Given to them |
101 (75%) 34 (25%) |
|
| If your doctor told you that you needed HIV medications, but they would only prescribe them if you allowed someone to observe you taking your medications, would you accept or refuse? | Accept Refuse |
118 (87%) 17 (13%) |
|
| If you agreed to have someone give you each of your medications, would you prefer them to come to you every day, or would you prefer to go to them every day? | Come to you Go to them |
76 (59%) 52 (41%) |
|
| How far would you travel to pick up your medications on a daily basis? | <One block | 21 (16%) | |
| 1–4 Blocks | 44 (33%) | ||
| Up to one mile | 56 (42%) | ||
| Have car | 13 (10%) | ||
| Of the following places, where would you be willing to go to get your HIV medications on a daily basis? | Methadone | Willing Not willing |
55 (40%) 84 (60%) |
| Doctor’s office | Willing | 119 (86%) | |
| Not willing | 20 (14%) | ||
| Mobile van | Willing | 120 (86%) | |
| Not willing | 19 (14%) | ||
| Pharmacy | Willing | 117 (84%) | |
| Not willing | 22 (16%) |
Note: Columns do not always add up to 141 subjects because some subjects did not answer all questions
Table 3 demonstrates that study participants, despite having been referred to this study for problematic adherence, placed high value on issues relating to personal health (need factor). Specifically, 52% of participants placed at least $4 (of the possible $20 available) on controlling HIV and 40% placed this amount for getting off drugs. Basic needs, such as living in a safe environment and having sufficient food to eat, were the next highest set of priorities. Table 4 lists the various adherence strategies (enabling factors) used by participants in the 3 months prior to the study entry. Electronic timing devices such as watches were the most commonly used technique, with over half (53%) of participants reporting their use.
Table 3.
Personal priorities: What subjects would do with $20 (n = 141)
| Item to spend the money on | Percentage of all money designated by subjects | Number of subjects placing ≥$4 on item (%) |
|---|---|---|
| Controlling HIV with medications | 21 | 74 (52%) |
| Getting off drugs and staying clean | 16 | 57 (40%) |
| Living in a safe environment | 14 | 44 (31%) |
| Having enough food to eat | 13 | 46 (33%) |
| Spirituality | 12 | 36 (26%) |
| Being free from pain and discomfort | 10 | 25 (18%) |
| Enjoying close personal relationships | 8 | 13 (9%) |
| Staying out of jail | 7 | 20 (14%) |
Table 4.
Adherence strategies used in the 3 months prior to the beginning of the study (n = 141)
| Strategy | Number (%) |
|---|---|
| Alarm clock/timer/watch | 74 (52%) |
| Linking with using drugs | 67 (48%) |
| Laying pills out | 59 (42%) |
| Linking with going to bed or getting up | 40 (28%) |
| Keeping pills in a specific spot | 34 (24%) |
| Chart | 18 (13%) |
| Notes | 18 (13%) |
| Pill box | 10 (7%) |
| Calendar | 9 (6%) |
| Linking with meals | 7 (5%) |
| No strategies identified | 26 (18%) |
Note: Subjects could check more than one strategy
Among all subjects, the mean score on the mental health component of the MOS-HIV (need factor) was 34.0 (standard deviation: 9.3; median: 33.9; interquartile range: 27.5–41.2); mean score on the physical health component of the MOS-HIV was 42.8 (standard deviation: 6.3; median: 43.0; interquartile range: 39.0–46.9). On the mental health component, 98 (70%) and 50 (35%) reported a score one and two standard deviations below the mean, respectively; 46 (33%) and 2 (1%) of all subjects, respectively, were one and two standard deviations below the mean on the physical health component. These scores, in both continuous and categorical analyses, did not differ between SAT and DAART recipients, nor between patients who completed the study and those who discontinued DAART.
Association Between Factors and Time-to-discontinuation
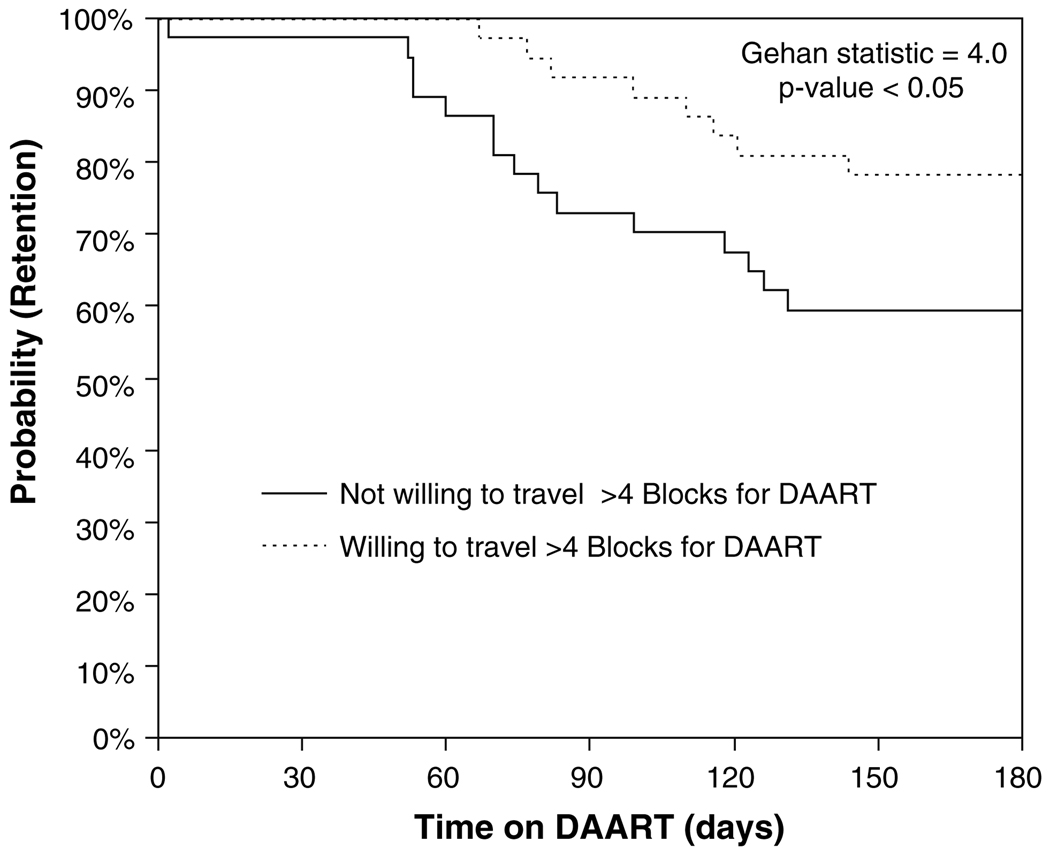
No pairwise differences emerged between those 37 participants who discontinued or refused DAART to those 51 participants who remained in the intervention on any demographic, psychosocial, or laboratory variables tested (predisposing and enabling factors; Table 1). Three factors did emerge, however, as significant predictors of time-to-discontinuation: (1) lack of willingness to travel more than four blocks to receive DAART (HR = 2.0; 95% CI: 1.0–3.9, Gehan statistic = 4.0; P-value < 0.05); (2) severe score on the CESD depression scale, (HR = 2.4; 95% CI: 1.0–6.0, Gehan statistic = 4.4; P-value < 0.05); and (3) lower score on the DAST scale (HR = 0.4; 95% CI: 0.1–0.9, Gehan statistic = 5.7; P-value < 0.05). Figure 2 provides a Kaplan–Meier plot for the DAART acceptance strata. The analyses for the other variables—quality of DAART care, age, sex, education, homelessness status, income level—did not significantly impact time-to-dropout and are not shown.
Fig. 2.
Proportion continuing DAART during the intervention period, stratified by willingness to travel to DAART program (n = 74). The analysis is restricted to those patients who accepted DAART. The survival analysis stratified by response to the question, “How far would you travel to pick up your medications on a daily basis?” dichotomizing the data based on whether the subject would be willing or unwilling to travel greater than four blocks to reach the site of DAART provision
Discussion
In this community-based clinical trial of DAART among HIV-infected drug users, 84% initiated the intervention, and 69% were retained throughout the entire six-month period. The intention-to-treat DAART adherence, including all subjects, was 54%. Mean adherence to observed doses was 73% when the patients were available and expected to come to the CHCV. Major reasons for missed doses included entry into drug-treatment, incarceration, switching care to visiting nurses association, and hospitalization. These numbers compare with those in the published literature: 48% retention among participants in a 12-month DAART program at a methadone clinic (Lucas et al. 2006b); 79% among a general (i.e., non-drug-using) population receiving 6 months of DAART through six-public clinics (Wohl et al. 2006); and 45% among a drug-using population in a six-month DAART program (Mitty et al. 2005). Similarly, the overall adherence is similar to the 83% median adherence of observed doses in the DAART program in which DAART was linked to receipt of methadone (Lucas et al. 2007).
The Behavioral Model provides insight into interpreting these results by framing DAART utilization as a product of predisposing, enabling, and need factors (Fig. 1). Of the major predisposing factors impacting utilization presented in Fig. 1, depressive symptomatology, the willingness to travel to DAART, and substance abuse severity impacted retention in bivariate survival analyses. A lack of willingness to travel was the only attitude about DAART that significantly predicted time-to-discontinuation. Similar findings have been reported regarding syringe exchange use by drug users in New York City (Rockwell et al. 1999). This finding suggests that DAART programs should be convenient and seriously consider a patient’s willingness and capacity to travel prior to establishing whether, and where, to provide DAART. It also provides some support for the modification of DAART as directly delivered therapy, an intervention that has recently shown some promise (Visnegarwala et al. 2006).
The major other predisposing factors associated with time to drop-out were evidence of presence of severe depressive symptomatology at baseline and low DAST scores. Since untreated or uncontrolled depression has been associated with poor adherence to antiretroviral therapy (Dalessandro et al. 2007; Lima et al. 2007), it is not surprising that depression similarly mediated retention in this DAART intervention. Future DAART interventions may need to incorporate effective pharmacotherapy and counseling as means to retain subjects.
The counter-intuitive finding of high levels of addiction severity in predicting increased retention may partially be related to the fact that groups with the greatest need received the most attention from the DAART Specialist. In a previous study, we demonstrated that high DAST scores had also predicted better virological outcomes (Smith-Rohrberg et al. 2006). A potential explanation is that those with lower DAST scores may have been better able to address their addiction and enter drug-treatment, which was a major reason for dropping out of the study. Alternatively, the increased social support provided by the DAART Specialist for those with the highest perceived need may have mediated better retention (Altice et al. 2001). In any case, these data suggest that, regardless of willingness to participate in drug-treatment, substance users can benefit from DAART.
Other predisposing factors assessing the acceptability of DAART did not affect retention. For example, 75% of the participants at baseline stated that they would prefer SAT to DAART, and, if they were to use DAART, 41% would prefer that DAART Specialists come to them. This result is similar to that seen in a hospital-based cross-sectional study, where only 17% of participants answered that they would prefer DAART to SAT (Santos et al. 2006). The finding of the poor acceptability of DAART if administered at methadone clinics has relevance to the design of programs there. While there are significant advantages in terms of adherence (Lucas et al. 2006b), owing in part to the positive pharmacological incentive of methadone, acceptability and initiation may be challenging in real-world implementation.
No enabling factors were found to impact time-to-discontinuation here; previously, however, we had shown that provision of medical and case management services positively impacted virological outcomes (Smith-Rohrberg et al. 2006). While we could not assess this in our study, some of the supportive activities provided by the DAART-Specialist in this study included: eliminating structural barriers to filling prescriptions, obtaining social services, overcoming the subject’s internalized resistance to consistently adhering to antiretroviral medications, linking subjects immediately to medical services if side effects reported, and assisting with transportation. All these enabling factors may help contribute to greater acceptance of the program, though further head-to-head evaluation is required.
Additional enabling factors that are often overlooked by providers are the adherence strategies that clients themselves are already using. It is important for DAART implementers to understand that even poorly adherent clients typically desire good health outcomes. Here, controlling HIV was the single most important priority reported among study participants at the beginning of the study. To achieve this, these participants were already using a variety of medication adherence strategies (Table 4). Some of these strategies may be counter-intuitive to staff, such as linking HIV medications with illicit drug use (48% of all participants).
Need factors are the final elements in the Behavior Model that impact utilization. Indeed, subjects enrolled in this DAART program with a high baseline burden and severity of comorbid conditions, as evidenced by participants’ severe scores on the MOS-HIV mental and physical health scales. Mental health scores were particularly low, with 35% of participants falling two standard deviations below the general population mean. As a result, the major causes of drop-out among our participants were hospitalization, incarceration, and entry into drug-treatment—all typically associated with poor physical and mental functioning. While this DAART intervention was limited in duration and does not reflect a lifetime of adherence, effective DAART programs will need to develop strategies to continue to effectively transfer patients on and off DAART, depending on the array of predisposing or need factors that reduce the subject’s likelihood of remaining adherent, particularly as they transfer into and out of these institutional settings.
There are several important limitations to this study. The study population was small and restricted to a single inner-city community, which limits the generalizability of these results. This sample size also limited our ability to perform multivariable adjustment to assess the robustness of the relationships seen between, for example, willingness to travel to DAART sites and time-to-drop-out. Furthermore, the analyses presented here were not part of pre-planned analyses comparing randomized groups. As such, the inferences made here must be considered as tentative and hypothesis-generating rather than definitive. The Behavioral Model has limitations as a theoretical framework, and indeed the lack of many significant predictors of our utilization outcome may indicate some weakness in its predictive power. Finally, much of our analysis was qualitative and descriptive, with the goal of generating ideas and hypotheses rather than testing them.
As DAART becomes more widely implemented in the community, the predisposing, enabling, and need factors discussed here require further study, preferably in the context of prospective cohort studies or randomized controlled trials.
Acknowledgments
The authors would like to acknowledge the National Institutes on Drug Abuse (R01 DA13805) for funding this study as well as providing career development awards for FLA (K24 DA 0170720), SAS (K23 DA 019381-02) and RDB (K23 DA 022143). DSR receives funding from the National Institutes of Health Medical Science Training Program (GM07205). The funding sources played no role in design of the study, data collection, analysis or interpretation of results or in the writing of the report. The authors would like to thank James Taylor, Rodolfo Lopez, Angel Ojeda and Natalie Laurenco for their contribution to the study implementation. Without their active participation and attention to detail, this study would not have been possible. We additionally would like to thank Paula Dellamura for administrative support. Most importantly, we would like to thank the participants in this study who dedicated time and energy to make this research possible.
Contributor Information
Duncan Smith-Rohrberg Maru, Email: paula.dellamura@yale.edu.
Frederick L. Altice, Email: raltice@aol.com.
References
- Altice FL, Friedland GH. The era of adherence to HIV therapy. Annals of Internal Medicine. 1998;129:503–505. doi: 10.7326/0003-4819-129-6-199809150-00015. [DOI] [PubMed] [Google Scholar]
- Altice FL, Mezger JA, Hodges J, Bruce RD, Marinovich A, Walton M, Springer S, Friedland GH. Developing a directly administered antiretroviral therapy intervention for HIV infected drug users: implications for program replication. Clinical Infectious Diseases. 2004;38:S376–S387. doi: 10.1086/421400. [DOI] [PubMed] [Google Scholar]
- Altice FL, Mostashari F, Friedland GH. Trust and the acceptance of and adherence to antiretroviral therapy. Journal of the Acquired Immune Deficiency Syndromes (1999) 2001;28:47–58. doi: 10.1097/00042560-200109010-00008. [DOI] [PubMed] [Google Scholar]
- Altice FL, Smith-Rohrberg D, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy compared to self-administered therapy among HIV-infected drug users: A randomized, controlled trial. Clinical Infectious Diseases. 2007;45:770–778. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Springer S, Buitrago M, Hunt DP, Friedland GH. Pilot study to enhance HIV care using needle exchange-based health services for out-of-treatment injecting drug users. Journal of Urban Health. 2003;80:416–427. doi: 10.1093/jurban/jtg053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RM. Revisiting the behavioral model and access to medical care: Does it matter? Journal of Health and Social Behavior. 1995;36:1–10. [PubMed] [Google Scholar]
- Andersen R, Aday LA. Access to medical care in the U.S.: Realized and potential. Medical Care. 1978;16:533–546. doi: 10.1097/00005650-197807000-00001. [DOI] [PubMed] [Google Scholar]
- Arnsten JH, Breg KM, Cooperman NA, Vinnanueva M, Li X, Parker F. A 6-month randomized controlled trial of directly observed therapy delivered in methadone clinics. Paper presented at the proceedings of the 2nd NIMH/IAPAC international conference on HIV treatment adherence; Jersey City, NJ. 2007. [Google Scholar]
- Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang CJ, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: Comparison of self-report and electronic monitoring. Clinical Infectious Diseases. 2001;33(8):1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. Journal of General Internal Medicine. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behforouz HL, Kalmus A, Scherz CS, Kahn JS, Kadakia MB, Farmer PE. Directly observed therapy for HIV antiretroviral therapy in an urban US setting. Journal of the Acquired Immune Deficiency Syndromes. 2004;36:642–645. doi: 10.1097/00126334-200405010-00016. [DOI] [PubMed] [Google Scholar]
- Carretero MD, Burgess AP, Soler P, Soler M, Catalan J. Reliability and validity of an HIV-specific health-related quality-of-life measure for use with injecting drug users. AIDS. 1996;10:1699–1705. doi: 10.1097/00002030-199612000-00015. [DOI] [PubMed] [Google Scholar]
- Conway B, Prasad J, Reynolds R, Farley J, Jones M, Jutha S, et al. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clinical Infectious Diseases. 2004;38:S402–S408. doi: 10.1086/421404. [DOI] [PubMed] [Google Scholar]
- Dalessandro M, Conti CM, Gambi F, Falasca K, Doyle R, Conti P, et al. Antidepressant therapy can improve adherence to antiretroviral regimens among HIV-infected and depressed patients. Journal of Clinical Psychopharmacology. 2007;27:58–61. doi: 10.1097/JCP.0b013e31802f0dd1. [DOI] [PubMed] [Google Scholar]
- Garland WH, Wohl AR, Valencia R, Witt MD, Squires K, Kovacs A, et al. The acceptability of a directly-administered antiretroviral therapy (DAART) intervention among patients in public HIV clinics in Los Angeles, California. AIDS Care. 2007;19:159–167. doi: 10.1080/09540120600911428. [DOI] [PubMed] [Google Scholar]
- Gelberg L, Andersen RM, Leake BD. The behavioral model for vulnerable populations: Application to medical care use and outcomes for homeless people. Health Services Research. 2000;34:1273–1302. [PMC free article] [PubMed] [Google Scholar]
- Greenberg B, Berkman A, Thomas R, Hoos D, Finkelstein R, Astemborski J, et al. Evaluating supervised HAART in late-stage HIV among drug users: A preliminary report. Journal of Urban Health. 1999;76:468–480. doi: 10.1007/BF02351504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozal MJ, Amico KR, Chiarella J, Cornman D, Fisher W, Fisher J, et al. HIV drug resistance and HIV transmission risk behaviors among active injection drug users. Journal of Acquired Immune Deficiency Syndromes. 2005;40(1):106–109. doi: 10.1097/01.qai.0000159666.95455.d2. [DOI] [PubMed] [Google Scholar]
- Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21(9):1175–1183. doi: 10.1097/QAD.0b013e32811ebf57. [DOI] [PubMed] [Google Scholar]
- Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Annals of Internal Medicine. 2001;134:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- Liu H, Miller LG, Hays RD, Golin CE, Wu T, Wenger NS, et al. Repeated measures longitudinal analyses of HIV virologic response as a function of percent adherence, dose timing, genotypic sensitivity, and other factors. Journal of Acquired Immune Deficiency Syndromes. 2006;41:315–322. doi: 10.1097/01.qai.0000197071.77482.6e. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. Journal of Acquired Immune Deficiency Syndromes. 2001;27:251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16(5):767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: A longitudinal study in the era of highly active antiretroviral therapy. American Journal of Epidemiology. 2006a;163(5):412–420. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Mullen BA, McCaul ME, Weidle PJ, Hader S, Moore RD. Adherence, drug use, and treatment failure in a methadone-clinic-based program of directly administered antiretroviral therapy. AIDS Patient Care and STDs. 2007;21(8):564–574. doi: 10.1089/apc.2006.0192. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Mullen BA, Weidle PJ, Hader S, McCaul ME, Moore RD. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clinical Infectious Diseases. 2006b;42(11):1628–1635. doi: 10.1086/503905. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Weidle PJ, Hader S, Moore RD. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: A nonrandomized comparative study. Clinical Infectious Diseases. 2004;38:S409–S413. doi: 10.1086/421405. [DOI] [PubMed] [Google Scholar]
- Macalino GE, Hogan JW, Mitty JA, Bazerman LB, Delong AK, Loewenthal H, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21:1473–1477. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]
- Macalino GE, Mitty JA, Bazerman LB, Singh K, McKenzie M, Flanigan T. Modified directly observed therapy for the treatment of HIV-seropositive substance users: lessons learned from a pilot study. Clinical Infectious Diseases. 2004;38:S393–S397. doi: 10.1086/421402. [DOI] [PubMed] [Google Scholar]
- Maru DSR, Kozal MJ, Bruce RD, Springer SA, Altice FL. Directly administered antiretroviral therapy for HIV-infected drug users does not impact antiretroviral resistance: results from a randomized, controlled trial. Journal of the Acquired Immune Deficiency Syndromes. 2007;46(5):555–563. doi: 10.1097/qai.0b013e318158c0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CG, Freels S, Creticos CM, Oltean A, Douglas R. Preliminary findings of an intervention integrating modified directly observed therapy and risk reduction counseling. AIDS Care. 2007;19(4):561–564. doi: 10.1080/09540120601040813. [DOI] [PubMed] [Google Scholar]
- Mitty JA, Macalino GE, Bazerman LB, Loewenthal HG, Hogan JW, MacLeod CJ, et al. The use of community-based modified directly observed therapy for the treatment of HIV-infected persons. Journal of Acquired Immune Deficiency Syndromes. 2005;39:545–550. [PubMed] [Google Scholar]
- Murri R, Fantoni M, Del Borgo C, Izzi I, Visona R, Suter F, et al. Intravenous drug use, relationship with providers, and stage of HIV disease influence the prescription rates of protease inhibitors. Journal of Acquired Immune Deficiency Syndromes. 1999;22:461–466. doi: 10.1097/00126334-199912150-00006. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Potoski B, Capitano B. Measurement of adherence to antiretroviral medications. Journal of Acquired Immune Deficiency Syndromes. 2002;31:S103–S106. doi: 10.1097/00126334-200212153-00003. [DOI] [PubMed] [Google Scholar]
- Rockwell R, Des Jarlais DC, Friedman SR, Perlis TE, Paone D. Geographic proximity, policy and utilization of syringe exchange programmes. AIDS Care. 1999;11:437–442. doi: 10.1080/09540129947811. [DOI] [PubMed] [Google Scholar]
- Santos CQ, Adeyemi O, Tenorio AR. Attitudes toward directly administered antiretroviral therapy (DAART) among HIV-positive inpatients in an inner city public hospital. AIDS Care. 2006;18:808–811. doi: 10.1080/09540120500448513. [DOI] [PubMed] [Google Scholar]
- Smith-Rohrberg D, Altice FL. Randomized, controlled trials of directly administered antiretroviral therapy for HIV-infected patients: Questions about study population and analytical approach. Clinical Infectious Diseases. 2006;43:1221–1222. doi: 10.1086/508357. [DOI] [PubMed] [Google Scholar]
- Smith-Rohrberg D, Mezger J, Walton M, Bruce RD, Altice FL. Impact of enhanced services on virologic outcomes in a directly administered antiretroviral therapy trial for HIV-infected drug users. Journal of Acquired Immune Deficiency Syndromes. 2006;43:S48–S53. doi: 10.1097/01.qai.0000248338.74943.85. [DOI] [PubMed] [Google Scholar]
- Tinoco I, Giron-Gonzalez JA, Gonzalez-Gonzalez MT, Vergara de Campos A, Rodriguez-Felix L, Serrano A, et al. Efficacy of directly observed treatment of HIV infection: Experience in AIDS welfare homes. European Journal of Clinical Microbiology and Infectious Diseases. 2004;23:331–335. doi: 10.1007/s10096-003-1099-8. [DOI] [PubMed] [Google Scholar]
- Tyndall M, McNally M, Lai C, Zhang R, Wood E, Kerr T, et al. Directly observed therapy programs for antiretroviral treatment among injection drug users in Vancouver: Access, adherence and outcomes. International Journal of Drug Policy. 2007;18(4):281–287. doi: 10.1016/j.drugpo.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Visnegarwala F, Rodriguez-Barradass MC, Graviss EA, Caprio M, Nykyforchyn M, Laufman L. Community outreach with weekly delivery of anti-retroviral drugs compared to cognitive-behavioural health care team-based approach to improve adherence among indigent women newly starting HAART. AIDS Care. 2006;18:332–338. doi: 10.1080/09540120500162155. [DOI] [PubMed] [Google Scholar]
- Wohl AR, Garland WH, Valencia R, Squires K, Witt MD, Kovacs A, et al. A randomized trial of directly administered antiretroviral therapy and adherence case management intervention. Clinical Infectious Diseases. 2006;42:1619–1627. doi: 10.1086/503906. [DOI] [PubMed] [Google Scholar]
- Wu AW. MOS-HIV health survey: Users manual. John Hopkins University; 1999. [Google Scholar]
- Wu AW, Revicki DA, Jacobson D, Malitz FE. Evidence for reliability, validity and usefulness of the medical outcomes study HIV health survey (MOS-HIV) Quality of Life Research. 1997;6:481–493. doi: 10.1023/a:1018451930750. [DOI] [PubMed] [Google Scholar]