Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used for the treatment of peripheral pain and inflammation, with millions of people using over-the-counter and prescription NSAIDs daily. The principal target of NSAIDs is cyclooxygenase (COX), the requisite enzyme for synthesis of potent lipid mediators called prostanoids. COX exists in two isoforms: COX-1 appears to be constitutively expressed in many tissues and is responsible for homeostatic production of prostanoids. In contrast, COX-2 is often considered an inducible isoform and a principal contributor to peripheral inflammation via production of prostaglandin E2 (PGE2) [30]. Since chronic COX-1 inhibition is associated with side effects such as gastrointestinal bleeding and ulcers [6,38], COX-2 inhibition became a mainstay therapy in treatment for rheumatoid arthritis, osteoarthritis, and other chronic inflammatory conditions [33].
The production of COX-2 by neurons and glia is a common characteristic of neuroinflammation associated with central nervous system injury [19], neurodegenerative disease [12,26,28], and aging [23,47]. Furthermore, changes in COX-2 expression and activity are associated with pathology-associated cognitive decline [2,7,27]. However, in the absence of inflammation, COX-2 appears to contribute to constitutive neural function as it is expressed in neurons [5,48], regulated by synaptic activity [8,22,32], and integral in hippocampal long-term potentiation (LTP) and depression (LTD) [9,29,32].
Surprisingly, only a handful of studies have investigated the role of COX-2 in normal learning and memory. Collectively, the results show that COX-2 inhibition via intracerebral or peripheral NSAID administration corresponds to diminished retention in hippocampal-dependent spatial memory tasks in adult male rats [36,40,43]. Considering the anticipation of cognitive assessment in transgenic mouse models of inflammation that incorporate NSAID treatment, the aim of the present study was to examine the role COX-2 in the spatial memory in mice to determine if systemic administration of the COX-2 selective inhibitor NS-398 influences hippocampus-dependent learning as demonstrated in rats. Our investigation included female mice to address reported sex differences in normal mnemonic processes [3,14,45] and the potential for sex to influence constitutive COX-2 activity.
All protocols complied with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the Santa Clara Univerisity Institutional Animal Care & Use Committee. Male (n =15) and female (n= 16) C57BL/6J mice (12 weeks-old) from Jackson Laboratory (Bar Harbor, ME) were utilized in the study. All mice were handled approximately five minutes per day for one week prior to behavioral assessment. Mice were grouped by sex (four per cage) in a 12-hour light/dark cycle room.
Mice were administered intraperitoneal injections (0.1 mL) of vehicle (10% dimethyl sulfoxide (DMSO) in 0.15 M phosphate buffer) or NS-398 (10 mg/kg in vehicle solution; Cayman Chemical, Ann Arbor, MI) six hours prior to behavioral assessment on each day. The testing apparatus was constructed using a black, plastic circular tub (80 cm diameter, 12 cm deep) filled with water (25±2°C) made opaque using non-toxic white tempera paint. A removable plexiglass 10 cm2 platform was placed in the water approximately one centimeter below the surface of the water. Visual cues were placed in various locations of the testing room (8′ × 8′). For two days preceding spatial training, mice were introduced to the water and allowed four 45-second swimming periods.
The spatial learning protocol was adapted from previous rodent studies [18,43] and consisted of one day of acquisition containing 8 trials separated into 2 sets of 4 trials. Inter-trial and inter-set intervals were 10 and 120 minutes. Retention of spatial learning was tested twenty-four hours following the onset of acquisition using the same trial schedule. For each trial, mice were placed in the maze at one of four designated drop points in a pseudo-random order that allowed each drop point to be represented within each set. A maximum of 45 seconds was allowed for discovery of the hidden platform. If the platform was not found within the allotted time, mice were guided to the platform. For all trials, latency (time to find the platform; seconds), path length (distance traveled; meters), swim speed (meters/second) and perimeter distance (percent of distance traveled in outer 50% of tank) were recorded using commercial tracking software (ANY-Maze®, Stoelting Co., Chicago, IL). In addition, total distance traveled on each testing day was calculated by summing path length measurements across the eight trials. Two-way ANOVAs with repeated measures (trials) were performed to assess the effects of sex and treatment on acquisition and retention days for each task (SPSS, Inc; Chicago, IL ). One-way ANOVAS were utilized to compare summed path length and perimeter travel indices. Post-hoc comparisons between the four experimental groups (Female Vehicle, Female NS-398, Male Vehicle, Male NS-398) were made using Tukey’s HSD multi-comparison test. An alpha level of 0.05 was necessary to reject the null hypothesis and to consider the data statistically significant.
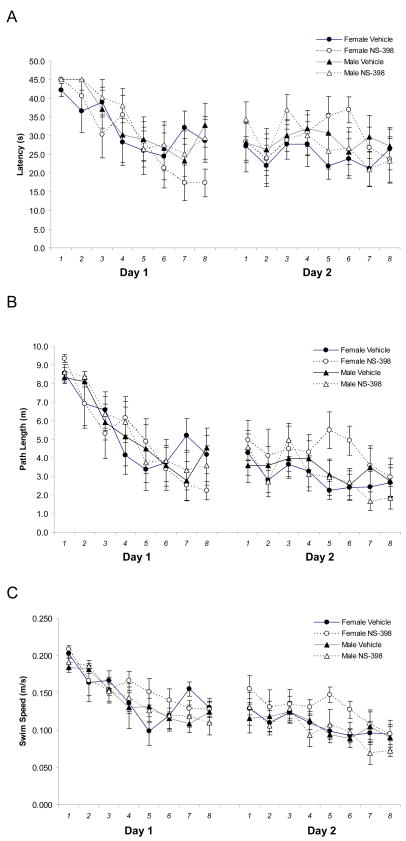
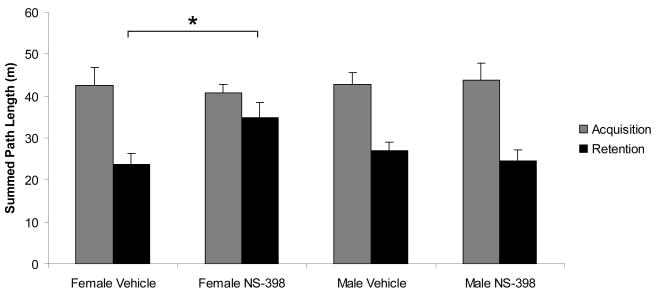
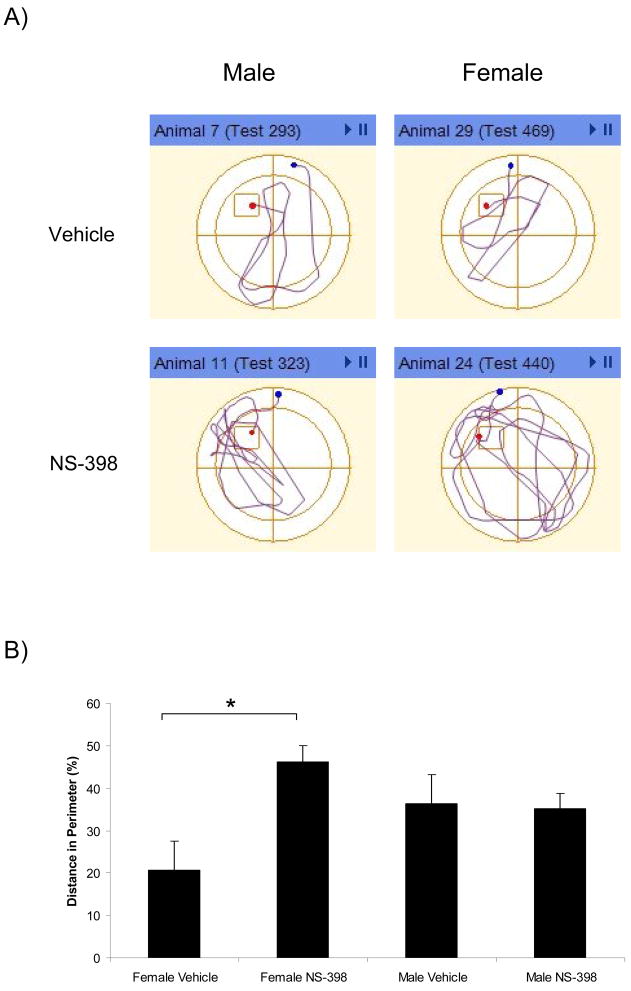
As reported in Table 1, we found no significant main or interaction effects of sex or treatment on the latency, path length, and swim speed on initial day of spatial training. Acquisition rate was similar in all groups as reflected in a significant trial effect for latency [F(7, 189)=10.883 P<0.001] and path length [F(7, 189)=16.389, P<0.001] with no interaction effects of sex or treatment (Figure 1). During the retention trials, an effect of sex on swim speed approached significance [F(1, 27)=3.967, P=0.057], with females demonstrating faster swim rates (Figure 1C). Therefore, path length was considered a more appropriate measure of retention as it was minimally affected by swimming speed. Although no main sex or treatment effect of sex was found on the retention day of spatial training (Table 1), we did find a significant sex × treatment interaction [F(1,27)=5.510, P=0.026] on retention trial path length with post-hoc Tukey analysis indicating that NS-398-treated female mice traveled greater path lengths across the 8 retention trials (34.8 ± 10.6 m) relative to vehicle-treated female mice (23.72±7.2 m; p=0.046) (Figure 2). Further analysis of spatial retention strategy revealed a significant sex × treatment interaction on proportion of path length traveled in the perimeter of the tank during the retention trials [F(1,27)=14.4, P=0.001] with NS-398 treatment associated with greater perimeter travel in female mice. Specifically, this distinction between female treatment groups was apparent on the initial retention trial (Vehicle: 20.8±10% vs. NS-398: 46.1±11%; p=0.012; Figure 3], suggesting an NS-398-related deficit in overnight memory retention and/or retrieval that persisted throughout the remaining trials. Our results suggest that administration of NS-398, a COX-2 inhibitor, prior to spatial training and retention performance impairs memory on a spatial task in female C57BL/6 mice without influencing male performance. To our knowledge, this is the first report of a sexual dimorphism of COX-2 influence in spatial memory in normal mice.
Table 1.
Results of analyses of variance
| Acquisition | Retention | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Treatment | Sex × Treatment | Trials 1 | Sex | Treatment | Sex × Treatment | Trials 1 | |||||||||
| F(1, 27) | p | F(1, 27) | p | F(1, 27) | p | F(7,189) | p | F(1, 27) | p | F(1, 27) | p | F(1, 27) | p | F(7,189) | p | |
| Latency | 1.953 | 0.174 | 0.127 | 0.725 | 0.578 | 0.454 | 10.883 | 0.000 | 0.226 | 0.638 | 0.534 | 0.471 | 1.160 | 0.291 | 1.171 | 0.321 |
| Path Length | 0.219 | 0.643 | 0.020 | 0.889 | 0.167 | 0.686 | 16.389 | 0.000 | 1.486 | 0.233 | 2.258 | 0.145 | 5.510 | 0.026 | 2.041 | 0.052 |
| Swim Speed | 1.862 | 0.184 | 0.790 | 0.382 | 0.263 | 0.612 | 13.414 | 0.000 | 3.967 | 0.057 | 1.318 | 0.261 | 3.537 | 0.071 | 4.567 | 0.000 |
repeated measure
Figure 1.
Mean latency (A), path length (B), and swim speed (C) of each trial during acquisition (Day 1) and retention (Day 2) of the spatial task. Each data point represents the mean (± SEM) for each experimental group.
Figure 2.
NS-398 treatment prior to spatial task performance increases path length across retention trials. Path lengths across eight trials were summed for each animal on each day. Each column represents the mean (± SEM) of total distance traveled on acquisition (grey) and retention (black) days. * indicates p<0.05 between the indicated experimental groups as determined by post-hoc analysis.
Figure 3.
NS-398 treatment increases perimeter travel in female C57/BL6J mice. A) Selected traces (ANY-maze, Stoelting Co. Chicago, IL) representing mean perimeter path lengths of first retention trial for each experimental group. B) Percent of retention trial distance traveled in perimeter of tank. Each column represents the retention trial mean (+ SEM) for each experimental group. * indicates p<0.05 between the indicated experimental groups as determined by post-hoc analysis.
Given the identified roles of PGE2 in hippocampal long-term potentiation [8,9,11], it is not surprising that inhibition of its production may influence hippocampal-dependent tasks such as spatial memory. Previous reports assessing rodent behavior have demonstrated the role of COX-2 in spatial memory performance in male rodents. Similar to data collected following bilateral intrahippocampal infusion of the COX-2-inhibiting celecoxib in male rats[40], Teather and colleagues [43] reported that intraperitoneal administration of a COX non-selective inhibitor (indomethacin) or a COX-2-selective inhibitor (NS-398) immediately following one day of training on the hidden platform task was associated with increased escape latencies on retention trials 24 hours later. Interestingly, no difference in retention rates were observed when inhibitors were given 2 hours following spatial training, suggesting a time-dependent role of COX-2 in memory consolidation [25]. Although we observed similar performance deficits in female C57BL/6 mice at 24 hours post-training, we did not see any effect of COX-2 inhibition in male mice. This sex-dependent effect of NS-398 may be due to a difference in drug metabolism with consideration to the critical window of its influence on memory processes. Pharmacokinetic studies in male rats estimate the terminal half-life of nimesulide, a methane sulfonanilide COX-2 inhibitor with similar chemical structure to NS-398, to be 4.5 hours and 6.1 hours following intravenous and oral administration, respectively [35]. This metabolic time frame is similar to that of the COX-2-specific inhibitor celecoxib in male rats; however, female rats have shown an extended celecoxib half-life of 12 hours [31]. Although no influence of gender on pharmacokinetic indices of nimesulide was found in humans [4], if NS-398 is sensitive to sex steroid-influenced metabolic enzymes as suggested by celecoxib, its levels at completion of acquisition training may have decreased below the criterion concentration necessary to impair memory consolidation in male mice [40,43]. Future investigation of NS-398 dose and dosing schedules is required to determine at what systemic dose, if any, male C57BL/6 mice exhibit COX-2-dependent memory dysfunction. Furthermore, inclusion of treatment groups that are only administered NS-398 to mice immediately following acquisition or prior to retention trials will distinguish the role of COX-2 in memory consolidation versus retrieval of spatial learning, respectively.
Although we did not find a treatment effect on acquisition of the spatial learning task, we must acknowledge that NS-398 was in circulation and may have had a subtle impact on learning that was not detected by our measurements. A previous report presented increased latency and path length for acquisition trials following intrahippocampal infusion of celecoxib in rats [36], suggesting that COX-2 is necessary for spatial learning. In addition to an assumed difference in dose given the variation in drug administration (systemic vs. intrahippocampal), our lack of NS-398 influence may be due to a species difference, as rate of spatial acquisition and acquisition strategy differ between mouse and rat [15,46]. In addition, our water maze apparatus had a search ratio (tank diameter: platform) smaller than comparable studies in rats, a factor that has been associated with steeper learning curves and greater difficulty in assessing learning [46].
The role of estrous stage on spatial memory in C57BL/6 mice has been addressed by other researchers [13]. Vaginal smears were collected twice during this experiment to assess estrous stage in a subset of the female mice (n=12). Cytological analysis of vaginal smears identified females as estrus (cornified epithelial cells) or metestrus/diestrus (presence of leukocytes with few nucleated epithelial cells ) on day of acquisition training. When considering estrous stage and drug as between-subjects factors, we found a notable interaction effect on path length of acquisition trials [p=0.07]. However, estrous stage did not have a significant impact on overall path length or perimeter swimming across the retention trials. Given the small sample sizes for each estrous stage and treatment group in this experiment, a larger scale study is necessary to accurately examine the factor of estrous stage in COX-2-mediated memory.
Sex differences in memory performance have been observed in young naïve rats and mice, however other groups, including this report, have reported no differences (for review, see [21]). Several mechanisms have been described as mediating the variations in performance, including circulating sex steroid hormones [17,20,39], estrogen receptors[16,37], and/or the cholinergic system [3,24,44]. These factors may intersect with prostaglandin production as COX-2 activity in brain is increased with estradiol administration [1] and mediates nAChR signaling in spatial memory [41]. Therefore, our results may represent an interaction between COX-2-dependent PGE2 production and sex hormone-influenced synaptic plasticity.
In conclusion, our data suggest that COX-2 activity influences mnemonic processes in C57BL/6 mice in a sex-dependent manner. Previous studies report sex as a factor in response to inflammatory-related conditions with particular attention given to estrogen [34,42] and COX-2 [10,27], encouraging research to consider female subjects in rodent models of inflammatory disorders. By suggesting that normal female mice, in absence of inflammation, exhibit memory impairment following NS-398 treatment, our results may be critical in designing and interpreting future experiments that examine the cognitive effects of NSAID treatment during inflammation in both sexes.
Acknowledgments
The authors would like to thank the consultation of Dr. Michelle Marvier. C.G. and A.M. were supported by a fellowship from the Clare Boothe Luce Foundation to encourage women in the natural sciences. K.G. and L.G. were supported by the Community of Science Scholars Initiative made possible by the Howard Hughes Medical Institute to support undergraduate research. Extramural support was also provided by NIH R03MH069746.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- 1.Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002;22:8586–96. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreasson KI, Savonenko A, Vidensky S, Goellner JJ, Zhang Y, Shaffer A, Kaufmann WE, Worley PF, Isakson P, Markowska AL. Age-dependent cognitive deficits and neuronal apoptosis in cyclooxygenase-2 transgenic mice. J Neurosci. 2001;21:8198–209. doi: 10.1523/JNEUROSCI.21-20-08198.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger-Sweeney J, Arnold A, Gabeau D, Mills J. Sex differences in learning and memory in mice: effects of sequence of testing and cholinergic blockade. Behav Neurosci. 1995;109:859–73. doi: 10.1037//0735-7044.109.5.859. [DOI] [PubMed] [Google Scholar]
- 4.Bernareggi A. Clinical pharmacokinetics of nimesulide. Clin Pharmacokinet. 1998;35:247–74. doi: 10.2165/00003088-199835040-00001. [DOI] [PubMed] [Google Scholar]
- 5.Breder CD, Dewitt D, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buttgereit F, Burmester GR, Simon LS. Gastrointestinal toxic side effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2-specific inhibitors. Am J Med. 2001;110(Suppl 3A):13S–9S. doi: 10.1016/s0002-9343(00)00728-2. [DOI] [PubMed] [Google Scholar]
- 7.Casolini P, Catalani A, Zuena AR, Angelucci L. Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat. J Neurosci Res. 2002;68:337–43. doi: 10.1002/jnr.10192. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Bazan NG. Endogenous PGE2 regulates membrane excitability and synaptic transmission in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;93:929–41. doi: 10.1152/jn.00696.2004. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol. 2002;87:2851–7. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- 10.Chillingworth NL, Morham SG, Donaldson LF. Sex differences in inflammation and inflammatory pain in cyclooxygenase-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R327–34. doi: 10.1152/ajpregu.00901.2005. [DOI] [PubMed] [Google Scholar]
- 11.Cowley TR, Fahey B, O’Mara SM. COX-2, but not COX-1, activity is necessary for the induction of perforant path long-term potentiation and spatial learning in vivo. Eur J Neurosci. 2008;27:2999–3008. doi: 10.1111/j.1460-9568.2008.06251.x. [DOI] [PubMed] [Google Scholar]
- 12.Esposito E, Di Matteo V, Benigno A, Pierucci M, Crescimanno G, Di Giovanni G. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp Neurol. 2007;205:295–312. doi: 10.1016/j.expneurol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav Neurosci. 2001;115:229–37. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- 14.Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–91. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- 15.Frick KM, Stillner ET, Berger-Sweeney J. Mice are not little rats: species differences in a one-day water maze task. Neuroreport. 2000;11:3461–5. doi: 10.1097/00001756-200011090-00013. [DOI] [PubMed] [Google Scholar]
- 16.Fugger HN, Cunningham SG, Rissman EF, Foster TC. Sex differences in the activational effect of ERalpha on spatial learning. Horm Behav. 1998;34:163–70. doi: 10.1006/hbeh.1998.1475. [DOI] [PubMed] [Google Scholar]
- 17.Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–9. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Harburger LL, Pechenino AS, Saadi A, Frick KM. Post-training progesterone dose-dependently enhances object, but not spatial, memory consolidation. Behav Brain Res. 2008;194:174–80. doi: 10.1016/j.bbr.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurley SD, Olschowka JA, O’Banion MK. Cyclooxygenase inhibition as a strategy to ameliorate brain injury. J Neurotrauma. 2002;19:1–15. doi: 10.1089/089771502753460196. [DOI] [PubMed] [Google Scholar]
- 20.Imwalle DB, Bateman HL, Wills A, Honda S, Harada N, Rissman EF. Impairment of spatial learning by estradiol treatment in female mice is attenuated by estradiol exposure during development. Horm Behav. 2006;50:693–8. doi: 10.1016/j.yhbeh.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav Rev. 2005;28:811–25. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:2317–21. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukiw WJ, Bazan NG. Strong nuclear factor-kappaB-DNA binding parallels cyclooxygenase-2 gene transcription in aging and in sporadic Alzheimer’s disease superior temporal lobe neocortex. J Neurosci Res. 1998;53:583–92. doi: 10.1002/(SICI)1097-4547(19980901)53:5<583::AID-JNR8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Lukoyanov NV, Andrade JP, Dulce Madeira M, Paula-Barbosa MM. Effects of age and sex on the water maze performance and hippocampal cholinergic fibers in rats. Neurosci Lett. 1999;269:141–4. doi: 10.1016/s0304-3940(99)00442-5. [DOI] [PubMed] [Google Scholar]
- 25.McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–8. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- 26.McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28:639–47. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Melnikova T, Savonenko A, Wang Q, Liang X, Hand T, Wu L, Kaufmann WE, Vehmas A, Andreasson KI. Cycloxygenase-2 activity promotes cognitive deficits but not increased amyloid burden in a model of Alzheimer’s disease in a sex-dimorphic pattern. Neuroscience. 2006;141:1149–62. doi: 10.1016/j.neuroscience.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–10. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- 29.Murray HJ, O’Connor JJ. A role for COX-2 and p38 mitogen activated protein kinase in long-term depression in the rat dentate gyrus in vitro. Neuropharmacology. 2003;44:374–80. doi: 10.1016/s0028-3908(02)00375-1. [DOI] [PubMed] [Google Scholar]
- 30.O’Banion MK, Miller JC, Chang JW, Kaplan MD, Coleman PD. Interleukin-1 beta induces prostaglandin G/H synthase-2 (cyclooxygenase-2) in primary murine astrocyte cultures. J Neurochem. 1996;66:2532–40. doi: 10.1046/j.1471-4159.1996.66062532.x. [DOI] [PubMed] [Google Scholar]
- 31.Paulson SK, Zhang JY, Breau AP, Hribar JD, Liu NW, Jessen SM, Lawal YM, Cogburn JN, Gresk CJ, Markos CS, Maziasz TJ, Schoenhard GL, Burton EG. Pharmacokinetics, tissue distribution, metabolism, and excretion of celecoxib in rats. Drug Metab Dispos. 2000;28:514–21. [PubMed] [Google Scholar]
- 32.Pepicelli O, Fedele E, Bonanno G, Raiteri M, Ajmone-Cat MA, Greco A, Levi G, Minghetti L. In vivo activation of N-methyl-D-aspartate receptors in the rat hippocampus increases prostaglandin E(2) extracellular levels and triggers lipid peroxidation through cyclooxygenase-mediated mechanisms. J Neurochem. 2002;81:1028–34. doi: 10.1046/j.1471-4159.2002.00897.x. [DOI] [PubMed] [Google Scholar]
- 33.Pisetsky DS, St Clair EW. Progress in the treatment of rheumatoid arthritis. Jama. 2001;286:2787–90. doi: 10.1001/jama.286.22.2787. [DOI] [PubMed] [Google Scholar]
- 34.Pozzi S, Benedusi V, Maggi A, Vegeto E. Estrogen action in neuroprotection and brain inflammation. Ann N Y Acad Sci. 2006;1089:302–23. doi: 10.1196/annals.1386.035. [DOI] [PubMed] [Google Scholar]
- 35.Rainsford KD. Nimesulide -- Actions and Uses. Springer; 2005. [Google Scholar]
- 36.Rall JM, Mach SA, Dash PK. Intrahippocampal infusion of a cyclooxygenase-2 inhibitor attenuates memory acquisition in rats. Brain Res. 2003;968:273–6. doi: 10.1016/s0006-8993(03)02248-0. [DOI] [PubMed] [Google Scholar]
- 37.Rissman EF, Wersinger SR, Fugger HN, Foster TC. Sex with knockout models: behavioral studies of estrogen receptor alpha. Brain Res. 1999;835:80–90. doi: 10.1016/s0006-8993(99)01452-3. [DOI] [PubMed] [Google Scholar]
- 38.Sakamoto C. Roles of COX-1 and COX-2 in gastrointestinal pathophysiology. J Gastroenterol. 1998;33:618–24. doi: 10.1007/s005350050147. [DOI] [PubMed] [Google Scholar]
- 39.Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulates performance on a spatial working memory task in male rats. Horm Behav. 2006;50:18–26. doi: 10.1016/j.yhbeh.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Sharifzadeh M, Naghdi N, Khosrovani S, Ostad SN, Sharifzadeh K, Roghani A. Post-training intrahippocampal infusion of the COX-2 inhibitor celecoxib impaired spatial memory retention in rats. Eur J Pharmacol. 2005;511:159–66. doi: 10.1016/j.ejphar.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 41.Sharifzadeh M, Tavasoli M, Naghdi N, Ghanbari A, Amini M, Roghani A. Post-training intrahippocampal infusion of nicotine prevents spatial memory retention deficits induced by the cyclo-oxygenase-2-specific inhibitor celecoxib in rats. J Neurochem. 2005;95:1078–90. doi: 10.1111/j.1471-4159.2005.03454.x. [DOI] [PubMed] [Google Scholar]
- 42.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–74. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 43.Teather LA, Packard MG, Bazan NG. Post-training cyclooxygenase-2 (COX-2) inhibition impairs memory consolidation. Learn Mem. 2002;9:41–7. doi: 10.1101/lm.43602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veng LM, Granholm AC, Rose GM. Age-related sex differences in spatial learning and basal forebrain cholinergic neurons in F344 rats. Physiol Behav. 2003;80:27–36. doi: 10.1016/s0031-9384(03)00219-1. [DOI] [PubMed] [Google Scholar]
- 45.Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–81. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- 46.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu D, Meydani SN. Mechanism of age-associated up-regulation in macrophage PGE2 synthesis. Brain Behav Immun. 2004;18:487–94. doi: 10.1016/j.bbi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–86. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]