Abstract
The wide chemical shift dispersion and long T1 of 13C have allowed determination of in vivo magnetization transfer effects caused by aspartate aminotransferase and lactate dehydrogenase reactions using 13C MRS. In this report, we demonstrate that these effects can be observed in the proton spectra by transferring the equilibrium magnetization of 13C via the one-bond scalar coupling between 13C and 1H using an inverse INEPT-based heteronuclear polarization transfer method. This inverse method allows a combination of the advantages of the long 13C T1 for maximum magnetization transfer and the high sensitivity of proton detection. The feasibility of this in vivo inverse polarization transfer approach was evaluated for detecting the 13C magnetization transfer effect of aspartate aminotransferase and lactate dehydrogenase reactions from a 72.5 μL voxel in the rat brain at 11.7 Tesla.
Keywords: Proton Detection, Carbon-13, Magnetization Transfer Effect
Introduction
Recently, in vivo 13C magnetization (saturation) transfer effect of aspartate aminotransferase (AAT, L-aspartate: α-ketoglutarate aminotransferase; or AST, aspartate transaminase, EC 2.6.1.1) [1], lactate dehydrogenase (LDH, EC 1.1.1.27) [2], malate dehydrogenase (MDH, EC 1.1.1.37) [3] and carbonic anhydrase (CA, EC 4.2.1.1) [4] reactions have been described. Using 13C spectroscopy methods and by saturating the carbonyl carbon of α-ketoglutarate (α-KG) at 206.0 ppm in α-chloralose-anesthetized adult rat brain, the pseudo first-order rate constant of the unidirectional Glutamate (Glu) → α-KG flux was determined to be 0.13 ± 0.01 sec−1 (mean ± SD, n = 11) following intravenous infusion of [1,6-13C2]glucose [1]. By infusion of [2-13C]glucose and saturation of pyruvate (Pyr) C2 at 207.9 ppm the pseudo first-order rate constant of the unidirectional lactate (Lac) → Pyr flux was determined to be 0.08 ± 0.01 sec−1 (mean ± SD, n = 4) in halothane-anesthetized adult rat brain during systemic administration of GABAA receptor antagonist bicuculline [2]. In addition, the AAT-catalyzed aspartate (Asp) ↔ oxaloacetate (OAA) half reaction [1,3], the MDH-catalyzed malate + NAD+ ↔ oxaloacetate + NADH + H+ reaction [3], and the CA-catalyzed bicarbonate (HCO3−) ↔ CO2 exchange reaction [4] have also been measured quantitatively in vivo. The LDH reaction and CA-catalyzed bicarbonate-carbon dioxide exchange have direct consequences to current 13C metabolic imaging using infusion of hyperpolarized [1-13C]pyruvate [5]. The fast exchange reactions Lac ↔ Pyr catalyzed by LDH and HCO3− ↔ CO2 by CA can obviously be measured with high spatial resolution using hyperpolarized 13C imaging combined with inversion transfer [6] or exchange spectroscopy (EXSY) methods [7].
13C MRS offers high spectral separation, thereby allowing clear discrimination of many metabolite resonances even at low magnetic field strength. Conventional 13C MRS, which derives signals from low gyromagnetic ratio Zeeman energy difference at room temperature, suffers from inherently low sensitivity. To overcome the low sensitivity of conventional 13C MRS, we used large tissue volumes for detection of the relatively small magnetization transfer effect (compared to that of the creatine kinase reaction in 31P MRS) in our previous work. In the case of the CA-catalyzed bicarbonate-carbon dioxide exchange, magnetization transfer effect is expected to be undetectable if direct proton saturation and detection is employed because the labile proton of bicarbonate is not observable in proton MRS. The magnitude of magnetization transfer is expected to reduce in the cases of AAT, LDH and MDH reactions if direct proton saturation and detection is used. This is because the relatively fast T1 relaxation processes of Glu, Asp and Lac protons which compete with the population difference caused by saturation of α-KG, OAA, Asp or Pyr protons. The crowded proton spectrum may also make it difficult to avoid RF spillover effect unless extreme care is exercised [8–10]. These factors may explain why magnetization transfer effect of AAT, LDH and MDH reactions has escaped detection although the offset-dependent magnetization transfer effect between tissue water and metabolites has been investigated extensively using proton MRS [11,12].
Significant improvement of this situation can be achieved by combining 13C magnetization transfer with high sensitivity 1H detection. The protons bound to 13C atoms can be detected by various techniques. There are three efficient ways to transfer 13C equilibrium magnetization to 1H using the one-bond J coupling between 13C and its proton(s) 1JCH: the insensitive nuclei enhanced by polarization transfer (INEPT) [13,14], the distortionless enhancement by polarization transfer (DEPT) [15] and heteronuclear Hartmann-Hahn transfer [16,17] methods. Note that techniques such as proton-observed-carbon-edited (POCE) [18], heteronuclear multiple-quantum coherence (HMQC) [19], or heteronuclear single-quantum coherence (HSQC) [20] spectroscopy methods should not be used because they detect equilibrium magnetization of 1H instead of 13C. Unlike DEPT or heteronuclear Hartmann-Hahn transfer techniques, INEPT forms intermediate longitudinal two-spin order states, which allow water and outer volume suppression (OVS) to be conveniently inserted into the sequence after creation of the longitudinal two-spin order. Here we developed an INEPT-based inverse 13C-to-1H heteronuclear polarization transfer technique for spatially localized detection of 13C magnetization transfer effect of AAT and LDH reactions in vivo. The long 13C T1 maximizes magnetization transfer and the wide 13C chemical shift dispersion minimizes RF spillover during saturation. In the meanwhile, the high sensitivity of proton detection allows detection of the enzyme reactions in a 72.5 μL localized volume in the rat brain. The Glu+Gln (Glx) H2 methine proton at 3.75–3.76 ppm was used for detecting the 13C magnetization transfer effect of the AAT reaction. Because of the close proximity between Lac H2 at 4.11 ppm and water as well as its quartet resonance structure, the more sensitive Lac H3 methyl protons was used for detection of the 13C magnetization transfer effect of the LDH reaction in this study.
Materials and Methods
MR Hardware
All experiments were performed on a Bruker microimaging spectrometer (Bruker Biospin, Billerica, MA) interfaced to an 11.7 Tesla 89-mm bore vertical magnet (Magnex Scientific, Abingdon, UK). The magnet is equipped with a 57-mm inner diameter gradient (Mini0.5, Bruker Biospin, Billerica, MA, with a maximum gradient strength of 3.0 G/mm and a rise time of 100 μs). The RF probe/animal handling system for the 13C-to-1H heteronuclear polarization transfer spectroscopy experiments of rat brain using the vertical 89-mm bore magnet have been described recently [21]. Briefly, the inner circular loop is the 1H coil with an inner diameter of 15 mm and a conductor width of 2.5 mm. The outer rectangular loop is the 13C coil with an inner width, length and conductor width of 22.6, 25.0 and 2.5 mm, respectively. The integrated RF coils/head holder system is capable of rat head fixation (with ear pins and a bite bar), body support, physiology maintenance, coil tuning and RF shielding. The structure of RF probe/animal handling system for 13C detection experiment was similar to that of the 1H detection system except that the 13C coil has an inner diameter and conductor width of 11.1 mm and 2.8 mm, respectively. The 1H coil has an inner diameter and conductor width of 24.2 mm and 3.2 mm, respectively.
Animal Preparation
Sixteen male adult Sprague-Dawley rats (body weight: 161–223 g) were studied according to procedures approved by the National Institute of Mental Health Animal Care and Use Committee. Eight rats were used in the measurements of the 13C magnetization transfer effects of ATT and LDH reactions by the INEPT-based inverse 13C-to-1H heteronuclear polarization transfer technique (four rats for the AAT reaction, four rats for the LDH reaction). The other eight rats were used to measure the same reactions using the direct 13C detection method (four rats for the AAT reaction, four rats for the LDH reaction). All rats were orally intubated and ventilated with a mixture of 70% N2O/30% O2 and 1.5% isoflurane. One femoral vein was cannulated for the intravenous infusion of 13C-labeled glucose ([1,6-13C2]glucose or [1-13C]glucose) (99% enriched, Cambridge Isotope Laboratories, Inc, Andover, MA). In the case of measuring the LDH reaction, a second femoral vein was cannulated for administration of bicuculline (Sigma-Aldrich Co., St Louis, MO). One artery was cannulated for intermittent sampling of arterial blood for measurement of plasma gases and glucose concentrations using a blood analyzer (Bayer Rapidlab 860, East Walpole, MA). After surgery, anesthesia was maintained using 1.5 % isoflurane. The coils were positioned approximately 0–1 mm posterior to bregma based on separate position calibrations. Plasma glucose level was rapidly raised to and maintained at 18.3 ± 5.3 mM. For detecting the LDH reaction, bicuculline (1 mg/kg) was injected 15 min after the start of [1,6-13C2]glucose or [1-13C]glucose infusion. Additional bicuculline (0.5 mg/kg per injection) was administered to maintain elevated brain lactate level during data acquisition. Rectal temperature was monitored and maintained at 37.5 ± 0.5 °C using an external pump for heat exchange by water circulation (BayVoltex, Modesto, CA, USA). During the AAT reaction measurement, the physiological variables were approximately maintained as following: arterial blood pO2 >120 mm Hg and pCO2 20 – 54 mm Hg, mean blood pressure 100 – 169 mm Hg, and pH 7.10–7.36. During the LDH reaction measurement, the physiological variables were similar to our previous study using [2-13C]glucose [2]. Bicuculline treatment led to a rapid increase in mean arterial blood pressure from 122–160 mm Hg (baseline) to 155–197 mm Hg and a rapid decrease in mean heart rate from 416–478 beats per min (baseline) to 350–430 beats per min. Both mean arterial blood pressure and heart rate returned to the baseline values ~ 4 min after bicuculline administration. Blood gasses were maintained within approximately normal physiology limits (pCO2 = 22–57 mm Hg, pO2 > 120 mm Hg) with few exceptions. Blood pH was found to be in the range of 6.92–7.35 due to nonrespiratory plasma acidosis caused by bicuculline-induced seizure activities [22].
Pulse Sequence Design and In Vivo MRS
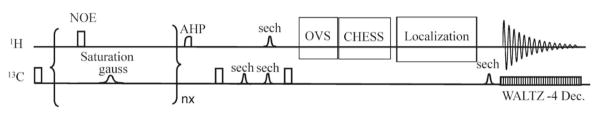
Three-slice (coronal, horizontal, and sagittal) scout rapid acquisition with relaxation enhancement (RARE) images (FOV = 2.5 cm, slice thickness = 1 mm, TR/TE = 200/15 ms, rare factor = 8, 128 × 128 data matrix) were acquired for positioning the RF coils/head holder systems such that the gradient isocenter approximately coincides with the coil center along the z direction. The rat brain was shimmed using automatic shimming methods described earlier [23]. The pulse sequence for 1H detection of the 13C magnetization transfer effect was shown in Fig. 1. The spectroscopy voxel (5 × 2.9 × 5 mm3) was placed along the brain midline in the neocortex. For localized proton detection of the LDH reaction, the pulse sequence uses spectrally selective RF saturation of Pyr C3 at 29.08 ppm by a continuous train of 2 ms nominally 180° Gaussian pulses in the 13C channel and a simultaneous train of nominally 180° 200 μs rectangular pulses spaced 200 ms apart in the 1H channel for spectrally nonselective pre-saturation of thermal equilibrium 1H signals to generate heteronuclear nuclear Overhauser enhancement (NOE) and to suppress water. The duration of the simultaneous spectrally nonselective 1H and spectrally selective 13C saturation was 9 sec. At the end of the saturation pulse trains, an optional 500 μs adiabatic half passage (AHP) pulse and crusher gradients was used for further water suppression. Then the equilibrium Lac C3 signal at 20.76 ppm was transferred to proton using an inverse, mostly adiabatic 13C-to-1H INEPT-based polarization transfer technique. The INEPT-based inverse polarization transfer method was optimized on a phantom sample containing 10 mM [3-13C]lactate. As shown in Fig. 1, a nonadiabatic 90°x pulse was used for spectrally and spatially nonselective excitation of 13C equilibrium signals. A pair of identical hyperbolic secant pulses (sech, 2.5 ms, μ = 3, 1% truncation) were used to generate an adiabatic double spin echo with TE1 = 8.3 ms [24]. A 1.5-ms sech (μ = 5, 1% truncation) was also applied to the proton channel to reintroduce heteronuclear J evolution. After a nominal delay of , a nonadiabatic 200 μs 90°±y pulse was used to convert the antiphase (21H1z13Cy + 21H2z13Cy + 21H3z13Cy) terms into longitudinal two-spin orders (21H1z13Cz + 21H2z13Cz + 21H3z13Cz). Phase cycling at the second nonadiabatic pulse facilitates cancellation of unwanted proton signals. Immediately after creation of 21Hz13Cz + 21H2z13Cz + 21H3z13Cz, proton outer volume suppression (OVS) using six nominal 90° sech pulses (2 ms, μ = 5, 1% truncation) along the x (10 mm slab), −x (10 mm slab), y (3 mm slab), −y (5 mm slab), z (10 mm slab), −z (10 mm slab) directions plus crusher gradients, and the chemical shift-selective (CHESS) water suppression (15 ms, Gaussian 90° pulse plus gradient crushers) were executed. Then, the single-shot, fully adiabatic proton localization sequence ([25, 26] and references therein) was used to convert longitudinal two-spin orders into antiphase transverse proton coherence and to achieve transverse spatial localization for selection of a 5 (x) × 2.9 (y) × 5 (z) mm3 (72.5 μL) voxel. A final hyperbolic secant pulse (2.5 ms, μ = 3, 1% truncation) was applied to the 13C channel to refocus heteronuclear J evolution [25, 26]. After a nominal delay of 1/21JCH, the in-phase transferred proton coherence is detected with simultaneous 13C decoupling using WALTZ4 (9.6 ms per cycle for a total duration of 192 ms, [18]) with carrier frequency of the decoupling pulses placed at Lac C3 at 20.8 ppm. TE2 = 22.6 ms. When control spectra were acquired, the saturating pulses was placed at an equal spectral distance from the observed spin but on the opposite side of Pyr C3. The saturated and control spectra were interleaved using scheme: (control-saturated)NA to minimize slow varying lactate concentration and motion artifacts. The pulse sequence used for detecting the 13C magnetization transfer effect of the AAT reaction in the proton channel was similar to that described above. It was optimized using a phantom sample containing 10 mM [2-13C]glutamate. Due to the large spectral separation between Glu C2 at 55.7 ppm and α-KG C2 at 206.0 ppm, a continuous wave pulse (CW, γB1sat = 158 Hz) was used for spectrally selective saturation of α-KG C2. TE1 = 10.2 ms with the nominal delay used for reintroducing heteronuclear J evolution in the 13C channel set to 1/41JCH. The same voxel size of 5 × 2.9 × 5 mm3 was employed. TE2 = 22.6 ms. The same WALTZ-4 decoupling sequence was used with decoupler frequency placed at Glu C2 at 55.7 ppm. The saturated and control spectra were also interleaved as in the case of the LDH reaction.
Fig. 1.
Pulse sequence for localized 1H detection of 13C magnetization transfer effect. For clarity, depiction of the gradient pulses was omitted. Details were given in the text. Briefly, for localized proton detection of the LDH reaction, Pyr C3 was irradiated using a continuous train of 2 ms nominally 180° Gaussian pulses and a simultaneous train of nominally 180° 200 μs rectangular pulses spaced 200 ms apart. At the end of the saturation pulse trains, an optional 500 μs adiabatic half passage (AHP) pulse and crusher gradients was used for further water suppression. Then a nonadiabatic 90°x pulse was used for excitation of 13C equilibrium signals. A pair of identical hyperbolic secant pulses (sech, 2.5 ms, μ = 3, 1% truncation) were used to generate an adiabatic double spin echo with an 13C echo time (TE1) of 8.3 ms. A 1.5-ms sech pulse was also applied to the proton channel to reintroduce heteronuclear J evolution. A nonadiabatic 200 μs 90°±y pulse was used to convert the antiphase terms into longitudinal two-spin orders. Then, proton outer volume suppression using six nominal 90° sech pulses (2 ms, μ = 5, 1% truncation) along the x (10 mm slab), −x (10 mm slab), y (3 mm slab), −y (5 mm slab), z (10 mm slab), −z (10 mm slab) directions plus crusher gradients, and CHESS water suppression (15 ms, Gaussian 90° pulse plus gradient crushers) were executed. Finally, the single-shot, fully adiabatic proton localization sequence was used to convert longitudinal two-spin orders into antiphase transverse proton coherence and to achieve transverse spatial localization for selection of a 5 (x) × 2.9 (y) × 5 (z) mm3 (72.5 μL) voxel. A hyperbolic secant pulse was applied to the 13C channel to refocus heteronuclear J evolution. After a nominal delay of 1/21JCH, the in-phase transferred proton coherence is detected with simultaneous 13C decoupling using WALTZ4. The 1H echo time TE2 = 22.6 ms.
The pulse sequences for direct 13C detection of the 13C magnetization transfer effect was similar to those described in our previous publications [1,2] and to the initial portion of the pulse sequence depicted in Fig. 1 for generation of heteronuclear NOE enhancement and 13C saturation transfer. The methyl carbon of Pyr C3 at 29.1 ppm and the carbonyl carbon of α-KG C2 at 206.03 ppm were selectively saturated using CW pulses with a nominal γB1sat of 79 and 158 Hz, respectively. When control spectra were acquired, the saturation pulse was placed at an equal spectral distance from the observed spin but on the opposite side of Pyr C3 or α-KG C2. The same AHP and WALTZ-4 pulses were used for excitation and subsequent proton decoupling, respectively. For determination of the Lac C3 signal in the control scan, the overlapping natural abundance background signals from subcutaneous lipids were subtracted prior to spectral quantification. All time domain spectral data were zerofilled to 16 K prior to Fourier transform.
Results
Fig. 2a shows the α-KG ↔ Glu 13C magnetization transfer spectra acquired using the localized (5 × 2.9 × 5 mm3) INEPT-based 13C-to-1H heteronuclear polarization transfer technique and [1,6-13C2]glucose infusion from one animal. Number of acquisition (NA) = 256. A total of NA × 2 interleaved scans were acquired. TR = 7.1 sec. Resolution-enhancing Lorentz-Gauss transformation (lb(exponential broadening factor) = −20, gb(Gaussian broadening factor) = 0.02) was applied before Fourier transform. Only zero order phase correction was necessary. In the symmetric control spectrum and spectrum acquired with saturation of α-KG C2 at 206.03 ppm, resonances from Glu H2 (3.75 ppm), Glu H4 (2.3–2.4 ppm) and residual water (4.65 ppm) were observed. The overlapping Gln H2 resonance at 3.76 ppm was also present because of the spectrally nonselective 200 μs 90° pulse employed for excitation of 13C resonances. The bandwidth of the rectangular 200 μs 90° 13C pulse covers a range of ±22.3 ppm at 11.7 Tesla in the 13C channel (The bandwidth is defined as the width of the excitation profile at 3 dB less than the maximum amplitude). Since the spectral separation between Glu C2 and Gln C2 is only 0.5 ppm, Gln C2, although it does not carry any saturation transfer effect due to the slow rate of glutamine synthetase, was fully excited and transferred to Glx H2 (Glx H2 = Glu H2 + Gln H2). In contrast, the spectral separation between Glu C2 and Glu C4 is 21.3 ppm. Only a very small portion of Glu C4 was excited and transferred to Glu H4 as expected and shown in Fig. 2a. The more distal Gln C4 at 31.9 ppm and Glx C3 are outside of the bandwidth of the 13C excitation pulse. Correspondingly, no Gln H4 (2.45 ppm) and Glx H3 (2.08–2.12 ppm) were detected in the proton spectrum shown in Fig. 2a. In Fig. 2a, the difference between the symmetric control spectrum and that with saturation of α-KG was shown in the bottom. The small Glu H4 signal was cancelled in the difference spectrum. Because α-KG C3 and C4 resonate at 31.40 and 36.66 ppm, which are far from the selectively saturated α-KG C2 resonance at 206.03 ppm, no magnetization transfer effect was expected for Glx H3 and Glx H4. The ratio of the intensity of Glu H2 in the difference spectrum to that of the Glx H2 signal in the control spectrum was determined to be 16 ± 1 % (mean ± SD, n = 4). Since difference between the fractional enrichment of Glu C2 and that of Gln C2 is small under our experimental condition [26], the ratio of the intensity of Glu H2 in the difference spectrum to that of the Glu H2 signal in the control spectrum can be calculated based on the known pool sizes of total Glu and Gln. Using previously determined total Glu and Gln concentration from perchloric acid extracts ([Glu] = 10.22 ± 0.30 μmol/g wet weight, [Gln] = 5.17 ± 0.31μmol/g wet weight) [26], (ΔM/Mn°sat)Glu was calculated to be 23 ± 2 %, corresponding to kGlu → α-KG = 0.12 ± 0.01 sec−1 for the pseudo first-order rate constant of the unidirectional Glu → α-KG flux. Fig. 2b shows the same 13C saturation transfer effect as shown in Fig. 2a. In Fig. 2b, the effect was measured using direct 13C detection with no spatial localization other than that provided by the 13C surface transceiver coil. Infusion of [1-13C]glucose was used. TR = 9.4 sec. A total of 256 × 2 scans were acquired per rat. The spectra shown in Fig. 2b were summed from four rats. The difference spectrum showed significant intensity changes in Glu C2 at 55.7 ppm only. The percent change in Glu C2 signal intensity due to 13C saturation transfer effect was found to be 22 ± 1 % (mean ± SD, n = 4), corresponding to kGlu → α-KG = 0.12 ± 0.01 sec−1. Note that a strong resolution-enhancing window function (lb= −30, gb = 0.2) was used in Fig 2b to resolve Glu C2 at 55.7 ppm from the overlapping Gln C2 at 55.2 ppm.
Fig. 2.
(a) The α-KG ↔ Glu 13C magnetization transfer spectra acquired using the localized (5 × 2.9 × 5 mm3) INEPT-based 13C-to-1H heteronuclear polarization transfer technique and [1,6-13C2]glucose infusion from one animal. A total of 256 × 2 interleaved scans were acquired. TR = 7.1 sec. lb = −20, gb = 0.02. In the upper and middle traces, resonances from Glx H2 (Glu H2 + Gln H2) (3.75–3.76 ppm), Glu H4 (2.3–2.4 ppm) and residual water (4.65 ppm) were observed. Note that the small Glu H4 signal was cancelled in the difference spectrum. (b) The same 13C saturation transfer effect as shown in (a) except that the effect was measured using direct 13C detection and infusion of [1-13C]glucose. TR = 9.4 sec. A total of 256 × 2 scans were acquired per rat. lb= −30, gb = 0.2. The spectra were summed from four rats. The difference spectrum showed significant intensity changes in Glu C2 at 55.7 ppm only.
The 13C magnetization transfer effect due to LDH-catalyzed exchange between Pyr and Lac in vivo during bicuculline-induced seizure in the rat brains is as follows: Fig. 3a shows the 1H results obtained after infusion of [1,6-13C2]glucose from the localized spectroscopy voxel (5 × 2.9 × 5 mm3) in rat brain using the INEPT-based inverse 13C-to-1H heteronuclear polarization transfer technique. TR = 9.2 sec. Total number of acquisitions was 384 × 2. Resolution-enhancing Lorentz-Gauss transformation (lb = −30, gb = 0.02) was applied before Fourier transform. In the control spectrum and spectrum acquired with saturation of Pyr C3 at 29.08 ppm, both Lac H3 resonance at 1.32 ppm and alanine (Ala) H3 signal at 1.47 ppm were observed. Again, this is because the use of spectrally nonselective excitation in the 13C channel, the close proximity between the resonance frequencies of Lac C3 (20.8 ppm) and Ala C3 (16.8 ppm), and elevation of Ala during bicuculline-induced seizure activities [2, 22, 27]. In the difference spectrum, the intensity change of the Lac H3 signal was found to be 16 ± 4 % (mean ± SD, n = 4). The T1 of Lac C3 was determined to be 5.6 ± 0.4 sec in a separate experiment (data not shown). The pseudo first-order rate constant for the unidirectional Lac → Pyr flux was calculated to be 0.054 ± 0.004 sec−1 according to procedures described in [2]. Cancellation of the elevated Ala signal at 1.47 ppm in the difference spectrum was incomplete due to partial saturation of the Ala C3 signal at 16.8 ppm during the control experiment with the irradiation frequency placed at 12.4 ppm. Note that the rate of alanine aminotransferase reaction is too low in brain tissue to generate any detectable magnetization transfer effect in vivo as explained earlier [2, 28]. Fig. 3b showed the same 13C saturation transfer effect measured using direct 13C detection with no spatial localization other than that provided by the 13C surface transceiver coil (TR = 8.9 sec, NA = 64 × 2, lb = −10, gb = 0.03, [1-13C]glucose infusion, summed from four rats). The percent change in Lac C3 signal intensity at 20.8 ppm due to saturation of Pyr C3 at 29.1 ppm was found to be 23 ± 4 % (mean ± SD, n = 4), corresponding to k Lac → Pyr = 0.09 ± 0.01 sec−1. The relative standard deviations of the reported percent change are in agreement with the predicted values using eq. [28] given by Alger and Shulman [29].
Fig. 3.

(a) The 1H results obtained after infusion of [1,6-13C2]glucose from the localized spectroscopy voxel (5 × 2.9 × 5 mm3) in rat brain using the INEPT-based inverse 13C-to-1H heteronuclear polarization transfer technique. TR = 9.2 sec. lb = −30, gb = 0.02. Total number of acquisitions was 384 × 2. In the control spectrum and spectrum acquired with saturation of Pyr C3 at 29.08 ppm, both Lac H3 resonance at 1.32 ppm and alanine Ala H3 signal at 1.47 ppm were observed. Cancellation of the elevated Ala signal at 1.47 ppm in the difference spectrum was incomplete due to partial saturation of the Ala C3 at 16.8 ppm signal during the control experiment with the irradiation frequency placed at 12.4 ppm. (b) The same 13C saturation transfer effect measured using direct 13C detection with no spatial localization other than that provided by the 13C surface transceiver coil (TR = 8.9 sec, NA = 64 × 2, lb = −10, gb = 0.03, [1-13C]glucose infusion, summed from four rats)
Discussion
Although 1H-to-13C polarization transfer methods have been extensively used for studying in vivo metabolism of glucose and other substrates (e.g., [30,31]), the inverse polarization transfer approach has not found in vivo applications until now. As in vivo magnetization transfer effects of more enzymes are found, which provide valuable information on specific enzymatic processes in tissue, it is imperative to evaluate the feasibility of combining 13C magnetization transfer preparation and 1H detection for characterizing these enzyme reactions in vivo. As shown by our results, the 13C magnetization transfer effect of the AAT and LDH reactions was successfully demonstrated in the proton spectra acquired from the rat brain in vivo using the INEPT-based 13C-to-1H polarization transfer technique which combines 13C saturation transfer and proton detection.
The large chemical shift dispersion (>18000 Hz at 11.7 T) between the α-carbons of amino acids and the carbonyl carbons of their cognate α-keto acids provides an ideal situation for saturation transfer experiments without any significant interference from the “RF spillover” effects between the saturated spins and the observed spins. In the carbon-13 magnetization transfer effect of rapid AAT reaction study, the chemical shift difference between α-KG C3 and Glu C3 is only 3.4 ppm. It is therefore more convenient to saturate the carbonyl carbon of α-KG at 206.03 ppm and allow magnetization (saturation) to transfer to the α-C of Glu at 55.7 ppm. The signal intensity change of the Glu H2 was calculated to be 23 ± 2 % in rats using the 13C-to-1H polarization transfer technique, in quantitative agreement with determination based on direct 13C detection of the Glu C2 (22 ± 1 %, Fig 2b) and with that reported previously using [1,6-13C2]glucose infusion and α-chloralose anesthesia [1]. Nonetheless, the presence of the overlapping Gln H2, although it carries no 13C saturation transfer information and is cancelled in the difference spectra, complicates quantification by affecting the direct measurement of total Glu H2 in Fig. 2. Due to significantly increased sensitivity and the advantage of spatial localization in the proton channel, which results in much smaller localization error because of its narrow chemical shift range, inconvenience due to the presence of the inert Gln H2 resonance therefore seems to be a small price to pay.
The detection of 13C magnetization transfer effect using Lac H3 is less straightforward, however. Since the signal of Lac H2 distributes among four resonance lines and it is very close to water, it is therefore impractical to use the Pyr C2 → Lac C2 → Lac H2 pathway. Instead, Pyr C3 was saturated for detection of 13C magnetization transfer of the LDH reaction using the methyl protons of Lac H3 as the reporter signal. The chemical shift separation between Pyr C3 and Lac C3 is only 8.3 ppm. More importantly, Pyr C3 is also a quartet due to its one-bond scalar coupling to the three methyl protons of Pyr, making it difficult to attain spectrally selective RF saturation. The presence of Ala C3 at 16.8 ppm further complicates the situation because it was partially saturated by the control saturation pulse placed at 12.4 ppm. As Ala C3 is within the bandwidth of the 200 μs 90° 13C excitation pulse centered at the frequency of Lac C3, Ala H3 was also observed in the proton spectra as shown in Fig. 3a. The k Lac → Pyr determined using the inverse 13C-to-1H polarization transfer method is significantly smaller than those determined using saturation of Pyr C2 at 207.9 ppm. This discrepancy could be due to incomplete saturation of the Pyr C3 quartet by using the train of 2-ms nominal 180° Gaussian pulses, leading to an underestimate of k Lac → Pyr. In addition to the Lac methyl signal at 1.32 ppm, a small Ala H3 methyl signal at 1.47 ppm was also detected. Based on our previous investigation on the LDH reaction [2], no significant signal change in Ala signal was observed in bicuculline-treated rats. Because the Ala C3 signal at 16.8 ppm is closer to the saturating pulse placed at 12.4 ppm during the control scans, a small negative signal of Ala was also detected in the difference spectrum in this study which may have also contributed to the apparent underestimation of k Lac → Pyr. On the other hand, the percent change in Lac C3 signal due to the magnetization transfer effect from saturating Pyr C3 was 23 ± 4 % (mean ± SD) based on direct 13C detection. The kLac → Pyr determined using the direct 13C MRS method (k Lac → Pyr = 0.09 ± 0.01 sec−1) agrees well with our previous measurement using [2-13C]glucose infusion and saturation of Pyr C2 [2]. This is explained in the following: In the direct 13C MRS method, a nominal γB1sat of 158 Hz was used for saturation of the Pyr C3 quartet. Unlike the Gaussian pulses, the width of the frequency response of the CW pulse has a strong dependence on B1 inhomogeneity. This dependence therefore allows more complete saturation of the Pyr C3 quartet in tissue volume closer to the coil. It should be pointed out, nonetheless, that [1,2-13C2]glucose infusion and a 13C-13C homonuclear polarization transfer process, commonly used in multidimensional high-resolution NMR spectroscopy studies of fully 13C-labeled proteins, could be employed to enable the Pyr C2 → Lac C2 → Lac C3 → Lac H3 pathway to overcome the difficulties in spectrally selective saturation of the Pyr C3 quartet as described above.
Finally, it should be noted that the α-H of Glu is also in exchange with water. This exchange, however, is very inefficient [32]. Based on previous deuterium exchange experiment [33], the rate of this proton exchange reaction is orders of magnitude slower than the unidirectional pseudo first-order rate constant kGlu → α-KG catalyzed by AAT. Therefore, basically no loss of Glu H2 to water is expected due to the rapid signal detection after conversion of Glu C2 to Glu H2 using the inverse polarization transfer method. The exchange of the α-H of Lac with water is similarly slow. The methyl protons of Lac, which was used as the reporter signal of the 13C magnetization transfer effect in this study, are considered to be non-labile protons. In summary, the exchange of protons of Glu and Lac with water can be safely ignored when using inverse 13C-1H polarization transfer method for detecting 13C magnetization transfer effect using proton reporter signals.
In conclusion, we have demonstrated the feasibility of detecting the 13C magnetization transfer effects caused by the rapid exchanges between α-KG and Glu catalyzed by AAT and between Pyr and Lac catalyzed by LDH in vivo in the proton spectra using the localized INEPT-based 13C-to-1H heteronuclear polarization transfer technique. Further extension of this strategy using inverse 13C-to-1H DEPT or Hartmann-Hahn transfer is also feasible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shen J. In vivo carbon-13 magnetization transfer effect. Detection of aspartate aminotransferase reaction. Magn Reson Med. 2005;54:1321–1326. doi: 10.1002/mrm.20709. [DOI] [PubMed] [Google Scholar]
- 2.Xu S, Yang J, Shen J. In vivo13C Saturation transfer effect of lactate dehydrogenase reaction. Magn Reson Med. 2007;57:258–264. doi: 10.1002/mrm.21137. [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Shen J. Relayed 13C magnetization transfer: Detection of malate dehydrogenase in vivo. J Magn Reson. 2007;184:344–349. doi: 10.1016/j.jmr.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J, Singh S, Shen J. In vivo detection of the rate of cerebral carbonic anhydrase reaction. Book of abstracts: Fifteenth Annual Meeting of the International Society of Magnetic Resonance in Medicine; Berkeley, CA: ISMRM; 2007. p. 3120. [Google Scholar]
- 5.Golman K, in ‘t Zandt R, Thaning M. Real-time metabolic imaging. Proc Natl Acad Sci USA. 2006;103:11270–11275. doi: 10.1073/pnas.0601319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh PS, Balaban RS. Saturation and inversion transfer studies of creatine kinase kinetics in rabbit skeletal muscle in vivo. Magn Reson Med. 1988;7:56–64. doi: 10.1002/mrm.1910070107. [DOI] [PubMed] [Google Scholar]
- 7.Kuchel PW, Bulliman BT, Chapman BE. Mutarotase equilibrium exchange kinetics studied by 13C-NMR. Biophys Chem. 1988;32:89–95. doi: 10.1016/0301-4622(88)85037-3. [DOI] [PubMed] [Google Scholar]
- 8.Kingsley PB, Monahan WG. Effects of off-resonance irradiation, cross-relaxation, and chemical exchange on steady-state magnetization and effective spin-lattice relaxation times. J Magn Reson. 2000;143:360–375. doi: 10.1006/jmre.2000.2018. [DOI] [PubMed] [Google Scholar]
- 9.Kingsley PB, Monahan WG. Corrections for off-resonance effects and incomplete saturation in conventional (two-site) saturation-transfer kinetic measurements. Magn Reson Med. 2000;43:810–819. doi: 10.1002/1522-2594(200006)43:6<810::aid-mrm6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Kingsley PB, Monahan WG. Correcting for incomplete saturation and off-resonance effects in multiple-site saturation-transfer kinetic measurements. J Magn Reson. 2000;146:100–109. doi: 10.1006/jmre.2000.2124. [DOI] [PubMed] [Google Scholar]
- 11.de Graaf RA, Kranenburg A, Nicolay K. Off-resonance metabolite magnetization transfer measurements on rat brain in situ. Magn Reson Med. 2000;41:1136–1144. doi: 10.1002/(sici)1522-2594(199906)41:6<1136::aid-mrm9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 12.Swanson SD. Protein mediated magnetic coupling between lactate and water protons. J Magn Reson. 1998;135:248–255. doi: 10.1006/jmre.1998.1535. [DOI] [PubMed] [Google Scholar]
- 13.Morris GA, Freeman R. Enhancement of nuclear magnetic resonance signals by polarization transfer. J Am Chem Soc. 1979;101:760–762. [Google Scholar]
- 14.Li S, Chen Z, Zhang Y, Lizak M, Bacher J, Innis RB, Shen J. In vivo single-shot, proton-localized 13C MRS of rhesus monkey brain. NMR Biomed. 2005;18:560–569. doi: 10.1002/nbm.993. [DOI] [PubMed] [Google Scholar]
- 15.Doddrell DM, Pegg DT, Bendall MR. Distortionless enhancement of NMR signals by polarization transfer. J Magn Reson. 1982;48:323–327. [Google Scholar]
- 16.Artemov D, Bhujwalla ZM, Glickson JD. Band-selective heteronuclear cross polarization in liquids. J Magn Reson B. 1995;107:286–288. doi: 10.1006/jmrb.1995.1091. [DOI] [PubMed] [Google Scholar]
- 17.Shen J. Doubly selective heteronuclear J cross polarization and its applications to measurement of long-range 1H-13C coupling constants. J Magn Reson A. 1996;119:101–104. [Google Scholar]
- 18.Yang J, Li CQ, Shen J. In vivo detection of cortical GABA turnover from intravenously infused [1-13C]D-glucose. Magn Reson Med. 2005;53:1258–1267. doi: 10.1002/mrm.20473. [DOI] [PubMed] [Google Scholar]
- 19.Müller L. Sensitivity enhanced detection of weak nuclei using heteronuclear multiple quantum coherence. J Am Chem Soc. 1979;101:4481–4484. [Google Scholar]
- 20.Bodenhausen G, Ruben DJ. Natural abundance nitrogen-15 NMR by enhanced heteronuclear spectroscopy. Chem Phys Lett. 1980;69:185–189. [Google Scholar]
- 21.Li S, Shen J. Integrated RF probe for in vivo multinuclear spectroscopy and functional imaging of rat brain using an 11.7 Tesla 89-mm bore vertical microimager. MAGMA. 2005;18:119–127. doi: 10.1007/s10334-005-0103-y. [DOI] [PubMed] [Google Scholar]
- 22.Chapman AG, Meldrum BS, Siesjo BK. Cerebral metabolic changes during prolonged epileptic seizures in rats. J Neurochem. 1977;28:1025–1035. doi: 10.1111/j.1471-4159.1977.tb10665.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Li SS, Yang J, Letizia D, Shen J. Measurement and automatic correction of high order B0 inhomogeneity in the rat brain at 11.7 Tesla. Magn Reson Imaging. 2004;22:835–842. doi: 10.1016/j.mri.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 24.Conolly S, Glover G, Nishimura D, Macovski A. A reduced power selective adiabatic spin-echo pulse sequence. Magn Reson Med. 1991;18:28–38. doi: 10.1002/mrm.1910180105. [DOI] [PubMed] [Google Scholar]
- 25.Shen J, Yang J, Li SS, Xu S. In vivo spectroscopy at 11.7 Tesla, MAGMA. 2004;16(Suppl 1):S53. [Google Scholar]
- 26.Yang J, Shen J. In vivo evidence for reduced cortical glutamate-glutamine cycling in rats treated with the antidepressant/antipanic drug phenelzine. Neuroscience. 2005;135:927–937. doi: 10.1016/j.neuroscience.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 27.Siesjo BK. Brain Energy Metabolism. John Wiley & Sons; Chichester: 1977. [Google Scholar]
- 28.Benuck M, Stern F, Lajtha A. Transamination of amino acids in homogenates of rat brain. J Neurochem. 1971;18:1555–1567. doi: 10.1111/j.1471-4159.1971.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 29.Alger JR, Shulman RG. NMR studies of enzymatic rates in vitro and in vivo by magnetization transfer. Q Rev Biophys. 1984;17:83–124. doi: 10.1017/s0033583500005266. [DOI] [PubMed] [Google Scholar]
- 30.Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, Petroff OAC, Shulman GI, Robert G, Shulman RG, Rothman DL. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo13C NMR. Proc Natl Acad Sci U S A. 1999;96:8235–8240. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu S, Shen J. In vivo dynamic turnover of cerebral 13C isotopomers from [U-13C]glucose. J Magn Reson. 2006;182:221–228. doi: 10.1016/j.jmr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Arnone A, Christen P, Jansonius JN, Metzler DE. Hypothetical mechanism of action of aspartate aminotransferases. In: Christen P, Metzler DE, editors. Transaminases. New York: John Wiley & Sons, Inc; 1985. pp. 326–362. [Google Scholar]
- 33.Moldes M, Cerdan S, Erhard P, Seelig J. 1H-2H exchange in the perfused rat liver metabolizing [3-13C]alanine and 2H2O as detected by multinuclear NMR spectroscopy. NMR Biomed. 1994;7:249–262. doi: 10.1002/nbm.1940070602. [DOI] [PubMed] [Google Scholar]