Abstract
Objective
Autosomal dominant parkinsonism, hypoventilation, depression and severe weight loss (Perry syndrome) is an early-onset rapidly progressive disease. At autopsy, previous studies have found severe neuronal loss in the substantia nigra without Lewy bodies. Transactive response DNA-binding protein of 43 kDa (TDP-43) has recently been identified as a major ubiquitinated constituent of neuronal and glial inclusions in frontotemporal lobar degeneration with ubiquitin-positive inclusions and in amyotrophic lateral sclerosis. This study reports clinical, genetic and neuropathologic investigations of Perry syndrome.
Methods
Clinical data and autopsy brain tissue samples were collected from eight patients from four genealogically unrelated kindreds with Perry syndrome. Brain tissue was studied with immunohistochemistry and biochemistry for TDP-43. Patients were screened for mutations in the progranulin (GRN) and TDP-43 (TARDBP) genes.
Results
The mean age at onset was 47 years (range: 40-56), and the mean age at death was 52 years (range: 44-64). In all patients, we identified TDP-43-positive neuronal inclusions, dystrophic neurites and axonal spheroids in a predominantly pallidonigral distribution, and we demonstrated changes in solubility and electrophoretic mobility of TDP-43 in brain tissue. The inclusions were highly pleomorphic and predominated in the extrapyramidal system, sparing the cortex, hippocampus and motor neurons. There were no mutations in GRN or TARDBP.
Interpretation
Perry syndrome displays unique TDP-43 pathology that is selective for the extrapyramidal system and spares the neocortex and motor neurons.
Keywords: autosomal dominant, axonal dystrophy, neuronal cytoplasmic inclusions, pallidonigral, parkinsonism, Perry syndrome, TARDBP, TDP-43
Introduction
Perry syndrome is characterized by rapidly progressive and fatal autosomal dominant parkinsonism, central hypoventilation, depression and severe weight loss [1]. Seven families have been described so far including the original Canadian family reported by Perry et al. [2-10]. The mean age of onset is 46 years (range: 30-56 years) and the mean disease duration is five years (range: 2-10 years). Most patients die from hypoventilation/respiratory complications, some unexpectedly at night, and some by suicide. Previous autopsy studies have shown severe neuronal loss in the substantia nigra (SN) and in the locus ceruleus, with few or more often no neuronal cytoplasmic inclusions with conventional staining. Recently, selective loss of putative respiratory neurons in the ventrolateral medulla and dorsal raphe nucleus was reported, which may represent the pathologic substrate of central hypoventilation [11]. Clinicopathological correlation has been poor so far, in that 1) the severity of SN neuronal loss outweighs the relatively mild parkinsonism observed in patients, and 2) no pathological lesion has been identified that would convincingly account for severe weight loss or depression.
Transactive response DNA-binding protein of 43 kDa (TDP-43), a nuclear protein implicated in transcription regulation and exon skipping, was recently identified as a major ubiquitinated protein constituent of neuronal inclusions found in sporadic and familial forms of frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U) with or without motor neuron disease (MND), and amyotrophic lateral sclerosis (ALS) [12-15].
Herein, we describe TDP-43-positive inclusions in eight patients with Perry syndrome from four genealogically unrelated families. Morphologically, the inclusions are comparable to those found in FTLD-U and ALS. In Perry syndrome, TDP-43 pathology has a distinct distribution that predominantly affects the extrapyramidal system, but spares the cortex, hippocampus and motor neurons.
Material and methods
Clinical and pathologic material for this study was collected through the international consortium established by Z.K.W. in 2001 [16]. The study was approved by the Institutional Review Board of the Mayo Clinic under the auspices of the Morris K. Udall Center for Excellence in Parkinson Disease Research, and by each individual Institutional Review Board through established collaborations.
Patients were examined by experienced movement disorders neurologists from France (three patients), Japan (two patients), the United States (two patients), and Canada (one patient). All patients were members of previously published families (French, Canadian, Japanese and West Virginia families) [2,3,5,6,8,10], and the neuropathology of one Japanese patient (from the same Japanese family) was reported recently [11]. Patient 1 from West Virginia and the Canadian patient have not been reported before (patients WV 1 and CA in Table 1).
Table 1.
Demographic and clinical data
| FR 1 | FR 2 | FR 3 | WV 1* | WV 2 | CA* | JP 1 | JP 2 | |
|---|---|---|---|---|---|---|---|---|
| Gender | M | F | F | M | F | M | M | M |
| AOO | 55 | 45 | 56 | 41 | 45 | 51 | 41 | 43 |
| AAD | 61 | 53 | 64 | 44 | 48 | 54 | 46 | 49 |
| Parkinsonism | (+) | + | + | + | + | (+) | + | + |
| Bradykinesia | + | + | + | + | Nr | - | + | + |
| T/RT | +/- | +/- | -/+ | +/- | Nr | +/+ | +/+ | +/+ |
| Rigidity | + | + | + | + | + | - | + | + |
| Hypo/resp | -/-1 | -/+ | -/- | -/+ | -/-2 | -3/+ | +/+ | +/+ |
| Weight loss | Nr | + | Nr | Nr | - | + | + | + |
| Depression | + | + | (+)4 | - | + | (+)4 | + | + |
| LD response | NA | - | - | (+) | + | NA | (+) | + |
Legend:
patients who were not reported before (the other patients were reported previously in [5, 6, 8, 10, 11] and are discussed and summarized in [1]).
died of respiratory insufficiency with pneumonia.
sudden and unexplained death.
severe central apnea, nocturnal tachypnea, mild hypoventilation.
apathy.
+, present. (+) mild or transient. -, absent. AAD, age at death. AOO, age of onset. CA, Canada. F, female. FR, France. Hypo, documented central hypoventilation or severe hypoventilation. JP, Japan. LD, levodopa. M, male. NA, not applicable. Nr, not reported. Resp, respiratory symptoms. RT, rest tremor. T, unspecified tremor or postural tremor. WV, West Virginia.
Pathologic material included fixed and frozen brains (two patients, one from Canada and one from West Virginia), fixed tissue embedded in paraffin (five patients, three from France and two from Japan), and unstained tissue on glass slides (one patient from West Virginia). Neuropathologic evaluation of the two fixed and frozen brains followed a standardized dissection and staining protocol [17]. Histologic stains included hematoxylin and eosin and thioflavin-S fluorescent microscopy. Immunohistochemistry for tau (CP13, Peter Davies, Albert Einstein College of Medicine, Bronx, NY), α-synuclein (NACP; Mayo Clinic), ubiquitin and α-internexin (both antibodies from EnCor Biotechnology, Alachua, FL) and TDP-43 (ProteinTech Group, Chicago, IL) was performed as previously described [18]. For TDP-43 biochemistry, tissue was homogenized and subjected to sequential fractionation [12]; the urea fraction was loaded on Tris-HCl polyacrylamide gels and transferred to polyvinylidene difluoride membranes after electrophoresis. Membranes were probed with rabbit antibody to TDP-43 (1:1,000, Protein Tech Group, Chicago, IL), followed by anti–rabbit antibody conjugated to IRDYE-800 (1:10,000; LI-COR BioSciences, Lincoln, NE) [18]. Blots were imaged using the Odyssey Infrared Imager (Licor BioSciences, Lincoln, NE). Semi-quantitative values were obtained for each type of TDP-43-positive pathology (neuronal cytoplasmic inclusions = NCI, neuronal intranuclear inclusions = NII, glial cytoplasmic inclusions and axonal spheroids) using a four point scale (0, none; 1, at least 1; 2, more than 1 but sparse; 3, moderate-to-frequent).
DNA was extracted from blood (four patients not included in the present study, one from the French family, and three from three unrelated Japanese families) and frozen brain (two patients, CA and WV 1) using standard methods. All genes linked to Parkinson disease or atypical parkinsonism, including LRRK2, SNCA, PRKN, PINK1, DJ-1, ATP13A2, MAPT, SCA2 and SCA3, were examined for mutations and excluded (unpublished data). For this study, direct gene sequencing was performed for GRN and TARDBP on an ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA). TARDBP copy-number analyses was performed using TaqMan gene expression assays (Applied Biosystems) to exons 2, 4 and 6 of TARDBP and to exon 5 of PSEN2 as endogenous control (primers available on request). Real-time PCR with 25 ng genomic DNA as template was performed on an ABI 7900 using the TaqMan method according to standard procedures. All samples were run in triplicate. The FAM-fluorescent signal was analyzed using SDS2.2.2 software, and genomic copy number determined by relative quantification.
Results
Genealogical and clinical description
Genealogical studies performed on all seven reported kindreds with Perry syndrome (including the four of the present study) identified no ancestral relationship between them. A summary of clinical and pathological data on these families was recently published [1]. Here we report two new cases from the West Virginia and Canadian families (Table 1, WV 1 and CA). Briefly, the West Virginia family currently consists of seven patients (six previously reported) over three generations [10]. The original Canadian family described by Perry et al. in 1975 currently consists of 13 patients (10 previously reported) over four generations [2,3].
Of the eight patients included in the present study, there were five men and three women, with a mean age of onset of 47 years (range: 40-56), and a mean age at death of 52 years (range: 44-64) (Table 1).
All patients presented with mild to moderate parkinsonism consisting of variable combinations of bradykinesia, rest tremor or postural tremor, rigidity and axial signs. Depression or apathy was present in all but one patient, and severe weight loss was apparent in four patients. Respiratory symptoms were present in five patients, and severe central hypoventilation was documented in two of them. Of three patients free of respiratory symptoms during disease progression, one died of acute pneumonia with respiratory insufficiency and another died suddenly at night of unexplained cause.
The previously unreported Canadian patient (Table 1, CA) presented at age 53 with a two-year history of weight loss (16 kg), shortness of breath, fatigue, loss of energy, sleep disturbance and mild postural tremor. His father was Patient III-3 in the original Perry family [3]. He had mild micrographia, hypomimia, bilateral postural and kinetic hand tremor, altered postural reflex and reduced arm swing. Over the next year, apathy worsened, and he developed intermittent rest tremor, dysarthria, dysphagia and shortness of breath even when sitting. Two polysomnograms established severe central sleep apnea with tachypnea, but only mild nocturnal hypoventilation; however, during the second exam performed at age 53, the patient slept for less than four hours. He died unexpectedly at age 54.
The West Virginia patient (Table 1, WV 1) was 42 when evaluated for a one-year history of apathy and lethargy, tremor when using his hands, and sleep disturbance with sleep apnea treated with chronic positive airways pressure. He was the son of Patient II-7 from the West Virginia family [10]. He displayed reduced facial expression, upper limbs and neck rigidity, decreased arm swing and mild postural instability. Over the following year, he became uncomfortable around people, socially withdrawn, and he complained of feeling restless. He became more motionless and rigid, and only marginally benefited from carbidopa/levodopa 25/100 mg three times a day. Neuropsychological exam showed a frontal dysexecutive syndrome with decreased initiation and speed, severe perseveration and a memory retrieval deficit. A single polysomnography performed at age 43 failed to confirm hypoventilation or significant sleep apnea; however, the patient only slept three hours during the exam. He died at age 44.
Neuropathology
Examination was conducted on eight patients, including fixed and frozen brain samples (two patients, one from Canada and one from West Virginia), fixed tissue embedded in paraffin (five patients, three from France and two from Japan), and unstained tissue on glass slides (one patient from West Virginia)
Macroscopically, there was no significant atrophy of the hemispheres, median structures or deep white matter and nuclei (Figure 1A, B). There was depigmentation of the SN and locus ceruleus (arrow in Figure 1C, D, respectively).
Figure 1.
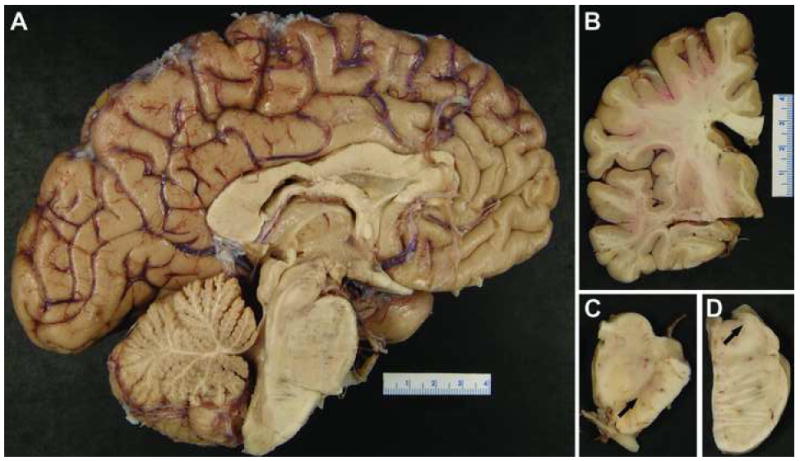
Macroscopic aspect of the brain of CA shows no significant abnormality on sagittal (A) and coronal (B) views, but marked depigmentation of the substantia nigra (C) and locus ceruleus (C) (arrows).
On histologic evaluation, there was severe neuronal loss and gliosis in the SN, with no Lewy bodies or neurofibrillary tangles. Some degree of neuronal loss was also present in the lentiform nucleus, hypothalamus, periaqueductal gray matter, locus ceruleus, dorsal raphe nucleus and brainstem reticular formation. Immunohistochemistry showed ubiquitin and TDP-43-positive neuronal inclusions, dystrophic neurites, glial cytoplasmic inclusions and axonal spheroids (Figure 2). The majority of abnormal TDP-43 immunoreactivity was in NCI (Figure 2A-C, E, F), but there were rare NII (Figure 2A, inset). NCI were morphologically pleomorphic with round, irregular, granular, rod-like and skein-like shapes (Figure 2A, B, B inset, C, E, F). Neurites were also detected, and they tended to be short lentiform processes, but were occasionally longer with thin and curvilinear profiles (Figure 2D, D inset). TDP-43 immunoreactive axonal spheroids, with a granular or foamy appearance, were common in the SN and globus pallidus. Scattered oval dense inclusions, presumably in astrocyte foot processes, were found abutting capillaries (Figure 2E, inset). The SN was most affected, followed by the globus pallidus and the substantia innominata (Table 2). TDP-43-immunoreactive inclusions were present in the inferior olivary nucleus, striatum, subthalamic nucleus and midbrain tegmentum; inconsistently found in the hippocampal dentate fascia, pontine and medullary tegmentum, hypothalamus, spinal cord gray matter and thalamus; negligible (with only a few dystrophic neurites) in the locus ceruleus, hippocampal pyramidal layer and spinal cord anterior horn; and absent in the frontal, temporal, parietal and entorhinal cortices, upper and lower motor neurons, amygdala, pontine base and hypoglossal nucleus (Table 2). The inclusions were negative for tau, α-synuclein and α-internexin, but positive for ubiquitin.
Figure 2.
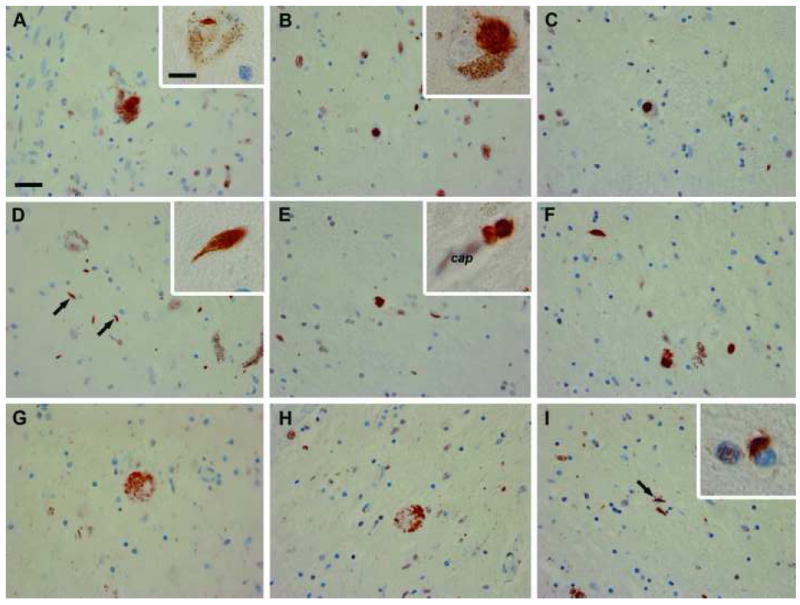
TDP-43 immunohistochemistry of the CA (A, D and G), WV 1 (B, E and H), and JP 1 (C, F and I) patients shows mesencephalic or substantia nigra NCI (A-C; higher magnification in inset of B), NII (inset in A), dystrophic neurites (D arrows, E and F; higher magnification in inset of D), axonal spheroids (G, H), glial cytoplasmic inclusions (I, arrow; higher magnification in inset of I), and a perivascular astrocytic inclusions (inset of E; cap= capillary). Scale bar: 25 μm (A-I), 10 μm (insets of A, B, D, E and I).
Table 2.
Distribution and severity of the TDP-43-positive inclusions
| NCI | DN | GCI | Spheroids | ||
|---|---|---|---|---|---|
| N | Pathologic score: Median (25%-, 75%-tile) | ||||
| Substantia nigra | 6 | 3 (3, 3) | 3 (3, 3) | 1 (0, 1) | 2 (2, 2) |
| Globus pallidus | 5 | 2 (1.5, 2) | 2 (1.75, 2) | 0 (0, 0) | 0 (0, 1) |
| Substantia innominata | 5 | 2 (1.5, 2) | 1 (1, 1) | 0 (0, 0) | -- |
| Subthalamic nucleus | 5 | 1 (0, 1) | 1 (1, 1) | 0 (0, 0) | -- |
| Midbrain tegmentum | 6 | 1 (0, 2) | 1 (0, 1) | 0 (0, 0) | -- |
| Inferior olivary nucleus | 4 | 1 (0, 2.5) | 1.5 (1, 2.5) | 0.5 (0, 1) | -- |
| Caudate/putamen | 5 | 1 (0, 1.25) | 1 (0, 1.5) | 0 (0, 0) | -- |
| Spinal cord gray matter | 2 | 0.5 (0, 1) | 0 (0, 0) | 0.5 (0, 1) | -- |
| Hypoglossal nucleus | 5 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | -- |
| Thalamus | 5 | 0 (0, 0.25) | 1 (0, 1) | 0 (0, 0) | -- |
| Pontine tegmentum | 7 | 0 (0, 1) | 1 (0.25, 1) | 0 (0, 0) | -- |
| Medullary tegmentum | 5 | 0 (0, 0.25) | 1 (0.75, 1) | 0 (0, 0) | -- |
| Temporal cortex | 5 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | -- |
| Spinal cord anterior horn | 2 | 0 (0, 0) | 0.5 (0, 1) | 0 (0, 0) | -- |
| Parietal cortex | 5 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | -- |
| Motor cortex | 2 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | -- |
| Locus ceruleus | 6 | 0 (0, 0) | 1 (1, 1) | 0 (0, 1) | -- |
| Hypothalamus | 3 | 0 (0, 0.75) | 1 (0.25, 1) | 0 (0, 0) | -- |
| Frontal cortex | 6 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | -- |
| Entorhinal cortex | 7 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | -- |
| Cerebellar dentate nucleus | 2 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | -- |
| Hippocampal dentate fascia | 7 | 0 (0, 0.75) | 0 (0, 0) | 0 (0, 0) | -- |
| Hippocampal pyramidal layer | 7 | 0 (0, 0) | 0 (0, 0.75) | 0 (0, 0) | -- |
| Pontine base | 7 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | -- |
| Amygdala | 5 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | -- |
Legend: The pathology was scored from 0 to 3 (0, none; 1, at least 1; 2, more than 1 but sparse; 3, moderate-to-frequent) for TDP-43 positive neuronal cytoplasmic inclusions (NCI), dystrophic neurites (DN), glial cytoplasmic inclusions (GCI) and axonal spheroids (only found in pallidonigral distribution). The number in the table is the median score of N available cases (25%-, 75%-tile) for each lesion type in each region.
In the CA and WV 1 patients, TDP-43 biochemistry showed the presence of pathologic hyperphosphorylated and truncated 25 kDa fragments in the urea fraction of the globus pallidus and SN, but not in the temporal cortex, in line with immunohistochemistry results (Figure 3). These pathologic forms of TDP-43 were similar to those found in the temporal cortex of FTLD-U and were absent in a neurologically normal control brain (Figure 3).
Figure 3.
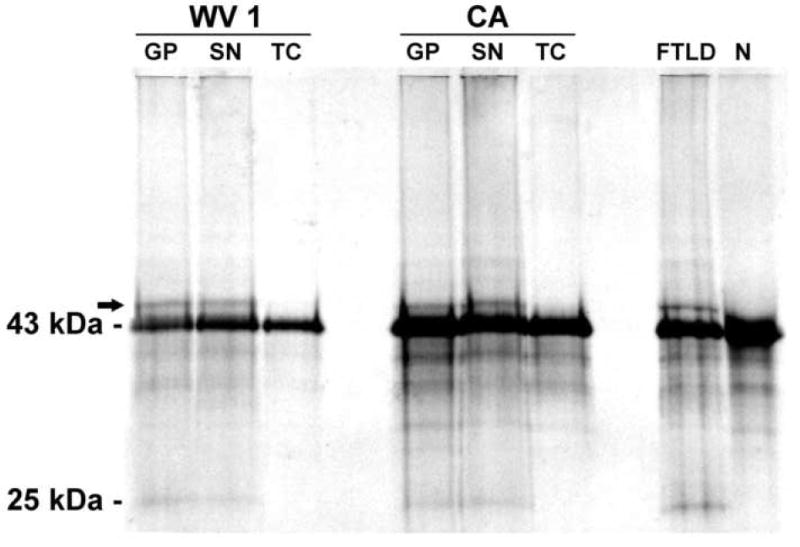
Western blot of Perry patients from WV 1 and CA, one patient with FTLD-U (FTLD) and one neurologically normal control (N) shows the normal 43 kDa TDP-43 band in all subjects. In Perry syndrome, pathologic 25 kDa fragment and hyperphosphorylated (arrow) TDP-43 bands are present in the globus pallidus (GP) and substantia nigra (SN), but not in the temporal cortex (TC). Abnormal bands are similar in Perry patients and FTLD-U, and absent in the control subject.
Genetic study
All coding and non-coding exons of GRN and TARDBP were screened for mutations in six patients from six genealogically unrelated families with Perry syndrome, including the two patients (CA and WV 1) with neuropathology described in this study. No mutations were identified. Copy-number analysis of TARDBP in the same patients did not show evidence of partial or complete gene deletions or duplications.
Discussion
The present study shows for the first time a molecular signature of Perry syndrome; namely, abnormal phosphorylated and truncated forms of TDP-43 associated with NCI, NII and dystrophic neurites. TDP-43 is a DNA binding protein found in the nucleus in physiological conditions, therefore its cytoplasmic localization strongly supports a pathologic role. Furthermore, the pathologic hyperphosphorylated and 25 kDa truncated forms of TDP-43 were detected in brain regions that also had immunohistochemical evidence of pathology (globus pallidus and SN), but not in histologically unaffected regions (temporal cortex). The electrophoretic and solubility profiles of TDP-43 were similar to those found in other TDP-43 proteinopathies [12,13,19]. Given that mutations in GRN [20,21] and TARDBP [22-24] are associated with TDP-43 pathology, these genes were excluded as causes of Perry syndrome.
TDP-43-positive NCI in Perry syndrome were highly pleomorphic, and some resembled the skein-like inclusions detected in motor neurons of ALS [23,25]. On the other hand, the distribution of NCI was different. In ALS motor neurons are affected, while in Perry syndrome NCI were most common in the dopaminergic neurons of the pars compacta of the SN. In addition to NCI, TDP-43 was also detected in lentiform shaped NII; however NII were detected in only four of the eight cases, and they were very sparse. NII are found in some cases of FTLD-U and are more frequent in familial disease [26]. For example, all cases with mutations in GRN [26,27] and the Valosin Containing Protein gene-associated FTLD have NII [28,29]. It should be emphasized, however, that not all cases with NII have a positive family history [27] and not all cases with a family history have NII. Perry syndrome falls into the latter category.
The morphology and distribution of TDP-43 immunoreactive NCI, NII and dystrophic neurites in cortex and hippocampus has served to delineate four subtypes of FTLD-U [30-32]. The TDP-43 pathology in Perry syndrome defies subclassification due to absence of lesions in the cortex and hippocampus and the consistent involvement of the SN and globus pallidus, which are brain regions not even considered in FTLD-U subclassification schemes. The presence of TDP-43 in axonal spheroids, particularly in the substantia nigra and globus pallidus, has not been emphasized in previous immunohistochemical studies using antibodies to TDP-43. While SN TDP-43-immunoreactive axonal spheroids were prominent in Perry syndrome, they are not specific to this condition. Out of 21 cases of pathologically confirmed FTLD-U with subtype 3 TDP-43 [31,32] pathology (12 men; mean age [±SD], 75 [±10] years), 16 (76%) had SN TDP-43-positive axonal spheroids (unpublished data). Glial inclusions similar to those noted in Perry syndrome are also found in FTLD-U, ALS and Guam Parkinson dementia complex [25,33,34]. Extramotor pathology is increasingly recognized in ALS, particularly in ALS with dementia, where inclusions have been described in the striatum, globus pallidus, neocortex, hippocampus, SN and inferior olivary nucleus [35,36]. The inferior olivary nucleus, which was frequently affected in Perry syndrome, is also commonly affected in FTLD-U, FTLD-MND and ALS [15,25].
In Perry syndrome the most distinctive pathologic feature is the distribution of the inclusions, which primarily involves the extrapyramidal system, but spares the cortex, hippocampus and upper and lower motor neurons. While hippocampal pathology is common in FTLD-U, it was almost absent in Perry syndrome. Sparse neuritic pathology in the pyramidal layer of the hippocampus and rare NCI in the dentate fascia were detected in only a few cases. None of the cases have involvement of the neocortex.
In Perry syndrome the distribution of the pathology only imperfectly explains the clinical features. The extrapyramidal system predominance, especially the severe involvement of the SN, likely accounts for parkinsonism in Perry syndrome patients. In addition, the basal ganglia involvement may explain the poor or transient response to levodopa. Recently, a neuropathologic study on one of the Japanese patients reported herein found selective loss of putative respiratory neurons in the ventrolateral medulla and in the dorsal raphe nucleus, which most probably represents the pathological substrate of hypoventilation [11]. Depression in Perry syndrome may stem from the loss of aminergic neurons in the locus ceruleus and ventral tegmental area. Frontal-type apathy, which better applies to some patients than true depression, likely results from reduced ventral tegmental area-frontal cortex dopaminergic projections [1,5]. No convincing pathological lesion has been found that would explain the severe weight loss displayed by many patients. We found mild pathology in the hypothalamus which may play a role; however, NCI were sparse and not associated with overt neuronal loss and gliosis.
While TDP-43 pathology was initially thought to be a specific marker for FTLD-U and ALS, subsequent studies have shown it in a variety of other disorders, including Guam Parkinson dementia complex [34], Pick’s disease [13], Alzheimer’s disease [18], hippocampal sclerosis [18], and Lewy body disease [37]. It remains unclear if TDP-43 plays a direct and pathologically relevant role in these diseases, or if it only represents a secondary phenomenon. In contrast to these conditions, no other pathological hallmark such as α-synuclein (Lewy bodies) or tau-positive (Pick bodies, tangles) pathology is present in Perry syndrome. Although further studies may challenge TDP-43 pathogenicity in Perry syndrome, the absence of other identifiable pathology supports its designation as a TDP-43 proteinopathy.
Given the lack of mutations in either GRN or TARDBP, the present results point to a missing link between the genetic defect and the deposition of TDP-43-positive inclusions in Perry syndrome. This missing link may include gene-gene, gene-protein or protein-protein interaction, whereby the mutated gene/protein influences the expression or translation of TARDBP or acts on the processing or posttranslational modification of TDP-43. A biochemical link between progranulin and TDP-43 was recently identified in a cell biology study, in which caspase-dependent cleavage of TDP-43 was produced by suppression of PGRN expression by small interfering RNA. Caspase-dependent cleavage of TDP-43 was associated with redistribution of TDP-43 from the nucleus to the cytoplasm, reminiscent of the pattern seen in FTLD-U and in Perry syndrome [38]. This observation supports a pathogenic role of TDP-43 which is likely to be established in a subset of neurodegenerative conditions with TDP-43-positive pathology [39].
Our study shows that Perry syndrome is a unique entity, with a distinct TDP-43-positive pathology and a characteristic clinical presentation. Further studies are needed to establish the role of TDP-43 in Perry syndrome and ultimately to identify its genetic cause.
Acknowledgments
We are grateful to all family members who participated in the study. We thank John Gonzalez for help in TDP-43 biochemistry. C.W. is supported by the Swiss National Science Foundation, Parkinson Switzerland, and the Robert H. and Clarice Smith and the M.L. Simpson Foundation Trust. Z.K.W., M.J.F. and D.W.D. are supported by the Morris K. Udall Center of Excellence for Parkinson Disease Research (P50-NS40256). D.W.D. and R.R. are supported by the Mayo Clinic ADRC grant P50-AG16574. Z.K.W., M.J.F., R.R., A.J.S. and D.W.D. are supported by the Pacific Alzheimer Research Foundation (PARF) grant C06-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wider C, Wszolek ZK. Rapidly progressive familial parkinsonism with central hypoventilation, depression and weight loss (Perry syndrome)--a literature review. Parkinsonism Relat Disord. 2008;14(1):1–7. doi: 10.1016/j.parkreldis.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Perry TL, Bratty PJ, Hansen S, Kennedy J, Urquhart N, Dolman CL. Hereditary mental depression and Parkinsonism with taurine deficiency. Arch Neurol. 1975;32(2):108–13. doi: 10.1001/archneur.1975.00490440058009. [DOI] [PubMed] [Google Scholar]
- 3.Perry TL, Wright JM, Berry K, Hansen S, Perry TL., Jr Dominantly inherited apathy, central hypoventilation, and Parkinson’s syndrome: clinical, biochemical, and neuropathologic studies of 2 new cases. Neurology. 1990;40(12):1882–7. doi: 10.1212/wnl.40.12.1882. [DOI] [PubMed] [Google Scholar]
- 4.Purdy A, Hahn A, Barnett HJ, Bratty P, Ahmad D, Lloyd KG, et al. Familial fatal Parkinsonism with alveolar hypoventilation and mental depression. Ann Neurol. 1979;6(6):523–31. doi: 10.1002/ana.410060611. [DOI] [PubMed] [Google Scholar]
- 5.Lechevalier B, Schupp C, Fallet-Bianco C, Viader F, Eustache F, Chapon F, et al. Familial parkinsonian syndrome with athymhormia and hypoventilation. Rev Neurol (Paris) 1992;148(1):39–46. [PubMed] [Google Scholar]
- 6.Lechevalier B, Chapon F, Defer G, Rivrain Y, Le Doze F, Schupp C, et al. Perry and Purdy’s syndrome (familial and fatal parkinsonism with hypoventilation and athymhormia) Bull Acad Natl Med. 2005;189(3):481–90. discussion 90-2. [PubMed] [Google Scholar]
- 7.Elibol B, Kobayashi T, Atac FB, Hattori N, Sahin G, Gurer G. Familial parkinsonism with apathy, depression and central hypoventilation (Perry’s syndrome) Boston, MA: Kluwer Academic/Plenum Publishers; 2002. [Google Scholar]
- 8.Tsuboi Y, Wszolek ZK, Kusuhara T, Doh-ura K, Yamada T. Japanese family with parkinsonism, depression, weight loss, and central hypoventilation. Neurology. 2002;58(7):1025–30. doi: 10.1212/wnl.58.7.1025. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia KP, Daniel SE, Marsden CD. Familial parkinsonism with depression: a clinicopathological study. Ann Neurol. 1993;34(6):842–7. doi: 10.1002/ana.410340614. [DOI] [PubMed] [Google Scholar]
- 10.Roy EP, 3rd, Riggs JE, Martin JD, Ringel RA, Gutmann L. Familial parkinsonism, apathy, weight loss, and central hypoventilation: successful long-term management. Neurology. 1988;38(4):637–9. doi: 10.1212/wnl.38.4.637. [DOI] [PubMed] [Google Scholar]
- 11.Tsuboi Y, Dickson DW, Nabeshima K, Schmeichel AM, Wszolek ZK, Yamada T, et al. Neurodegeneration involving putative respiratory neurons in Perry syndrome. Acta Neuropathol. 2008;115(2):263–8. doi: 10.1007/s00401-007-0246-1. [DOI] [PubMed] [Google Scholar]
- 12.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 13.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351(3):602–11. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 14.Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, et al. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171(1):227–40. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown S, Du Plessis D, Neary D, et al. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol. 2007;113(5):521–33. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- 16.Wszolek ZK, Tsuboi Y, Farrer M, Uitti RJ, Hutton ML. Hereditary tauopathies and parkinsonism. Adv Neurol. 2003;91:153–63. [PubMed] [Google Scholar]
- 17.Josephs KA, Dickson DW. Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord. 2003;18(9):1018–26. doi: 10.1002/mds.10488. [DOI] [PubMed] [Google Scholar]
- 18.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61(5):435–45. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwong LK, Neumann M, Sampathu DM, Lee VM, Trojanowski JQ. TDP-43 proteinopathy: the neuropathology underlying major forms of sporadic and familial frontotemporal lobar degeneration and motor neuron disease. Acta Neuropathol. 2007;114(1):63–70. doi: 10.1007/s00401-007-0226-5. [DOI] [PubMed] [Google Scholar]
- 20.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442(7105):916–9. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 21.Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442(7105):920–4. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 22.Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D, et al. TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol. 2008;63(4):535–8. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668–72. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40(5):572–4. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 25.Dickson DW, Josephs KA, Amador-Ortiz C. TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol. 2007;114(1):71–9. doi: 10.1007/s00401-007-0234-5. [DOI] [PubMed] [Google Scholar]
- 26.Mackenzie IR. The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol. 2007;114(1):49–54. doi: 10.1007/s00401-007-0223-8. [DOI] [PubMed] [Google Scholar]
- 27.Josephs KA, Ahmed Z, Katsuse O, Parisi JF, Boeve BF, Knopman DS, et al. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol. 2007;66(2):142–51. doi: 10.1097/nen.0b013e31803020cf. [DOI] [PubMed] [Google Scholar]
- 28.Forman MS, Mackenzie IR, Cairns NJ, Swanson E, Boyer PJ, Drachman DA, et al. Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containing protein gene mutations. J Neuropathol Exp Neurol. 2006;65(6):571–81. doi: 10.1097/00005072-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Neumann M, Mackenzie IR, Cairns NJ, Boyer PJ, Markesbery WR, Smith CD, et al. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J Neuropathol Exp Neurol. 2007;66(2):152–7. doi: 10.1097/nen.0b013e31803020b9. [DOI] [PubMed] [Google Scholar]
- 30.Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006;112(5):539–49. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, et al. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169(4):1343–52. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114(1):5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann M, Kwong LK, Truax AC, Vanmassenhove B, Kretzschmar HA, Van Deerlin VM, et al. TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J Neuropathol Exp Neurol. 2007;66(3):177–83. doi: 10.1097/01.jnen.0000248554.45456.58. [DOI] [PubMed] [Google Scholar]
- 34.Geser F, Winton MJ, Kwong LK, Xu Y, Xie SX, Igaz LM, et al. Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol. 2008;115(1):133–45. doi: 10.1007/s00401-007-0257-y. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Tan CF, Mori F, Tanji K, Kakita A, Takahashi H, et al. TDP-43-immunoreactive neuronal and glial inclusions in the neostriatum in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol. 2008;115(1):115–22. doi: 10.1007/s00401-007-0285-7. [DOI] [PubMed] [Google Scholar]
- 36.Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61(5):427–34. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- 37.Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007;114(3):221–9. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 38.Zhang YJ, Xu YF, Dickey CA, Buratti E, Baralle F, Bailey R, et al. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci. 2007;27(39):10530–4. doi: 10.1523/JNEUROSCI.3421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickson DW. TDP-43 immunoreactivity in neurodegenerative disorders: disease versus mechanism specificity. Acta Neuropathol. 2008;115(1):147–9. doi: 10.1007/s00401-007-0323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


