Abstract
We present a longitudinal study using the rhesus monkey to determine biochemical and histological changes in vastus lateralis (VL) muscle fibers and whether these changes correlate with muscle mass loss. Dual energy x-ray absorptiometry (DXA) was used to determine body weight, body fat and to estimate upper leg muscle mass in twelve adult male rhesus monkeys over 12 years. Muscle mass (MM) was evaluated at years six, nine and twelve of the study. Concurrently, VL muscle biopsy samples were collected. Muscle tissue was sectioned, stained and individual muscle fibers were analyzed for fiber type, cross-sectional area (CSA) and mitochondrial electron transport system (ETS) enzyme abnormalities. The animals body weight did not change over time, however a significant increase in DXA-measured percent body fat was observed. Significant MM loss occurred in the upper leg over 12 years. A reduction in muscle fiber CSA significantly contributed to the MM loss observed in the VL of middle-aged rhesus monkeys. An age-dependent increase in muscle fibers developing mitochondrial enzyme abnormalities due to mitochondrial DNA deletion mutations was observed. The longitudinal approach of this study demonstrated that significant muscle changes occurred during middle age in a cohort of aging rhesus monkeys.
Keywords: rhesus monkey, longitudinal, sarcopenia, DXA
Introduction
Sarcopenia is the inevitable decline in muscle mass and function that occurs with age. It negatively impacts the daily activities of a significant portion of the elderly population contributing to billions of dollars in added health care costs per year (Janssen, 2006). Sarcopenia is increasingly recognized as a biomarker of aging, predicting an increased risk for disability and mortality (Faulkner et al., 2007; Melton et al., 2000; Roubenoff and Castaneda, 2001). Developing standardized criteria to define sarcopenia, and especially to identify the early stages, is crucial given the strong evidence that people in the early stages of sarcopenia are most likely to benefit from interventions (Lauretani et al., 2003).
The age-dependent loss of skeletal muscle mass results from the contributions of both muscle fiber atrophy and fiber loss. It is not clear if the two processes are linked. In cross-sections of autopsied whole vastus lateralis (VL) from healthy men, Lexell et al. (1988) determined that approximately 10% of the muscle area was lost by 50 years of age. After age 50 the reduction accelerated such that, by 80 years of age, almost half of the muscle had wasted. Further analyses revealed that a reduction in muscle fiber cross-sectional area, specifically type II fibers, contributed to muscle area loss as early as 50 years of age. Fiber loss occurred at older ages with significant reductions observed after 70 years of age (Lexell et al., 1988). Fischer × Brown Norway hybrid rats (mean life span 33 months, maximum life span 43 months) show a similar trend of gradual fiber atrophy between 15- and 30-months followed by significant fiber atrophy and 30%-46% fiber loss at very old age (33- to 36-months of age) (Lushaj et al., in press).
The proposed mechanisms of sarcopenia are multi-factorial and include the loss and reorganization of neuromuscular junctions (Delbono, 2003; Larsson, 1995), contraction-induced injuries (Rader and Faulkner, 2006), satellite cell deficiencies (Carlson, 1995; Collins et al., 2007; Jejurikar et al., 2006), alterations in gonadal hormones (Lee et al., 2007; Roubenoff and Hughes, 2000), oxidative stress (Muller et al., 2007; Weindruch, 1995) and age-dependent changes in the mitochondrial genome (mtDNA) (Dirks et al., 2006; McKenzie et al., 2002). A more complete understanding of these mechanisms may lead to successful treatment of sarcopenia.
Our work has focused on the hypothesis that mitochondrial DNA deletion mutations in aging skeletal muscle fibers ultimately results in permanent fiber loss (Aiken et al., 2002; Herbst et al., 2007). Age-dependent alterations to the mitochondrial genome results in the removal of large portions of mtDNA that encode crucial subunits of electron transport system (ETS) enzymes that are required for normal oxidative phosphorylation. These deletion mutations induce phenotypic alterations in mitochondrial enzyme activities (ETS abnormalities) that can be observed on histological sections, specifically, the absence of cytochrome c oxidase (COXneg) activity and a concomitant hyper-reaction of succinate dehydrogenase (SDHhyp) activity. Using consecutive sections of muscle tissue, single muscle fibers can be followed from slide to slide tracking phenotypic and genotypic changes. Utilizing this histological approach to evaluate the relationship between mitochondrial genotype and muscle fiber phenotype, mtDNA deletion mutation accumulation was correlated with dysfunctional cellular phenotypes and ultimately with muscle fiber loss (Bua et al., 2006; Herbst et al., 2007).
In this study, we employ a longitudinal study design in a non-human primate model, rhesus monkey (Macaca mulatta), to characterize age-specific changes in muscle. The rhesus monkey more closely models human aging, compared to rodent models, due to its phylogenetic proximity to humans, physiologic similarities and a rate of aging approximately 2.5 – 3 times that of humans (Colman et al., 2005). Male rhesus monkeys reach their maximum total body lean mass at 15.6 ± 2.5 years of age with a 20% reduction in muscle mass by 23 years of age (Colman et al., 2005). Male human lean mass stabilizes by the mid- 40's (Roubenoff and Hughes, 2000). A 23% decline in estimated skeletal muscle was observed in human males between 18 to 34 and >75 years age groups (Kyle et al., 2001). The purpose of this study was to characterize biochemical and histological changes in the vastus lateralis (VL) muscle and determine whether these changes correlate with muscle mass loss as rhesus monkeys age through their middle years (mean age 16.1 ± 1.6y) into early old age (22.1 ± 1.6y). VL muscle mass loss was estimated in 12 adult rhesus monkeys using dual energy X-ray absorptiometry (DXA) scans over 12 years. VL muscle biopsies collected at six, nine and twelve years of the study were sectioned and stained to determine muscle fiber cross-sectional area and muscle fiber type, as well as, the abundance and mitochondrial genotype of muscle fibers bearing mitochondrial electron transport system (ETS) enzyme abnormalities. These data provide unique insights into the early stages of muscle mass loss in an important model of human aging.
Materials and Methods
The animals in this longitudinal study are part of an ongoing study at the Wisconsin National Primate Research Center (WNPRC) to systematically define the effects of aging in rhesus monkeys (Colman et al., 2005; Colman et al., 2007; Kemnitz et al., 1993; Ramsey et al., 2000). The mean and maximum life spans for rhesus monkeys at the WNPRC are 27y and 41y, respectively (Colman et al., 1998; Colman et al., in press). A cohort of 15 males, between 8 and 14 years of age, was assigned to the study in 1989. The animals were housed individually and fed ad libitum. Three animals died early in the study; we report on the remaining 12 rhesus monkeys using data collected between 1990 and 2002. All animal procedures were performed at the WNPRC with approved protocols from the Institutional Animal Care and Use Committee of the Graduate School at the University of Wisconsin, Madison.
Appendicular lean mass, fat mass and body weight were assessed biannually using DXA (Model DXP-L, GE/Lunar Corp., Madison, WI) whole body scans as previously described (Colman et al., 1998; Colman et al., 1999; Colman et al., in press). Estimated skeletal muscle mass (ESM) of the upper leg was determined by summing the lean mass from the thigh region of both limbs. Upper leg muscle mass loss was determined by dividing the upper leg lean mass at each time point by the maximum upper leg lean mass measured in the 12 years of the study. Fat mass, determined by DXA measurements, was used to calculate percent body fat (%BF = [fat mass / body weight] × 100).
In 1996, 1999 and 2002 (6, 9 and 12 years post-initiation of the study) VL biopsies were performed on alternating legs following the DXA measurements. Biopsy tissue was bisected with one half of the sample flash frozen in liquid nitrogen and the other embedded in Optimal Cutting Temperature Medium (OCT, Sakura Inc., Torrance, CA) and frozen in liquid nitrogen. Both halves were stored at -80°C until use. Frozen muscle biopsies were brought to -20°C and blocks of tissue were sectioned using a cryostat. For each biopsy, 200 consecutive 10μm-thick sections were cut. Cryosections were stored at -80°C.
Two slide sections were stained with hematoxylin and eosin (H and E; Sheehan and Hrapchak, 1980) for muscle morphology and muscle fiber counts. A digital composite image of the entire muscle biopsy was created by taking reading frame images of the muscle and interlacing those images using ImagePro Plus software (MediaCybernetics, Silver Spring, MD). Muscle fiber counts were obtained from the composite images by annotating each muscle fiber using Photoshop followed by ImagePro Plus to sum the total number of fibers annotated.
Using standard procedures for indirect immunoenzymatic staining (Sheehan and Hrapchak, 1980) with monoclonal antibody MY32 (Sigma St. Louis, MO) and DAB reagent for detection, ‘fast’ Type II muscle fibers were identified on histological sections. Type II muscle fibers were counted on each section and percentage determined from the total number of fibers present on each biopsy section.
General muscle fiber atrophy was assessed using slides from the MY32 immunohistochemistry assay for fiber type. Five 10× images per section were taken. Type II fibers were identified and cross-sectional area (CSA) was measured using ImagePro. Data were imported into Excel for analysis. At least 200 Type II muscle fibers were measured from each biopsy.
Histochemical staining for mitochondrial enzyme activities, cytochrome c oxidase (COX) and succinate dehydrogenase (SDH) were performed on air-dried sections according to Seligman et al. (1968) and Dubowitz (1985), respectively. Dual staining for COX and SDH provides high contrast to identify abnormal mitochondrial enzyme activities (Taylor et al., 2003; Figure 1). Twenty-nine slide triplicates (the 2nd, 3rd and 4th, the 9th, 10th and 11th etc.) from the 200 slide sections from each biopsy were stained for COX, SDH and dual COX and SDH enzyme activity staining. Intermediate slides were used for the mtDNA analyses.
Figure 1.
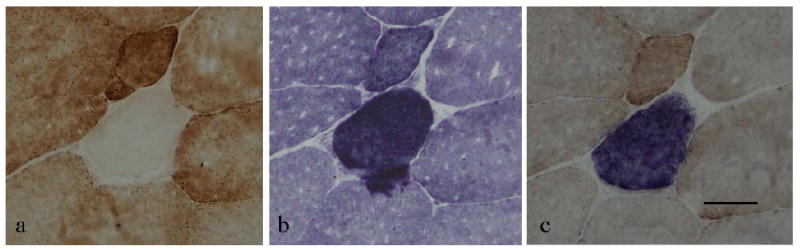
ETS abnormal muscle fiber from a 21y male rhesus monkey. Consecutive slide sections stained for a) COX activity, b) SDH activity and c) dual stained for COX and SDH. Note the lack of COX activity in panel (a), hyper-reactivity for SDH in panel (b) and the characteristic blue against a brown background for dual stained sections. Black bar represents 50μm.
ETS abnormal muscle fibers were initially identified on COX/SDH dual-stained slide sections by the characteristic blue staining for a COX negative muscle fiber. The specific COX and SDH phenotypes were then resolved on the single-stained sections. The number of unique ETS abnormal fibers observed in the 2000μm of tissue was divided by the number of muscle fibers counted in each biopsy to determine the proportion of fibers with ETS abnormalities for each animal at the three time points. Once an abnormal fiber was identified, it was followed along its length, every 70μm, using the remaining 28 slide triplicates described above. The COX and SDH phenotypes of the fiber were recorded for each set of slides and the cross-sectional area (CSA) of the fiber measured at each interval (ImagePro). The CSA measurements along the length of ETS normal and ETS abnormal fibers were used to determine intra-fiber atrophy. A cross-sectional area ratio was determined for each fiber where the minimum CSA of the ETS abnormal region (or minimum CSA for normal fibers) was divided by the mean CSA of the normal region. Abnormal fibers with a CSA ratio less than or greater than the normal distribution of normal fiber CSA ratios were defined as atrophic or hypertrophic, respectively.
Laser capture microdissection (LCM) was used to isolate 10μm thick sections of ETS abnormal and normal muscle fibers to determine the mitochondrial genotype. Frozen sections adjacent to those used for identification of ETS abnormal phenotypes were stained for SDH activity, dehydrated in ethanol and cleared in xylenes. Muscle fibers of interest were microdissected using a PixCell II laser capture microscope (Arcturus Bioscience, Inc., Mountainview, CA, USA) as previously described (Gokey et al., 2004).
Total DNA was extracted by incubating microdissected muscle sections with 1ul DNA extraction solution (10mM EDTA, 0.5% SDS, 2 mg/ml proteinase K, 50mM Tris; pH 8.0) for 30 minutes at 37°C. Ten microliters of ddH2O was added to the extraction solution and 1ul of the DNA solution was used for PCR reactions. Primary PCR reactions were performed using the following mtDNA primers specific to the rhesus mitochondrial genome (Gokey et al., 2004): Rh 725R (5′-GTGCTTGATGCTTGCTCCTCTTGGT-3′) and Rh 1176F (5′-CCACCCCACCCTCTCTTGCTCA-3′) using the following conditions: 94°C for 30s, 58°C for 30s, 72°C for 6min, 32 cycles. Nested PCR reactions were performed using the primers Rh 16245R (5′-GGGAACCATGTTATGTGTTACTGTTG-3′) and Rh 3499F (5-CATTGCCCTCCTCCTATGAACCCC-3′) at 94°C for 30s, 60°C for 30s, 72°C for 5 min, 32 cycles.
PCR products were gel-extracted and purified using QIAquick Gel Extraction Kit (Quiagen Sciences, Maryland, USA) and sequenced using the Big Dye terminator cycle sequencing at the University of Wisconsin-Madison sequencing facility.
A linear mixed model approach was used to estimate longitudinal trends while accounting for the dependency in the data due to multiple observations per subject. The Roy (2006) multivariate linear mixed model approach, which treats the multiple outcome variables as a random effect, was used to estimate the correlation among the repeatedly measured outcomes assuming an autoregressive process over time. SAS PROC MIXED was used for all analyses.
Results
Body weight and percent body fat (%BF) were determined for 12 rhesus monkeys over the six years of the study. No significant changes were observed in body weight over time (r = 0.234l p = 0738; Table 1). An age-dependent increase in %BF was observed. The correlation between %BF and age, adjusted for repeated measures, was statistically significant (r = 0.423, p = 0.005).
Table 1.
Morphometrics of the aging rhesus monkey
| Animal | Biopsy Yr | Age @ Biopsy (yrs) |
Weight (kg) |
% Body Fat | % MM of Maxa |
|---|---|---|---|---|---|
| 1 | 6 | 20 | 15.97 | 28.31 | 92.9 |
| 1 | 9 | 23 | 15.80 | 30.58 | 64.3 |
| 1 | 12 | 26 | 14.93 | 32.64 | 54.6 |
| 2 | 6 | 18 | 12.25 | 16.52 | 94.6 |
| 2 | 9 | 21 | 13.98 | 25.12 | 93.2 |
| 2 | 12 | 24 | 13.96 | 26.74 | 82.6 |
| 3 | 6 | 17 | 18.52 | 31.55 | 96.9 |
| 3 | 9 | 20 | 19.09 | 33.54 | 88.6 |
| 3 | 12 | 23 | 19.09 | 34.64 | 85.4 |
| 4 | 6 | 16 | 11.85 | 27.25 | 93.6 |
| 4 | 9 | 19 | 14.69 | 39.39 | 85.8 |
| 4 | 12 | 22 | 14.79 | 44.14 | 71.5 |
| 5 | 6 | 16 | 14.48 | 19.36 | 99.0 |
| 5 | 9 | 19 | 15.06 | 23.42 | 88.7 |
| 5 | 12 | 22 | 14.56 | 24.62 | 90.3 |
| 6 | 6 | 16 | 14.70 | 26.38 | 94.9 |
| 6 | 9 | 19 | 13.90 | 26.93 | 88.4 |
| 6 | 12 | 22 | 13.06 | 27.11 | 78.2 |
| 7 | 6 | 15 | 13.26 | 22.54 | 96.3 |
| 7 | 9 | 18 | 14.00 | 30.82 | 81.8 |
| 7 | 12 | 21 | 13.46 | 30.54 | 74.6 |
| 8 | 6 | 15 | 13.67 | 21.42 | 88.4 |
| 8 | 9 | 18 | 16.70 | 26.62 | 82.0 |
| 8 | 12 | 21 | 15.47 | 27.32 | 77.8 |
| 9 | 6 | 15 | 15.33 | 24.85 | 98.9 |
| 9 | 9 | 18 | 14.68 | 30.58 | 69.1 |
| 9 | 12 | 21 | 11.58 | 24.11 | 60.5 |
| 10 | 6 | 15 | 10.96 | 16.79 | 95.3 |
| 10 | 9 | 18 | 10.69 | 17.55 | 90.7 |
| 10 | 12 | 21 | 8.32 | 9.46 | 82.0 |
| 11 | 6 | 15 | 12.92 | 22.54 | 94.9 |
| 11 | 9 | 18 | 12.02 | 20.43 | 87.9 |
| 11 | 12 | 21 | 11.61 | 24.15 | 84.5 |
| 12 | 6 | 15 | 11.10 | 15.65 | 94.6 |
| 12 | 9 | 18 | 14.58 | 29.52 | 83.5 |
| 12 | 12 | 21 | 13.30 | 30.93 | 77.1 |
| r values | 0.234 | 0.423 | -0.702 | ||
| p values | 0.738 | 0.005 | < 0.001 | ||
%MM of Max = (muscle mass of upper leg / maximum mass of upper leg) × 100
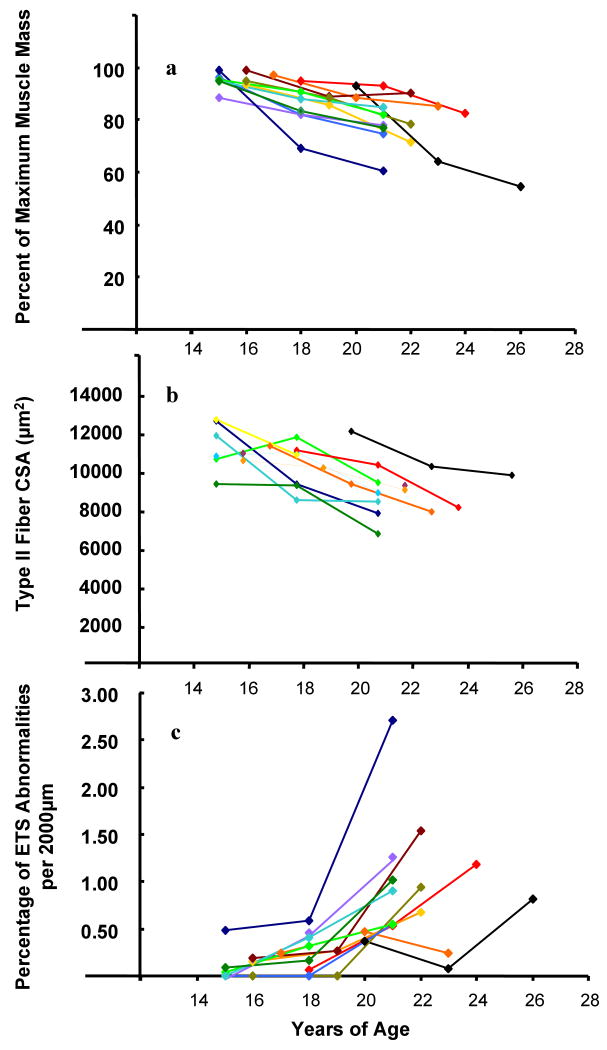
DXA was used to estimate upper leg muscle mass in 12 rhesus monkeys over 12 years of the study. The maximum upper leg muscle mass attained for each animal during this 12 year period was used to determine the percent upper leg muscle mass loss for each animal at years 6, 9 and 12 of the study. At year 6, the majority of animals (n=9) were 15-16 years old. Average muscle mass of the upper leg among this group was 95.1 ± 2.0% of their maximum (Figure 2a). The average upper leg muscle loss for this group was 15 ± 4.6% at year 9 (n=9, 18-19y) and 22.6 ± 5.7% at year 12 (n=9, 21-22y). Among these animals, one (15 year old) displayed an accelerated rate of muscle mass loss: 29% between years 6 and 9 of the study, followed by an additional 11% loss between years 9 and 12. Muscle mass loss among the two monkeys aged 17y and 18y at year 6 of the study was not significantly different from that observed among the majority. The eldest monkey in the study was 20y at year 6, he also exhibited an accelerated rate of muscle mass loss, 36% loss between years 6 and 9 and an additional 8% between years 9 and 12. The correlation between muscle mass loss in the Table 1 as and increasing age, examining all animals over the length of the study (12 monkeys, 3 time points each, between the ages of 15y and 26y), adjusted for repeated measures was statistically significant (Table 1; r = -0.702; p < 0.001).
Figure 2.
Longitudinal analyses of VL muscle from 12 aging rhesus monkeys using three time points over 6 years. Panel a) upper leg muscle mass loss, b) Type II fiber cross-sectional area and c) percentage of muscle fibers in 2000μm of tissue that were ETS abnormal. Connected dots represent the same animal over time, like colored lines represent the same animal in each panel.
Type II muscle fibers were identified on sectioned biopsies, the percentage of Type II fibers determined and CSAs measured (Table 2; Figure 2b). The proportion of Type II muscle fibers declined from 96.4 ± 4.8% to 88.0 ± 6.3% over the course of the study (r = -0.2990; p = 0.024). Type II muscle fiber CSA decreased with age. The average CSA was 11124 ± 1293μm2 for 15-16y rhesus monkeys (n=9) at year 6; the same monkeys at year 9 of the study had an average muscle fiber CSA of 10366 ± 1335μm2, a 7% decline in CSA. At year 12 of the study, muscle fiber CSA was 8621 ± 1071μm2 for monkeys now 21-22 years old, representing overall, a 33% decline in CSA. One monkey (15 years of age at year 6 of the study) had increased muscle fiber CSA between year 6 and 9 of the study. Among all animals in the study, the correlation between fiber CSA and age, adjusted for repeated measures, was statistically significant (r = -0.253, p < 0.001). An average CSA decline of 457μm2 per year between the ages of 15y and 24y was estimated using a standard linear mixed model (F = 30.13; p = 0.0002). The correlation between fiber CSA decline and muscle mass loss, adjusted for repeated measures, was statistically significant (r = 0.071, p = 0.01).
Table 2.
Age-dependent changes in rhesus monkey vastus lateralis muscle fibers
| Animal | Biopsy Yr | Age @ Biopsy (yrs) |
% Type II | Type II Fiber CSAa (μm2) |
% ETS Abnb per 2000μm |
|---|---|---|---|---|---|
| 1 | 6 | 20 | 99.63 | 12570 ± 2740 | 0.374 |
| 1 | 9 | 23 | 95.59 | 10584 ± 2319 | 0.082 |
| 1 | 12 | 26 | 93.82 | 10073 ± 5112 | 0.808 |
| 2 | 6 | 18 | 94.94 | 11483 ± 2479 | 0.057 |
| 2 | 9 | 21 | 94.02 | 10610 ± 2011 | 0.533 |
| 2 | 12 | 24 | 88.00 | 8132 ± 3034 | 1.180 |
| 3 | 6 | 17 | 99.11 | 11712 ± 2405 | 0.236 |
| 3 | 9 | 20 | 88.26 | 9574 ± 3730 | 0.469 |
| 3 | 12 | 23 | 92.03 | 7881 ± 1922 | 0.243 |
| 4 | 6 | 16 | 95.32 | 10894 ± 2853 | 0.188 |
| 4 | 9 | 19 | 94.63 | 10450 ± 1653 | 0.268 |
| 4 | 12 | 22 | 92.11 | 9182 ± 4064 | 0.669 |
| 5 | 6 | 16 | 98.68 | 11301 ± 2051 | 0.188 |
| 5 | 9 | 19 | nd | nd | 0.271 |
| 5 | 12 | 22 | 78.94 | 9482 ± 3646 | 1.541 |
| 6 | 6 | 16 | nd | nd | 0.000 |
| 6 | 9 | 19 | nd | nd | 0.000 |
| 6 | 12 | 22 | nd | nd | 0.938 |
| 7 | 6 | 15 | 97.56 | 11168 ± 2769 | 0.000 |
| 7 | 9 | 18 | 94.48 | 11410 ± 3961 | 0.000 |
| 7 | 12 | 21 | 95.40 | 9030 ± 4071 | 0.545 |
| 8 | 6 | 15 | 98.05 | 13288 ± 2306 | 0.000 |
| 8 | 9 | 18 | 93.36 | 11224 ± 2490 | 0.461 |
| 8 | 12 | 21 | 87.08 | long | 1.259 |
| 9 | 6 | 15 | 96.40 | 13207 ± 2113 | 0.480 |
| 9 | 9 | 18 | 90.99 | 9490 ± 2199 | 0.579 |
| 9 | 12 | 21 | 81.86 | 7867 ± 2442 | 2.710 |
| 10 | 6 | 15 | 99.14 | 10970 ± 1870 | 0.039 |
| 10 | 9 | 18 | 89.27 | 12232 ± 2580 | 0.322 |
| 10 | 12 | 21 | 96.76 | 9651 ± 1432 | 0.541 |
| 11 | 6 | 15 | 84.99 | 12382 ± 2103 | 0.000 |
| 11 | 9 | 18 | 92.07 | 8574 ± 1563 | 0.407 |
| 11 | 12 | 21 | 85.12 | 8520± 1432 | 1.063 |
| 12 | 6 | 15 | 98.69 | 9495 ± 1695 | 0.088 |
| 12 | 9 | 18 | 99.96 | 9182 ± 1885 | 0.160 |
| 12 | 12 | 21 | 86.33 | 6615 ± 3141 | 1.015 |
| r values | -0.299 | -0.253 | 0.367 | ||
| p values | 0.024 | < 0.001 | <0.001 | ||
CSA=Cross-sectional area
Percentage of electron transport system enzyme abnormalities observed in 2000μm of tissue
Thirty-six biopsies were sectioned, stained and scored for mitochondrial enzyme activities and the percentage of ETS abnormal fibers per 2000μm of sectioned tissue was determined. There were, on average, 2115 ± 1008 muscle fibers per biopsy. The age-dependent accumulation of ETS abnormalities observed in each of the 12 rhesus monkeys, over time, is presented in Figure 2c and Table 2. By year 12 of the study, monkeys ranged in age from 21y to 26y and approximately 1.0% of the muscle fibers in 2000μm of tissue contained an ETS abnormality. One monkey, 15 years of age at year 6 of the study, had nearly twice as many ETS abnormalities (2.7%) at age 21y compared to other animals at year 12 of the study. The correlation between the accumulation of ETS abnormalities and age in rhesus VL muscle was statistically significant (r = 0.367; p < 0.001), as was the relationship between ETS abnormal muscle fibers and muscle mass loss (r = -0.497; p < 0.001).
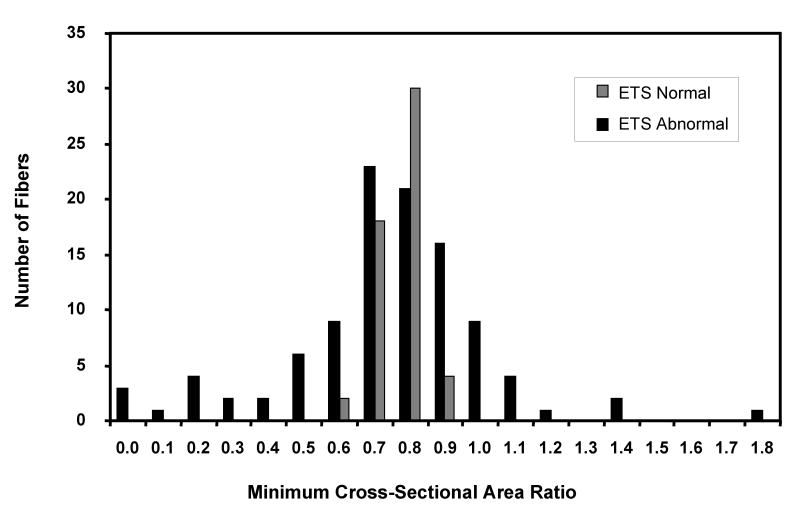
A total of 446 ETS abnormal fibers were observed in 36 muscle biopsies, 63% were COXneg / SDHhyp, 31% COXneg / SDHnl and 6% presented other abnormal COX and SDH phenotypes such as COXlow / SDHhigh, COXhyp / SDHnor or COXnor / SDHhyp. One hundred fifteen ETS abnormal muscle fibers (COXneg / SDHhyp and COXneg / SDHnl) were followed along their length. The CSA was measured and COX/SDH phenotypes recorded. CSA ratios of ETS abnormal fibers describe the changes in fiber area along 2000μm. Minimum CSA ratios provide an indication of the degree of atrophy within an ETS abnormal region compared to the ETS normal region of the same fiber. These ratios were compared to the minimum CSA ratios determined for normal fibers. ETS abnormal fiber minimum CSA ratios ranged from 0 to 1.8, normal fibers had a more narrow range (0.6 to 0.9) (Figure 3). Only 58% of the ETS abnormal fibers had CSA ratios within the normal range; 26% had CSA ratios less than normal, and 16% had CSA ratios greater than normal. Although the mean minimum CSA ratio was similar between ETS normal and abnormal muscle fibers, there was a significant difference in the variance between the two groups. Regression analysis of the minimum CSA ratio for ETS abnormal fibers indicated a significant correlation between intra-fiber atrophy and age (r = -0.35; p= 0.015).
Figure 3.
The distribution of the minimum cross-sectional area ratio for ETS normal and ETS abnormal muscle fibers.
Sections of single fibers were obtained by LCM and analyzed for mitochondrial genotype. The mitochondrial genomes from seven ETS abnormal and 17 ETS normal fibers from a 24 year-old rhesus monkey were analyzed for the presence of mtDNA deletions. Mitochondrial deletion mutations were present in all ETS abnormal muscle fiber regions sampled. Deletion mutations were large, between 4.5 and 10.6kb, and 6 out of 7 mutations had direct repeats at the breakpoints (Table 3). Two fibers contained deletion mutations with identical breakpoints. Deletion mutations were not detected in ETS normal muscle fibers.
Table 3.
Mitochondrial mtDNA deletion mutation analysis of ETS abnormal fibers.
| Fiber | Phenotype | Breakpoint | Direct Repeat Size (bp) | Deletion Size (bp) |
|---|---|---|---|---|
| 1 | COXneg/SDHhyp | 6116 – 10611 | 4 | 4528 |
| 2 | COXlow/SDHhyp | 10596 – 16063 | 6 | 5466 |
| 3 | COXlow/SDHhi | 5047 – 15667 | 4 | 10620 |
| 4 | COXneg/SDHhyp | 9081 – 14828 | 14 | 5797 |
| 5 | COXneg/SDHhyp | 9081 – 14828 | 14 | 5797 |
| 6 | COXneg/SDHhyp | 3323 – 12573 | 6 | 9250 |
| 7 | COXneg/SDHhyp | 3224 – 10680 | - | 7456 |
Discussion
Much of our understanding of skeletal muscle aging has been garnered from rodent models. While there are clear advantages to working in relatively inexpensive, well-characterized and short-lived mammals, there is an obvious challenge in extrapolating data generated in rodents to human muscle aging. In contrast, data generated in the closely related primate species, rhesus macaque, are more readily translatable to human aging; characterization of this model is imperative for elucidation of novel mechanistic insights.
Sarcopenia is a gradual process culminating in significant losses in muscle mass and function. To investigate the early stages of this process we monitored upper leg muscle mass (DXA) in 12 adult monkeys over a twelve year period. At the cellular level, we measured muscle fiber atrophy (CSA of individual fibers), changes in muscle fiber type and the accumulation of muscle fiber mitochondrial abnormalities at years six, nine and twelve. The combination of repeated measures for gross muscle changes over time with tandem analyses at the muscle fiber level in a primate model provides a unique data set that typical cross-sectional studies in rodent models or even longitudinal studies in humans have not provided. This study assessed the pattern of muscle aging in the cohort, as a whole, as well as in individuals and demonstrated that chronological age does not necessarily reflect aging changes.
We confirm that upper leg muscle mass loss contributes to the decline in peripheral lean muscle observed in aging rhesus monkeys (Colman et al., 2005). Ten of the 12 monkeys in this study lost 13 ± 4% upper leg muscle mass by year nine of the study and a total of 20 ± 6% loss by year twelve. The rate of muscle mass loss between maximum mass (15-16y) and 21-22 years of age was ∼3% per year. Observations in male humans approximate a 1-2% lean mass loss per year past the age of 50 (Hughes et al., 2001; Thomas, 2007). Losses specific to the leg muscle in 68 – 78 year old men averaged 3.6% over a two year period (Zamboni et al., 2003). If we extrapolate the ages of the monkeys in this study to human years, the majority of monkeys were equivalent to men as they progress through their early 40's, 50's and 60's.
Two monkeys experienced accelerated muscle mass loss. One, 15y at year six of the study, lost 30% of its upper leg muscle mass by age 18y and 39% by 21y. The second monkey, the oldest in the study, was 20y at year six of the study. At 20y upper leg muscle mass was only 7% less than its maximum, compared to an average of 20% loss for the other 21-24 year olds, however, at 23y a 36% loss was observed and at 26y, 45% of the muscle had wasted. These two monkeys were defined as sarcopenic as their muscle mass loss was greater than two standard deviations (>2 sd) from the mean muscle mass of similar age animals (Lauretani et al., 2003). This critical level was reached within year nine of the study at two distinct ages, 18y and 23y, respectively, emphasizing the considerable age variability in the initiation of sarcopenic changes.
The VL is one of the largest fast-twitch muscles of the upper leg and is vulnerable to sarcopenia (rat: Bua et al., 2002; human: [Lexell et al., 1988]). It is primarily composed of Type II muscle fibers, the largest of the skeletal muscle fiber types, however, they are more prone to fiber atrophy and loss with age than Type I muscle fibers (Lee et al., 2006; Proctor et al., 1995). We observed an age-dependent shift in the proportion of Type II fibers from 96% to 88%. Rhesus monkey VL Type II fibers were extremely large (11124 ± 1293μm2), 2-3 times larger than that reported for humans (4113μm2 ± 957 for males 19 – 35 years of age, [Lexell and Taylor, 1991]). Jouffroy et al. (1999) report that rhesus monkey Type II VL muscle fiber CSA was 6810 ± 1457μm2, smaller than we report, however, only one animal was represented, age unknown, these were deep tissue samples and the cadaver had been formalin fixed. They found that Type II muscle fibers in nonpostural muscles of mixed fiber type were significantly larger than Type I fibers. The average Type II muscle fiber CSA in 15 -16y rhesus monkeys was ∼11000μm2. In the same animals three years later, the value was ∼9800μm2 and, at 21 - 22 years of age, ∼8000μm2. This represents a 32% decline over the six years of the study, approximately 5% per year. A similar decline was observed in humans, where Type II muscle fiber CSA in young men (21 - 30y) was 4508 ± 1168μm2 compared to 2637 ± 658μm2 in older (51-62y) men (Proctor et al., 1995), a 42% loss in fiber CSA. Reductions in muscle mass and fiber CSA in both human and rhesus begin soon after peak estimates. We conclude that muscle fiber atrophy significantly contributes to and is an initial event in the development of sarcopenia.
A sedentary lifestyle affects muscle physiology and biochemistry in humans and may be a contributing factor in this study. Body composition changes with aging in rhesus monkeys closely parallel those in humans with an increase in fat mass and a decline in lean body mass (Schwartz and Kemnitz, 1992). We did not observe any change in overall body weight, however, a significant increase in %BF and a significant decrease in upper leg lean mass were observed among the monkeys over the course of the study. While we have conducted activity profiles for the animals described here (unpublished data); we have not examined age-dependent differences in muscle strength and endurance. The majority of strength loss can be accounted for by decreased muscle mass (Doherty, 2003).
We have suggested that mtDNA deletion mutations and their phenotypic effects on single muscle fibers have a role in muscle fiber loss (Herbst et al., 2007). Muscle fiber numbers are not yet available for the rhesus monkeys in this study due to the longitudinal experimental design. The number of ETS abnormal muscle fibers, however, significantly increased in VL muscles within the same animals over time. Extrapolating from the percentage of ETS abnormal fibers found in 2000μm (Lopez et al., 2000) of a VL biopsy sample from 21-22 year old rhesus monkeys, to the entire length of the VL (∼4cm; Roy et al., 1991), we predict that ∼20% of the VL muscle fibers in these animals contain mitochondrial abnormalities somewhere along their length. Even though a significant number of muscle fibers were affected by mitochondrial abnormalities, intra-fiber atrophy (minimum CSA ratio) for abnormal fibers was not different from that of normal muscle fibers. The variability range in CSA ratios for the ETS abnormal fibers, in rhesus monkeys, was considerable and a significant negative correlation between the minimum CSA ratio of ETS abnormal regions and age was observed. Some fibers atrophied so that, within the ETS abnormal region, the fiber broke. MtDNA deletion mutation accumulation has been linked to fiber splitting, atrophy and breakage, with the more severe cellular pathologies resulting from higher mutation abundance (Herbst et al., 2007). Permanent muscle fiber loss appears to be a sarcopenic event occurring in the very aged; in hybrid rats at 33-36 months of age (Lushaj et al., in press) and in humans in their 80's (Lexell et al., 1988).
The work described here is the first longitudinal non-human primate study to characterize changes at the cellular level leading to the onset and development of sarcopenia. Sarcopenia is an age-dependent process resulting in reduced skeletal muscle mass and function due to two distinct phenomena, muscle fiber loss and fiber atrophy. In agreement with rodent studies, defects in mitochondrial ETS were detected with increasing age in a manner consistent with their effects on fiber loss. Extension of these studies into a primate species places new emphasis on the early stages of sarcopenia and reveals that a reduction in muscle fiber CSA significantly contributes to muscle mass loss in rhesus monkeys. A critical insight of this study is the identification of early muscle fiber changes indicative of sarcopenia. This knowledge facilitates early diagnosis of individuals susceptible to sarcopenia and allows the opportunity for treatment before significant muscle loss has occurred.
Acknowledgments
The authors gratefully acknowledge the excellent technical assistance provided by S. Baum, J. A. Adriansjach, C. E. Armstrong, E. Aiken and the Animal Care and Veterinary Staff of the Wisconsin National Primate Research Center. This work was supported by grants P01 AG-11915 and P51 RR000167. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01.
The authors would like to thank Ms. J. Johnson, Dr. R. Anderson and Dr. D. McKenzie for critical review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiken J, Bua E, Cao Z, Lopez M, Wanagat J, McKenzie D, McKiernan S. Mitochondrial DNA deletion mutations and sarcopenia. Ann N Y Acad Sci. 2002;959:412–423. doi: 10.1111/j.1749-6632.2002.tb02111.x. [DOI] [PubMed] [Google Scholar]
- Bua E, McKiernan SH, Wanagat J, McKenzie D, Aiken JM. Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J Appl Physiol. 2002;92:2617–2624. doi: 10.1152/japplphysiol.01102.2001. [DOI] [PubMed] [Google Scholar]
- Bua E, Johnson J, Herbst A, Delong B, McKenzie D, Salamat S, Aiken JM. Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Amer J Human Gen. 2006;79:469–480. doi: 10.1086/507132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BM. Factors influencing the repair and adaptation of muscle in aged individuals: satellite cells and innervation. J Gerontol A Biol Sc Med S. 1995;50:96–100. doi: 10.1093/gerona/50a.special_issue.96. [DOI] [PubMed] [Google Scholar]
- Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–994. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Kemnitz JW. Aging experiments in nonhuman primates. In: Yu BP, editor. Methods in Aging Research. CRC Press; Boca Raton, FL: 1998. pp. 249–267. [Google Scholar]
- Colman RJ, Roeker EB, Ramsey JJ, Kemnitz JW. The effect of dietary restriction on body composition in adult male and female Rhesus macaques. Aging Clin Exp Res. 1998;10:83–92. doi: 10.1007/BF03339642. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Ramsey JJ, Roecker EB, Havighurst T, Hudson JC, Kemnitz JW. Body fat distribution with long-term dietary restriction in adult male Rhesus macaques. J Gerontol Biol Sci. 1999;54A:B283–B290. doi: 10.1093/gerona/54.7.b283. [DOI] [PubMed] [Google Scholar]
- Colman RJ, McKiernan SH, Aiken JM, Weindruch R. Muscle mass loss in rhesus monkeys: Age of onset. Exp Gerontol. 2005;40:573–581. doi: 10.1016/j.exger.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci. doi: 10.1093/gerona/63.6.556. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2:21–29. doi: 10.1046/j.1474-9728.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- Doherty TJ. Invited review: aging and sarcopenia. Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Dubowitz V. Muscle biopsy: a practical approach. Bailliere Tindall; London: 1985. [Google Scholar]
- Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev. 2006;5:179–195. doi: 10.1016/j.arr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharm Phys. 2007;34:1091–1096. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- Gokey NG, Cao Z, Pak J, Lee D, McKiernan SH, McKenzie D, Weindruch R, Aiken JM. Molecular analyses of mtDNA deletion mutations in microdissected skeletal muscle fibers from aged Rhesus monkeys. Aging Cell. 2004;3:319–326. doi: 10.1111/j.1474-9728.2004.00122.x. [DOI] [PubMed] [Google Scholar]
- Herbst A, Pak J, McKenzie D, Bua E, Bassiouni M, Aiken J. Accumulation of mitochondrial DNA deletion mutations in aged muscle fibers: Evidence for a causal role in muscle fiber loss. J Gerontol. 2007;62A:235–245. doi: 10.1093/gerona/62.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Singh MAF. Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity and health. J Gerontol. 2001;56A:B209–B217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- Janssen I. Influence of sarcopenia on the development of physical disability: The cardiovascular health study. JAGS. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- Jejurikar SS, Henkelman EA, Cederna PS, Marcelo CL, Urbanchek MG, Kuzon WM. Aging increases the susceptibility of skeletal muscle derived satellite cells to apoptosis. Exp Gerontol. 2006;41:828–836. doi: 10.1016/j.exger.2006.06.053. [DOI] [PubMed] [Google Scholar]
- Jouffroy FK, Stern JT, Jr, Medina MF, Larson SG. Function and cytochemical characteristics of postural limb muscles of the rhesus monkey: A telemetered EMG and immunofluorescence study. Folia Primatol. 1999;70:235–253. doi: 10.1159/000021703. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Weindruch R, Roecher EB, Crawford K, Kaufman PL, Ershler WB. Dietary restriction of adult male rhesus monkeys: design, methodology, and preliminary findings from the first year of study. J Gerontol Biol Sci. 1993;48:B17–B26. doi: 10.1093/geronj/48.1.b17. [DOI] [PubMed] [Google Scholar]
- Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. J Am Geriatr Soc. 2001;49:1633–1640. doi: 10.1038/sj.ejcn.1601198. [DOI] [PubMed] [Google Scholar]
- Larsson L. Motor units: remodeling in aged animals. J Gerontol A Biol Sci Med Sci. 1995;50:91–95. doi: 10.1093/gerona/50a.special_issue.91. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Russo CR, Bandinelli S, Baraili B, Cavazzini C, DiIorio A, Carsi AM, Rantanen T, Guralnick FM, Ferricci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- Lee WS, Cheung WH, Qin L, Tang N, Leung KS. Age-associated decrease of type IIa/B human skeletal muscle fibers. Clin Ortho Rel Res. 2006;450:231–237. doi: 10.1097/01.blo.0000218757.97063.21. [DOI] [PubMed] [Google Scholar]
- Lee CE, McArdle A, Griffiths RD. The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr. 2007;26:524–534. doi: 10.1016/j.clnu.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? J Neuro Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC. Variability in muscle fibre areas in whole human quadriceps muscle: effects of increasing age. J Anat. 1991;174:239–249. [PMC free article] [PubMed] [Google Scholar]
- Lopez M, VanZeeland NL, Dahl DB, Weindruch RW, Aiken JM. Cellular phenotypes of age-associated skeletal muscle mitochondrial abnormalities in rhesus monkey. Mut Res. 2000;452:123–138. doi: 10.1016/s0027-5107(00)00059-2. [DOI] [PubMed] [Google Scholar]
- Lushaj EB, Johnson JK, McKenzie D, Aiken JM. Sarcopenia accelerates at advanced ages in Fisher 344 × Brown Norway rats. J Gerontol. doi: 10.1093/gerona/63.9.921. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie D, Bua E, McKiernan S, Cao Z, Wanagat J, Aiken JM. Mitochondrial DNA deletion mutations: A causal role in sarcopenia. Eur J Biochem. 2002;269:2010–2015. doi: 10.1046/j.1432-1033.2002.02867.x. [DOI] [PubMed] [Google Scholar]
- Melton LJ, Khosla S, Crowso CS, O'Connor M, O'Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Amer Geriat Soc. 2000;48:625–630. [PubMed] [Google Scholar]
- Muller FL, Song W, Jang YC, Kiu Y, Sabia M, Richardson A, VanRemmen H. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Com Physiol. 2007;293:R1159–R1168. doi: 10.1152/ajpregu.00767.2006. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PWR. Oxidative capacity of human muscle fiber types: effect of age and training status. J Appl Physiol. 1995;48:2033–2038. doi: 10.1152/jappl.1995.78.6.2033. [DOI] [PubMed] [Google Scholar]
- Rader EP, Faulkner JA. Recovery from contraction-induced injury is impaired in weight-bearing muscles of old male mice. J Appl Physiol. 2006;100:656–661. doi: 10.1152/japplphysiol.00663.2005. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Hughes VA. Sarcopenia: Current concepts. J Gerontol MS. 2000;55:M716–M724. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Castaneda C. Sarcopenia – understanding the dynamics of aging muscle. JAMA. 2001;286:1230–1231. doi: 10.1001/jama.286.10.1230. [DOI] [PubMed] [Google Scholar]
- Roy A. Estimating correlation coefficient between two variables with repeated observations using mixed effects model. Biom J. 2006;48:286–301. doi: 10.1002/bimj.200510192. [DOI] [PubMed] [Google Scholar]
- Roy RR, Bodine-Fowler SC, Kim J, Hawue N, deLeon D, Rudolph W, Edgerton VR. Architectural and fiber type distribution properties of selected rhesus leg muscles: feasibility of multiple independent biopsies. Acta Anat. 1991;140:350–356. doi: 10.1159/000147081. [DOI] [PubMed] [Google Scholar]
- Seligman AM, Karnovsky MJ, Wasserkrug HL, Hanker JS. Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB) J Cell Biol. 1968;38:1–14. doi: 10.1083/jcb.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DC, Hrapchak BB. Theory and Practice of Histotechnology. Battellle Press; Columbus, Ohio: 1980. [Google Scholar]
- Schwartz SM, Kemnitz JW. Age- and gender-related changes in body size, adiposity, and endocrine and metabolic parameters in free-ranging Rhesus macaques. Am J Phys Anthropol. 1992;89:109–121. doi: 10.1002/ajpa.1330890110. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Barron MJ, Borthwick GM, Gospel A, Chinnery PF, Samuels DC, Taylor GA, Plusa SM, Needham SJ, Greaves LC, Kirkwood TBL, Turnbull DM. Mitochondrial DNA mutations in human colonic crypt stem cells. J Clin Invest. 2003;112:1351–1360. doi: 10.1172/JCI19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26:389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Weindruch R. Interventions based on the possibility that oxidative stress contributes to sarcopenia. J Gerontol A Biol Sci Med Sci. 1995;50:157–161. doi: 10.1093/gerona/50a.special_issue.157. [DOI] [PubMed] [Google Scholar]
- Zamboni M, Zoico E, Scartezzini T, Mazzali G, Tosoni P, Zivelonghi A, Gallagher D, DePergola G, DiFrancesco V, Bosello O. Body composition changes in stable-weight elderly subjects: the effect of sex. Aging Clin Exp Res. 2003;15:321–327. doi: 10.1007/BF03324517. [DOI] [PubMed] [Google Scholar]