Abstract
Background
Inducibility of atrial fibrillation (AF) with burst pacing after pulmonary vein (PV) isolation is associated with recurrent AF.
Objective
This study evaluated whether an external 30 Joule (J) shock synchronized to the R wave, during the vulnerable period of atrial repolarization, is able to risk-stratify patients further for AF recurrence after PV isolation.
Methods
One hundred and sixteen consecutive patients underwent PV isolation for AF. Atrial burst pacing was performed after PV isolation. In patients without AF induced by burst pacing, a biphasic external 30 J shock synchronized to the R wave was delivered as a further test for inducible AF. Patients were followed for a mean of 16 months, and recurrent AF was defined as more than 10 sec of AF on ambulatory monitoring.
Results
AF was induced in 19 (16%) of patients with burst pacing. Eighty-one patients who were noninducible with burst pacing had a 30 J shock administered, which induced AF in 16 (20%). In follow-up, 21% of patients who were noninducible with burst pacing or low-energy shock vs 54% who were inducible with either test developed recurrent AF at one year (HR 3.18, P = 0.0004 on multivariate analysis). Among patients who were noninducible with burst pacing, 18% who were noninducible with a low-energy shock vs 60% who were inducible with shock developed recurrent AF at one year (HR = 4.63, P = 0.0006 on multivariate analysis).
Conclusion
Inducibility of AF by a 30 J shock delivered during atrial repolarization after PV isolation may predict AF recurrence. Evaluation of inducibility of AF with burst pacing and a biphasic external synchronized shock after PV isolation may help guide postprocedure management.
Keywords: atrial fibrillation, catheter ablation, pulmonary vein isolation, electrophysiology study, biphasic electrical shock
Introduction
Catheter ablation of atrial fibrillation (AF) has become a widely accepted treatment in the management of AF refractory to medical therapy. Since Haissaguerre et al. first introduced the concept of ablation of pulmonary vein triggers of AF in 1998, the technique of catheter ablation has been modified extensively.1 Current techniques used for ablation of AF aim to eliminate both the triggers of AF and the mechanisms that perpetuate AF.2–9
Since AF ablation employs somewhat empiric techniques, predictors of procedural success have been sought by a number of investigators. The presence of paroxysmal versus persistent or permanent AF has been a widely recognized predictor of freedom from AF in follow-up.2,10 Left atrial scar, atrial size, and age have been reported as predictors of procedural success.11 We and others have also shown that inducibility of AF by burst pacing of the atrium is correlated with an increased incidence of recurrent AF in follow-up.12,13
Despite the increasing popularity and ongoing refinement of these procedures, recent reported success rates range from 52% to 88%, and additional predictors of procedural success are needed to guide increasingly complex ablation strategies.2,7,14–16 This study was performed to determine whether a novel technique—inducibility of AF by a low-energy shock during the vulnerable period of atrial repolarization—is predictive of recurrent AF in follow-up.
Methods
Study Subjects
One hundred and sixteen consecutive patients with symptomatic paroxysmal (44%), persistent (34%), and permanent (22%) AF undergoing pulmonary vein (PV) isolation (PV) by a single operator at our institution were studied. AF was classified as persistent if it was not self-terminating within 7 days or was cardioverted electrically or pharmacologically and classified as permanent if it lasted 6 months and cardioversion failed or was not attempted.17 Among the 25 patients with permanent AF, 10 had failed cardioversion without any return of sinus rhythm, 4 had developed recurrent AF less than one week after cardioversion, and 11 had been in AF for longer than 6 months. All patients had episodes of AF in the 6 months prior to PV isolation. Patients had failed therapy with a mean of 1.3 ± 1 antiarrhythmic agents prior to ablation. Patients, except those who had recently been treated with amiodarone (17 patients), discontinued antiarrhythmic drug therapy prior to the electrophysiologic study. Seventeen patients included in this study were undergoing a repeat ablation procedure (6 underwent their prior ablation at other institutions and 11 at our institution).
All patients gave written informed consent prior to the procedure and the collection of patient data in this study was approved by the Committee on Clinical Investigations of Beth Israel Deaconess Medical Center.
Electrophysiologic Study
Circumferential antral ablation around the PVs using bidirectional PV isolation as an endpoint was performed on all patients as described previously.18 All procedures were performed under general anesthesia. Three-dimensional electroanatomic mapping and ablation outside the PV ostia were performed using an 8 mm NaviStar catheter (Biosense Webster, Diamond Bar, CA, USA, used in 111 patients) or 3.5 mm Navistar Thermocool irrigated catheter (Biosense Webster, used in five patients). Ablation was performed approximately 5–10 mm outside each PV ostium until conduction block was achieved. As previously described, bidirectional block was defined by PV entrance block with the loss of PV potentials on the circumferential catheter and exit block with failure to capture the left atrium during sinus rhythm by pacing (at 10 mA and 2.5 ms pulse width) each of the bipolar pairs of electrodes of the circumferential catheter placed at the ostium of each pulmonary vein.18 Testing for bidirectional block was repeated at least 20 min after ablation at each PV. All PVs were isolated in all patients routinely in sinus rhythm. Patients in AF at the onset of the study were cardioverted internally to sinus rhythm during initial PV isolation.
Inducibility Testing
Inducibility testing was performed following bidirectional PV isolation. As previously described, induction of AF was attempted by burst pacing at a cycle length of 250 ms with sequential decrement down to 200 ms (as long as 1:1 capture was maintained) for 5–10 sec at least three times from the coronary sinus catheter and then repeated from the right atrium.12 Isoproterenol up to 20 mcg/min IV was then administered to identify triggers or reconnection, and burst atrial pacing was repeated. If AF was induced by burst pacing, isolation of all PVs was reassessed. If recurrent PV conduction was observed, further ablation was performed until bidirectional block was again established. Final inducibilty testing with burst pacing was performed after persistent electrical PV isolation was confirmed. Fifteen (19%) patients with left atrial tachycardia induced by burst pacing underwent attempted ablation of the tachycardia as indicated. Four (5%) of these patients underwent creation of an ablation line from the left inferior pulmonary vein to the mitral annulus when activation suggested the possibility of a macroreentrant rhythm.
Patients who were noninducible with burst atrial pacing underwent further evaluation for inducibility during continued infusion of isoproterenol 10–20 mcg/min IV. A 30 J external biphasic shock (LifePak 12, Medtronic Emergency Response, Redmond, WA, USA) synchronized to the R wave was delivered between external defibrillator pads (PadTac, TZ Medical, Portland, OR, USA) placed in the anterior–posterior configuration. The external defibrillator was programmed to deliver a standard synchronized shock timed to the peak of the R wave, though practical testing and observation has shown that at this setting the shock is delivered during the last 20–40 ms of the QRS complex. Three of the first patients were administered a 50 J shock, but subsequent patients were tested with a 30 J shock. The shock was delivered during sinus rhythm or coronary sinus pacing at a cycle length of 600 ms (in patients with an accelerated junctional rhythm on isoproterenol). Patients were considered “shock-inducible” if AF lasting longer than 10 sec occurred after the external shock (see Fig. 1). If AF was induced, all PVs were again interrogated for entrance block, and if all were found to be isolated, the patient was cardioverted and no further left atrial ablation was performed. If reconnection was found, further ablation was performed until complete bidirectional block was demonstrated. If this was done, after restoration of sinus rhythm the 30 J synchronized shock was repeated to evaluate inducibility of AF.
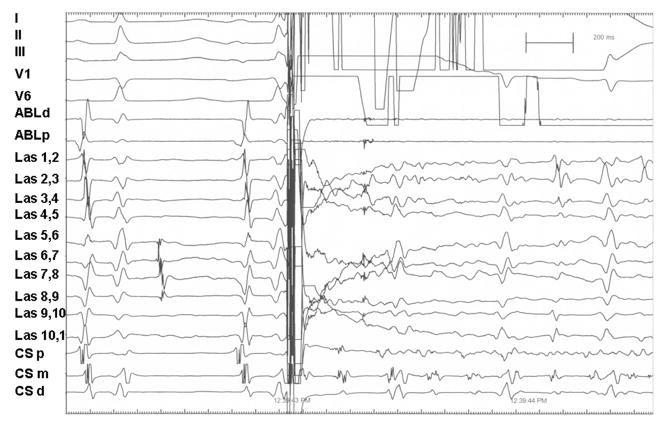
Figure 1.
External 30 J shock during sinus rhythm induces atrial fibrillation. During sinus rhythm a 30 J external biphasic shock synchronized to the R wave is delivered, which induces atrial fibrillation. Note the dissociated pulmonary vein electrogram seen on Lasso catheter poles 5–9 prior to the external shock, demonstrating successful exit and entrance block to this pulmonary vein. Surface leads I, II, III, V1, and V6 are shown as labeled. Intracardiac recordings from an 8 mm tip ablation catheter at the proximal and distal electrodes, 10 overlapping bipolar recordings from a circumferential catheter (Lasso 1–10) placed in the left inferior pulmonary vein, and bipolar recordings from the distal (CSd), mid- (CSm), and proximal (CSp) coronary sinus catheter are shown at a paper speed of 100 mm/sec.
The right atrial cavotricuspid isthmus was ablated to achieve bidirectional block in 62 (53%) patients with a history of typical atrial flutter or inducible typical isthmus-dependent atrial flutter observed during the procedure. In addition, 19 (16%) patients with inducible AF underwent ablation in the region of the superior vena cava-right atrial junction guided by the circumferential catheter and empirically along the septum in the region of Bachman’s bundle, to affect interatrial conduction. Right atrial tachycardias were not routinely mapped.
Follow-Up
After ablation, patients were observed overnight and started on warfarin with an international normalized ratio goal of 2–3 for at least 6 months postprocedure. In addition, patients with persistent or permanent AF or with risk factors for stroke were treated with subcutaneous enoxaparin twice daily until the international normalized ratio value was >2.0. Antiarrhythmic medications were continued for at least one month in 50 (43%) patients and reinitiated in 10 patients with early (less than 30 days) recurrence of AF. Antiarrhythmic drugs (AAD) were continued in patients who remained inducible after ablation or who developed recurrent AF in early follow-up and discontinued in follow-up for patients free of arrhythmias. Seventeen (15%) patients were treated with amiodarone immediately after ablation (all of these patients had been on amiodarone prior to ablation), and this was continued after 30 days in 16 (14%) patients. AAD therapy with other agents was continued during follow-up in an additional 29 patients due to recurrence of AF or atrial tachycardia. Evaluation for atrial fibrillation in follow-up was performed using a 2-week transtelephonic event recorder with routine twice-daily and symptomatic transmissions upon discharge from the hospital. In addition, patients were scheduled to undergo 7–14 day mobile continuous outpatient telemetry at 1, 3, 6, and 12 months postprocedure. For patients with symptoms, additional monitoring was performed. The primary outcome of recurrent AF was defined as any symptomatic or asymptomatic episode of AF lasting longer than 10 sec occurring more than 30 days postprocedure.19 Recurrent AF in the first 30 days postprocedure was not considered in the outcome analysis since 30–57% of patients with early recurrence of AF may be free of late AF.20,21 Patients with documented atrial tachycardia without any evidence of AF on monitoring were not considered to have met the primary endpoint of recurrent AF. Once a patient developed recurrent AF after 30 days, they were considered to have reached the endpoint of the study, and were not considered free of AF even if they developed no further AF with a change in medical therapy.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation. Differences among groups of continuous variables were evaluated by analysis of variance (ANOVA). Dichotomous variables were compared using chi-square analysis. Cumulative AF-free survival was determined using the Kaplan-Meier method, and differences in AF free survival were evaluated using the log-rank test. We evaluated predictors of AF-free survival using first using univariate and then multivariate Cox regression analysis. Variables evaluated included age, sex, paroxysmal vs persistent or permanent AF, hypertension, coronary artery disease, mitral regurgitation, valvular heart disease, left atrial size, diabetes, ablation procedure duration, ablation time, prior AF ablation procedure, and medication use (antiarrhythmic drugs, rate control drugs, ACE inhibitors, angiotensin receptor blockers, and statins). Final multivariable regression models were chosen using a stepwise selection process requiring a significant P value (0.05) for a variable to be retain in the model. All significance tests were two-sided, and a P value < 0.05 was considered statistically significant. All statistical analyses were performed using SAS Release 8.2 statistical software package (SAS Institute Inc., Cary, NC, USA).
Results
Baseline patient characteristics are presented in Table 1. All patients underwent circumferential PV antral isolation with confirmation of bidirectional block in 113 of 116 (97%).
TABLE 1.
Patient Characteristics
| Entire Cohort (n = 116) |
Patients Undergoing Shock (n = 81)* |
||||||
|---|---|---|---|---|---|---|---|
| All Patients | Inducible by Burst Pacing or Shock | Non-Inducible by Burst or Shock | P-Value | Inducible by Shock | Non-Inducible by Shock | P-Value | |
| (n = 116) | (n = 35) | (n = 81) | (n = 16) | (n = 65) | |||
| Age (years) | 56 ± 12 | 57 ± 11 | 55 ± 12 | 0.529 | 54 ± 10 | 54 ± 12 | 0.800 |
| Male gender | 90 (78%) | 28 (80%) | 62 (77%) | 0.682 | 15 (94%) | 47 (72%) | 0.070 |
| Paroxysmal AF | 51 (44%) | 13 (37%) | 38 (47%) | 0.331 | 7 (44%) | 32 (49%) | 0.694 |
| Persistent AF | 40 (35%) | 10 (29%) | 30 (37%) | 0.379 | 4 (25%) | 21 (32%) | 0.570 |
| Permanent AF | 25 (22%) | 12 (34%) | 13 (16%) | 0.028 | 5 (31%) | 12 (18%) | 0.261 |
| Valvular heart disease | 22 (19%) | 10 (29%) | 12 (15%) | 0.083 | 4 (25%) | 11 (17%) | 0.362 |
| Mitral regurgitation | 70 (60%) | 22 (63%) | 48 (59%) | 0.716 | 4 (25%) | 6 (9%) | 0.519 |
| Left ventricular hypertrophy | 31 (27%) | 12 (34%) | 19 (23%) | 0.226 | 4 (25%) | 15 (23%) | 0.871 |
| Structural heart disease | 47 (41%) | 17 (49%) | 30 (37%) | 0.254 | 8 (50%) | 30 (46%) | 0.338 |
| Ejection fraction | 0.56 ± 0.07 | 0.55 ± 0.09 | 0.56 ± 0.05 | 0.282 | 0.56 ± 0.11 | 0.58 ± 0.06 | 0.099 |
| LA diameter (cm) | 4.2 ± 0.8 | 4.4 ± 0.9 | 4.2 ± 0.8 | 0.092 | 4.5 ± 0.8 | 4.1 ± 0.7 | 0.080 |
| Prior AF ablation | 17 (15%) | 5 (14%) | 12 (15%) | 0.941 | 1 (6%) | 10 (15%) | 0.339 |
| Procedure duration (min) | 242 ± 60 | 240 ± 48 | 243 ± 65 | 0.836 | 234 ± 47 | 236 ± 61 | 0.918 |
| Ablation time (min) | 58 ± 19 | 67 ± 19 | 55 ± 18 | 0.002 | 62 ± 17 | 52 ± 17 | 0.067 |
Only patients that were noninducible by burst pacing underwent testing of inducibity by shock.
Inducibility Testing
Of the 116 patients undergoing PV isolation, 19 (16%) had AF inducible with burst pacing despite confirmation of electrical PV isolation and additional left atrial ablation, while 97 (84%) were not inducible with burst pacing. Three of the 19 patients inducible by burst pacing were only inducible during isoproterenol infusion. Nine patients who were initially inducible by burst atrial pacing were rendered noninducible by subsequent additional ablation and were categorized as noninducible by burst pacing. Twenty-six patients had atrial tachycardia induced by burst pacing, but after additional ablation, 20 of these patients had no inducible atrial tachyarrhythmias and were classified as noninducible with burst pacing.
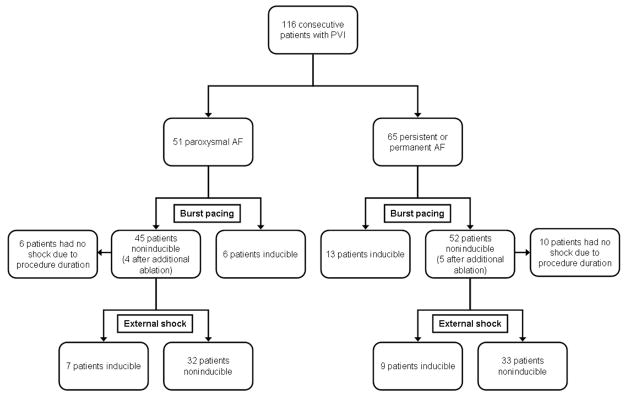
Eighty-one patients in whom AF was not inducible with burst pacing underwent subsequent evaluation with a synchronized external shock (see Table 1). AF was induced in 16 (20%) of these patients with a synchronized external shock, and 65 (80%) were noninducible. The sequence of inducibility testing is shown in Figure 2. Sixteen patients did not undergo inducibility testing with an external shock due to prolonged procedure times.
Figure 2.
Flow diagram of consecutive patients undergoing catheter ablation of AF. All patients enrolled are divided into paroxysmal and persistent or permanent AF groups. The response to burst atrial pacing and, in patients who were noninducible by pacing, synchronized external shock is shown. PVI = Pulmonary vein isolation, AF = atrial fibrillation.
Outcomes
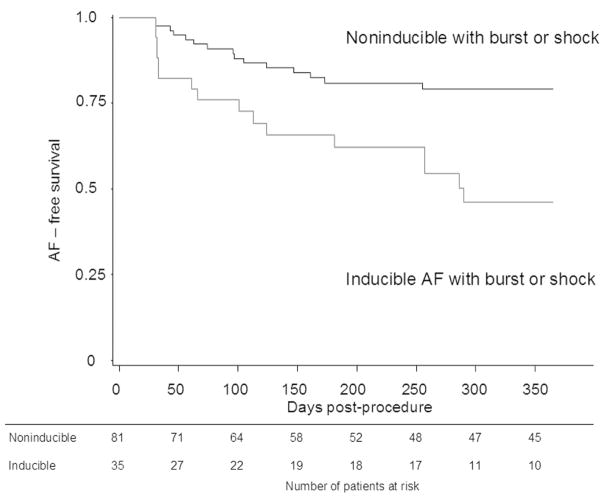
Mean follow-up duration was 16 ± 9 months. At study exit, 45 (39%) of study patients remained on AAD. Patients with recurrent AF were more likely to continue AAD than patients who remained free of AF (51% vs 32%, P = 0.050). Among the 19 patients with AF inducible with burst atrial pacing, 49% developed recurrent AF at 1 year, compared with 27% of patients who were noninducible with burst pacing (P = 0.049). When inducibility by either burst pacing or synchronized shock was considered, the rate of recurrent AF was again significantly greater in inducible vs. noninducible patients. Survival curves for inducibility by burst or shock are shown in Figure 3. These demonstrate a significantly greater rate of AF in inducible patients, with 54% vs 21% of patients developing recurrent AF at one year on Kaplan-Meier analysis for patients who were inducible with burst or shock vs those who were noninducible by either method (P= 0.0005). Prevalence of atrial tachycardia in follow-up was similar among patients who were inducible and noninducible (11% vs 15%, P = 0.773).
Figure 3.
Kaplan-Meier curves showing AF-free survival at 12 months according to inducibility of AF by either burst atrial pacing or synchronized external shock. The curves demonstrate a significantly greater rate of recurrent AF in patients who were inducible with either burst atrial pacing or a synchronized external shock compared with patients who were not inducible by either method (P = 0.0005). The table below the graph represents the number of patients at risk in each group at the beginning of each time interval.
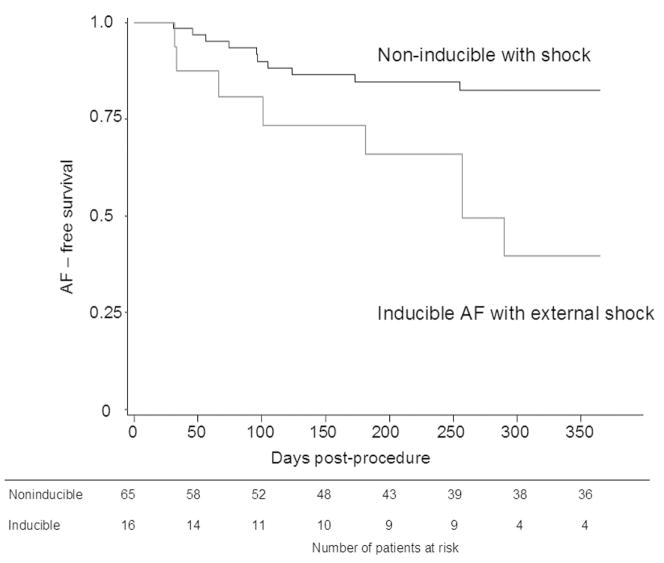
Among patients that were not inducible by burst pacing, inducibility by synchronized shock significantly predicted AF recurrence. Survival curves are shown in Figure 4, which demonstrate a significantly greater rate of recurrent AF (60% vs 18%) at one year in patients who were inducible with a synchronized external shock (P = 0.0005) compared with those who were not inducible. Prevalence of atrial tachycardia in follow-up was also similar among patients who were inducible and noninducible by shock (13% vs 19%, P = 0.725).
Figure 4.
Kaplan-Meier curves showing AF-free survival at 12 months according to inducibility of AF by a synchronized external shock. The curves demonstrate a significantly greater rate of recurrent AF in patients who were not inducible with burst atrial pacing but had AF induced by a synchronized external shock compared with patients who were not inducible by burst pacing or synchronized shock (P = 0.0005). The table below the graph represents the number of patients at risk in each group at the beginning of each time interval.
Predictors of AF Recurrence
Among the entire cohort of patients undergoing PV isolation in this study, univariate analysis revealed that inducible AF by burst pacing, inducible AF by a synchronized external shock or burst pacing, paroxysmal AF (vs persistent or permanent AF), and mitral regurgitation were the only variables significantly associated with recurrent AF in follow-up. In multivariate analysis evaluating inducibility by burst pacing, inducibility, paroxysmal AF, and mitral regurgitation were significantly associated with the primary outcome (HR = 2.79, 95% confidence intervals [CI] 1.30–6.00, P = 0.008 for burst pacing, HR = 0.38, CI 0.19–0.78, P = 0.008 for paroxysmal AF, and HR = 2.50, CI 1.17–5.34, P = 0.018 for mitral regurgitation). Likewise, in multivariate analysis evaluating inducibility by burst or shock, inducibility, paroxysmal AF, and mitral regurgitation were the only significant predictors of AF recurrence (HR = 3.18, CI 1.67–6.08, P = 0.0004 for burst or shock inducibility, HR = 0.37, CI 0.18–0.76, P = 0.007 for paroxysmal AF, and HR = 2.43, CI 1.14–5.17, P = 0.022 for mitral regurgitation). Only five patients underwent treatment with an externally irrigated catheter; therefore, the effect of catheter selection on recurrence rates could not be adequately assessed.
Among the subset of 81 patients who underwent testing with a synchronized shock, univariate analysis demonstrated that inducible AF with shock and age were the only variables associated with recurrent AF. In multivariate analysis, only shock-inducibility and age were significant predictors of recurrent AF in follow-up (HR = 4.63, CI 1.93–11.09, P = 0.0006 for shock inducibility, and HR = 0.95, CI 0.92–0.99, P = 0.013 for each year of age).
Complications
Significant complications occurred in 6 of 116 patients. Pericardial effusion was documented in two patients and progressed to tamponade in one patient, requiring placement of a percutaneous drain. Major bleeding requiring blood transfusion occurred in two patients. One patient had blurred vision in one eye with a normal brain MRI, and was diagnosed with transient retinal artery occlusion. Another patient developed partial paralysis of the right hemidiaphragm. No pulmonary vein stenosis (defined as >70% reduction in diameter on follow-up imaging), atrio-esophageal fistulas, or deaths occurred.
Discussion
The main finding of this study was that inducibility of AF with a synchronized external biphasic shock delivered during the vulnerable period of atrial refractoriness is associated with recurrent AF after PV isolation. This predictor of AF recurrence provides additive information over inducibility with burst atrial pacing alone. This test provides a simple, rapid method of determining a patient’s likelihood of developing recurrent AF that may be used in planning postprocedure management.
Mechanism of Inducibility
A low to intermediate electrical shock delivered at the peak of the T wave during the relative refractory period of the ventricular myocardium will induce ventricular fibrillation in most subjects. Atrial myocardium also has a period of vulnerability during the relative refractory period during which an electrical shock will induce AF. Prior human studies have demonstrated that low energy shocks delivered during this vulnerable period are effective at inducing AF.22,23 One of these studies reported that the vulnerable period was significantly longer and that AF was more inducible at higher energies in patients with a history of AF compared with study subjects without prior AF.22 Upper and lower limits of vulnerability for the induction of AF were proposed to be 31 and 4 J in a study of patients undergoing ICD testing using epicardial electrodes.24 The 30 J external shock delivered in this study is consistent with this proposed range.
The mechanism of induction of fibrillation with an electrical shock during the relative refractory period is based on heterogeneous repolarization during this phase of atrial activation. The dispersion of atrial refractoriness at the time of electrical stimulation leads to incomplete depolarization and variable and irregular wavefronts of excitation that may spread and deteriorate into AF. Compared with normal subjects, patients with AF have shorter effective refractory periods but slower conduction and a greater dispersion of refractoriness, characteristics which may predispose to irregular impulse propagation during a longer relative refractory period.25,26
The likely explanation for the lack of inducibility immediately after catheter ablation is that the substrate facilitating perpetuation of AF has been significantly modified with catheter ablation. Therefore, the inability of the atrial myocardium to sustain AF after ablation may be the property tested with the synchronized external shock. Patients with persistent or permanent AF may have a different atrial substrate than patients with paroxysmal AF, and may have a different mechanism of AF, yet shock inducibility results were similar in each group. The similar results of this test across different types of AF reflects the generalizability of shock inducibility testing to evaluate the efficacy of ablation.
Utility of Testing for Inducibility
The finding of inducible AF may be used to guide more extensive catheter ablation in search of more effective substrate modification. A more common finding, however, is acute reconnection of the pulmonary veins in patients who are found to be inducible after PV isolation, and further ablation resulting in persistent isolation will often render patients noninducible.27 Many patients may have a durable success with pulmonary vein isolation alone, but some patients require more extensive ablation of nonpulmonary vein triggers of AF. Testing for inducibility of AF after isolation of the pulmonary veins may allow the operator to guide the ablation procedure in stages. This “tailored” method of catheter ablation of AF has been recently advocated in the literature.5,28 Many groups use administration of isoproterenol to identify triggers and guide further ablation. This study supports using more aggressive maneuvers to identify the presence of a substrate able to sustain AF. Rapid burst pacing followed by pacing on high-dose isoproterenol may identify patients with persistent inducible AF. In patients who pass this initial test, a synchronized biphasic external shock may reveal additional patients who have sufficient substrate to maintain AF.
Inducible AF by burst pacing or synchronized external shock may also be used to guide postprocedure management. In patients who remain inducible after pulmonary vein isolation, the likelihood of recurrent AF in follow-up is higher. Therefore, continuation of antiarrhythmic medication after AF ablation may be warranted in these patients.
Limitations
One limitation of this study is that baseline shocks to assess inducibility were not performed, and, therefore, the possibility exists that the results of inducibility testing did not reflect the effects of ablation, but rather an intrinsic property of the myocardium. Even if this is the case, the predictive capacity in patients undergoing this procedure remains valid. In addition, no control group of patients was administered synchronized external shocks, and varying energy shocks were not tested, so it is possible that a higher or lower energy shock may have had a better association with outcomes.
While the external shock was synchronized by the external defibrillator to the peak of the R wave, PR interval varies among patients, thus placing the shock in relatively different points of atrial repolarization, which could affect inducibility of AF. Unfortunately, we were unable to determine whether the coupling interval from the P wave to the shock was related to inducibility. In addition, review of the electrograms of the synchronized shock show that the shock was generally timed near the end of the QRS complex (see Figs. 1 and 2). However, prior studies of the use of shocks to induce AF demonstrated a fairly wide zone of inducibility from the upslope of the R wave to near the end of the QRS.22 Finally, patient characteristics such as obesity and thoracic height may change the resistance of the body cavity and therefore alter the effective energy delivery to the heart which may affect AF inducibility. A comparison of body mass index in patients with and without shock-inducible AF showed no significant difference, however.
Conclusion
This study suggests that inducibility of AF with a synchronized low-energy shock delivered during atrial refractoriness after PV isolation predicts recurrent AF in follow-up. Using a synchronized shock to determine inducibility of AF provides additional predictive information beyond burst pacing. Further research is indicated to determine the most effective ablation strategy and management for patients who remain inducible despite effective pulmonary vein isolation.
Acknowledgments
Dr. Essebag is the recipient of a Clinician Scientist Award from the Canadian Institutes of Health Research (CIHR). Dr. Reynolds is the recipient of grant K23 HL077171 from the NHLBI.
References
- 1.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Oral H, Knight BP, Tada H, Ozaydin M, Chugh A, Hassan S, Scharf C, Lai SW, Greenstein R, Pelosi F, Jr, Strickberger SA, Morady F. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–1081. doi: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 3.Karch MR, Zrenner B, Deisenhofer I, Schreieck J, Ndrepepa G, Dong J, Lamprecht K, Barthel P, Luciani E, Schomig A, Schmitt C. Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: A randomized comparison between 2 current ablation strategies. Circulation. 2005;111:2875–2880. doi: 10.1161/CIRCULATIONAHA.104.491530. [DOI] [PubMed] [Google Scholar]
- 4.Haissaguerre M, Jais P, Shah DC, Garrigue S, Takahashi A, Lavergne T, Hocini M, Peng JT, Roudaut R, Clementy J. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000;101:1409–1417. doi: 10.1161/01.cir.101.12.1409. [DOI] [PubMed] [Google Scholar]
- 5.Arruda M, Natale A. The adjunctive role of nonpulmonary venous ablation in the cure of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:S37–S43. [Google Scholar]
- 6.Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000;102:2619–2628. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- 7.Pappone C, Manguso F, Vicedomini G, Gugliotta F, Santinelli O, Ferro A, Gulletta S, Sala S, Sora N, Paglino G, Augello G, Agricola E, Zangrillo A, Alfieri O, Santinelli V. Prevention of iatrogenic atrial tachycardia after ablation of atrial fibrillation: A prospective randomized study comparing circumferential pulmonary vein ablation with a modified approach. Circulation. 2004;110:3036–3042. doi: 10.1161/01.CIR.0000147186.83715.95. [DOI] [PubMed] [Google Scholar]
- 8.Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu LF, Garrigue S, Takahashi Y, Rotter M, Sacher F, Scavee C, Ploutz-Snyder R, Jalife J, Haissaguerre M. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 9.Callans DJ. Comparing different strategies for catheter ablation of atrial fibrillation. Circulation. 2005;111:2866–2868. doi: 10.1161/CIRCULATIONAHA.105.541904. [DOI] [PubMed] [Google Scholar]
- 10.Bourke JP, Dunuwille A, O’Donnell D, Jamieson S, Furniss SS. Pulmonary vein ablation for idiopathic atrial fibrillation: Six month outcome of first procedure in 100 consecutive patients. Heart. 2005;91:51–57. doi: 10.1136/hrt.2003.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, Schweikert RA, Saliba W, Cummings J, Burkhardt JD, Bhargava M, Belden WA, Abdul-Karim A, Natale A. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: An independent predictor of procedural failure. J Am Coll Cardiol. 2005;45:285–292. doi: 10.1016/j.jacc.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Essebag V, Baldessin F, Reynolds MR, McClennen S, Shah J, Kwaku KF, Zimetbaum P, Josephson ME. Non-inducibility post-pulmonary vein isolation achieving exit block predicts freedom from atrial fibrillation. Eur Heart J. 2005;26:2550–2555. doi: 10.1093/eurheartj/ehi577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oral H, Chugh A, Lemola K, Cheung P, Hall B, Good E, Han J, Tamirisa K, Bogun F, Pelosi F, Jr, Morady F. Noninducibility of atrial fibrillation as an end point of left atrial circumferential ablation for paroxysmal atrial fibrillation: A randomized study. Circulation. 2004;110:2797–2801. doi: 10.1161/01.CIR.0000146786.87037.26. [DOI] [PubMed] [Google Scholar]
- 14.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 15.Arruda M, Natale A. The adjunctive role of nonpulmonary venous ablation in the cure of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:S37–S43. [Google Scholar]
- 16.Essebag V, Wylie JV, Josephson ME. Effectiveness of catheter ablation of atrial fibrillation. Eur Heart J. 2006;27:130–131. doi: 10.1093/eurheartj/ehi625. [DOI] [PubMed] [Google Scholar]
- 17.McNamara RL, Brass LM, Drozda JP, Jr, Go AS, Halperin JL, Kerr CR, Levy S, Malenka DJ, Mittal S, Pelosi F, Jr, Rosenberg Y, Stryer D, Wyse DG, Radford MJ, Goff DC, Jr, Grover FL, Heidenreich PA, Malenka DJ, Peterson ED, Redberg RF. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Data Standards on Atrial Fibrillation) Circulation. 2004;109:3223–3243. doi: 10.1161/01.CIR.0000131893.41821.D1. [DOI] [PubMed] [Google Scholar]
- 18.Essebag V, Wylie JV, Jr, Reynolds MR, Baldessin F, McClennen S, Shvilkin A, Germano J, Richardson A, Zimetbaum PJ, Josephson ME. Bi-directional electrical pulmonary vein isolation as an endpoint for ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2006;17:111–117. doi: 10.1007/s10840-006-9057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: Recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Oral H, Knight BP, Ozaydin M, Tada H, Chugh A, Hassan S, Scharf C, Lai SW, Greenstein R, Pelosi F, Jr, Strickberger SA, Morady F. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol. 2002;40:100–104. doi: 10.1016/s0735-1097(02)01939-3. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, Yu WC, Huang JL, Ueng KC, Cheng JJ, Ding YA, Chen SA. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2004;10:221–226. doi: 10.1023/B:JICE.0000026915.02503.92. [DOI] [PubMed] [Google Scholar]
- 22.Tse HF, Lau CP, Ayers GM. Atrial fibrillation induction and determination of atrial vulnerable period using very low energy synchronized biatrial shock in normal subjects and in patients with atrial fibrillation. Pacing Clin Electrophysiol. 2000;23:469–476. doi: 10.1111/j.1540-8159.2000.tb00829.x. [DOI] [PubMed] [Google Scholar]
- 23.Euler DE, Scanlon PJ. Acetylcholine release by a stimulus train lowers atrial fibrillation threshold. Am J Physiol. 1987;253:H863–868. doi: 10.1152/ajpheart.1987.253.4.H863. [DOI] [PubMed] [Google Scholar]
- 24.Katz A, Evans JJ, Fogel RI, Schier JJ, Matheny RG, Baranowski GM, Prystowsky EN. Atrial fibrillation/flutter induced by implantable ventricular defibrillator shocks: Difference between epicardial and endocardial energy delivery. J Cardiovasc Electrophysiol. 1997;8:35–41. doi: 10.1111/j.1540-8167.1997.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 25.Simpson RJ, Jr, Amara I, Foster JR, Woelfel A, Gettes LS. Thresholds, refractory periods, and conduction times of the normal and diseased human atrium. Am Heart J. 1988;116:1080–1090. doi: 10.1016/0002-8703(88)90163-9. [DOI] [PubMed] [Google Scholar]
- 26.Kojodjojo P, Peters NS, Davies DW, Kanagaratnam P. Characterization of the electroanatomical substrate in human atrial fibrillation: The relationship between changes in atrial volume, refractoriness, wavefront propagation velocities, and AF burden. J Cardiovasc Electrophysiol. 2007;18:269–275. doi: 10.1111/j.1540-8167.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 27.Callans DJ, Gerstenfeld EP, Dixit S, Zado E, Vanderhoff M, Ren JF, Marchlinski FE. Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:1050–1055. doi: 10.1046/j.1540-8167.2004.04052.x. [DOI] [PubMed] [Google Scholar]
- 28.Oral H, Chugh A, Good E, Sankaran S, Reich SS, Igic P, Elmouchi D, Tschopp D, Crawford T, Dey S, Wimmer A, Lemola K, Jongnarangsin K, Bogun F, Pelosi F, Jr, Morady F. A tailored approach to catheter ablation of paroxysmal atrial fibrillation. Circulation. 2006;113:1824–1831. doi: 10.1161/CIRCULATIONAHA.105.601898. [DOI] [PubMed] [Google Scholar]