Abstract
The house sparrow (Passer domesticus) is a common and abundant amplifying host of West Nile virus (WNV) and many survive infection and develop humoral immunity. We experimentally inoculated house sparrows with WNV and monitored duration and protection of resulting antibodies. Neutralizing antibody titers remained relatively constant for ≥ 36 months (N = 42) and provided sterilizing immunity for up to 36 months post-inoculation in 98.6% of individuals (N = 72). These results imply that immune house sparrows are protected from WNV infection for multiple transmission seasons. Additionally, individuals experiencing WNV-associated mortality reached significantly higher peak viremia titers than survivors, and mortality during acute infection was significantly higher in caged versus free-flight sparrows. A better understanding of the long-term immunity and mortality rates in birds is valuable in interpreting serosurveillance and diagnostic data and modeling transmission and disease dynamics.
INTRODUCTION
West Nile virus (WNV; family Flaviviridae, genus Flavivirus) is endemic in much of the United States,1 and birds played a pivotal role in its rapid geographic expansion and establishment.2–5 Since its arrival to the Western Hemisphere, WNV has caused mortality of many thousands of birds,6 whereas survivors overcome infection and produce anti-WNV antibodies.7 West Nile virus seroprevalence rates of various avian species have been documented within numerous regions of the United States,8–12 whereas antibody duration has been assessed in captive birds.13–15
The level of protection provided by primary immunity to WNV over multiple transmission seasons has yet to be characterized in birds. This information is important for understanding transmission dynamics, and long-term effects of WNV on avian populations. In addition, data regarding long-term duration of antibodies and response to secondary exposure in a variety of avian species will aid in understanding the epidemiology and ecology of WNV and in interpretation of serosurvey data. Naturally induced WNV neutralizing antibodies were detectable and showed relatively little variation over ∼1 year in rock pigeons (Columba livia) and fish crows (Corvus ossifragus), and > 4 years in raptors.13–15
We performed a 36-month controlled study of WNV infection in the house sparrow (Passer domesticus), an abundant and ubiquitous passerine that is a competent reservoir host of WNV.11,16 Our major objectives were to monitor WNV neutralizing antibody titers of experimentally inoculated house sparrows for up to 36 months, to assess the protectiveness of these antibodies over time, and to measure serologic responses to primary and secondary exposure. Secondary objectives were to assess for contact transmission among communally housed sparrows, to compare mortality rates in sparrows caged and handled through the period of acute WNV infection with rates in sparrows in a free-flight aviary and not captured, and to compare viremic responses and viral titers in tissues of birds that succumb with those that survive infection.
METHODS
House sparrow capture and husbandry
From January to March of 2005, 179 adult house sparrows (hereafter, sparrows) were captured by mist net in northern Colorado. Upon arrival, birds were leg-banded, weighed, and bled from the jugular vein.
Sparrows were housed free-flight, divided equally between two rooms (each 3.7 m width × 3.7 m height × 5.5 m length) containing branches, stumps, ropes, sand baths, cuttlefish bone, and multiple food and water stations. Fresh water and food were provided ad libitum; food consisted of a dry mix of millet, milo, cracked corn, cracked sunflower seed, and oats (in equal parts), as well as live mealworms 1–2 times/week. Birds were acclimated to captivity for 2–12 weeks before WNV inoculation. Birds caged for daily bleedings were housed 2–5 individuals per cage (each 0.4–0.5 m width × 0.4 m height × 0.6–0.8 m length).
Birds exhibiting clinical signs (lethargy, fluffed feathers, anorexia) before or during the study were euthanized via sodium pentobarbital overdose administered intravenously. This study was performed in accordance with regulations established by the Institutional Animal Care and Use Committee at Colorado State University.
Experimental groups and inoculation
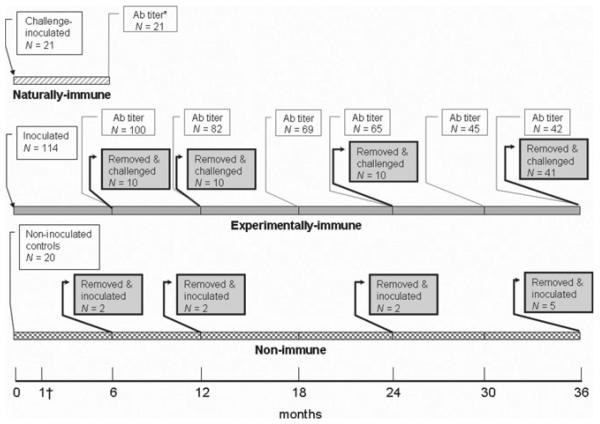
Sparrows were divided into three experimental groups based on initial WNV serostatus (Figure 1). Groups included WNV seronegative birds for experimental inoculation (hereafter, deemed “experimentally immune;” N = 114), naturally infected birds with pre-existing anti-WNV antibodies (hereafter, deemed “naturally immune;” N = 21), and WNV seronegative birds to serve as antibody-negative controls (hereafter, deemed “non-immune;” N = 20). The former two groups were experimentally inoculated subcutaneously with 1,000–2,000 plaque forming units (PFU) of WNV strain NY99-4132 administered in 0.1 mL BA1 (M199-Hank's salts, 1% bovine serum albumin, 350 mg/L sodium bicarbonate, 100 units/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL amphotericin B in 0.05 M Tris, pH 7.6). The latter group was not experimentally inoculated with the initial groups but remained among the inoculated birds as non-immune contact controls in the free-flight room to assess for potential contact transmission. Some of these seronegative sparrows were housed in separate cages from experimentally immune sparrows and inoculated as non-immune controls at 6, 12, 24, and 36 months post-inoculation (PI).
Figure 1.
Timeline of West Nile virus experimental inoculation of three experimental groups of house sparrows. * Antibody (Ab) titer indicates when serum samples were titrated to determine WNV PRNT90 antibody titers. † All birds were bled at 1 month post-inoculation to confirm seroconversion and assess antibody titers.
Sample collection and preparation
After initial inoculation, all but 14 sparrows were housed free-flight within rooms. These 14 sparrows (seven naturally immune and seven non-immune) were caged and bled 0.1 mL via jugular venipuncture from 1 to 6 days PI and then released into the room with the remainder of the sparrows.
All sparrows were caught by hand-held nets and bled 0.2 mL via jugular venipuncture at 1, 6, 12, 18, 24, 30, and 36 months PI. At 6 months PI the 21 naturally immune sparrows that had been inoculated 6 months prior were bled and euthanized.
Challenge experiments (i.e., re-inoculation or secondary exposure) occurred at 6, 12, 24, and 36 months PI. Sparrows were placed into cages for several days and then needle-inoculated subcutaneously with 2,500–3,500 PFU of WNV strain NY99-4132. After challenge inoculation (or initial inoculation for non-immune controls), blood samples were collected from 1–7 and on 14 days PI, when birds were euthanized.
Blood samples were either added to BA1 with 20% fetal bovine serum (FBS) in cryovials for an approximate 1:10 serum dilution (for viremia analysis) or dispensed undiluted into serum separator tubes (for antibody analysis). Blood samples were held at room temperature for 20–30 minutes for coagulation, centrifuged for 10 minutes at 6,000 × G and sera frozen to −80°C (diluted samples) or for 3 minutes at 12,000 × G and sera frozen to −20°C (undiluted samples).
Sparrows that died or were euthanized as a result of morbidity < 10 days PI, any non-immune controls that succumbed during the study, and eight non-immune controls euthanized at 14 days PI were necropsied, at which time oropharyngeal swabs, spleen, kidney, heart, and brain were collected and placed in 1 mL BA1 with 20% FBS (tissues were weighed for a 10% suspension). Tissues were processed as previously described17 and tested for WNV by plaque assay. These birds were considered to have experienced acute WNV-associated mortality if WNV was isolated from multiple tissues.
Vero cell plaque assay and plaque reduction neutralization test
Sera collected from 1 to 7 days PI, as well as oral swabs and tissue homogenates from birds dying < 10 days PI, were tested for infectious WNV by Vero cell plaque assay as previously described.18 Representative plaques were confirmed as WNV through reisolation and testing by VecTest WNV Antigen Assay (Medical Analysis Systems, Camarillo, CA) as previously described.17 The detection thresholds for WNV were 101.7 PFU/mL serum and 100.7 PFU/swab or mL of tissue homogenate.
Sera were tested for neutralizing antibodies to WNV using the plaque reduction neutralization test (PRNT)19 with the same WNV strain as for inoculation of sparrows. Sera that neutralized ≤ 60% of WNV PFU were considered negative for antibodies, whereas sera that neutralized > 90% were considered positive (no serum samples neutralized between 60–90% of viral plaques). Antibody positive serum samples were serially diluted 2-fold and tested in duplicate to determine reciprocal endpoint 90% neutralization (PRNT90) titers. Anamnestic antibody responses to challenge were considered significant when a ≥ 4-fold increase in PRNT90 titer was observed within ∼2–4 weeks of challenge.
Mathematical and statistical analyses
To assess the variation in PRNT90 titers of all sparrows alive for at least two consecutive time points, the multiple-fold change in titer for each individual at a given time point and the one immediately following was represented by a numerical value (e.g., −2 for a 2-fold decrease, 0 for no change in titer, 2 for a 2-fold increase). These values were averaged among all individuals to determine average changes in titer at each time period (Table 1). This analysis avoided eliminating individuals that were not present throughout all time points.
Table 1.
Antibody profiles over time in house sparrows after experimental inoculation with West Nile virus
| Time post-inoculation (months) | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 6 | 12 | 18 | 24 | 30 | 36 | |
| N* | 104 | 100 | 82 | 69 | 65 | 45 | 42 |
| PRNT90 range; median, mode | 40–2,560; 320, 320 | 10–2,560; 160, 80 | < 10–320; 80, 80 | 10–640; 80, 80 | 20–1,280; 160, 160 | 10–640; 80, 40 | 10–640; 80, 80 |
| PRNT90 from 20–160 | 26.9% (28/104) | 62.0% (62/100) | 79.3% (65/81) | 84.1% (58/69) | 81.5% (53/65) | 86.7% (39/45) | 85.7% (36/42) |
| Overall change in PRNT90† | – | −4.7 | −2.4 | 0.0 | 1.1 | −1.6 | 0.3 |
N represents the number of sparrows still alive during each time point and included in plaque reduction neutralization test PRNT90 analyses.
The overall change in PRNT90 reflects the number-fold increase or decrease in titer from the closest previous time point to the current time point.
A χ2 test (α = 0.05) was used to compare mortality rates (as proportions) of caged sparrows that were frequently captured and sampled with those of free-flight sparrows not handled after inoculation. Peak viremia titers in log10 PFU/mL serum (the dependent variable) were analyzed as a function of disposition (death versus survival; the fixed variable) using general linear model procedure (Proc GLM). This method was also used to compare tissue titers in log10 PFU/0.5 cm3 (the dependent variable), analyzed as a function of days PI when death occurred (5–6 days PI versus 7–9 days PI; the fixed variable). Viral titers below the threshold of detection were considered zero. Statistics were calculated in SAS/STAT MULTTEST software, version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Initial serology and mortality
Thirty-one of 179 (17.3%) free-ranging sparrows had WNV-neutralizing antibodies before initiation of the study. A total of 125 seronegative sparrows were inoculated (114 at initiation of the study, plus 11 controls during subsequent challenge time points), 14 of which experienced acute WNV-associated mortality after experimental inoculation. An additional 32 deaths occurred over the 36-month study, including four seronegative control birds. Twelve experimentally immune sparrows were euthanized at pre-determined time points for a separate study. All deaths that occurred beyond the acute phase of infection were attributed to natural causes, bird-induced trauma, or husbandry- or sampling-related causes.
Contact transmission
No serologic evidence of contact transmission was observed in non-immune control sparrows (N = 20) throughout the study. Ten of these were alive at 36 months PI, six were euthanized during challenge experiments, and four died during the study (the latter had no evidence of acute WNV infection).
Acute responses to infection in non-immune sparrows
After inoculation with WNV, a total of 18 non-immune sparrows were caged and bled daily to evaluate viremia, whereas 107 birds were released into large rooms and not handled until 1 month PI. Five of 18 (27.8%) caged sparrows had visible clinical signs after inoculation, including lethargy, fluffed feathers, anorexia, and/or hind limb rigidity; these birds died or were euthanized between 5–9 days PI. Mortality attributed to WNV infection (death at < 10 days PI and WNV isolated from multiple tissues) was significantly greater among the caged sparrows handled daily (5/18; 27.8%) compared with free-flying sparrows that were not handled (9/107; 8.4%) (N = 125, χ2 = 5.81, P = 0.016; 95% confidence interval [CI]: 0.069, 0.823).
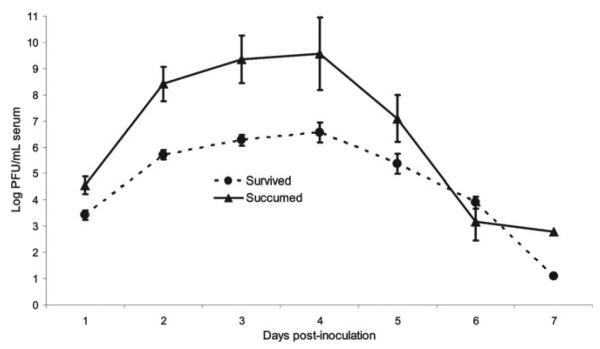
All 18 non-immune control sparrows caged and bled daily after inoculation developed viremia of ≥ 3 days duration. The peak viremia titers of sparrows that experienced WNV-associated morbidity and mortality (105.5–10.2 PFU/mL serum; mean 109.7) were significantly higher than in those that survived acute infection and showed no clinical signs (104.5–7.6 PFU/mL serum; mean 106.6) (N = 18, P = 0.006, 95% CI: 0.694, 3.401) (Figure 2). Death occurred from 1 to 6 days after peak viremia, and viremia titers decreased ∼200–300,000-fold before death in 4/5 (80.0%) sparrows.
Figure 2.
Average daily viremia titers among house sparrows experimentally inoculated with West Nile virus that succumbed and those that survived infection. Standard error bars are provided for 1–6 days post-inoculation.
All of 14 necropsied sparrows that died of acute infection had WNV isolated from oropharyngeal swab (102.2–6.6 PFU/swab), heart (101.7–6.5 PFU/0.5 cm3), and kidney (100.7–7.1 PFU/0.5 cm3); 13/14 (92.9%) also had virus isolated from brain (104.2–6.7 PFU/0.5 cm3) and 12/14 (85.7%) from spleen (103.8–7.1 PFU/0.5 cm3). Viral titers in all tissues were significantly higher in birds that succumbed to acute infection earlier (5–6 days PI) versus after this time (N = 14; oropharyngeal swab, P = 0.014, 95% CI: 0.380, 2.778; heart, P < 0.001, 95% CI: 1.394, 3.610; kidney, P < 0.001, 95% CI: 2.822, 5.120; spleen, P = 0.001, 95% CI: 1.863, 5.573; brain, P = 0.006, 95% CI: 0.903, 4.206). West Nile virus was isolated from tissues collected from three of eight necropsied individuals that remained healthy until euthanasia at 14 days PI (spleen from two individuals at 101.3–2.0 PFU/cm3, and kidney 101.0 PFU/0.5 cm3 and heart 100.7 PFU/0.5 cm3 from another individual).
Acute responses to challenge in immune sparrows
All but one of 71 (98.6%) house sparrows challenged by needle-inoculation at 6–36 months PI demonstrated sterilizing immunity; one sparrow had low-titered viremia from 3 to 5 days after challenge (Table 2). Antibody titers in this bird increased 256-fold by 14 days post-challenge. Anamnestic rises in antibody titers of ≥ 4-fold by 14 days post-challenge were observed in 72.9% (51/70) of experimentally immune sparrows, and rises were pronounced in some cases (up to 512-fold).
Table 2.
Serologic responses among immune and non-immune house sparrows after experimental inoculation with West Nile virus
| Pre-inoculation |
Viremia profiles |
14 days post-inoculation |
||||
|---|---|---|---|---|---|---|
| Experimental group, time PI* | N | PRNT90† range | % viremic | Peak range (PFU‡/mL serum) | PRNT90 titer range | % ≥ 4-fold increase |
| Non-immune controls | 18 | < 10 | 100 | 104.5–10.2 | 40–2,560 | – |
| Naturally- immune | 7 | 10–320 | 0 | < 101.7 | 80–2,560§ | 38 |
| Experimentally immune, 6 mo PI | 10 | 10–80 | 10 | 101.7–2.4 | 80–2,560 | 80 |
| Experimentally immune, 12 mo PI | 10 | < 10–80 | 0 | < 101.7 | 20–2,560 | 80 |
| Experimentally immune, 24 mo PI | 10 | 10–320 | 0 | < 101.7 | 40–2,560 | 60 |
| Experimentally immune, 36 mo PI | 41 | 10–640 | 0 | < 101.7 | 80–20,480 | 73 |
PI = post-inoculation.
PRNT90 = endpoint 90% neutralization titer (PRNT90 < 10 is considered seronegative).
PFU = plaque forming units.
Post-inoculation PRNT90 titers for the naturally immune group were determined at one month PI, and 21 birds were included in this analysis.
No experimentally immune sparrows exhibited morbidity or mortality after challenge except for one bird that died 5 days post-36-month challenge. This individual had no detectable viremia after challenge, and heart, spleen, brain, and kidney were negative by virus isolation, though a low titer (101.7 PFU/swab) of infectious WNV was isolated from the oropharyngeal swab collected after death.
None of the naturally immune sparrows (N = 21) exhibited morbidity or mortality after challenge inoculation, and none of the seven sparrows bled from 1 to 6 days after challenge inoculation had detectable viremia.
Chronic responses to challenge in immune sparrows
All non-immune sparrows that survived inoculation seroconverted and 55% (55/100) had a ≥ 4-fold decrease in antibody titer from 1 to 6 months PI. Thereafter, little variation in PRNT90 titers was observed (Table 1), as titers of most sparrows (41/42; 97.6%) did not vary ≥ 2-fold over the subsequent 30 months.
Approximately 38% (8/21) of naturally immune sparrows exhibited a ≥ 4-fold increase in PRNT90 antibody titer 1 month after challenge (Table 2). At 6 months PI, titers ranged from 40 to 1,280, with 19.0% (4/21) exhibiting a ≥ 4-fold decrease from 1 to 6 months post-challenge.
DISCUSSION
An understanding of the duration and protection provided by WNV immunity in passerine birds is important because numerous members of this large taxonomic group are virus-amplifying hosts20 that are commonly fed upon by mosquitoes.21–23 Some passerines, such as the house sparrow, reside primarily or exclusively in areas where humans are present,16 suggesting that their WNV reservoir competence and immune responses could have implications for public health.11 Although many passerines experience relatively high viremia titers after WNV infection, some also mount an effective immune response and survive infection.8–11,20 In addition, WNV immunity is long-lasting in some birds.13–15 However, the ability of anti-WNV antibodies to protect against viremia upon subsequent infection in birds, thereby effectively rendering a potential amplifying host into a dead-end host, remains unknown.
Elevated WNV transmission likely corresponds to relatively high proportions of infected birds, and the resulting widespread immunity among survivors could potentially dampen transmission.24 If immune birds survive multiple transmission seasons and their immunity persists, the protective effect against infection among the remainder of the population (e.g., herd immunity) may be relatively long-lasting and lead to a reduction in disease incidence.13,25 Humans and other vertebrates may also benefit from long-lasting protective WNV immunity among birds. Annual adult survival of house sparrows is 57% and longevity has reached > 13 years in the wild.16 In addition, house sparrows were recaptured after an average of 559 days (range 502–649) in southern California,26 supporting the notion that some free-ranging sparrows survive multiple transmission seasons. However, herd immunity may be unattainable in some avian species based on life history traits such as population turnover rate and life span. For example, the house sparrow often rears multiple broods of up to eight chicks per brood in a given season, leading to an influx of naive offspring into the population at regular intervals. Furthermore, annual survival of hatch-year house sparrows is estimated at only 20%.16 Collectively, these factors may make it difficult to attain a sufficient proportion of immune individuals to protect the remainder of the population.
Along with duration, the level of protection provided by anti-WNV antibodies affects transmission dynamics and avian population health. Antibodies to other flaviviruses for which birds also serve as amplifying hosts, such as St. Louis encephalitis virus (SLEV), have variable persistence in birds, sometimes declining after 3 months PI and becoming undetectable by 6–12 months PI. However, even with undetectable SLEV-neutralizing antibodies, some experimentally inoculated house finches (Carpodacus mexicanus) and house sparrows were protected from viremia at 6, 12, and 24 months PI with anamnestic rises in titers in those challenged at 12 months PI.24,27,28 In the present study, nearly all sparrows were protected from challenge for up to 36 months PI, as evidenced by sterilizing immunity. The significance of the single experimentally immune house sparrow that experienced a relatively low-titered viremia after challenge is unknown, but this bird apparently retained partial immunity, resulting in titers below those observed in non-immune sparrows. West Nile virus was unlikely associated with the death of one experimentally immune sparrow after challenge inoculation because of a lack of virus detection in serum and tissues. However, low levels of virus were detected in the oropharyngeal cavity upon death, the significance of which is also unknown.
Understanding patterns of anamnestic antibody responses subsequent to initial infection in birds is useful for interpretation of serosurveillance and diagnostic data. Traditionally, a ≥ 4-fold increase in antibody titer over several weeks to months indicates a recent infection.19 However, in the present study, ∼27% of experimentally immune and ∼62% of naturally immune sparrows failed to meet this criterion. Similarly, 5/6 (83.3%) SLEV-immune house finches failed to demonstrate a > 2-fold rise in anti-SLEV antibody titer 2 and 6 weeks after homologous challenge; however, all of four WNV-immune finches exhibited a ≥ 4-fold increase in anti-WNV antibody titer when challenged with WNV.7 Perhaps in some cases, existing immunity is sufficient to neutralize challenge virus or rises in post-challenge titers are delayed. Alternately, needle-inoculation could fail to stimulate a rise in antibody titer, though needle versus mosquito inoculation did not lead to a difference in overall patterns of arbovirus infection observed in chickens or house finches.29,30 In the present study, most house sparrows experienced a ≥ 4-fold decrease in antibody titer between 1 and 6 months PI, likely reflecting a decline after the initial peak that follows primary infection, a pattern that may suggest relatively recent WNV infection in some birds.14
The significance and extent of bird-to-bird WNV transmission in nature remains unknown, but the probability of contact transmission is likely dependent upon multiple factors, such as bird behavior, habitat, and environmental conditions. Although bird-to-bird WNV transmission has been observed in corvids, gulls, and domestic birds in controlled experiments,20,31–33 no house sparrows in the present study became infected through contact transmission. Unlike most previous studies, in which birds were caged, housing in the present study was more similar to a natural setting, consisting of spacious rooms with hundreds of perching options and numerous food and water stations. The likelihood and frequency of bird-to-bird WNV transmission in nature remain unknown.
Much of the currently available information regarding avian mortality rates associated with North American strains of WNV derives from experimental infection studies involving wild-caught birds subsequently caged and frequently handled for sampling. These birds likely undergo intensified and frequent rises in stress levels because of confinement and repeated close contact and handling by humans that differ from the more prolonged and continual stress associated with life in the wild to which they are presumably more accustomed (e.g., underlying competition for food and territories, constant need for foraging and vigilance against predators, unfavorable climate, etc.). Thus, captive studies may lead to overestimates of WNV-attributed morbidity and mortality rates in free-ranging birds. Mortality rates of caged house sparrows bled daily after experimental inoculation with WNV NY99 have ranged from ∼38 to 50%.20,34 In the present study, the mortality rate of caged birds handled daily was significantly higher than that observed among birds in a free-flight aviary and spared the stress of capture, restraint, and blood collection (27.8 versus 7.5%, respectively).
Although marked differences in responses to WNV infection among North American bird species have been observed,20 intra-species differences suggest that individual variation is also an important factor in infection outcome. Results from the present study suggest that individuals that are unable to control viral replication in tissues, including blood, are less likely to recover from infection. Some sparrows succumbed to infection near the period of peak viremia, whereas others succumbed up to 6 days after viremia titers began to decline. This inability to control virus replication and dissemination may be associated with immune deficiencies, as was observed in antibody, IgM, and B cell-deficient mice that had higher viremia titers, higher viral loads in the central nervous system, and were more vulnerable to lethal WNV infection than wild-type mice.35,36 However, some birds that succumb to WNV infection begin to mount WNV-neutralizing antibody responses prior to death.37 Studies are needed that examine the potential underlying immune deficits that may be associated with higher viremia titers, widespread viral dissemination, and eventual death in birds.
In conclusion, successful transmission of WNV in nature is dependent upon avian amplifying hosts. Therefore, knowledge of patterns of immunity in birds will aid in understanding and predicting future transmission patterns. Long-lasting protective immunity to WNV infection in birds could potentially dampen transmission rates both within bird populations, and in humans and other susceptible vertebrates.
Acknowledgments
We thank Nathan Roberts, Nick Panella, Kate Huyvaert, and Nick Komar for providing sparrows. We are grateful to Paul Gordy, Louise Ansell, Angela Bosco-Lauth, Jenny Bervin, Christina Ndaluka, Kristin Jones, Ginger Young, and others for their assistance in catching and sampling sparrows. Paul Gordy provided logistical support and Robert McLean editorial comments.
Financial support: This research was funded by NIH contract N01-AI25489.
REFERENCES
- 1.Bertolotti L, Kitron U, Goldberg TL. Diversity and evolution of West Nile virus in Illinois and the United States, 2002–2005. Virol. 2007;360:143–149. doi: 10.1016/j.virol.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 2.Owen J, Moore F, Panella N, Edwards E, Bru R, Hughes M, Komar N. Migrating birds as dispersal vehicles for West Nile virus. EcoHealth. 2006;3:79–85. [Google Scholar]
- 3.Peterson AT, Vieglais DA, Andreasen JK. Migratory birds modeled as critical transport agents for West Nile virus in North America. Vector Borne Zoonotic Dis. 2003;3:27–37. doi: 10.1089/153036603765627433. [DOI] [PubMed] [Google Scholar]
- 4.Rappole JH, Compton BW, Leimgruber P, Robertson J, King DI, Renner SC. Modeling movement of West Nile virus in the western hemisphere. Vector Borne Zoonotic Dis. 2006;6:128–139. doi: 10.1089/vbz.2006.6.128. [DOI] [PubMed] [Google Scholar]
- 5.Rappole JH, Hubálek Z. Migratory birds and West Nile virus. J Appl Microbiol. 2003;94:47S–58S. doi: 10.1046/j.1365-2672.94.s1.6.x. [DOI] [PubMed] [Google Scholar]
- 6.Marra PP, Griffing S, Caffrey C, Kilpatrick AM, McLean R, Brand C, Saito E, Dupuis AP, Kramer L, Novak R. West Nile virus and wildlife. Bioscience. 2004;54:393–402. [Google Scholar]
- 7.Fang Y, Reisen WK. Previous infection with West Nile or St. Louis encephalitis viruses provides cross protection during reinfection in house finches. Am J Trop Med Hyg. 2006;75:480–485. [PubMed] [Google Scholar]
- 8.Beveroth TA, Ward MP, Lampman RL, Ringia AM, Novak RJ. Changes in seroprevalence of West Nile virus across Illinois in free-ranging birds from 2001 through 2004. Am J Trop Med Hyg. 2006;74:174–179. [PubMed] [Google Scholar]
- 9.Gibbs SEJ, Allison AB, Yabsley MJ, Mead DG, Wilcox BR, Stallknecht DE. West Nile virus antibodies in avian species of Georgia, USA: 2000–2004. Vector Borne Zoonotic Dis. 2006;6:57–72. doi: 10.1089/vbz.2006.6.57. [DOI] [PubMed] [Google Scholar]
- 10.Godsey MS, Blackmore MS, Panella NA, Burkhalter K, Gottfried K, Halsey LA, Rutledge R, Langevin SA, Gates R, Lamonte KM, Lambert A, Lanciotti RS, Blackmore CGM, Loyless T, Stark L, Oliveri R, Conti L, Komar N. West Nile virus epizootiology in the southeastern United States, 2001. Vector Borne Zoonotic Dis. 2005;5:82–89. doi: 10.1089/vbz.2005.5.82. [DOI] [PubMed] [Google Scholar]
- 11.Komar N, Panella NA, Burns JE, Dusza SW, Mascarenhas TM, Talbot TO. Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg Infect Dis. 2001;7:621–625. doi: 10.3201/eid0704.010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komar N, Panella NA, Langevin SA, Brault AC, Amador M, Edwards E, Owen JC. Avian hosts for West Nile virus in St. Tammany Parish, Louisiana, 2002. Am J Trop Med Hyg. 2005;73:1031–1037. [PubMed] [Google Scholar]
- 13.Gibbs SEJ, Hoffman DM, Stark LM, Marlenee NL, Blitvich BJ, Beaty BJ, Stallknecht DE. Persistence of antibodies to West Nile virus in naturally infected rock pigeons (Columba livia) Clin Diagn Lab Immunol. 2005;12:665–667. doi: 10.1128/CDLI.12.5.665-667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemeth NM, Kratz GE, Bates R, Scherpelz JA, Bowen RA, Komar N. Naturally induced humoral immunity to West Nile virus infection in raptors. EcoHealth. 2008;5:298–304. doi: 10.1007/s10393-008-0183-z. [DOI] [PubMed] [Google Scholar]
- 15.Wilcox BR, Yabsley MJ, Ellis AE, Stallknecht DE, Gibbs SEJ. West Nile virus antibody prevalence in American crows (Corvus brachyrhynchos) and fish crows (Corvus ossifragus) in Georgia, USA. Avian Dis. 2007;51:125–128. doi: 10.1637/0005-2086(2007)051[0125:WNVAPI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Lowther PE, Cink CL. House sparrow (Passer domesticus) In: Poole A, Stettenheim P, Gill F, editors. The Birds of North America. The Academy of Natural Sciences, The American Ornithologist's Union; Washington, DC: 1992. pp. 1–20. [Google Scholar]
- 17.Nemeth NM, Beckett S, Edwards E, Klenk K, Komar N. Avian mortality surveillance for West Nile virus in Colorado. Am J Trop Med Hyg. 2007;76:431–437. [PubMed] [Google Scholar]
- 18.Bunning ML, Bowen RA, Cropp CB, Sullivan KG, Davis BS, Komar N, Godsey MS, Baker D, Hettler DL, Holmes DA, Biggerstaff BJ, Mitchell CJ. Experimental infection of horses with West Nile virus. Emerg Infect Dis. 2002;8:380–386. doi: 10.3201/eid0804.010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaty BJ, Calisher CH, Shope RE. Diagnostic procedures for viral, rickettsial, and chlamydial infections. In: Lennette EH, Lennette DA, Lennette ET, editors. Arboviruses. American Public Health Association; Washington, DC: 1995. pp. 189–212. [Google Scholar]
- 20.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apperson CS, Hassan HK, Harrison BA, Savage HM, Aspen SE, Farajollahi A, Crans W, Daniels TJ, Falco RC, Benedict M, Anderson M, McMillen L, Unnasch TR. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoonotic Dis. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. Host heterogeneity dominates West Nile virus transmission. Proc R Soc B: Biol Sci. 2006;273:2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savage HM, Aggarwal D, Apperson CS, Katholi CR, Gordon E, Hassan HK, Anderson M, Charnetzky D, McMillen L, Unnasch EA, Unnasch TR. Host choice and West Nile virus infection rates in blood-fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee, 2002–2003. Vector Borne Zoonotic Dis. 2007;7:365–386. doi: 10.1089/vbz.2006.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reisen WK, Chiles BE, Green EN, Fang Y, Mahmood F. Previous infection protects house finches from re-infection with St. Louis encephalitis virus. J Med Entomol. 2003;40:300–305. doi: 10.1603/0022-2585-40.3.300. [DOI] [PubMed] [Google Scholar]
- 25.Fine PEM. Herd immunity: history, theory, practice. Epidemiol Rev. 1993;15:265–302. doi: 10.1093/oxfordjournals.epirev.a036121. [DOI] [PubMed] [Google Scholar]
- 26.Gruwell JA, Fogarty CL, Bennett SG, Challet GL, Vanderpool KS, Jozan M, Webb JP. Role of peridomestic birds in the transmission of St. Louis encephalitis virus in southern California. J Wildl Dis. 2000;36:13–34. doi: 10.7589/0090-3558-36.1.13. [DOI] [PubMed] [Google Scholar]
- 27.McLean RG, Mullenix J, Kerschner J, Hamm J. The house sparrow (Passer domesticus) as a sentinel for St. Louis encephalitis virus. Am J Trop Med Hyg. 1983;32:1120–1129. doi: 10.4269/ajtmh.1983.32.1120. [DOI] [PubMed] [Google Scholar]
- 28.Reisen WK, Kramer LD, Chiles RE, Green EGN, Martinez VM. Encephalitis virus persistence in California birds: preliminary studies with house finches. J Med Entomol. 2001;38:393–399. doi: 10.1603/0022-2585-38.3.393. [DOI] [PubMed] [Google Scholar]
- 29.Reisen WK, Chiles RE, Kramer LD, Martinez VM, Eldridge BF. Method of infection does not alter response of chicks and house finches to western equine encephalomyelitis and St. Louis encephalitis viruses. J Med Entomol. 2000;37:250–258. doi: 10.1603/0022-2585-37.2.250. [DOI] [PubMed] [Google Scholar]
- 30.Styer LM, Bernard KA, Kramer LD. Enhanced early West Nile virus infection in young chickens infected by mosquito bite: effect of viral dose. Am J Trop Med Hyg. 2006;75:337–345. [PubMed] [Google Scholar]
- 31.Langevin SA, Bunning M, Davis B, Komar N. Experimental infection of chickens as candidate sentinels for West Nile virus. Emerg Infect Dis. 2001;7:726–729. doi: 10.3201/eid0704.010422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLean RG, Ubico SR, Docherty DE, Hansen WR, Sileo L, McNamara TS. West Nile virus transmission and ecology in birds. Ann NY Acad Sci. 2001;951:54–57. doi: 10.1111/j.1749-6632.2001.tb02684.x. [DOI] [PubMed] [Google Scholar]
- 33.Swayne DE, Beck JR, Smith CS, Shieh WJ, Zaki SR. Fatal encephalitis and myocarditis in young domestic geese (Anser anser domesticus) caused by West Nile virus. Emerg Infect Dis. 2001;7:751–753. doi: 10.3201/eid0704.010429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langevin SA, Brault AC, Panella NA, Bowen RA, Komar N. Variation in virulence of West Nile virus strains for house sparrows (Passer domesticus) Am J Trop Med Hyg. 2005;72:99–102. [PubMed] [Google Scholar]
- 35.Diamond MS, Shrestha B, Marri A, Mahan D, Engle M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol. 2003;77:2578–2586. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, Engle M. Critical role for induced IgM in the protection against West Nile virus infection. J Exp Med. 2003;198:1853–1862. doi: 10.1084/jem.20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nemeth NM, Hahn DC, Gould DH, Bowen RA. Experimental West Nile virus infection in Eastern Screech Owls (Megascops asio) Avian Dis. 2006;50:252–258. doi: 10.1637/7466-110105R1.1. [DOI] [PubMed] [Google Scholar]