Abstract
Introduction
Administrative claims data have a limited ability to identify persons with high compliance to oral bisphosphonates. We tested whether adding information on compliance with other drugs used to treat chronic, asymptomatic conditions would improve the predictive ability of administrative data to identify adherent individuals.
Methods
Using data from a large, U.S. healthcare organization, we identified new bisphosphonate users and their 1 year compliance to oral bisphosphonates, quantified by the Medication Possession Ratio (MPR). Multivariable logistic regression models evaluated the relationship between high bisphosphonate compliance (MPR >= 80%) and patient demographics, comorbidities, and health services utilization. To these logistic regression models, we evaluated the incremental change in the area under the receiver operator curve (AUC) after adding information regarding compliance with other drug classes. These included anti-hyperlipidemics (statins), anti-hypertensives, anti-depressants, oral diabetes agents, and glaucoma medications. Results from the logistic regression models were evaluated in parallel using recursive partitioning trees with 10-fold cross-validation.
Results
Among 101,038 new bisphosphonate users, administrative data identified numerous non-medication factors (e.g. age, gender, use of preventive services) significantly associated with high bisphosphonate compliance at 1 year. However, all these factors in aggregate had low discriminant ability to identify persons highly adherent with bisphosphonates (AUC = 0.62). For persons who were new users of ≥ 1 of the other asymptomatic condition drugs, MPR data on the other drugs substantially improved the prediction of high bisphosphonate compliance. The impact on prediction was largest for concomitant statin users (AUC = 0.70).
Conclusions
Information on compliance with drugs used to treat chronic asymptomatic conditions improves the prediction of compliance with oral bisphosphonates. This information may help identify persons who should receive targeted interventions to promote compliance to osteoporosis medications.
Keywords: bisphosphonate, adherence, compliance, osteoporosis
Introduction
Long term compliance with medications used to treat chronic, asymptomatic conditions such as osteoporosis, hyperlipidemia, and hypertension is poor [1-17]. Most studies have demonstrated that approximately one-half of patients discontinue therapy for these conditions within 1-2 years after treatment initiation. Factors previously shown to be strongly associated with high compliance include age, comorbidities, and events and diagnostic tests associated with the disease state (e.g. for osteoporosis, a fracture or bone mineral density testing).
Being able to identify prospectively patients who are less likely to adhere to these therapies would have important public health implications. It might allow one to tailor certain medications, treatment and follow-up strategies, or interventions to particular individuals that were at greatest risk of non-compliance. The Morisky scale [18] has been shown to predict compliance accurately and has been specifically evaluated in osteoporosis [19]. However, this patient-based, self-reported instrument is generally infeasible to routinely administer to large populations outside the context of a research study. In contrast, administrative claims data are routinely collected by large health systems and insurers and offer the opportunity to evaluate medication compliance in large populations. However, accurately predicting compliance using these data sources in order to tailor follow-up strategies, particular therapies or interventions to promote compliance has proved exceedingly challenging. In osteoporosis for example, one study found eight demographic, clinical, and health services utilization factors that were significantly associated with high compliance to bisphosphonates, the most commonly prescribed medications used to prevent fractures [8]. However, even considering all these factors together yielded a poor ability to discriminate between osteoporosis patients who had good versus poor compliance, with area under the receiver-operator curve (AUC) as low as 0.58. Another study that evaluated osteoporosis medication compliance using a continuous measure, the medication possession ratio (MPR [20]), found that the 19 factors that were significantly associated with compliance explained only 6% of the variation in MPR [9].
Despite most studies of adherence with osteoporosis medications reporting numerous factors that are significantly associated with compliance, these factors collectively may provide only a limited ability to predict adherence accurately; only a few of these studies provide any detail regarding model fit or discrimination.
Using a large administrative claims database, we sought to improve the prediction of compliance with bisphosphonates, the most commonly used osteoporosis medications, by incorporating information about prior compliance with other classes of medications used to treat chronic conditions. We hypothesized that for persons who used at least one of these other medications, our ability to identify those individuals with high bisphosphonate compliance one year after starting therapy would be substantially improved with this additional information.
Methods
Data Source and Eligible Population
After institutional review board approval, we used the administrative claims databases of a U.S. health care organization covering approximately 17 million persons living in 8 U.S. census regions with commercial insurance. We identified persons with medical and pharmacy benefits filling prescriptions for oral bisphosphonates (i.e. alendronate, risedronate, or ibandronate) from January 1998 to July 2005. Although there are other medications used to treat osteoporosis (e.g. raloxifene, teriparatide), we focused on bisphosphonates given they are the most commonly used drugs to treat osteoporosis and based on concerns that the severity of osteoporosis might confound the relationship between receipt of the other drugs and associated compliance to them. We identified new bisphosphonate users as those initiating therapy after at least a six month period without any bisphosphonate prescription. The date of the first filled bisphosphonate prescription after this six month period was defined as the bisphosphonate index date. Baseline demographic characteristics, specific disease diagnoses and a summed comorbidity count [21], and health services utilization were examined in the six months prior to the index date.
Identification of Concomitant Medication Use
For these new bisphosphonate users, we further identified those persons who filled at least one medication used for the treatment of several other chronic, asymptomatic conditions, anticipating that long term use of medications for these conditions was likely. These conditions included hypertension, hyperlipidemia, diabetes, depression, and glaucoma. The classes of medications of interest and their associated National Drug Codes (NDC) were grouped as angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), beta-blockers (BB), calcium channel blockers (CCB), thiazide diuretics, 3-hydroxy-3-methylglutaryl coenzyme A (HMG co-A) reductase inhibitors (statins) and other anti-hyperlipidemics, oral diabetes agents (excluding insulin), selective serotonin uptake inhibitors (SSRI), and glaucoma agents (topical or oral). Persons were considered new users of each drug class if they had a six month period of time with no filled prescription for any drug in that class. The date of first use of a medication in each class of drugs defined a unique, drug class-specific index date.
Calculation of Compliance
Recognizing that prescription claims database analyses lack precise details of dosing frequency and patient medication-taking behavior, compliance with bisphosphonates and the other drug classes was quantified by computing a drug class-specific MPR. This was calculated by summing the total amount of medication filled after the index date and dividing it by the calendar time since the index date [20]. MPR was computed for every day of observation. Observation time was censored at the time of disenrollment from the health plan or the end of the study period. A filled prescription for any medication within a therapeutic class was considered equivalent to any other; thus, individuals were allowed to switch to other drugs within the same class and still be considered adherent. The number of new bisphosphonate users that were continuing or new users of each other drug class was then evaluated. The median MPR for each drug class among these new users was plotted graphically. We further identified which individuals were new users of the other drugs and also initiated those drugs prior to or within six months of the bisphosphonate index date.
Outcome and Statistical Analysis
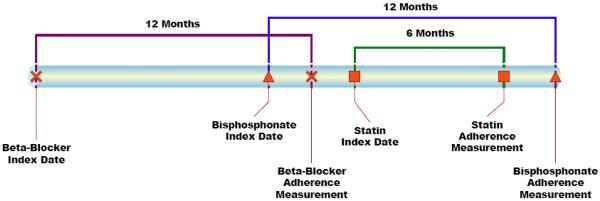
Our primary outcome of interest was high compliance with bisphosphonates one year after initiating therapy. Recognizing that a threshold for compliance with any medication is somewhat arbitrary, we followed the convention of prior studies that have used an MPR ≥ 80% [1, 11]. Moreover, some past studies have shown an inverse relation between an MPR ≥ 80% and fracture risk [3, 22]. Our primary independent variable of interest was the compliance at 1 year with each of the other drug classes. We first reported the pair-wise agreement between MPR with bisphosphonates at 1 year and MPR with each of the other drug classes at 1 year using kappa statistics with 95% confidence intervals. Because persons who initiated the other drugs after they began bisphosphonates would not have their 12 month other drug MPR prior to their 12 month bisphosphonate MPR, we used their 6 month other drug MPR instead (19% of the cohort) – see Figure 1. Since persons who initiated the other drugs more than 6 months after beginning bisphosphonates would not have a 6 month MPR at the time of the 12 month bisphosphonate MPR, they were not included in the compliance analyses for that drug class.
Figure 1.
Example of Relationships between Bisphosphonate Compliance Measurement (12 month Medication Possession Ratio) and the Measurement of Compliance with other Drug Classes (6 or 12 month Medication Possession Ratio)
Note: 6 rather than 12 month compliance measurements for the other drug classes are used when the index date for the other drugs is later than the bisphosphonate index date
We then developed multivariable logistic regression models to identify persons with a 1 year bisphosphonate MPR ≥ 0.80. Based on clinical knowledge and review of the literature, we included all demographic, clinical variables and health services utilization variables considered to be relevant and computed the area under the receiver operator curve (AUC) or c statistic, a measure of discrimination [23]. An AUC of 0.50 – 0.59 is considered poor, 0.60 to 0.69 fair, 0.70 to 0.79 good, and 0.80 and above, excellent. The Hosmer-Lemeshow goodness of fit test was used to assess the fit of the models [24]. To this baseline model, we then added the single compliance variables for each of the other drug classes and evaluated the change in the AUCs with this one additional variable. Separate logistic regression models were created for each drug class. For persons that were new users of multiple drug classes, we further added the corresponding MPR variables for these additional drugs and evaluated the incremental change in the AUCs with the addition of each new MPR variable. These analyses were performed using SAS 9.1 (SAS Institute, North Carolina).
Model validation
Because results from these prediction models might be overly optimistic, we further evaluated our results using a recursive partitioning tree approach that incorporated a validation step. Recursive partitioning creates a decision tree that strives to correctly classify members of the study population (i.e. highly adherent or not adherent) based on multiple dichotomous independent variables. The classification tree determines optimal splits by minimizing the heterogeneity in the outcome proportions (“impurity”) of the daughter nodes of a split compared their proportions in the parent node. The amount of impurity is proportional to the chi-square test statistic for the corresponding 2 × 2 table. The relative importance of each of the variables in the tree is assessed by determining the change in impurity in the entire tree after excluding each variable one at a time. Unlike logistic regression, this technique allows one to assess the importance of each variable relative to other variables in the model in correctly classifying persons. We built separate, drug class-specific classification trees using the identical variables in the corresponding logistic regression models.
A ten-fold cross-validation step was used to derive and evaluate our model by randomly partitioning the data into ten smaller datasets, training the model on nine of them and testing it on the 10th, and repeating the process ten times to derive a final model. This process generates separate ROC curves for the training and testing datasets. It also allows one to minimize the problem of ‘overfitting’, whereby prediction models created using one data set do not perform as well when applied to another data set. The classification trees were built and validated using CART 6.0 (Salford Systems, San Diego).
Sensitivity Analyses
Based upon the concern that hospitalized individuals might have different correlations between drug compliance, we conducted a sensitivity analysis that restricted the eligible individuals to those persons who were not hospitalized in the year after starting bisphosphonates or the other drug classes. We conducted a second sensitivity analysis in recognition that the indication for use of some drug classes is heterogeneous, and the indication for the drug might affect compliance patterns. We therefore restricted the ACEI/ARBs, BBs, CCBs, and thiazide users to those individuals who had a claim with a physician diagnosis of hypertension in the six months prior to the one year observation period for these drug classes.
Results
Among 101,038 new bisphosphonate users, 70% of the cohort used a medication from at least one of the other drug classes (e.g. statins, ACEI/ARBs), and 55% were identified as new users of at least one of these drug classes. The most common drug classes used were the statins (36% any use, 25% new users) and the ACEI/ARBs (28% any use, 17% new users). A total of 38,205 (38%) persons were new users of at least one of the other drug classes of interest and also had a 6 or 12 month MPR for that drug class before the one year bisphosphonate MPR measurement. Table 1 shows the demographic characteristics, comorbidities, health services utilization, and medication use of these individuals compared to those who were not new users with a 6 or 12 month MPR of any of the other medications. The new users of at least one of the other drugs were older, had a higher burden of comorbidity, were more likely to be hospitalized, and visited their physician more frequently. The mean ± standard deviation (SD) length of post-index observation time for all individuals in the cohort was 26.7 ± 17 months.
Table 1.
Demographic Characteristics, Comorbidities, and Health Services Utilization of Persons Initiating Bisphosphonate Therapy Who Were or Were Not Concomitant New Users of Medications Used to Treat Chronic, Asymptomatic Conditions (n = 101,038)
| On One or More Concomitant Therapies* (n = 38,205) |
Not On Any Concomitant Therapy (n = 62,833) |
P value | |
|---|---|---|---|
| Demographics | |||
| Age | |||
| 45-49 | 429 (1) | 1,353 (2) | < .0001 |
| 50-54 | 5,976 (16) | 12,875 (22) | < .0001 |
| 55-59 | 9,321 (24) | 17,613 (28) | < .0001 |
| 60-64 | 8,170 (21) | 13,322 (21) | N.S. |
| 65-69 | 4,039 (11) | 5,877 (9) | < .0001 |
| 69-74 | 3,571 (9) | 4,048 (6) | < .0001 |
| ≥ 75 | 6,699 (18) | 6,745 (11) | < .0001 |
| Women | 35,752 (94) | 59,989 (95) | < .0001 |
| Prior Fracture | |||
| Hip | 423 (1.1) | 433 (0.7) | < .0001 |
| Wrist/Forearm | 406 (1.1) | 620 (1) | N.S. |
| Clinical Vertebral | 743 (2) | 755 (1.2) | < .0001 |
| Non-hip, non-vertebral | 1,016 (2.7) | 1,361 (2.2) | < .0001 |
| Any non-vertebral | 1,246 (3.3) | 1,610 (2.6) | < .0001 |
| Unspecified or Other | 1,449 (3.8) | 1,997 (3.2) | < .0001 |
| Other Selected Comorbidities | |||
| Osteoporosis | 15,844 (41.2) | 26,761 (42.6) | < .001 |
| Diabetes | 4,258 (11.2) | 2,541 (4) | < .0001 |
| Rheumatoid Arthritis | 1,126 (3) | 1,721 (2.7) | 0.05 |
| Hyperlipidemia | 14,678 (38.4) | 14,346 (22.8) | < .0001 |
| Smoking | 504 (1.3) | 752 (1.2) | N.S. |
| Hyperthyroidism | 655 (1.7) | 757 (1.2) | < .0001 |
| Charlson Comorbidity Index | 1 (1.2) | 0 (0.8) | < .0001 |
| Prior Use of Selected Medications | |||
| Systemic Estrogen | 7,256 (19) | 14,555 (23.2) | < .0001 |
| Teriparatide | 29 (1) | 29 (0.1) | N.S. |
| Raloxifene | 2,009 (5.3) | 3,740 (6) | < .0001 |
| Nasal calcitonin | 1,226 (3.2) | 1,953 (3.1) | N.S. |
| Glucocorticoid | 4,033 (10.6) | 5,363 (8.5) | < .0001 |
| Health Services Utilization | |||
| Outpatient visits | 4 (3.45) | 3 (2.8) | < .0001 |
| Any hospitalization | 2,893 (7.6) | 2,321 (3.7) | < .0001 |
| Bone mineral density test | 21,440 (56.1) | 37,137 (59.1) | < .0001 |
| Other Screening Tests | 12,141 (31.8) | 22,207 (35.3) | < .0001 |
| Mammography | 2,281 (6) | 3,555 (5.7) | 0.04 |
| Colonoscopy | 7,486 (19.6) | 14,381 (22.9) | < .0001 |
| Fecal Occult Blood Test | 248 (0.7) | 482 (0.8) | 0.03 |
| Flexible Sigmoidscopy | 438 (1.2) | 541 (0.9) | < .0001 |
| Prostate Specific Antigen screening | |||
| Initial Bisphosphonate Use | |||
| Alendronate Daily | 3,846 (10.1) | 9,531 (15.2) | < .0001 |
| Alendronate Weekly | 22,718 (59.5) | 36,096 (57.5) | < .0001 |
| Risedronate Daily | 1,289 (3.4) | 2,261 (3.6) | 0.06 |
| Risedronate Weekly | 10,265 (26.9) | 14,811 (23.6) | < .0001 |
| Ibandronate Monthly | 87 (0.0) | 134 (0.0) | N.S. |
Data shown are n (%) or mean +− standard deviation. All factors were assessed in the 6 months prior to first bisphosphonate use.
these individuals also had available a 6 or 12 month compliance assessment for at least one of the other medication classes of interest
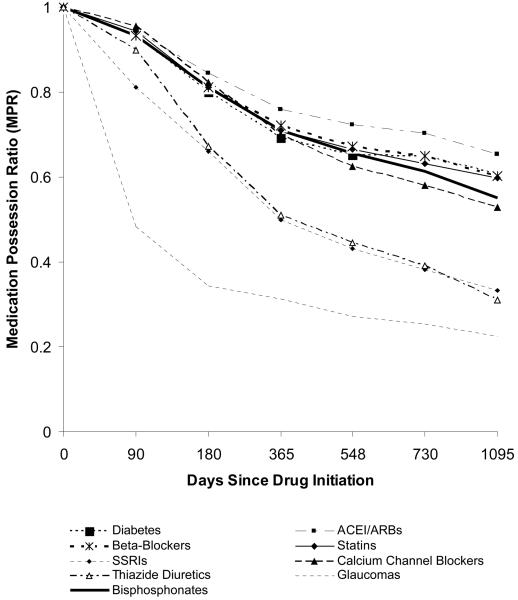
Although long term compliance with all of the drug classes was suboptimal (Figure 2), persons starting ACEI/ARBs or statin therapy were somewhat more adherent than users of the other drug classes; measured compliance was lowest for glaucoma medications. At 1 year, the proportion of persons with high bisphosphonate compliance (MPR >= 80%) was 44%. Agreement between the 1 year bisphosphonate MPR and 1 year MPR for the other drug classes was generally fair, with kappas in the range of 0.2 – 0.4. Pairwise agreement between high compliance for the other drug classes with each other was also in this range.
Figure 2.
Longitudinal Adherence among New Users of Medications Used to Treat Chronic Conditions
Multivariable logistic regression indicated that factors significantly associated with high bisphosphonate compliance included age, sex, and weekly or monthly bisphosphonate dosing (Table 2). Receipt of BMD testing and other types of preventive testing (e.g. colonoscopy) were also significantly associated with high compliance. More physician visits and a greater burden of comorbidity was associated with lower compliance. Despite the inclusion of numerous statistically significant factors, this model had low ability to identify persons with high 1 year compliance to bisphosphonates, with an AUC of 0.62. This model demonstrated poor calibration and failed the Hosmer-Lemeshow Goodness of Fit test (p = 0.049, consistent with a lack of model fit).
Table 2.
Multivariable-adjusted Factors associated with High Bisphosphonate Compliance at 1 Year (n = 38205 users)
| Factor | Odds Ratio (95% CI) |
|---|---|
| Age (years) | |
| 45-50 | 1.0 (referent) |
| 50-55 | 1.10 (0.99 – 1.22) |
| 55-60 | 1.21 (1.10 – 1.35) |
| 60-65 | 1.31 (1.18 – 1.45) |
| 65-70 | 1.17 (1.05 – 1.30) |
| 70-75 | 1.05 (0.94 – 1.17) |
| ≥ 75 | 1.05 (0.94 – 1.16) |
| Sex | |
| Men | 1.0 (referent) |
| Women | 0.80 (0.75 – 0.86) |
| Comorbidities | |
| Rheumatoid Arthritis | 0.91 (0.84 – 1.00) |
| Osteoporosis | 1.14 (1.11 – 1.17) |
| Diabetes | 0.91 (0.86 – 0.94) |
| Smoking | 0.67 (0.59 – 0.75) |
| Charlson Comorbidity Index | 0.89 (0.85 – 0.93) |
| Vertebral Fracture | 1.18 (1.06 – 1.32) |
| Hip Fracture | 1.30 (1.02 – 1.67) |
| Health Services Utilization | |
| BMD | 1.61 (1.56 – 1.66) |
| Mammography | 1.24 (1.20 – 1.28) |
| Colonoscopy | 1.14 (1.08 – 1.21) |
| Fecal Occult Blood Testing | 1.14 (1.11 – 1.18) |
| # of Outpatient Visits | |
| 0-1 | 1.0 (referent) |
| 2-4 | 0.90 (0.87 – 0.93) |
| ≥ 5 | 0.81 (0.78 – 0.85) |
| Medication Use | |
| Bisphosphonate Frequency | |
| Weekly/monthly use | 1.0 (referent) |
| Daily use | 0.73 (0.71 – 0.76) |
| Estrogen use | 1.28 (1.24 – 1.32) |
| Raloxifene use | 1.53 (1.44 – 1.62) |
| Nasal calcitonin use | 1.44 (1.34 – 1.56) |
| Glucocorticoid use | 0.89 (0.85 – 0.94) |
| # of unique Medications | |
| 0-1 | 1.0 (referent) |
| 2-5 | 0.95 (0.92 – 0.99) |
| ≥ 6 | 0.86 (0.82 – 0.89) |
All factors except age and gender were assessed in the 6 months prior to initiating bisphosphonates
additional factors assessed in the 6 months prior to the index date that were adjusted for but that were not statistically significant included non-vertebral fracture, non-vertebral/non-hip fracture, wrist fracture, other fracture, hyperlipidemia, hyperthyroidism, flexible sigmoidoscopy, prostate specific antigen testing, teriparatide use, nursing home stay, and number of hospitalizations.
To this base model we next added one additional variable, the 12 (or 6) month MPR with each of the other drug classes. As demonstrated in Table 3, the AUC increased with inclusion of this one additional variable and was highest for users of statins (AUC = 0.70). Except for a model with SSRI added, incorporating the new compliance variable resulted in all the models being properly calibrated and passing the Hosmer-Lemeshow Goodness of Fit Test.
Table 3.
Incremental Gain in Model Discrimination and Calibration After Adding Information Regarding Compliance to Other Medications
| N | AUC | Goodness of Fit* |
|
|---|---|---|---|
| Base model | 95,168 | 0.62 | 0.05 |
|
Base model + Compliance Measure for a Single Drug Class | |||
| Statins | 13,619 | 0.70 | 0.20 |
| ACEI/ARBs | 9,622 | 0.67 | 0.96 |
| SSRIs | 9,259 | 0.64 | 0.02 |
| Beta-Blockers | 8,062 | 0.66 | 0.42 |
| Calcium channel blockers |
5,641 | 0.67 | 0.26 |
| Thiazide diuretics | 5,520 | 0.65 | 0.13 |
| Glaucoma medications |
2,241 | 0.65 | 0.58 |
| Oral Diabetes agents | 1,711 | 0.67 | 0.63 |
|
Base model + Compliance Measures for Multiple Drug Classes | |||
| Statins + ACEI/ARBs | 2,946 | 0.73 | 0.17 |
| Statins + ACEI/ARBs + SSRIs | 462 | 0.78 | 0.54 |
AUC = area under the receiver operator curve of the prediction model
p values ≤ 0.05 are consistent with poor model calibration
Results from our recursive partitioning tree for the statin users showed similar results to the logistic regression results and yielded an AUC of 0.72 in the training set of data and 0.69 in the testing set (data not shown). Based upon the of variable importance table results from CART, the statin MPR variable was the most significant predictor of 1 year bisphosphonate compliance, followed by age and prior receipt of BMD testing. Results from the other classification trees were similar and showed that the drug class-specific compliance variables were consistently the most important predictors of bisphosphonate compliance compared to all the other factors in Table 2.
For persons that initiated drugs from multiple classes, we then sequentially added the corresponding MPR variables to our base compliance model (Table 3, last 2 rows). The numbers of persons with this pattern of usage decreased the sample size appreciably. However, the incremental benefit in the identification of persons with high bisphosphonate compliance with the addition of each compliance variable was moderate and reached a maximum of 0.78 for the combination of drugs that we evaluated (chosen based upon maximizing the sample size).
Finally, table 4 shows the relationship between high (MPR ≥ 80%) or moderate (MPR 50-80%) compliance with each of the other drug classes and high bisphosphonate compliance at 1 year, after adjusting for all the factors listed in Table 3. As shown, high compliance with each of the other drug classes was significantly associated with high bisphosphonate compliance; moderate compliance with each of the other drug classes was weaker and was significantly associated with high bisphosphonate compliance only for some drug classes. Associations using the 12 month MPR variables for the other drug classes were somewhat stronger than for the 6 month MPR variables (data not shown).
Table 4.
Multivariable Adjusted* Associations between High or Moderate Compliance to Other Drug Classes and High Bisphosphonate Compliance
| Drug Class | Odds Ratio (95% CI) |
|
|---|---|---|
| High Compliance** MPR >= 80% |
Moderate Compliance** (MPR 50 – 80%) |
|
| Statins | 4.08 (3.73 – 4.47) | 1.52 (1.37 – 1.69) |
| ACEI/ARBs | 2.65 (2.38 – 2.94) | 1.22 (1.07 – 1.39) |
| SSRIs | 1.80 (1.63 – 1.99) | 1.26 (1.12 – 1.42) |
| Beta-blockers | 2.06 (1.85 – 2.30) | 0.98 (0.85 – 1.13) |
| Calcium Channel Blockers | 2.35 (2.07 – 2.68) | 1.07 (0.90 – 1.27) |
| Thiazide Diuretics | 1.89 (1.67 – 2.14) | 0.98 (0.84 – 1.14) |
| Glaucoma | 2.24 (1.66 – 3.04) | 1.60 (1.30 – 1.97) |
| Diabetes | 2.38 (1.84 – 3.09) | 1.21 (0.88 – 1.66) |
BP = Bisphosphoonate; MPR = Medication Possession Ratio;
ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker
adjusted for all factors listed in Table 2
referent to low compliance (i.e. MPR < 50%) for that drug class
Results from our sensitivity analyses excluding persons hospitalized between initiation of bisphosphonates or the other drug classes and the corresponding compliance measurement did not change our primary results (data not shown). Also, our results were similar after restricting users of anti-hypertensive medications to only those individuals who had a physician diagnosis of hypertension in the six month preceding initiation of these therapies, although these diagnoses were found only for a minority of individuals.
Discussion
Many factors available in administrative claims data have been associated with high compliance to bisphosphonates. However, even in aggregate, the ability of these factors to discriminate accurately between persons with high and low compliance is quite limited. We demonstrated that for the substantial number of persons that are prescribed one or more other drugs used to treat chronic, typically asymptomatic conditions, evaluating prior compliance with these medications is significantly associated with oral bisphosphonate compliance in our study population and in fact, outweighs all other variables in importance.
Our results have practical implications when applied to a population or health system in that prospective identification of persons likely or not likely to be adherent with oral bisphosphonates may allow for targeted interventions. These might include a more aggressive follow-up regimen, more frequent contact with allied health care providers (e.g. a nurse case manager), or feedback regarding physiologic measures of efficacy such as bone turnover markers. These strategies have been shown to result in better compliance with osteoporosis medications [25, 26]. Treatment regimens that are more under the control of physicians and the healthcare system (e.g. parenteral bisphosphonates) may also be an attractive option for individuals less likely to be adherent. Although physicians probably have a clinical sense of their patients' likelihood of future compliance, the use of administrative data to select out persons at risk for low compliance could allows for this identification to happen not on a case-by-case basis but rather for a large population.
Our results are similar to prior work that identified a number of factors significantly associated with compliance (Table 2), including the use of preventive services that may represent a ‘healthy user’ effect [16]. However, as has been previously shown [8, 9], in our data these variables were collectively suboptimal to identify individuals with high compliance. Of all the drug class-specific MPR variables, compliance with anti-hyperlipidemics added the most information to our models. We suspect that this is because this drug class is inclusive of all therapies typically used for hyperlipidemia, and patients typically receive only one drug from this class at a time. Thus, compliance with this drug class is likely measured with the least error. Measured compliance with glaucoma medications was lowest, probably because a majority of the drugs are topical and unlike oral medications, a single prescription may last longer than 30 days. For the drugs that we classified as anti-hypertensives, the pattern of expected use may be more variable since the prescribing indications for these drugs extend to other conditions beyond hypertension. Unfortunately, restricting our population using these drugs to just those persons with a diagnosis code for hypertension yielded too small a sample size to evaluate whether this strategy could result in a more homogeneous group. Moreover, patients with hypertension may switch between drug classes and thus may appear non-adherent to that class of medications, despite being adherent to the overall treatment regimen. However, as a potential solution, collapsing the anti-hypertensives into a single drug class is infeasible since patients may be prescribed multiple anti-hypertensives simultaneously. In this situation, 50% compliance with two drugs cannot be easily distinguished from 100% compliance with one drug. Patients who are instructed to cut their tablets in half (for certain drugs) could similarly have their compliance misclassified. More complex claims-based algorithms might be developed to differentiate these patterns of use.
Although we identified new users of osteoporosis medications, as defined by at least six months with no use (a ‘clean period’), it is possible that patients previously received an osteoporosis medication before this time. Indeed, for statin users, the median interval of time between discontinuation and re-initiation is approximately one year [27]. However, the mean amount of observation time in our dataset was between two and three years, which limited our ability to identify recurrent use after prolonged treatment gaps. Data sources with more longitudinal information might be used to evaluate prior adherence with medications prescribed for the same indication. It may also be useful to require ‘clean periods’ for new drug users that are longer than 6 months to separate true incident drug users from those re-initiating therapy after a prolonged period of discontinuation. These latter patients may be channeled to particular therapies that have a lower risk of non-compliance; a failure to detect this channeling may introduce bias in studies that evaluate comparative compliance between medications.
Our drug class compliance variables might seem to offer some attractiveness for use as instrumental variables [28, 29] since some (e.g. ACEI/ARB compliance) are associated with exposure (i.e. bisphosphonate compliance) but have no biologically plausible effect on fracture outcomes. However, it would be necessary to assume that the unmeasured confounders (e.g. smoking, use of calcium and vitamin D, physical activity) associated with bisphosphonate compliance that one is trying to control for with the use of the instrumental variable are not associated with compliance to the other drug classes. This may or may not be true, although this assumption is testable. Rather than use them as instrumental variables, the drug class-specific compliance variables also might be used as covariates to control for confounding factors related to overall non-compliance that are typically unmeasured in administrative claims data.
We are not aware of other reports that have evaluated the prediction of high compliance with bisphosphonates on the basis of compliance with other medications used to treat chronic, asymptomatic conditions where long term therapy is required. Moreover, our approach has high generalizability to health systems that have ready access to administration data. To verify that our logistic regression models were not overly optimistic, we evaluated the performance of our approach using recursive partitioning trees with cross-validation derived in parallel using the same variables as our logistic regression models and obtained similar results.
One issue regarding the generalizability of our approach is that information regarding compliance to other drugs besides bisphosphonates is available for some but not all individuals. In our sample, 70% used at least one of the target drugs of interest, more than half were identified as new users, and 38% had available at least a six month MPR for the other drug classes prior to the one year bisphosphonate MPR assessment. Our population was commercially insured, and health systems with lower turnover and longer follow-up times (e.g. Medicaid, Medicare, Veterans Administration, self-contained health maintenance organizations) and/or greater burdens of comorbidities that require long term drug use may be able to identify an even higher proportion of new users of these drug classes. Because our results may or may not be generalizable to these settings, our approach needs to be evaluated in other populations.
Incorporating compliance with other drug classes besides those we studied (e.g. once daily aspirin, warfarin, digoxin) may also be useful, although these drugs would need to be used with a high enough prevalence to warrant their evaluation. Finally, we did not include a number of other factors that may be associated with compliance, including out of pocket drug costs, patient knowledge of osteoporosis, severity of disease (e.g. results from bone mineral density testing), physician factors (e.g. specialty), frequency of contact between the patient and physician prescriber [27], or claims for gastrointestinal (GI) diagnoses potentially associated with oral bisphosphonate intolerance (e.g. dyspepsia). In administrative databases similar to the one that we used, many of these factors are not consistently available or informative. For example, based upon past work we have done [15], the aforementioned GI diagnoses may be under-coded or minimally related to bisphosphonate compliance.
In conclusion, we propose that the evaluation of compliance with any specific set of medications should include not only the demographic factors, comorbidities, and health services utilization that have typically been examined but also should incorporate information regarding compliance patterns with medications used for other conditions. In this large U.S. health care organization population, we found that the addition of additional drug data provided an incremental benefit in identifying persons with high compliance and may prove worthwhile for future targeting of interventions aimed to promote patient compliance.
Acknowledgments
Funding
This project was funded by the Arthritis Foundation, a Phrma Foundation Research Grant in Health Outcomes, and Novartis Pharmaceuticals. Some of the investigators (JRC, KGS) also receive support from the National Institutes of Health (AR053351, AR052361). The authors independently developed the analysis plan, extracted the data, conducted the analysis, and interpreted the results.
Footnotes
Disclosures
JC: research grants: Novartis, Amgen, Merck, Proctor & Gamble, Eli Lilly; consulting: Roche; speakers bureau: Merck, Procter and Gamble, Eli Lilly, Roche
AW: research grants: Novartis
HC: research grants: Amgen
KL: research grants: Novartis, Amgen, Alliance for Better Bone health; consulting: Novartis, Procter & Gamble, Merck, Amgen, GTx, Lilly, GSK, Bone Medical Ltd.; patent: Use Patent investor; “methods for preventing or reducing secondary fractures after hip fracture”, US patent application 20050272707; provisional patent application: “medication kits and formulations for preventing, treating, or reducing secondary fractures after previous fracture”
KGS: research grants : Novartis, Amgen, Aventis, Merck, Procter & Gamble, Eli Lilly, Roche; consulting or speaking: Merck, Proctor and Gamble, Eli Lilly, Roche, Novartis, Amgen
ED: research grants: Amgen
References
- 1.Penning-van Beest FJ, Erkens JA, Olson M, Herings RM. Loss of treatment benefit due to low compliance with bisphosphonate therapy. Osteoporos Int. 2007 doi: 10.1007/s00198-007-0466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weycker D, Macarios D, Edelsberg J, Oster G. Compliance with drug therapy for postmenopausal osteoporosis. Osteoporos Int. 2006;17(11):1645–52. doi: 10.1007/s00198-006-0179-x. [DOI] [PubMed] [Google Scholar]
- 3.Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81(8):1013–22. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- 4.Rossini M, Bianchi G, Di Munno O, et al. Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int. 2006;17(6):914–21. doi: 10.1007/s00198-006-0073-6. [DOI] [PubMed] [Google Scholar]
- 5.Penning-van Beest FJ, Goettsch WG, Erkens JA, Herings RM. Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther. 2006;28(2):236–42. doi: 10.1016/j.clinthera.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone. 2006;38(6):922–8. doi: 10.1016/j.bone.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Gold DT, Silverman S. Review of adherence to medications for the treatment of osteoporosis. Curr Osteoporos Rep. 2006;4(1):21–7. doi: 10.1007/s11914-006-0011-8. [DOI] [PubMed] [Google Scholar]
- 8.Lo JC, Pressman AR, Omar MA, Ettinger B. Persistence with weekly alendronate therapy among postmenopausal women. Osteoporos Int. 2006;17(6):922–8. doi: 10.1007/s00198-006-0085-2. [DOI] [PubMed] [Google Scholar]
- 9.Solomon DH, Avorn J, Katz JN, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165(20):2414–9. doi: 10.1001/archinte.165.20.2414. [DOI] [PubMed] [Google Scholar]
- 10.Caro JJ, Salas M, Speckman JL, Raggio G, Jackson JD. Persistence with treatment for hypertension in actual practice. Cmaj. 1999;160(1):31–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzo JA, Simons WR. Variations in compliance among hypertensive patients by drug class: implications for health care costs. Clin Ther. 1997;19(6):1446–57. doi: 10.1016/s0149-2918(97)80018-5. discussion 1424-5. [DOI] [PubMed] [Google Scholar]
- 12.Parris ES, Lawrence DB, Mohn LA, Long LB. Adherence to statin therapy and LDL cholesterol goal attainment by patients with diabetes and dyslipidemia. Diabetes Care. 2005;28(3):595–9. doi: 10.2337/diacare.28.3.595. [DOI] [PubMed] [Google Scholar]
- 13.Perreault S, Lamarre D, Blais L, et al. Persistence with treatment in newly treated middle-aged patients with essential hypertension. Ann Pharmacother. 2005;39(9):1401–8. doi: 10.1345/aph.1E548. [DOI] [PubMed] [Google Scholar]
- 14.Gibson TB, Mark TL, McGuigan KA, Axelsen K, Wang S. The effects of prescription drug copayments on statin adherence. Am J Manag Care. 2006;12(9):509–17. [PubMed] [Google Scholar]
- 15.Curtis JR, Westfall AO, Allison JJ, Freeman A, Saag KG. Channeling and adherence with alendronate and risedronate among chronic glucocorticoid users. Osteoporos Int. 2006;17(8):1268–74. doi: 10.1007/s00198-006-0136-8. [DOI] [PubMed] [Google Scholar]
- 16.Brookhart MA, Patrick AR, Dormuth C, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166(3):348–54. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 17.Briesacher B, Andrade S, Fouayzi H, Chan K. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28(4):437–43. doi: 10.1592/phco.28.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Turbi C, Herrero-Beaumont G, Acebes JC, et al. Compliance and satisfaction with raloxifene versus alendronate for the treatment of postmenopausal osteoporosis in clinical practice: An open-label, prospective, nonrandomized, observational study. Clin Ther. 2004;26(2):245–56. doi: 10.1016/s0149-2918(04)90023-9. [DOI] [PubMed] [Google Scholar]
- 20.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11(7):449–57. [PubMed] [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 22.Curtis J, Westfall A, Cheng H, Lyles K, Saag K, Delzell E. The Benefit of Adherence with Bisphosphonates Depends on Age and Fracture Type: Results From an Analysis of 101,038 New Bisphosphonate Users. Journal Bone Mineral Research. 2008 doi: 10.1359/JBMR.080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosmer JDW, Lemeshow S. Applied Logistic Regression. 2nd Edition John Wiley and Sons; 2000. [Google Scholar]
- 24.Lemeshow S, Hosmer JDW. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- 25.Clowes JA, Peel NF, E R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89(3):1117–23. doi: 10.1210/jc.2003-030501. [DOI] [PubMed] [Google Scholar]
- 26.Delmas PD, Vrijens B, Eastell R, et al. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2007;92(4):1296–304. doi: 10.1210/jc.2006-1526. [DOI] [PubMed] [Google Scholar]
- 27.Brookhart MA, Patrick AR, Schneeweiss S, et al. Physician follow-up and provider continuity are associated with long-term medication adherence: a study of the dynamics of statin use. Arch Intern Med. 2007;167(8):847–52. doi: 10.1001/archinte.167.8.847. [DOI] [PubMed] [Google Scholar]
- 28.Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000;29(4):722–9. doi: 10.1093/ije/29.4.722. [DOI] [PubMed] [Google Scholar]
- 29.Angrist J, Imbens G, Rubin D. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996;81:444–55. [Google Scholar]