Abstract
To study the substrate specificity of enzymes, we use the amidohydrolase and enolase superfamilies as model systems; members of these superfamilies share a common TIM barrel fold and catalyze a wide range of chemical reactions. Here, we describe a collaboration between the Enzyme Specificity Consortium (ENSPEC) and the New York SGX Research Center for Structural Genomics (NYSGXRC) that aims to maximize the structural coverage of the amidohydrolase and enolase superfamilies. Using sequence- and structure-based protein comparisons, we first selected 535 target proteins from a variety of genomes for high-throughput structure determination by X-ray crystallography; 63 of these targets were not previously annotated as superfamily members. To date, 20 unique amidohydrolase and 41 unique enolase structures have been determined, increasing the fraction of sequences in the two superfamilies that can be modeled based on at least 30% sequence identity from 45% to 73%. We present case studies of proteins related to uronate isomerase (an amidohydrolase superfamily member) and mandelate racemase (an enolase superfamily member), to illustrate how this structure-focused approach can be used to generate hypotheses about sequence–structure–function relationships.
Electronic supplementary material
The online version of this article (doi:10.1007/s10969-008-9056-5) contains supplementary material, which is available to authorized users.
Keywords: Amidohydrolase and enolase superfamilies, Structural genomics, Structure annotation, Target selection
Introduction
A long-standing challenge in biology is to predict the molecular function of proteins from their sequences and/or structures. This task is facilitated by a limited number of domain folds [1], restricting the set of structural types that must be studied in deducing a much larger set of functions. Special challenges, however, exist for functional prediction in different classes of proteins. For example, the function of an enzyme often cannot be correctly predicted because there are no clear links from the domain fold to the catalytic function and substrate specificity. Off-setting these problems, studies of genomes and sets of homologous proteins demonstrate that some aspects of catalysis are often conserved between evolutionarily-related proteins, even when these proteins catalyze different overall reactions [2–4]. This empirical observation restricts the functional space that must be considered, further facilitating prediction and leading to definitions of homologous sets of enzymes in terms of protein superfamilies and families based not only on structural conservation, but also on functional conservation [5]: Superfamily members share a common ancestor and potentially some aspects of function, while members of the same family are isofunctional, catalyzing the same overall reaction(s).
The large and diverse amidohydrolase and enolase superfamilies provide a particularly attractive opportunity to study the problem of predicting substrate specificity and enzymatic mechanisms from evolutionary and physical perspectives. These superfamilies are attractive targets because significant knowledge about the specificity of many of their members already exists, while there are still large areas of their sequence space where we do not have any structural or functional information.
Members of the amidohydrolase superfamily catalyze the hydrolysis of a wide range of substrates bearing amide or ester functional groups at carbon and phosphorus centers [6, 7]. A common feature for this superfamily is a mononuclear or binuclear metal center coordinated in a (β/α)8-barrel (TIM barrel) polypeptide chain fold. The active site is formed by loops at the C-terminal ends of the β-strands. Currently, 36 named families have been identified based on the experimentally verified catalytic reactions. The set of superfamily sequences has been clustered into 90 subgroups based on sequence and in some cases active site similarities (the Structure-Function Linkage Database [8]: http://sfld.rbvi.ucsf.edu). In some subgroups, additional information about chemical reactions catalyzed by subgroup members is available; for many of the subgroups, however, no information about functional specificity is available.
Enolase superfamily members catalyze the abstraction of a proton α to a carboxylic acid to form an enolate anion intermediate [9, 10]. Members of this superfamily share an N-terminal α+β capping domain, as well as a C-terminal (β/α)7β-barrel domain (modified TIM barrel). The active site is formed by loops at the C-terminal ends of the TIM barrel β-strands and two flexible loops from the capping domain; the active site also includes a Mg2+ ion [11]. Reactions catalyzed by enolases are less diverse than those of the amidohydrolases. The enolases are currently organized into 16 named families and 6 subgroups [8]. Approximately 50% of the sequences in the superfamily are of unknown function.
The amidohydrolase and enolase superfamilies are the focus of our Enzyme Specificity Consortium (ENSPEC), members of which include protein crystallographers, enzymologists, and computational biologists. We aim to predict the substrate specificity of an enzyme based on its experimentally determined and/or modeled structure [2–4, 7, 10–42]. This goal has been enabled by determination of crystallographic structures representing many of the amidohydrolase and enolase families.
To maximize the number of experimentally determined structures, ENSPEC has collaborated with the New York SGX Research Center for Structural Genomics (NYSGXRC), which is one of the four large-scale production centers of the Protein Structure Initiative (PSI) (http://www.nigms.nih.gov/Initiatives/PSI; [43]). NIH guidelines mandate that 70% of the PSI targets come from diverse protein families selected by and shared among the four production centers [43]. About 15% of the targets are reserved for proteins of biomedical relevance defined by each center, and the remaining 15% are “community-nominated” targets. Several hundred of the NYSGXRC community targets are amidohydrolases and enolases nominated by ENSPEC. To date, the collaboration has determined 25 amidohydrolase and 50 enolase structures, contributing substantially to the total of 154 amidohydrolase and 89 enolase structures in the Protein Data Bank (PDB; 6/16/08) [44].
We begin by outlining the data sources and methods used for target selection and structure-based functional annotation (Materials and Methods). Second, we present the results of the target selection process, the status of the selected targets in the structural genomics pipeline, and the improvement in the modeling of the amidohydrolase and enolase superfamilies made possible by the new crystallographic structures (Results and Discussion). We conclude by discussing the biological impact of two sample target structures.
Materials and methods
Target selection
Target selection begins by identifying sequences of known members of the superfamilies (seed sequences), followed by filtering to obtain an initial target list. To identify additional members, we applied sequence- and structure-based expansion methods, followed by filtering for source organisms preferred by NYSGXRC. Superfamily membership for the additional targets was verified by expert curators by inspecting their sequences for probable catalytic residues. A web-based target selection tool was also constructed for further manual filtering to obtain the final target list.
Seed sequence sources
Verified amidohydrolase and enolase superfamily sequences (i.e., seed sequences) were obtained from the Structure Function Linkage Database (SFLD; http://sfld.rbvi.ucsf.edu/) [8]. The SFLD database is a manually constructed database that classifies enzymes hierarchically, based on specific sequence, structure, and functional criteria. The database is updated by a semi-automated method that detects new superfamily members by matching their sequences to Hidden Markov Models trained using the sequences of verified superfamily members, with subsequent manual inspection to verify the presence of catalytic residues. In June 2005, when our target list was constructed, the SFLD contained 3,701 amidohydrolases and 1,795 enolases.1
Filtering of seed sequences
PSI guidelines require that structural genomics targets share ~30% or less amino acid sequence identity to a known thee-dimensional structure. To satisfy this condition, the seed amidohydrolase and enolase sequences were processed using the automated comparative modeling server MODWEB (http://salilab.org/modweb) [45]. Sequences with more than 30% sequence identity to any structure in the PDB over at least 70% of their length were identified and excluded from further consideration.
Sequence-based expansion of amidohydrolase and enolase superfamily members
For each seed amidohydrolase and enolase, homologous sequences in the UNIPROT database [46] were identified by the BUILD_PROFILE routine of MODELLER-9 [45]. BUILD_PROFILE is an iterative database-searching tool that relies on local dynamic programming to generate alignments and a robust estimate of their statistical significance. This method identified additional potential amidohydrolase and enolase sequences that were not present in the seed sequence pools.
Structure-based expansion of amidohydrolase superfamily members
In addition to the SFLD entries, we also used the known amidohydrolase superfamily structures to find additional potential amidohydrolase superfamily members (this expansion was not performed for the enolase superfamily). We began by splitting 100 PDB files containing known amidohydrolase structures (June 2005) into separate monomeric structures and clustering them at 80% sequence identity. The resulting 45 non-redundant structures were used for comparative modeling using the automated modeling server MODWEB [45].
First, each structure sequence was used as a query to find its homologs in UNIPROT using PSIBLAST [47]. Second, these homologs were modeled using the corresponding structure as a template. All models were deposited in our comprehensive MODBASE database of comparative protein structure models (http://salilab.org/modbase/; direct links to the datasets can be found in the supplemental materials) [48]. In addition, the amidohydrolase homologs found in UNIPROT were filtered by removing known amidohydrolase superfamily members, and then subjected to standard comparative modeling with MODWEB using all non-redundant chains in the PDB as potential templates. This step allowed us to eliminate sequences that are likely members of other superfamilies, judged by sequence identity and coverage.
Filtering by organism
While seed sequences could come from any genome, the additional amidohydrolase sequences identified by sequence- and structure-based expansions were filtered for ease of cloning to include only 79 organisms with genomic DNA available to NYSGXRC in 2005 and the marine metagenome from the Sargasso Sea sequencing project (formerly called environmental sequences) [49]. For simplicity, we call the 79 genomes plus the marine metagenome the “NYSGXRC genomes” (Table 1). The NYSGXRC reagent genomes have since been expanded to include over 115 organisms.
Table 1.
List of 80 NYSGXRC genomes (as of June 2005)
| Organism | Taxonomy ID | Organism | Taxonomy ID |
|---|---|---|---|
| Aeropyrum pernix | 56636 | Listeria monocytogenes | 1639 |
| Aquifex aeolicus | 63363 | Metagenome sequences (Gene synthesis) | 256318 |
| Arabidopsis thaliana | 3702 | Methanococcus jannaschi | 2190 |
| Archaeoglobus fulgidus | 2234 | Mus musculus | 10090 |
| Bacillus cereus | 1396 | Mycobacterium turberculosis H37Rv | 83332 |
| Bacillus halodurnas | 86665 | Mycoplasma pneumonia | 2104 |
| Bacillus subtilis | 1423 | Neisseria gonorrhoeae | 485 |
| Bacillus thuringiensis | 1428 | Neisseria meningitidis | 487 |
| Bartonella henselae | 38323 | Nostoc | 1180 |
| Bordetella pertussis | 520 | Oryctolagus cuniculus | 9986 |
| Borrelia burgdorferi | 139 | Oryza sativa | 4530 |
| Bos taurus | 9913 | Ovis aries | 9940 |
| Caenorhabditis elegans | 6239 | Porphyromonas gingivalis | 837 |
| Campylobacter jejuni | 197 | Pseudomonas aeruginosa | 287 |
| Candida albicans | 5476 | Pseudomonas putida | 303 |
| Canis familiaris | 9615 | Pyrococcus furiosus | 2261 |
| Capra hircus | 9925 | Pyrococcus horikoshii | 53953 |
| Caulobacter vibrioides | 155892 | Rattus norvegicus | 10116 |
| Clostridium acetobutylicum | 1488 | Rhodobacter sphaeroides | 1063 |
| Corynebacterium diphtheriae | 1717 | Saccharomyces cerevisiae | 4932 |
| Cryptococcus neoformans | 5207 | Salmonella typhimurium | 602 |
| Cryptosporidium parvum | 5807 | Schizosaccharomyces pombe | 4896 |
| Deinococcus radiodurans | 1299 | Shigella Flexneri type 2a | 42897 |
| Desulfovibrio vulgaris | 881 | Simian immunodeficiency virus | 11723 |
| Dictyostelium discoideum | 44689 | Staphylococcus aureus | 1280 |
| Drosophila melanogaster | 7227 | Staphylococcus epidermidis | 1282 |
| Enterobacter cloacae | 550 | Streptococcus mutans | 1309 |
| Enterococcus faecalis | 1351 | Streptococcus pneumoniae | 1313 |
| Equus caballus | 9796 | Streptococcus pyogenes | 1314 |
| Escherichia coli | 562 | Sulfolobus solfataricus | 2287 |
| Escherichia coli 0157:H7 | 83334 | Sus scrofa | 9823 |
| Felis catus | 9685 | Takifugu rubripes | 31033 |
| Gallus gallus | 9031 | Thermoplasma acidophilum | 2303 |
| Haemophilus influenzae | 727 | Thermoplasma volcanium | 50339 |
| Halobacterium sp. NRC-1 | 64091 | Thermotoga maritima | 2336 |
| Helicobacter pylori | 210 | Ureaplasma urealyticum | 2130 |
| Homo sapiens | 9606 | Vibrio cholerae | 666 |
| Human immunodeficiency virus type 1 | 11676 | Xenopus laevis | 8355 |
| Klebsiella pneumoniae | 573 | Xylella fastidiosa | 2371 |
| Legionella pneumophila | 446 | Zea mays | 4577 |
Verification of catalytic residues
The putative amidohydrolase sequences resulting from the sequence- and structure-based expansions were aligned to existing amidohydrolase Hidden Markov Models (HMMs) in the SFLD and manually inspected for probable catalytic residues. The final target list only includes sequences with at least 70% of the catalytic residues present.
Target selection tool
For final manual filtering of the target list, we constructed a web-based target selection tool. The tool comprises a combination of MySQL database tables with an interactive web-interface using LAMP [50]. It contains information about the sequences, including UNIPROT annotation, organism, sequence length, closest known structure, sequence identity to other cluster members, and domain boundaries for the TIM barrel domain obtained from SFLD. The interface allows searching for project datasets, organism groups, homologs based on sequence identity, and clusters of related sequences; the resulting sequences can be flagged for rejection or inclusion into the final target list.
Analysis of the target structures
The amidohydrolase and enolase superfamilies were annotated using computational tools. Cytoscape clustering gives an overview of how the targets are distributed across the superfamily [51]. Also, template-based modeling determines how many new sequences can be modeled with the new structural information [45].
Sequence clustering of amidohydrolase superfamily by cytoscape
The time required to perfom BLAST searches against the NCBI non-redundant database (NR) of protein sequences [52] was prohibitive due to the size and complexity of the superfamily. Thus, a custom database was created containing only the amidohydrolase sequences in the SFLD. To generate the all-by-all connections for cytoscape clustering, BLAST searches were then performed against this database at an E-value cutoff of 10−10, using each sequence in the set as a query. Because this custom database contained only sequences known to be members of the amidohydrolase superfamily, the generation of E-values is biased. Consequently, the E-values from this analysis cannot be directly compared to those calculated by BLAST against the NCBI NR database. A cytoscape [51] network was created from these BLAST results. In the absence of established statistical techniques for selecting the E-value cutoff, we examined the superfamily networks at a number of different E-value cutoffs, and present here only one of the corresponding networks, at an E-value cutoff of 10−10. Further discussion regarding choosing and interpreting E-value cutoffs for sequence similarity networks may be found in [53]. Each node in the network represents a single sequence and each edge represents the pairwise connection between two sequences with the most significant BLAST E-value (better than the cut-off) connecting the two sequences. Lengths of edges are not meaningful, except that sequences in tightly clustered groups are more similar to each other than sequences with few connections. The nodes were arranged using the yFiles organic layout provided in Cytoscape version 2.4. Tools for visualization of protein networks were created by the UCSF Resource for Biocomputing, Visualization, and Informatics (http://www.rbvi.ucsf.edu).
Sequence clustering of enolase superfamily by cytoscape
To generate the all-by-all connections for cytoscape clustering, BLAST analysis was performed against the NR database, using the sequences in the mandelate racemase-like, glucarate dehydratase-like, mannonate dehydratase-like, and muconate cycloisomerase-like subgroups of the SFLD enolase superfamily. The enolase subgroup was not included in this analysis. Almost all of the enolase subgroup members are in the enolase family, the sequences of which are all isofunctional, i.e. they all perform the well-characterized enolase reaction, important in glycolysis. Only hits in the aforementioned subgroups were used for further analysis. The cytoscape network was created as described above, but using an E-value cutoff for this superfamily of 10−40.
Template-based modeling by MODWEB
Automated comparative modeling of all known protein sequences using the new NYSGXRC crystallographic structures as templates was performed with MODWEB [45]. We relied on the MODWEB option that allows using a protein structure as input and results in models for all of the identifiable sequence homologs of the input structure from the NCBI NR database; these homologs were identified during ten PSI-BLAST iterations of the template sequence against NR (E-value cutoff is 0.0001). The results are available at http://salilab.org/modbase/models_nysgxrc_latest.html (Table 2).
Table 2.
Summary of new enolase and amidohydrolase X-ray crystal structures and automated template-based modeling results, including subgroup and family assignments
| PDB code | Database accession number (Genpept GI IDs) | No of sequences in Psi-blast alignment | No of sequences with acceptable models and/or fold assignments | No of models >50% seq. ID (min 50% template coverage) | No of models 30–50% seq. ID (min 50% template coverage) | No of models <30% seq. ID (min 50% template coverage) | Subgroup assignment | Family assignment |
|---|---|---|---|---|---|---|---|---|
| Enolase super family | ||||||||
| 2GL5 | 16420812 | 2,863 | 2,777 | 0 | 98 | 2,462 | Mandelate racemase-like | Galactonate dehydratase |
| 2GDQ | 2633433 | 2,234 | 2,129 | 1 | 0 | 2,036 | Mandelate racemase-like | None |
| 2GSH | 16420830 | 2,588 | 2,286 | 16 | 9 | 2,110 | Mandelate racemase-like | l-Fuconate dehydratase |
| 2HNE | 21115341 | 2,746 | 2,712 | 83 | 20 | 2,527 | Mandelate racemase-like | None |
| 2HZG | 77386310 | 2,667 | 2,341 | 1 | 1 | 2,248 | Mandelate racemase-like | None |
| 2I5Q | 15832389 | 2,566 | 2,340 | 21 | 13 | 2,206 | Mandelate racemase-like | None |
| 2NQL | 17743914 | 2,849 | 2,470 | 2 | 1 | 2,356 | Mandelate racemase-like | None |
| 2O56 | 16767118 | 3,016 | 2,968 | 15 | 127 | 2,735 | Mandelate racemase-like | None |
| 2OQH | 21225834 | 2,690 | 2,668 | 2 | 32 | 2,630 | Glucarate dehydratease-like | None |
| 2OQY | 23100298 | 2,700 | 2,631 | 1 | 0 | 3,004 | Muconate cycloisomerase-like | None |
| 2OVL | 21221904 | 2,670 | 2,656 | 1 | 97 | 2,534 | Mandelate racemase-like | None |
| 2OG9 | 91786345 | 2,669 | 2,664 | 10 | 75 | 2,553 | Mandelate racemase-like | l-Talarate/galactarate dehydratase |
| 2OLA | 88195610 | 2,719 | 2,697 | 5 | 3 | 2,652 | Muconate cycloisomerase-like | o-Succinylbenzoate synthase |
| 2OO6 | 91778214 | 3,271 | 3,221 | 3 | 2 | 3,111 | Mandelate racemase-like | None |
| 2OKT | 57650581 | 2,723 | 2,705 | 5 | 3 | 2,664 | Muconate cycloisomerase-like | o-Succinylbenzoate synthase |
| 2OPJ | 72161814 | 2,562 | 1,855 | 19 | 31 | 1,712 | Mandelate racemase-like | o-Succinylbenzoate synthase |
| 2OX4 | 56552160 | 2,733 | 2,639 | 11 | 136 | 2,449 | Mandelate racemase-like | None |
| 2OZ3 | 67154209 | 2,743 | 2,656 | 38 | 25 | 2,567 | Mandelate racemase-like | None |
| 2OZ8 | 13475907 | 2,821 | 2,674 | 0 | 0 | 2,641 | Mandelate racemase-like | None |
| 2POI | 46136735 | 2,747 | 2,661 | 13 | 52 | 2561 | Mandelate racemase-like | None |
| 2OZT | 22294898 | 2,816 | 2,726 | 0 | 16 | 2,722 | Muconate cycloisomerase-like | o-Succinylbenzoate synthase |
| 2PCE | 83951697 | 2,693 | 2,683 | 1 | 16 | 2,635 | Muconate cycloisomerase-like | None |
| 2PGE | 51244103 | 2,779 | 2,767 | 1 | 19 | 2,768 | Muconate cycloisomerase-like | o-Succinylbenzoate synthase |
| 2PGW | 16263250 | 2,781 | 2,743 | 1 | 3 | 2,694 | Mandelate racemase-like | None |
| 2PMQ | 114764387 | 2,881 | 2,760 | 3 | 14 | 2,723 | Muconate cycloisomerase-like | None |
| 2POD | 53723090 | 2,745 | 2,732 | 12 | 97 | 2,585 | Mandelate racemase-like | Galactonate dehydratase |
| 2POZ | 13488170 | 2,861 | 2,836 | 1 | 162 | 2,687 | Mandelate racemase-like | None |
| 2PPG | 16262827 | 2,947 | 2,755 | 2 | 66 | 2,707 | Mandelate racemase-like | None |
| 2PS2 | 83774494 | 2,777 | 2,753 | 3 | 16 | 2,712 | Muconate cycloisomerase-like | None |
| 2QDE | 56478643 | 2,930 | 2,670 | 1 | 62 | 2,595 | Muconate cycloisomerase-like | None |
| 2QGY | 110347373 | 2,988 | 2,899 | 0 | 1 | 2,912 | Mandelate racemase-like | None |
| 2QQ6 | 108803396 | 3,238 | 3,216 | 0 | 201 | 3,081 | Mandelate racemase-like | Galactonate dehydratase |
| 2QYE | 83951695 | 3,128 | 3,121 | 0 | 21 | 3,110 | Muconate cycloisomerase-like | None |
| 3BJS | 6791043 | 3,261 | 2,897 | 4 | 82 | 2,810 | Mandelate racemase-like | None |
| 2QDD | 83951694 | 2,868 | 2,852 | 0 | 20 | 2,849 | Muconate cycloisomerase-like | o-Succinylbenzoate synthase |
| 3CAW | 42522147 | 2,220 | 2,139 | 0 | 0 | 2,137 | Muconate cycloisomerase-like | o-Succinylbenzoate synthase |
| 3CT2 | 70731221 | 3,483 | 2,771 | 84 | 77 | 2,667 | Muconate cycloisomerase-like | Muconate cycloisomerase |
| 3CYJ | 108805509 | 3,551 | 2,879 | 8 | 35 | 2,838 | Mandelate racemase-like | None |
| 3DDM | 33575875 | 3,603 | 3,576 | 6 | 27 | 3,591 | Mandelate racemase-like | None |
| 3BSM | 92115090 | 3,372 | 3,359 | 86 | 165 | 3,097 | Mannonate dehydratase-like | Mannonate dehydratase |
| Total (unique sequences) | 7,013 | 5,804 | 398 | 766 | 5,190 | |||
| Amidohydrolase superfamily | ||||||||
| 2GOK | 17742376 | 3,001 | 2,943 | 96 | 103 | 2,678 | Imidazolonepropionase-like | Imidazolonepropionase |
| 2OOD | 27378991 | 3,609 | 3,572 | 0 | 160 | 3,440 | Guanine deaminase-like | None |
| 2OOF | 83646866 | 3,588 | 3,578 | 142 | 154 | 3,270 | Imidazolonepropionase-like | Imidazolonepropionase |
| 2I5G | 9951721 | 569 | 448 | 28 | 50 | 340 | None | None |
| 2I9U | 15023121 | 3,386 | 3,363 | 5 | 96 | 3,198 | Newfam59 | None |
| 2ICS | 29342885 | 3,433 | 3,334 | 3 | 28 | 3,209 | Unknown18 | None |
| 2IMR | 9911007 | 3,790 | 3,502 | 1 | 3 | 3,498 | None | None |
| 2OGJ | 17741648 | 3,527 | 3,510 | 5 | 18 | 3,395 | Newfam71 | None |
| 2P9B | 23466009 | 3,319 | 3,302 | 2 | 37 | 3,230 | Unknown41 | None |
| 2PAJ | 91783796 | 3,264 | 3,252 | 4 | 116 | 3,128 | Unknown55 | None |
| 2QO1 | 13422863 | 460 | 263 | 35 | 14 | 172 | Uronate isomerase-like | Uronate isomerase |
| 2Q6B | 15615056 | 306 | 189 | 3 | 0 | 167 | Uronate isomerase-like | Uronate isomerase |
| 2QS8 | 114773165 | 3,508 | 3,497 | 15 | 144 | 3,280 | Unknown43 | None |
| 2QT3 | 32455889 | 3,723 | 3,693 | 1 | 49 | 3,606 | Unknown95 | None |
| 2RAG | 16126978 | 911 | 602 | 7 | 53 | 502 | Newfam32 | None |
| 2R8C | 4447959 | 3,649 | 3,632 | 19 | 195 | 3,359 | Unknown47 | None |
| 2I9U | 150231121 | 3,386 | 3,363 | 5 | 96 | 3,198 | Newfam59 | None |
| 2OOF | 83646866 | 3,588 | 3,578 | 142 | 154 | 3,270 | Imidazolonepropinase-like | Imidazolonepropionase |
| 3B40 | 9948434 | 1,149 | 656 | 16 | 34 | 504 | Newfam190 | None |
| 3CJP | 15896580 | 3,289 | 3,286 | 1 | 1,467 | 1,851 | Newfam63 | None |
| 3BE7 | 4436882 | 3,697 | 3,198 | 4 | 112 | 3,042 | Unknown42 | None |
| Total (unique sequences) | 12,101 | 11,628 | 302 | 2,429 | 8,912 | |||
Only one entry is shown for structures determined in different crystal forms or ligand binding states. An acceptable model is defined to be based on a significant PSI-BLAST E-value (0.0001) or a favorable GA341 model score (>0.7) [60]
Results and discussion
We first present the results of the target selection procedure. We also describe the current snapshot of the progress of the targets through our structural genomics pipeline (June 2008). We then indicate how the resulting crystallographic structures are distributed across the two superfamilies. Next, we determine the number of protein sequences in the comprehensive sequence databases that are detectably related to these protein structures (i.e., the modeling leverage). Finally, for each of the two superfamilies, we describe an example target with interesting biological features.
Target selection
Given the capacities of ENSPEC and NYSGXRC, the goal was to identify approximately 500 target sequences, approximately evenly distributed between the two superfamilies. These targets were obtained by selecting representatives from previously identified superfamily members as well as by identifying new superfamily members in a select set of genomes (Materials and Methods).
Targets for the amidohydrolase superfamily
From the SFLD, we obtained a list of 3,701 amidohydrolase superfamily members. The first filtering step resulted in 1,918 sequences with less than 30% sequence identity to a known structure and at least 250 amino acid residues in length, originating from 424 organisms. We chose the 30% sequence identity limit, in congruence with NIH PSI guidelines, to concentrate our efforts on protein sequences with limited structural knowledge; sequences related at less than 30% sequence identity to the closest known structure are frequently modeled inaccurately due to errors in the corresponding target-template alignments [54–56].
These 1,918 sequences were further filtered manually using the target selection tool to obtain the reduced set of 224 target sequences. The selected amidohydrolase superfamily members are evenly distributed among the various clades of the superfamily, thus representing the diversity within the superfamily. Preference was given to the NYSGXRC genomes, but other organisms were also considered.
The 224 targets can be divided into 76 clusters with less than 30% sequence identity between any pair of sequences from two different clusters, 126 clusters at 50% sequence identity, and 177 clusters at 80% sequence identity. The amidohydrolase superfamily members all contain the defining conserved TIM barrel domain with some variation in their lengths; all targets are between 224 and 628 amino acid residues long, with 90% of them shorter than 500 residues. The length variation stems mostly from loops that connect the main secondary structure elements of the TIM barrel fold and is consistent with the previously observed size range for TIM barrel domains (150 to 500 residues [57]).
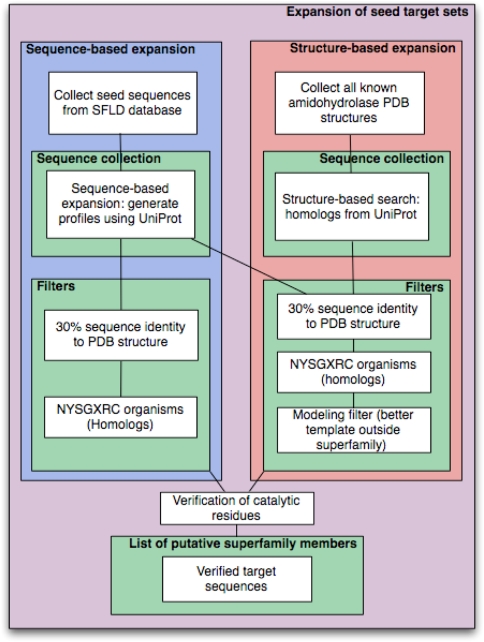
In addition to the known superfamily members, the sequence- and structure-based expansions detected 63 putative amidohydrolase superfamily members that were not initially in the SFLD (Table 3). These new potential targets fall into two categories: (i) divergent sequences that were detected by the sequence-based approach (Fig. 1, blue box) and (ii) divergent sequences that were detected by the structure-based approach (Fig. 1, orange box). Of the 63 putative amidohydrolase superfamily sequences, 50 were subsequently verified using the SFLD update procedure. The presence of probable catalytic residues for the remaining 13 targets was verified manually. Nine of these 13 sequences were detected by both the sequence- and structure-based approaches, and four sequences were only detected by the structure-based approach. Thus, the sequence- and structure-based approaches yielded 13 additional targets that could not be identified as amidohydrolase superfamily members using previously available protocols (corresponding to 21% of the new putative members of the amidohydrolase superfamily).
Table 3.
Putative amidohydrolase superfamily members
| Database ID (GenPept GI IDs) | Method | Organism | Length | Annotation available at target selection | Verification |
|---|---|---|---|---|---|
| 7462218 | Structure-based | Thermotoga maritima | 434 | Conserved hypothetical protein | HMM |
| 7497374 | Structure-based | Caenorhabditis elegans | 818 | Hypothetical protein C44B7.10 | HMM |
| 7500805 | Structure-based | Caenorhabditis elegans | 313 | T21966 hypothetical protein F38E11.3—Caenorhabditis elegans | HMM |
| 9948434 | Structure-based | Pseudomonas aeruginosa PAO1 | 448 | Probable dipeptidase precursor (Pseudomonas aeruginosa) | HMM |
| 10173106 | Structure-based | Bacillus halodurans | 427 | BH0493 | HMM |
| 10175729 | Structure-based | Bacillus halodurans | 571 | DNA-dependent DNA polymerase beta chain | HMM |
| 13700943 | Structure-based | Staphylococcus aureus subsp. aureus N315 | 570 | DNA-dependent DNA polymerase beta chain | HMM |
| 14600641 | Structure-based | Aeropyrum pernix | 313 | 313aa long hypothetical microsomal dipeptidase | HMM |
| 14601853 | Template | Aeropyrum pernix | 394 | Hypothetical protein (Aeropyrum pernix) | HMM |
| 14602106 | Structure-based | Aeropyrum pernix | 327 | Hypothetical protein (Aeropyrum pernix) | HMM |
| 15600589 | Structure-based | Pseudomonas aeruginosa PAO1 | 325 | D82971 hypothetical protein PA5396 (imported)—Pseudomonas aeruginosa (strain PAO1) | HMM |
| 15612748 | Structure-based | Bacillus halodurans | 448 | BH0185 | HMM |
| 15614834 | Structure-based | Bacillus halodurans | 310 | Dipeptidase | HMM |
| 15791917 | Structure-based | Campylobacter jejuni subsp. jejuni NCTC | 265 | Hypothetical protein Cj0556 | HMM |
| 15805850 | Structure-based | Deinococcus radiodurans R1 | 418 | Hydrolase, putative | HMM |
| 15896580 | Structure-based | Clostridium acetobutylicum | 262 | Predicted amidohydrolase (dihydroorotase family) | HMM |
| 15898656 | Structure-based | Sulfolobus solfataricus | 314 | Microsomal dipeptidase | HMM |
| 15925570 | Structure-based | Staphylococcus aureus subsp. aureus N315 | 336 | Conserved hypothetical protein | HMM |
| 16125737 | Structure-based | Caulobacter vibrioides | 487 | Uronate isomerase (EC 5.3.1.12) (Glucuronate isomerase) (UronicDE isomerase) | HMM |
| 16126978 | Structure-based | Caulobacter vibrioides | 417 | Dipeptidase | HMM |
| 16127409 | Structure-based | Caulobacter vibrioides | 353 | Hypothetical protein | HMM |
| 16130781 | Structure-based | Escherichia coli K12 | 464 | Soluble protein involved in cell viability at the beginning of stationary phase; soluble protein involved in cell viability at the beginning of stationary phase, contains urease domain | HMM |
| 16410647 | Structure-based | Listeria monocytogenes EGD-e | 570 | lmo1231 | HMM |
| 17556402 | Structure-based | Caenorhabditis elegans | 352 | Hypothetical protein Y71D11A.3a | HMM |
| 19705473 | Structure-based | Rattus norvegicus | 336 | 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase | HMM |
| 19911227 | Structure-based | Homo sapiens | 336 | 2-amino-3-carboxylmuconate-6-semialdehyde decarboxylase | HMM |
| 19911231 | Structure-based | Caenorhabditis elegans | 401 | 2-amino-3-carboxylmuconate-6-semialdehyde decarboxylase | HMM |
| 24379660 | Structure-based | Streptococcus mutans UA159 | 267 | conserved hypothetical protein | HMM |
| 33592291 | Structure-based | Bordetella pertussis Tohama I | 284 | Putative 2-pyrone-4,6-dicarboxylic acid hydrolase | HMM |
| 33593502 | Structure-based | Bordetella pertussis Tohama I | 341 | Putative dipeptidase | HMM |
| 39976001 | Sequence- and structure-based | Magnaporthe grisea 70–15 | 417 | Hypothetical protein | HMM |
| 42527610 | Structure-based | Treponema denticola ATCC 35405 | 371 | Dihydroorotase, putative | HMM |
| 42631159 | Structure-based | Haemophilus influenzae | 330 | Hypothetical protein | HMM |
| 51012913 | Structure-based | Saccharomyces cerevisiae | 313 | YMR262W | HMM |
| 51968376 | Structure-based | Arabidopsis thaliana | 346 | Unnamed protein product | HMM |
| 51968996 | Structure-based | Arabidopsis thaliana | 346 | Unnamed protein product | HMM |
| 55980841 | Structure-based | Thermus thermophilus HB8 | 369 | Amidohydrolase family protein | HMM |
| 60279993 | STRUCTURE-based | Pseudomonas aeruginosa | 403 | PvdM HMM | |
| 66807941 | Structure-based | Dictyostelium discoideum | 359 | Hypothetical protein | HMM |
| 66808659 | Structure-based | Dictyostelium discoideum | 322 | Hypothetical protein | HMM |
| 1065989 | Sequence-based | Bacillus subtilis subsp. subtilis str. 1 | 577 | Adenine deaminase | HMM |
| 15023784 | Sequence-based | Clostridium acetobutylicum | 570 | Adenine deaminase | HMM |
| 24636152 | Structure-based | Caenorhabditis elegans | 403 | Hypothetical protein C44B7.12 | HMM |
| 29377069 | Structure-based | Enterococcus faecalis V583 | 444 | Chlorohydrolase family protein | HMM |
| 40788915 | Structure-based | Homo sapiens | 777 | Q93075_chr3:10265710-10295706_H233R_V272I_L374P PUTATIVE DEOXYRIBONUCLEASE KIAA0218 (EC 3.1.21.-) | HMM |
| 45446932 | Sequence- and structure-based | Drosophila melanogaster | 774 | CG32626-PA, isoform A | HMM |
| 56203368 | Sequence- and structure-based | Homo sapiens | 776 | Adenosine monophosphate deaminase 1 (isoform M | HMM |
| 56203369 | Sequence-based | Homo sapiens | 780 | OTTHUMP00000059283 | HMM |
| 57230710 | Structure-based | Filobasidiella neoformans | 469 | Hydrolase, putative | HMM |
| 63055053 | Structure-based | Homo sapiens | 761 | TatD DNase domain containing 2 | HMM |
| 68250266 | Structure-based | Haemophilus influenzae | 251 | Conserved putative deoxyribonuclease | HMM |
| 429129 | Sequence-based | Saccharomyces cerevisiae | 797 | YB9Z_YEAST HYPOTHETICAL 92.9 KD PROTEIN IN SSH1-APE3 INTERGENIC REGION | Manual |
| 7293948 | Sequence-based | Drosophila melanogaster | 520 | CG5998-PA | Manual |
| 11463854 | Sequence-based | Drosophila melanogaster | 561 | Male-specific IDGF | manual |
| 14602062 | Structure-based | Aeropyrum pernix | 375 | Hypothetical protein [Aeropyrum pernix] | Manual |
| 15898896 | Structure-based | Sulfolobus solfataricus | 269 | Conserved hypothetical protein | Manual |
| 16264026 | Template | Sinorhizobium meliloti | 466 | HYPOTHETICAL PROTEIN | Manual |
| 17646150 | Sequence- and structure-based | Drosophila melanogaster | 506 | Adenosine deaminase-related growth factor C | Manual |
| 23093239 | Sequence-based | Drosophila melanogaster | 561 | CG32178-PA | Manual |
| 25009707 | Sequence-based | Drosophila melanogaster | 561 | AT05468p | Manual |
| 33593596 | Structure-based | Bordetella pertussis Tohama I | 523 | Conserved hypothetical protein | Manual |
| 40744823 | Structure-based | Aspergillus nidulans FGSC A4 | 562 | HYPOTHETICAL protein | Manual |
| 47678365 | Sequence-based | Homo sapiens | 511 | Cat eye syndrome critical region protein 1 [Homo sapiens] | Manual |
| 49116836 | Sequence- and structure-based | Xenopus laevis | 510 | Hypothetical protein | Manual |
Tables listing all amidohydrolase and enolase superfamily targets can be found at http://salilab.org/projects/enspec/ (HMM Hidden Markov Model verification)
Fig. 1.
Flowchart of the target expansion strategy of sequence-based target expansion (left) and structure-based target expansion (right)
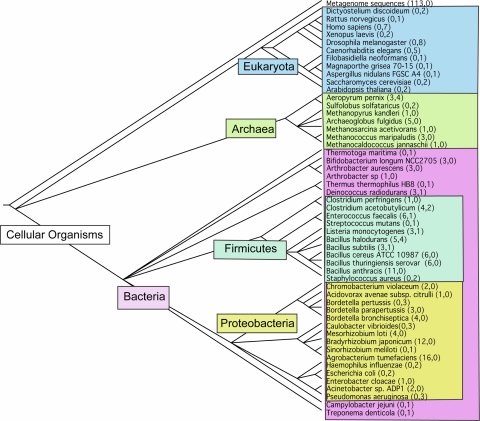
In summary, the final amidohydrolase target list includes 224 previously identified amidohydrolase superfamily members, as well as the 63 newly identified sequences. The final list includes 287 sequences from 53 organisms that cover 22 (61%) of the named families in the superfamily (Fig. 2).
Fig. 2.
Phylogenetic tree of the organisms for the selected amidohydrolase targets. The numbers in parentheses represent the number of targets for confirmed (first number) and putative (second number) amidohydrolase superfamily members. The tree was generated using the NCBI Taxonomy Browser [61]
Targets for the enolase superfamily
We used a simpler selection scheme for the enolase superfamily members, because previous detailed studies have effectively found all of the superfamily members in publicly available sequence and structure databases (data not shown). Of the 1,795 sequences already established as enolase superfamily members, we selected as targets the 255 sequences with less than 30% sequence identity to a known structure over at least 250 residues in length, originating from 98 organisms. These targets form 74 clusters at the 30% sequence identity cutoff, 126 clusters at 50% sequence identity, and 196 clusters at 80% sequence identity. The length distribution is 200 to 656 amino acid residues, with 90% of the sequences between 200 and 405 residues in length.
A complete list of the selected amidohydrolase and enolase superfamily targets can be found at http://salilab.org/projects/enspec/.
Structural genomics pipeline attrition
To date, 254 amidohydrolase (88%) and 206 enolase (80%) superfamily members have been attempted using the NYSGXRC/ENSPEC X-ray crystallographic structure determination pipeline. Progress to date and attrition rate at each stage of the pipeline are documented in Table 4 (June 2008). The project has not yet been completed, and a number of targets are still progressing through the pipeline. Also, a few targets in the target list have not yet been entered in the experimental pipeline. Therefore, the final overall success rate should be higher than that presented in Table 4. Experimental results for all NYSGXRC Community-nominated targets are updated weekly in PepcDB (http://pepcdb.pdb.org/).
Table 4.
Success rates for the steps in the structural genomics pipeline as of June 2008
| Step | Amidohydrolase superfamily | Enolase superfamily | Both superfamilies | |||
|---|---|---|---|---|---|---|
| Total | Fraction (%) | Total | Fraction (%) | Total | Fraction (%) | |
| In pipeline | 279 | 222 | 501 | |||
| Cloned | 254 | 91 | 206 | 93 | 460 | 92 |
| Expressed | 225 | 88 | 177 | 86 | 402 | 87 |
| Soluble | 167 | 74 | 112 | 63 | 279 | 69 |
| Purified | 110 | 66 | 67 | 60 | 177 | 63 |
| Crystallized | 63 | 57 | 44 | 66 | 107 | 60 |
| Unique structures | 20 | 32 | 41 | 93 | 61 | 57 |
| All structures | 25 | 50 | 75 | |||
Clear trends are observed in the success rates of crystallization and subsequent crystallographic structure determination for the amidohydrolase and enolase superfamily members. While only 38% of the purified targets were members of the enolase superfamily, they comprise 67% of the unique experimental structures. If crystals are obtained for an enolase superfamily member, there is a good chance that its structure will be successfully determined. On the other hand, for at least a quarter of the amidohydrolase proteins, we observed unusually broad peaks in the electrospray ionization (ESI) mass spectra of the intact proteins, indicative of heterogeneity in the preparation. Proteolytic digestion followed by tandem mass spectrometry analysis was carried out on the heterogeneous proteins; multiple sites of oxidation and methylation were identified with 90% of the protein sequence typically identified. These modifications were the source of the sample heterogeneity, and thus one reason for the limited success in obtaining usable crystallographic datasets from crystals of these amidohydrolases.
Of structural and functional interest was the fact that the oxidation sites were primarily located at histidine residues adjacent to Fe2+ ions in the presumed active sites of the amidohydrolases. Excess oxidation can be avoided using an alternate expression system (e.g. baculovirus) or adding excess Mn2+ and an iron chelator such as 2,2′-dipyridyl prior to induction during E. coli expression. In contrast, oxidation was not been observed in members of the enolase superfamily, since these proteins bind only a divalent metal ion such as Mg2+ or Mn2+ and not iron.
Analysis of the resulting crystallographic structures
Leverage of new crystallographic structures by modeling
To determine the impact of a structure on the structural mapping of the protein sequence space, we determine how many known protein sequences can be modeled based on the structure (i.e., the modeling leverage) (Table 2). Each enolase structure is a useful template for calculating comparative models for 2,500 to 3,200 other protein sequences in the NR database; a template is considered useful when the resulting model is based on a significant PSI-BLAST E-value (0.0001) or a favorable GA341 model score (>0.7). In contrast, the amidohydrolase superfamily structures fall into two categories: most are detectably related to 3,000–3,800 other proteins, but five structures (PDB Codes: 2I5G, 2Q01, 2Q6E, 2RAG, and 3B40) are related to a significantly smaller number of sequences (approximately 300–1,000).
A comparison of these numbers to the template-based modeling results for all NYSGXRC structures as of May 2007 (Table 5) shows that the average number of models per structure is significantly higher for the amidohydrolase and enolase superfamilies than for all structures determined by NYSGXRC (2,681 vs. 1,964). This difference reflects the relatively large sizes of the amidohydrolase and enolase superfamilies; according to the Superfamily database (http://supfam.org, [58]), across all of the superfamilies in the database, there are on average 1,770 protein sequences per superfamily.
Table 5.
Comparison of template-based modeling statistics for the 61 ENSPEC/NYSGXRC structures and all 327 NYSGXRC structures (May 2007)
| Amidohydrolase and enolase superfamily members | All | |
|---|---|---|
| Average number of sequences with acceptable models | 2,681 | 1,964 |
| Minimum/maximum number of sequences with acceptable models | 189/3693 | 30/6320 |
| Average number of sequences with >50% sequence identity, at least 50% coverage | 15 | 20 |
| Average number of sequences with 30–50% sequence identity, at least 50% coverage | 59 | 113 |
| Average number of sequences with <30% sequence identity, at least 50% coverage | 2,572 | 1,400 |
An acceptable model is defined to be based on a significant PSI-BLAST E-value (0.0001) or a favorable GA341 model score (>0.7)
Breaking down the modeling leverage by sequence identity reveals that the modeling leverage for the amidohydrolase and enolase superfamily structures is higher and lower than that for all NYSGXRC structures below and above the sequence identity cutoff of 30%, respectively. These differences are likely due in part to the relatively high diversity in the amidohydrolase and enolase superfamilies.
Upon initiation of the ENSPEC/NYSGXRC effort in June 2005, 45% of all known members of the amidohydrolase and enolase superfamilies were related to a known structure with a sequence identity higher than 30%. Due to the increased number of templates from the amidohydrolase and enolase superfamilies contributed by our consortia, this number increased to from 45% to 73%.
The total number of unique sequences modeled using the new amidohydrolase and enolase superfamily structures is 11,097, approximately 30% more than the number of known sequences from the amidohydrolase and enolase superfamilies. Among these additional sequences, we expect both members of other superfamilies with the TIM barrel fold, as well as currently unidentified members of the amidohydrolase and enolase superfamilies, because the sequence databases have been growing by approximately 50% since 2005, and also because we concentrated on selecting only targets from the NYSGXRC genomes in the target selection process for this project.
Distribution of targets over the amidohydrolase and enolase superfamilies
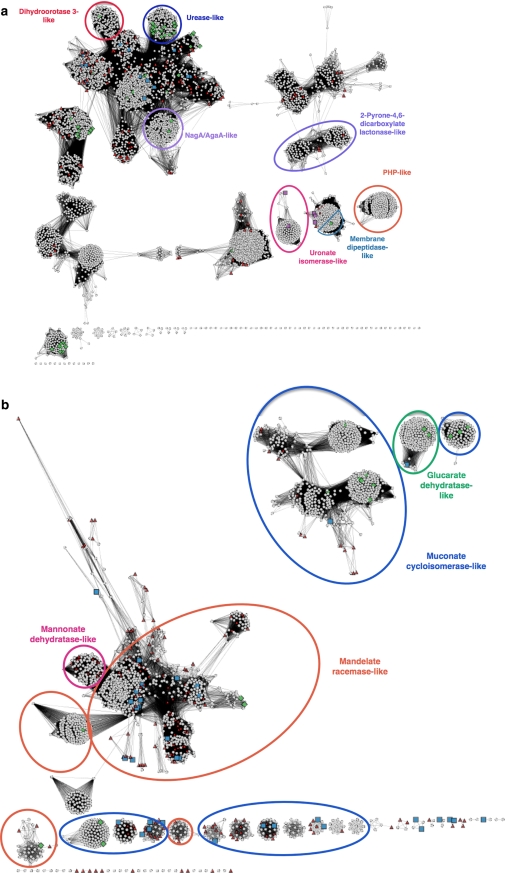
For large groups of related sequences, such as the amidohydrolase superfamily network-based visualization of their relationships is helpful in generating hypotheses about how various enzymes in the superfamily evolved, and on how closely the subgroups are related to each other. We have plotted cytoscape networks for the amidohydrolase and enolase superfamilies, based on clustering by sequence similarity, and marked previously known structures, and the final targets and the structures from this project (Fig. 3). For clarity, we circled a few distinct subgroups. Another network representation with all sub-group assignments can be found in the supplemental materials.
Fig. 3.
a Cytoscape clustering for the amidohydrolase superfamily. The most homogeneous subgroups have been named. An additional figure with full subgroup coloring is available in Supplemental Materials. Green diamonds Structures determined prior to the start of the ENSPEC/NYSGXRC project in June 2005. Red triangles Superfamily members in the target list. Purple squares Five divergent structures determined by ENSPEC/NYSGXRC. Blue squares All other structures determined by ENSPEC/NYSGXRC. Ovals indicate subgroups: red dihydroorotase 3-like; dark blue urease-like; purple NagA/AgaA-like; light-blue: 2-Pyrone-4,6-dicarboxylate lactonase-like; pink uronate-isomerase-like; orange PHP-like; delft-blue membrane dipeptidase-like. b Cytoscape clustering for the enolase superfamily. Subgroup clusters are marked for four subgroups. The full subgroup assignments can be found in Supplemental Materials. Green diamonds Structures determined prior to the start of the ENSPEC/NYSGXRC project in June 2005. Red triangles Superfamily members in the target list. Blue squares All structures determined by ENSPEC/NYSGXRC. Ovals indicate subgroups: pink mannonate dehydratase-like; orange mandelate racemase-like; blue muconate cycloisomerase-like; green glucarate dehydratase-like
Many subgroups in the large amidohydrolase superfamily, such as the urease-like subgroup and the uronate isomerase-like subgroup, are distinctly separated from the other superfamily members. This separation can most simply be interpreted as the result of the extreme divergence of these subgroups; thus, they are “outliers” in the overall context of the superfamily (see below for further discussion of this subgroup).
Four of the five divergent amidohydrolase structures with a considerably smaller number of homologs are separated from the main amidohydrolase network, even at the relatively non-stringent E-value cut-off of 10−10 required to visualize connections between nodes. Two of them (2Q01, 2Q6E) belong to the uronate isomerase-like subgroup. Another two of these structures (2RAG, 3B40) are clustered together with a number of unclassified sequences as well as several membrane dipeptidase-like amidohydrolase superfamily members, possibly indicating that these targets are additional members of the membrane dipeptidase subgroup. This subgroup membership is also supported by their annotation as putative dipeptidases in UniProt.
For the enolase superfamily, we chose to generate a cytoscape network that represents only four subgroups, containing the majority of the targets. The targets were mostly chosen from the mandelate racemase-like subgroup, because it is the largest subgroup with little previous structural coverage, and from the more divergent muconate cycloisomerase subgroup. The cytoscape networks illustrate that the targets and the resulting structures are indeed concentrated in regions of superfamily sequence space that lacked structural characterization prior to the start of the project, as desired for our target selection.
Examples of biological impact resulting from new structures obtained in this study
Amidohydrolase superfamily example: atypical uronate isomerase Bh0493
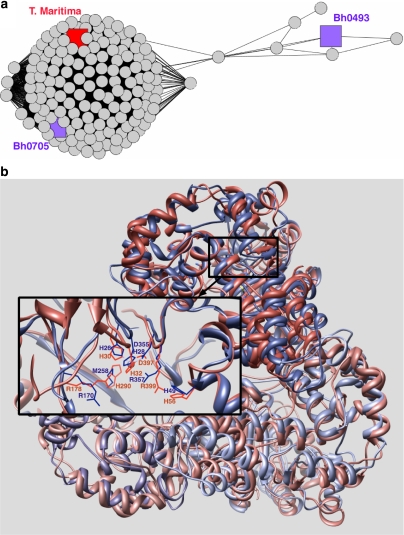
The enzymes in the uronate isomerase family are members of the amidohydrolase superfamily, although they are extremely diverged from other clusters of the amidohydrolase superfamily network (Fig. 3a). Target 9247a (gi 10173106, Bh0493) from Bacillus halodurans was identified by our structure-based expansion as a putative member of the amidohydrolase superfamily and has recently been experimentally confirmed as a uronate isomerase [29]. In most organisms, both glucuronic acid and galacturonic acid are first isomerized by a single uronate isomerase, followed by further modification by several sugar specific dehydrogenases and dehydratases. In B. halodurans, as in several other organisms, two uronate isomerase genes are found, in operons containing dehydrogenase as well as dehydratase enzymes, consistent with this assignment of activity. We characterized both uronate isomerase genes, a “typical” uronate isomerase, Bh0705, and Bh0493, an “outlier” relative to other characterized members of this family (Fig. 4a). Although the results showed that each enzyme can isomerize both substrates, galacturonate and glucuronate, the Bh0705 uronate isomerase preferentially isomerizes glucuronic acid (approximately 100 times faster than galacturonic acid). In contrast, Bh0493 isomerizes glucuronic acid and galacturonic acid almost equally efficiently. These observations indicate that in B. halodurans, the “typical” uronate isomerase (Bh0705) has specialized its catalytic activity to preferentially isomerize glucuronic acid, perhaps because the isomerization of galacturonic acid is sufficiently achieved by Bh0493.
Fig. 4.
a Cytoscape network showing the uronate isomerase family. The E-value threshold for displaying edges is 10−10. The large cluster represents the “typical” uronate isomerases; sequences in this cluster are more similar to other members of the amidohydrolase superfamily than is Bh0493. Bh0705 is shown in purple and the structurally characterized enzyme from Thermotoga maritima is shown in red. On the right, the outlier uronate isomerase, Bh0493, is shown in purple along with a small number of sequences of unknown function. b Ribbon diagram [62] of a superposition of the trimeric structures of Bh0493 (2Q6E, blue) and a uronate isomerase from Thermotoga maritima (1J5S, red). The detailed box shows the active site residues of chain A including a Zn2+ ion for 2Q6E
To gain further insight into the structural differences between Bh0493 and the “typical” uronate isomerases (and between uronate isomerases and other members of the amidohydrolase superfamily), and in the absence of a structure of Bh0705, we compared the structure of Bh0493 (PDB codes 2Q08 and 2Q6E) to another “typical” uronate isomerase from Thermotoga maritima (PDB code 1J5S). As shown in Fig. 4b, the functionally important residues Arg170, Arg357 and His49, are conserved and cluster together within the enzyme active site both in the T. maritima enzyme and Bh0493. However, an additional metal-coordinating histidine that is usually found at the end of β-strand five in “typical” uronate isomerases (H290 in the 1J5S) is missing in Bh0493, which has a Met (M258) in that position. The Zn2+ ion is coordinated by two histidine residues (His28 and His26) plus Asp355. Guided by these structures, further biochemical and computational studies to examine the differences between these two types of uronate isomerases, and how they may be related to their different specificities, are currently in progress.
Enolase superfamily example: mandelate racemase subgroup
The SFLD currently describes 17 different families in the enolase superfamily, each performing a different overall reaction associated with different substrates and products. For the approximately 50% of the superfamily sequences whose functions are yet unknown, we estimate that roughly 15–20 novel functions (i.e. new families) will be identified. Across the superfamily, the sequences whose functions are not yet identified can be clustered into three primary subgroups and several smaller ones based on sequence and structural differences, including differences in the constellations of active site residues involved in binding specificity and catalysis [10]. In the mandelate racemase subgroup, most of the enzymes with characterized reactions are dehydratases acting on acid sugars, with the “outlier” enzyme being mandelate racemase itself. All structurally characterized members of the subgroup can be distinguished by a His-Asp dyad at the ends of β-strands six and seven that is associated with proton abstraction of substrates in the R-configuration [59]. Mandelate racemase and several acid sugar dehydratases that were previously structurally and functionally characterized also have a conserved Lys-X-Lys motif on β-strand two, with the second Lys in this motif involved in proton abstraction of substrates in the S-configuration [42]. Within this subgroup, we also observe divergence in this motif among several members of both known [32] and unknown function.
Three members of the mandelate racemase subgroup whose structures were determined by NYSGXRC, 2GL5 and 2O56 from Salmonella typhimurium and 2OX4 from Zymomonas mobilis, were found to have a Lys-Val-Asp sequence motif at this position, possibly indicating a different catalytic mechanism or yet other novel function(s). The three structures align within 50% sequence identity to each other. The next closest structures (30% sequence identity) are also members of the mandelate racemase subgroup: 2POZ from Mesorhizobium loti and 2POD from Burkholderia pseudomallei have Lys-Phe-Tyr and Lys-Ile-Trp motifs at this position, respectively, providing further evidence for divergent catalytic function(s). Their structures reveal details of differences relative to that of well-characterized subgroup members containing a “canonical” Lys-X-Lys motif, providing information expected to be useful in identifying their functions. Figure 5 shows superpositions of mandelate racemase with 2GL5 and 2POD, illustrating the differences in this motif. Guided by these new structures, these enzymes are now being further analyzed computationally and experimentally.
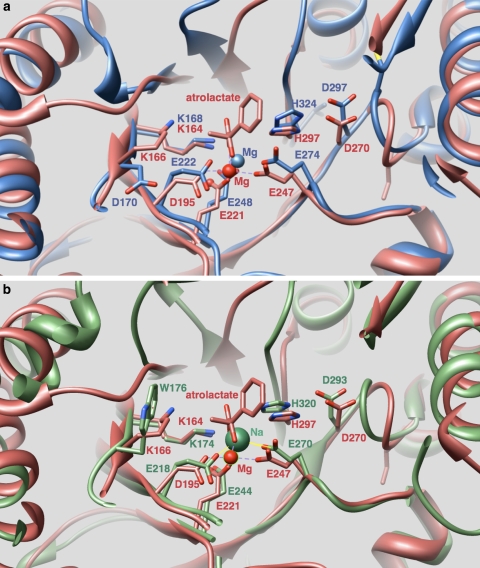
Fig. 5.
Mandelate racemase bound to a substrate analog, atrolactate, (1MDR: red), is shown superimposed with two structures of unknown function. In both superpositions, active site metal ligands D195, E221, E247, the active site His-Asp dyad (H297, D270), and a Lys-X-Lys motif (K164, K166) conserved in 1MDR and other members of the mandelate racemase subgroup are labeled (1MDR numbering). a Superposition of 2GL5 (blue) with 1MDR shows conservation of all of these active site residues, except for the second Lys in the Lys-X-Lys motif of 1MDR, which is replaced in 2GL5 by Asp170. This residue faces away from the active site in 2GL5. b Superposition of 2POD (green) with 1MDR also shows conservation of all of listed residues, except for the second Lys in the Lys-X-Lys motif that is replaced in 2POD by W176
Conclusion
The Enzyme Specificity Consortium and the New York SGX Research Center for Structural Genomics made significant progress towards characterizing the structures and functions in the amidohydrolase and enolase superfamilies. New members of the amidohydrolase superfamily have been identified through a combination of sequence- and structure-based expansions of the pool of known superfamily members. The structure-based expansion was particularly successful in identifying previously unrecognized superfamily members. The 63 crystallographic structures from the structural genomics pipeline increased the fraction of the sequences in these two superfamilies that can be modeled based on at least 30% sequence identity from 45% to 73%.
As an annotation tool for the targets in the two superfamilies, template-based modeling of all sequences that had detectable homology to a known structure in the amidohydrolase or enolase superfamily allowed us to suggest previously un-annotated amidohydrolase sequences, several of which were subsequently verified by experiment, as shown for Bh0493 in this paper. This demonstrates the power of combining sequence- and structure-based approaches for the structural genomics of two large and diverse enzyme superfamilies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by NIH (U54 GM074945 (Principal Investigator: Stephen K. Burley) and P01 GM71790), the Sandler Family Supporting Foundation, Fight for Mike Foundation, Ron Conway, Hewlett-Packard, NetApp, IBM, and Intel. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Basic Energy Sciences. Use of the SGX-CAT beam line facilities at Sector 31 of the APS was provided by SGX Pharmaceuticals, which constructed and operates the facility. Use of the NSLS beamline X29 was supported by the DOE and P41-EB-01979. Molecular graphics images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIH P41 RR-01081).
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- PDB
Protein Data Bank
- NYSGXRC
New York SGX Research Center for Structural Genomics
- ENSPEC
Enzyme specificity consortium
- SFLD
Structure function linkage database
- PSI
Protein structure initiative
- NR
Non-redundant database of protein sequences
- ESI
Electrospray ionization
- HMM
Hidden Markov Model
Footnotes
The numbers of sequences in the publicly accessible version of the SFLD differ from those cited here because large numbers of sequences are undergoing curation at any given time and are therefore not yet listed on the public site.
Current affiliation of SGX Pharmaceuticals is Eli Lilly and Company, 10505 Roselle Street, San Diego, CA 92121, USA.
Electronic supplementary material
The online version of this article (doi:10.1007/s10969-008-9056-5) contains supplementary material, which is available to authorized users.
Contributor Information
Ursula Pieper, Phone: +1-415-5144227, FAX: +1-415-5144231, Email: ursula@salilab.org.
Andrej Sali, Phone: +1-415-5144227, FAX: +1-415-5144231, Email: sali@salilab.org.
References
- 1.Andreeva A, Murzin AG (2006) Evolution of protein fold in the presence of functional constraints. Curr Opin Struct Biol 16:399–408 [DOI] [PubMed]
- 2.Gerlt JA, Babbitt PC (2001) Divergent evolution of enzymatic function: mechanistically diverse superfamilies and functionally distinct suprafamilies. Annu Rev Biochem 70:209–246 [DOI] [PubMed]
- 3.Glasner ME, Gerlt JA, Babbitt PC (2006) Evolution of enzyme superfamilies. Curr Opin Chem Biol 10:492–497 [DOI] [PubMed]
- 4.Todd AE, Orengo CA, Thornton JM (2001) Evolution of function in protein superfamilies, from a structural perspective. J Mol Biol 307:1113–1143 [DOI] [PubMed]
- 5.Pegg SC, Brown S, Ojha S, Huang CC, Ferrin TE, Babbitt PC et al (2005) Representing structure-function relationships in mechanistically diverse enzyme superfamilies. Pac Symp Biocomput 358–369 [PubMed]
- 6.Holm L, Sander C (1997) An evolutionary treasure: unification of a broad set of amidohydrolases related to urease. Proteins 28:72–82 [DOI] [PubMed]
- 7.Seibert CM, Raushel FM (2005) Structural and catalytic diversity within the amidohydrolase superfamily. Biochemistry 44:6383–6391 [DOI] [PubMed]
- 8.Pegg SC, Brown SD, Ojha S, Seffernick J, Meng EC, Morris JH, Chang PJ, Huang CC, Ferrin TE, Babbitt PC (2006) Leveraging enzyme structure–function relationships for functional inference and experimental design: the structure–function linkage database. Biochemistry 45:2545–2555 [DOI] [PubMed]
- 9.Babbitt PC, Hasson MS, Wedekind JE, Palmer DR, Barrett WC, Reed GH, Rayment I, Ringe D, Kenyon GL, Gerlt JA (1996) The enolase superfamily: a general strategy for enzyme-catalyzed abstraction of the alpha-protons of carboxylic acids. Biochemistry 35:16489–16501 [DOI] [PubMed]
- 10.Gerlt JA, Babbitt PC, Rayment I (2005) Divergent evolution in the enolase superfamily: the interplay of mechanism and specificity. Arch Biochem Biophys 433:59–70 [DOI] [PubMed]
- 11.Vick JE, Gerlt JA (2007) Evolutionary potential of (beta/alpha)8-barrels: stepwise evolution of a “new” reaction in the enolase superfamily. Biochemistry 46:14589–14597 [DOI] [PubMed]
- 12.Akana J, Fedorov AA, Fedorov E, Novak WR, Babbitt PC, Almo SC, Gerlt JA (2006) D-Ribulose 5-phosphate 3-epimerase: functional and structural relationships to members of the ribulose-phosphate binding (beta/alpha)8-barrel superfamily. Biochemistry 45:2493–2503 [DOI] [PubMed]
- 13.Almo SC, Bonanno JB, Sauder JM, Emtage S, Dilorenzo TP, Malashkevich V, Wasserman SR, Swaminathan S, Eswaramoorthy S, Agarwal R, Kumaran D, Madegowda M, Ragumani S, Patskovsky Y, Alvarado J, Ramagopal UA, Faber-Barata J, Chance MR, Sali A, Fiser A, Zhang ZY, Lawrence DS, Burley SK (2007) Structural genomics of protein phosphatases. J Struct Funct Genomics 8:121–140 [DOI] [PMC free article] [PubMed]
- 14.Bonanno JB, Almo SC, Bresnick A, Chance MR, Fiser A, Swaminathan S, Jiang J, Studier FW, Shapiro L, Lima CD, Gaasterland TM, Sali A, Bain K, Feil I, Gao X, Lorimer D, Ramos A, Sauder JM, Wasserman SR, Emtage S, D’Amico KL, Burley SK (2005) New York-Structural GenomiX Research Consortium (NYSGXRC): a large scale center for the protein structure initiative. J Struct Funct Genomics 6:225–232 [DOI] [PubMed]
- 15.Brown SD, Gerlt JA, Seffernick JL, Babbitt PC (2006) A gold standard set of mechanistically diverse enzyme superfamilies. Genome Biol 7:R8 [DOI] [PMC free article] [PubMed]
- 16.Gerlt JA (2007) A protein structure (or function?) initiative. Structure 15:1353–1356 [DOI] [PubMed]
- 17.Glasner ME, Fayazmanesh N, Chiang RA, Sakai A, Jacobson MP, Gerlt JA, Babbitt PC (2006) Evolution of structure and function in the o-succinylbenzoate synthase/N-acylamino acid racemase family of the enolase superfamily. J Mol Biol 360:228–250 [DOI] [PubMed]
- 18.Glasner ME, Gerlt JA, Babbitt PC (2007) Mechanisms of protein evolution and their application to protein engineering. Adv Enzymol Relat Areas Mol Biol 75:193–239 xii–xiii [DOI] [PubMed]
- 19.Hall RS, Brown S, Fedorov AA, Fedorov EV, Xu C, Babbitt PC, Almo SC, Raushel FM (2007) Structural diversity within the mononuclear and binuclear active sites of N-acetyl-D-glucosamine-6-phosphate deacetylase. Biochemistry 46:7953–7962 [DOI] [PubMed]
- 20.Hermann JC, Ghanem E, Li Y, Raushel FM, Irwin JJ, Shoichet BK (2006) Predicting substrates by docking high-energy intermediates to enzyme structures. J Am Chem Soc 128:15882–15891 [DOI] [PubMed]
- 21.Hermann JC, Marti-Arbona R, Fedorov AA, Fedorov E, Almo SC, Shoichet BK, Raushel FM (2007) Structure-based activity prediction for an enzyme of unknown function. Nature 448:775–779 [DOI] [PMC free article] [PubMed]
- 22.Imker HJ, Fedorov AA, Fedorov EV, Almo SC, Gerlt JA (2007) Mechanistic diversity in the RuBisCO superfamily: the “enolase” in the methionine salvage pathway in Geobacillus kaustophilus. Biochemistry 46:4077–4089 [DOI] [PubMed]
- 23.Irwin JJ, Raushel FM, Shoichet BK (2005) Virtual screening against metalloenzymes for inhibitors and substrates. Biochemistry 44:12316–12328 [DOI] [PubMed]
- 24.Li Y, Raushel FM (2005) Inhibitors designed for the active site of dihydroorotase. Bioorg Chem 33:470–483 [DOI] [PubMed]
- 25.Liao RZ, Yu JG, Raushel FM, Himo F (2008) Theoretical investigation of the reaction mechanism of the dinuclear zinc enzyme dihydroorotase. Chemistry 14:4287–4292 [DOI] [PubMed]
- 26.Marti-Arbona R, Raushel FM (2006) Mechanistic characterization of N-formimino-L-glutamate iminohydrolase from Pseudomonas aeruginosa. Biochemistry 45:14256–14262 [DOI] [PubMed]
- 27.Marti-Arbona R, Thoden JB, Holden HM, Raushel FM (2005) Functional significance of Glu-77 and Tyr-137 within the active site of isoaspartyl dipeptidase. Bioorg Chem 33:448–458 [DOI] [PubMed]
- 28.Marti-Arbona R, Xu C, Steele S, Weeks A, Kuty GF, Seibert CM, Raushel FM (2006) Annotating enzymes of unknown function: N-formimino-L-glutamate deiminase is a member of the amidohydrolase superfamily. Biochemistry 45:1997–2005 [DOI] [PubMed]
- 29.Nguyen TT, Brown S, Fedorov AA, Fedorov EV, Babbitt PC, Almo SC, Raushel FM (2008) At the periphery of the amidohydrolase superfamily: Bh0493 from Bacillus halodurans catalyzes the isomerization of D-galacturonate to D-tagaturonate. Biochemistry 47:1194–1206 [DOI] [PubMed]
- 30.Nowlan C, Li Y, Hermann JC, Evans T, Carpenter J, Ghanem E, Shoichet BK, Raushel FM (2006) Resolution of chiral phosphate, phosphonate, and phosphinate esters by an enantioselective enzyme library. J Am Chem Soc 128:15892–15902 [DOI] [PubMed]
- 31.Porter TN, Li Y, Raushel FM (2004) Mechanism of the dihydroorotase reaction. Biochemistry 43:16285–16292 [DOI] [PubMed]
- 32.Rakus JF, Fedorov AA, Fedorov EV, Glasner ME, Vick JE, Babbitt PC, Almo SC, Gerlt JA (2007) Evolution of enzymatic activities in the enolase superfamily: D-Mannonate dehydratase from Novosphingobium aromaticivorans. Biochemistry 46:12896–12908 [DOI] [PubMed]
- 33.Sakai A, Xiang DF, Xu C, Song L, Yew WS, Raushel FM, Gerlt JA (2006) Evolution of enzymatic activities in the enolase superfamily: N-succinylamino acid racemase and a new pathway for the irreversible conversion of D- to L-amino acids. Biochemistry 45:4455–4462 [DOI] [PubMed]
- 34.Song L, Kalyanaraman C, Fedorov AA, Fedorov EV, Glasner ME, Brown S, Imker HJ, Babbitt PC, Almo SC, Jacobson MP, Gerlt JA (2007) Prediction and assignment of function for a divergent N-succinyl amino acid racemase. Nat Chem Biol 3:486–491 [DOI] [PubMed]
- 35.Thoden JB, Taylor Ringia EA, Garrett JB, Gerlt JA, Holden HM, Rayment I (2004) Evolution of enzymatic activity in the enolase superfamily: structural studies of the promiscuous o-succinylbenzoate synthase from Amycolatopsis. Biochemistry 43:5716–5727 [DOI] [PubMed]
- 36.Tyagi R, Eswaramoorthy S, Burley SK, Raushel FM, Swaminathan S (2008) A common catalytic mechanism for proteins of the HutI family. Biochemistry 47:5608–5615 [DOI] [PMC free article] [PubMed]
- 37.Vick JE, Schmidt DM, Gerlt JA (2005) Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a “new” reaction in the enolase superfamily. Biochemistry 44:11722–11729 [DOI] [PubMed]
- 38.Weeks A, Lund L, Raushel FM (2006) Tunneling of intermediates in enzyme-catalyzed reactions. Curr Opin Chem Biol 10:465–472 [DOI] [PubMed]
- 39.Williams L, Nguyen T, Li Y, Porter TN, Raushel FM (2006) Uronate isomerase: a nonhydrolytic member of the amidohydrolase superfamily with an ambivalent requirement for a divalent metal ion. Biochemistry 45:7453–7462 [DOI] [PMC free article] [PubMed]
- 40.Yew WS, Fedorov AA, Fedorov EV, Rakus JF, Pierce RW, Almo SC, Gerlt JA (2006) Evolution of enzymatic activities in the enolase superfamily: L-fuconate dehydratase from Xanthomonas campestris. Biochemistry 45:14582–14597 [DOI] [PubMed]
- 41.Yew WS, Fedorov AA, Fedorov EV, Wood BM, Almo SC, Gerlt JA (2006) Evolution of enzymatic activities in the enolase superfamily: D-tartrate dehydratase from Bradyrhizobium japonicum. Biochemistry 45:14598–14608 [DOI] [PubMed]
- 42.Yew WS, Fedorov AA, Fedorov EV, Almo SC, Gerlt JA (2007) Evolution of enzymatic activities in the enolase superfamily: L-talarate/galactarate dehydratase from Salmonella typhimurium LT2. Biochemistry 46:9564–9577 [DOI] [PubMed]
- 43.Norvell JC, Berg JM (2007) Update on the protein structure initiative. Structure 15:1519–1522 [DOI] [PubMed]
- 44.Berman H, Henrick K, Nakamura H, Markley JL (2007) The worldwide Protein Data Bank (wwPDB): ensuring a single, uniform archive of PDB data. Nucleic Acids Res 35:D301–D303 [DOI] [PMC free article] [PubMed]
- 45.Eswar N, John B, Mirkovic N, Fiser A, Ilyin VA, Pieper U, Stuart AC, Marti-Renom MA, Madhusudhan MS, Yerkovich B, Sali A (2003) Tools for comparative protein structure modeling and analysis. Nucleic Acids Res 31:3375–3380 [DOI] [PMC free article] [PubMed]
- 46.Wu CH, Apweiler R, Bairoch A, Natale DA, Barker WC, Boeckmann B, Ferro S, Gasteiger E, Huang H, Lopez R, Magrane M, Martin MJ, Mazumder R, O’Donovan C, Redaschi N, Suzek B (2006) The Universal Protein Resource (UniProt): an expanding universe of protein information. Nucleic Acids Res 34:D187–D191 [DOI] [PMC free article] [PubMed]
- 47.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402 [DOI] [PMC free article] [PubMed]
- 48.Pieper U, Eswar N, Davis FP, Braberg H, Madhusudhan MS, Rossi A, Marti-Renom M, Karchin R, Webb BM, Eramian D, Shen MY, Kelly L, Melo F, Sali A (2006) MODBASE: a database of annotated comparative protein structure models and associated resources. Nucleic Acids Res 34:D291–D295 [DOI] [PMC free article] [PubMed]
- 49.Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, Wu D, Paulsen I, Nelson KE, Nelson W, Fouts DE, Levy S, Knap AH, Lomas MW, Nealson K, White O, Peterson J, Hoffman J, Parsons R, Baden-Tillson H, Pfannkoch C, Rogers YH, Smith HO (2004) Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66–74 [DOI] [PubMed]
- 50.Lee J, Ware B (2003) Open source web development with LAMP: using Linux, Apache, MySQL, Per, and PHP. Addison-Wesley, Boston
- 51.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504 [DOI] [PMC free article] [PubMed]
- 52.Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL (2008) GenBank. Nucleic Acids Res 36:D25–D30 [DOI] [PMC free article] [PubMed]
- 53.Atkinson HJ, Morris JH, Ferrin TE, Babbitt PC et al (2009) Using sequence similarity networks for visualization of relationships across diverse protein superfamilies. PLoS One (submitted) [DOI] [PMC free article] [PubMed]
- 54.Martin AC, MacArthur M W, Thornton JM et al (1997) Assessment of comparative modeling in CASP2. Proteins, Suppl 1:14–28 [DOI] [PubMed]
- 55.Sanchez R, Sali A (1997) Advances in comparative protein-structure modelling. Curr Opin Struct Biol 7:206–214 [DOI] [PubMed]
- 56.Vitkup D, Melamud E, Moult J, Sander C (2001) Completeness in structural genomics. Nat Struct Biol 8:559–566 [DOI] [PubMed]
- 57.Nagano N, Orengo CA, Thornton JM (2002) One fold with many functions: the evolutionary relationships between TIM barrel families based on their sequences, structures and functions. J Mol Biol 321:741–765 [DOI] [PubMed]
- 58.Gough J, Karplus K, Hughey R, Chothia C (2001) Assignment of homology to genome sequences using a library of hidden Markov models that represent all proteins of known structure. J Mol Biol 313:903–919 [DOI] [PubMed]
- 59.Schafer SL, Barrett WC, Kallarakal AT, Mitra B, Kozarich JW, Gerlt JA, Clifton JG, Petsko GA, Kenyon GL (1996) Mechanism of the reaction catalyzed by mandelate racemase: structure and mechanistic properties of the D270 N mutant. Biochemistry 35:5662–5669 [DOI] [PubMed]
- 60.Melo F, Sanchez R, Sali A (2002) Statistical potentials for fold assessment. Protein Sci 11:430–448 [DOI] [PMC free article] [PubMed]
- 61.Wheeler DL, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, Church DM, Dicuccio M, Edgar R, Federhen S, Feolo M, Geer LY, Helmberg W, Kapustin Y, Khovayko O, Landsman D, Lipman DJ, Madden TL, Maglott DR, Miller V, Ostell J, Pruitt KD, Schuler GD, Shumway M, Sequeira E, Sherry ST, Sirotkin K, Souvorov A, Starchenko G, Tatusov RL, Tatusova TA, Wagner L, Yaschenko E (2008) Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 36:D13–D21 [DOI] [PMC free article] [PubMed]
- 62.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.