Summary
Glioblastomas are lethal cancers characterized by florid angiogenesis promoted in part by glioma stem cells (GSCs). As hypoxia regulates angiogenesis, we examined hypoxic responses in GSCs. We now demonstrate that hypoxia-inducible factor HIF2α and multiple HIF-regulated genes are preferentially expressed in GSCs in comparison to nonstem tumor cells and normal neural progenitors. In tumor specimens, HIF2α co-localizes with cancer stem cell markers. Targeting HIFs in GSCs inhibits self-renewal, proliferation and survival in vitro, and attenuates tumor initiation potential of GSCs in vivo. Analysis of a molecular database reveals that HIF2A expression correlates with poor glioma patient survival. Our results demonstrate that GSCs differentially respond to hypoxia with distinct HIF induction patterns and HIF2α may represent a promising target for anti-glioblastoma therapies.
Significance
Recent evidence supports the presence of cancer stem cell populations that contribute to tumor progression through preferential resistance to radiation and chemotherapy, and promotion of tumor angiogenesis, invasion, and metastasis. Therefore, the elucidation of molecular regulators of cancer stem cells may translate into improved anti-neoplastic therapies. Our work demonstrates that cancer stem cells derived from glioblastomas differentially respond to hypoxia with a distinct induction of HIF2α. We find that HIFs are critical to cancer stem cell maintenance and angiogenic drive, and that expression of HIF2α is significantly associated with poor glioma patient survival. These data further suggest that anti-angiogenic therapies can be designed to target cancer stem cell specific molecules involved in neoangiogenesis, including HIF2α and its regulated factors.
Introduction
Cancer stem cells, which have been also described as tumor initiating cells or tumor propagating cells, are tumor cells that self renew and propagate tumors phenotypically similar to the parental tumor. Cancer stem cells from glioblastomas share some characteristics with normal neural stem cells including the expression of neural stem cell markers, the capacity for self renewal and long term proliferation, the formation of neurospheres, and the ability to differentiate into multiple nervous system lineages (neurons, astrocytes, and oligodendrocytes) (Hemmati et al., 2003; Singh et al., 2003; Galli et al., 2004; Singh et al., 2004; Vescovi et al., 2006; Bao et al., 2006a). However, brain tumor stem cells exhibit significant distinctions from normal stem cells in frequency, proliferation, aberrant expression of differentiation markers, chromosomal abnormalities, and tumor formation (Quintana et al., 2008; Reya et al., 2001; Vescovi et al., 2006). The potent tumorigenic capacity of cancer stem cells coupled with increasing evidence of radioresistance and chemoresistance suggests that cancer stem cells contribute to tumor maintenance and recurrence and that targeting cancer stem cells may offer new avenues of therapeutic intervention (Wulf et al., 2001; Bao et al., 2006a; Hambardzumyan et al., 2006; Jin et al., 2006; Liu et al., 2006; Blazek et al., 2007; Todaro et al., 2007; Bao et al., 2008). This hypothesis has been recently validated in clinical trial of breast cancer in which patients undergoing treatment with cytotoxic chemotherapy experienced an increase in breast cancer stem cells in the surviving tumor while the use of a targeted therapeutic against the stem cell population stabilized the cancer stem cell population (Li et al., 2008).
While the precise mechanisms responsible for the differential tumorigenic capacity of cancer stem cells have yet to be determined, previous studies have demonstrated that nonstem brain cancer cells can survive xenotransplantation but fail to form tumors (Singh et al., 2004). Although multiple mechanisms may be responsible for lack of tumor initiation, we previously demonstrated that glioma stem cells (GSCs) have a greater ability to promote tumor angiogenesis through secretion of elevated levels of vascular endothelial growth factor (VEGF) (Bao et al., 2006b). However, the upstream regulators responsible for up-regulating VEGF in GSCs remain to be defined. Hypoxia is a well-known regulatory factor for the “angiogenic switch” and regulates stem cell biology (Danet et al., 2003; Gassmann et al., 1996; Ezashi et al., 2005; Parmar et al., 2007; Blazek et al., 2007; Keith and Simon, 2007; Platet et al., 2007). Low oxygen levels promote maintenance of embryonic stem cell pluripotent potential and block differentiation (Ezashi et al, 2005). Moreover, the fraction of brain tumor cells expressing a stem cell marker is increased under hypoxia in vitro (Blazek et al, 2007; Platet et al, 2007). Thus, hypoxia may be a critical component of a cancer stem cell niche (Gilbertson and Rich, 2007; Keith and Simon, 2007). We therefore hypothesized that there are unique hypoxia responses in cancer stem cells which contributes to the tumor initiation and maintenance of cancer stem cells.
Cellular responses to hypoxia are commonly regulated by the hypoxia inducible factor (HIF) family of transcriptional factors (Harris, 2002; Keith and Simon, 2007). HIFs function as heterodimers consisting of an oxygen sensitive HIFα subunit and a constitutively expressed HIFβ subunit. Under normoxic conditions, HIFα is ubiquinated by the Von Hippel-Lindau (VHL) tumor suppressor gene product and then targeted for proteasomal degradation, but under hypoxia the interaction between HIFα and VHL is abrogated. As a result, HIFα is stabilized, dimerizes with HIFβ and then binds to hypoxia responsive elements (HREs) in the promoters of hypoxia regulated genes. The HIF dimer activates the transcription of hundreds of downstream genes which modulate cell survival, motility, metabolism and angiogenesis (Harris, 2002). Two HIFα proteins, HIF1α and HIF2α, are highly homologous and bind to similar HRE sequences. As HIF1α is universally expressed while HIF2α shows a more restricted expression pattern, relatively few studies have determined the role of HIF2α in cancer initiation or tumor progression (Covello et al, 2006; Holmquist-Mengelbier et al, 2006; Hu et al, 2006; Raval et al, 2005). However, it is now clear that HIF1α and HIF2α can often play nonoverlapping biological roles due to their unique target genes as well as different requirement of oxygen for activation (Holmquist-Mengelbier et al., 2006). The identification of the stem cell regulator Oct4 as a HIF2α target gene directly links HIF2α to stem cell biology (Covello et al., 2006). Moreover, in a renal carcinoma model, HIF2α enhances the transcriptional activity of another stem cell factor c-Myc, while HIF1α destabilizes Myc complexes (Gordan et al., 2007). Another family member, HIF3α, lacks the transcriptional activation domain and functions as a dominant negative regulator of the hypoxia response due to sequestration of HIFβs (Kaur et al., 2005). Together, these data differentially link HIFs to stem cell biology and angiogenesis. We therefore sought to determine HIF expression and its biological consequence in the context of the GSC and nonstem glioma cell subpopulations.
Results
mRNA Levels of HIF2A and Other Hypoxia Response Genes Are Differentially Expressed in Glioma Stem Cells
To determine if the angiogenic drive of GSCs is regulated by specific molecular responses to hypoxia, we created short term cultures enriched or depleted for cancer stem cells directly from glioblastoma surgical biopsy specimens or xenografts derived from brain tumor specimens (patient characteristics presented in Table S1 and Figure S1) using our previously described methodology (Bao et al., 2006a; Bao et al., 2006b). The neoplastic origin of these cells was confirmed by fluorescent in situ hybridization (FISH) analysis (Figure S2). For these cultures, we validated the enrichment or depletion of cancer stem cells using functional assays, including propagation of tumors with characteristics of the parental sample (Table S2, S3; Figure S2) and stem cell marker expression (Figure S3). Using matched cultures of GSCs or nonstem cells, we compared the mRNA levels of hypoxia-regulated genes in GSCs or nonstem cancer cells under normoxia (20% O2) and hypoxia (1% O2). Multiple hypoxia responsive genes were strongly differentially regulated between GSCs and nonstem cells isolated from the same tumor specimens (Table S4), including HIF2A (but not HIF1A).
Using semi-quantitative real-time PCR, we confirmed a strong basal and hypoxia-induced upregulation ofHIF2A (but not HIF1A) mRNA in GSCs as compared to matched nonstem cancer cells or fetal human neural progenitors (Figures 1A/B, 2A/B, S4A/B, S5A/B, S6A/B). Similar patterns of mRNA expression were detected using the iron chelator, desferrioxamine (DFX) [which induces molecular hypoxic responses with similar kinetics to hypoxia (Wang and Semenza, 1993)] (Figures 1A/B, S4A/B, S5A/B), or atmospheric hypoxia (1% O2) (Figures 2A/B, S6A/B). Minimal HIF2A expression was also detected in normal adult murine neural progenitors under normoxia or hypoxia (Figure S5D). These data demonstrate that HIF2A, but not HIF1A, is a hypoxia responsive gene dramatically up-regulated in GSCs.
Figure 1. Glioblastoma Stem and Nonstem Cells Differentially Expressed Hypoxia Response Genes.
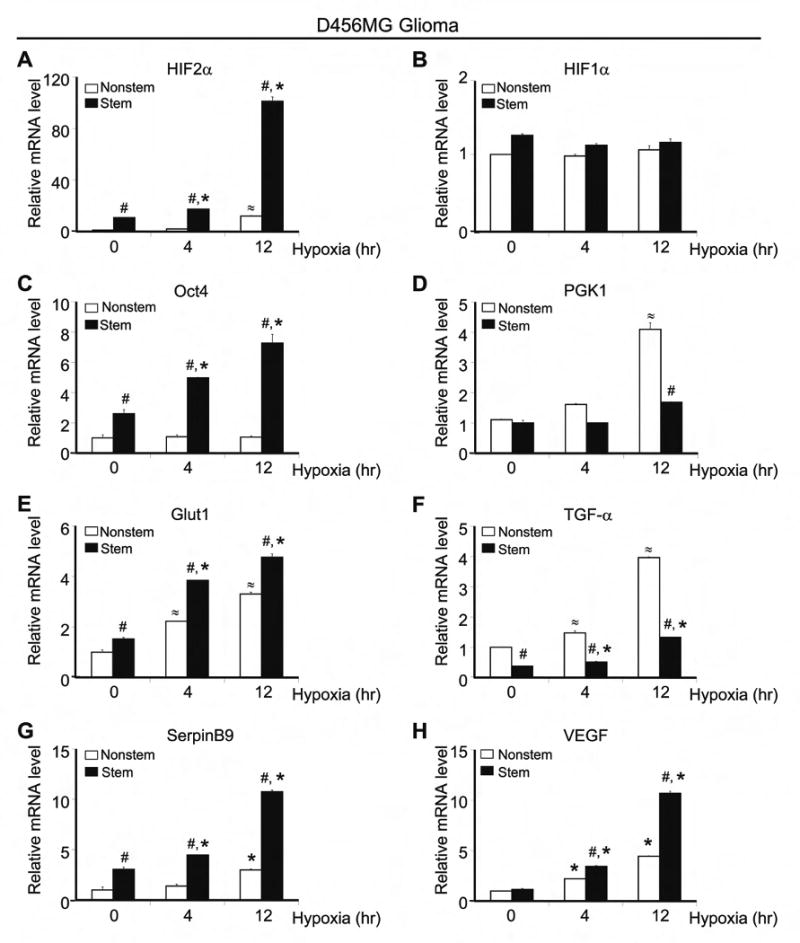
Glioblastoma stem and nonstem isolated from the glioblastoma xenograft D456MG were treated with desferrioxamine (DFX) to mimic hypoxia for the time indicated. RT-PCR was performed with primers specific for HIF2α (A), HIF1α (B), Oct4 (C), phosphoglycerate kinase 1, PGK1 (D), glucose transporter type 1, Glut1 (E), transforming growth factor alpha, TGF-α (F), SerpinB9 (G), and vascular endothelial growth factor, VEGF (H). Data were normalized to GAPDH, Ubiquitin C, and SDHA. #, P < 0.01 with ANOVA comparison of stem cells to nonstem cells with identical treatments. *, P < 0.01 with ANOVA comparison of stem cells under hypoxia vs. normoxia. ≈, P < 0.01 with ANOVA comparison of on-stem cells under hypoxia vs. normoxia.
Figure 2. Glioma Stem Cells and Normal Neural Progenitors Differentially Expressed Hypoxia Response Genes.
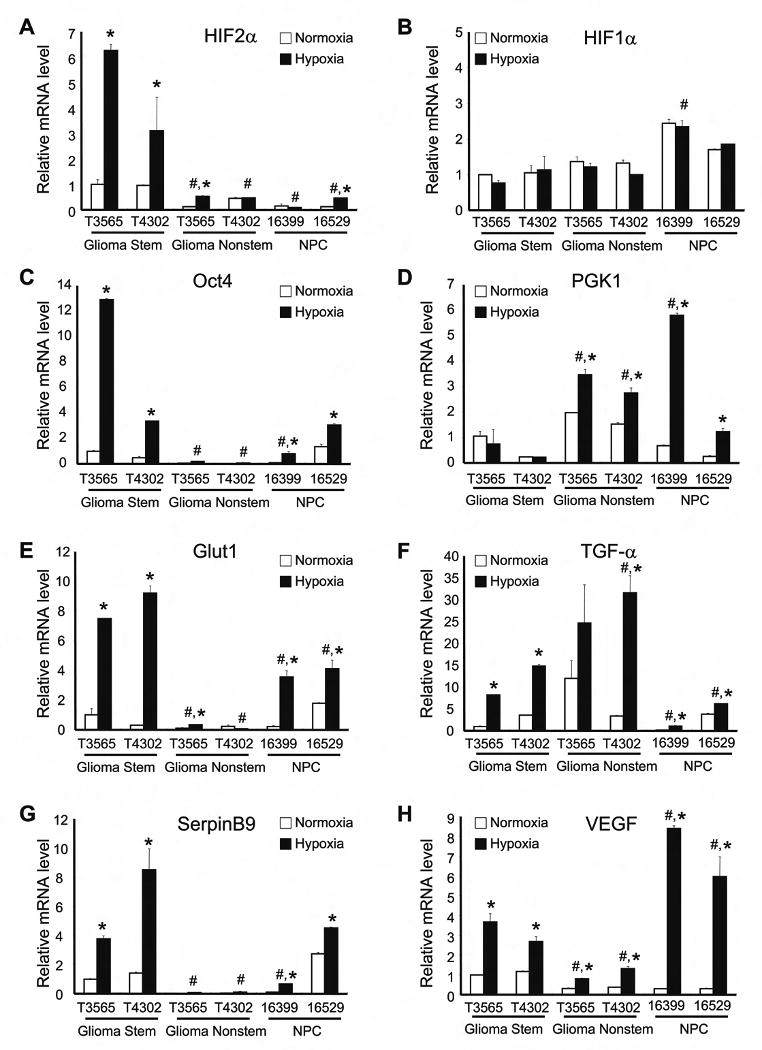
Cells from T3565 and T4302 glioblastoma samples and two different normal neural progenitor cell preparations were cultured in normoxia (20% oxygen) or hypoxia (1% oxygen) for 24 hr. RT-PCR analysis was performed with primers specific for HIF2α (A), HIF1α (B), Oct4 (C), PGK1 (D), Glut1 (E), TGF-α (F), SerpinB9 (G), and VEGF (H). Data were normalized to β-actin levels. *, P < 0.01 with ANOVA comparison of hypoxia treated cells to identically prepared normoxia controls. #, P < 0.01 with ANOVA comparison of indicated hypoxia treated cells to both hypoxia treated GSCs.
The elevated HIF2A mRNA levels in GSCs may result from enhanced transcription or increased mRNA stability. Surprisingly, the half-life of HIF2A was shorter in GSCs in comparison to matched nonstem glioma cells (Figure S7A), suggesting that the increase in HIF2A mRNA levels is not due to a difference in mRNA stabilization. In contrast, de novo mRNA synthesis is required as blocking mRNA transcription by Actinomycin D abrogated the induction of HIF2A in GSCs upon hypoxia treatment (Figure S7B). To determine the relative levels of transcription of the HIF2A promoter in the tumor subpopulations, we performed RNA Polymerase II chromatin immunoprecipitation. GSCs under both normoxia and hypoxia had a greater enrichment of RNA Polymerase II binding to the HIF2A promoter than nonstem glioma cells (Figure S7C). Together, these data demonstrate that HIF2A mRNA is up-regulated in GSCs with enhanced transcription.
Considering the differential expression of HIF2A and HIF1A in GSC and nonstem cancer cell subpopulations and normal neural progenitors, we determined the mRNA expression of genes known to be specifically regulated by HIF2α or HIF1α (Figures 1C-H, 2C-H, S4C-H, S5C, S6C-H). Genes reported to be HIF2α dependent [Oct4 (Covello et al., 2006; Keith and Simon, 2007), glucose transporter type 1 (Glut1) (Keith and Simon, 2007), and SerpinB9 (Holmquist-Mengelbier et al., 2006)] were expressed at significantly higher levels in GSCs as compared to matched nonstem cancer cells under hypoxia (Figures 1C/E/G, 2C/E/G, S4C/E/G, S6C/E/G). In contrast, the HIF1α-regulated gene phosphoglycerate kinase 1 (PGK1) (Keith and Simon, 2007) was strongly up-regulated in nonstem glioma cells under hypoxia as compared to matched GSCs (Figures 1D, 2D, S4D, S6D). Analysis of VEGF, a gene regulated by HIF1α and/or HIF2α in a cell specific manner (Harris, 2002; Holmquist-Mengelbier et al., 2006; Keith and Simon, 2007), demonstrated that hypoxia induced a greater increase in VEGF levels in GSCs than in matched nonstem cancer cells (Figures 1H, 2H, S4H, S6H; Table S4). HIF target genes in GSCs were variable relative to normal neural progenitors, with Glut1 demonstrating a consistent elevation in GSCs (Figures 2C-H, 5C).
Figure 5. HIF Knockdown Altered Glioma Stem Cell Neurosphere Formation.
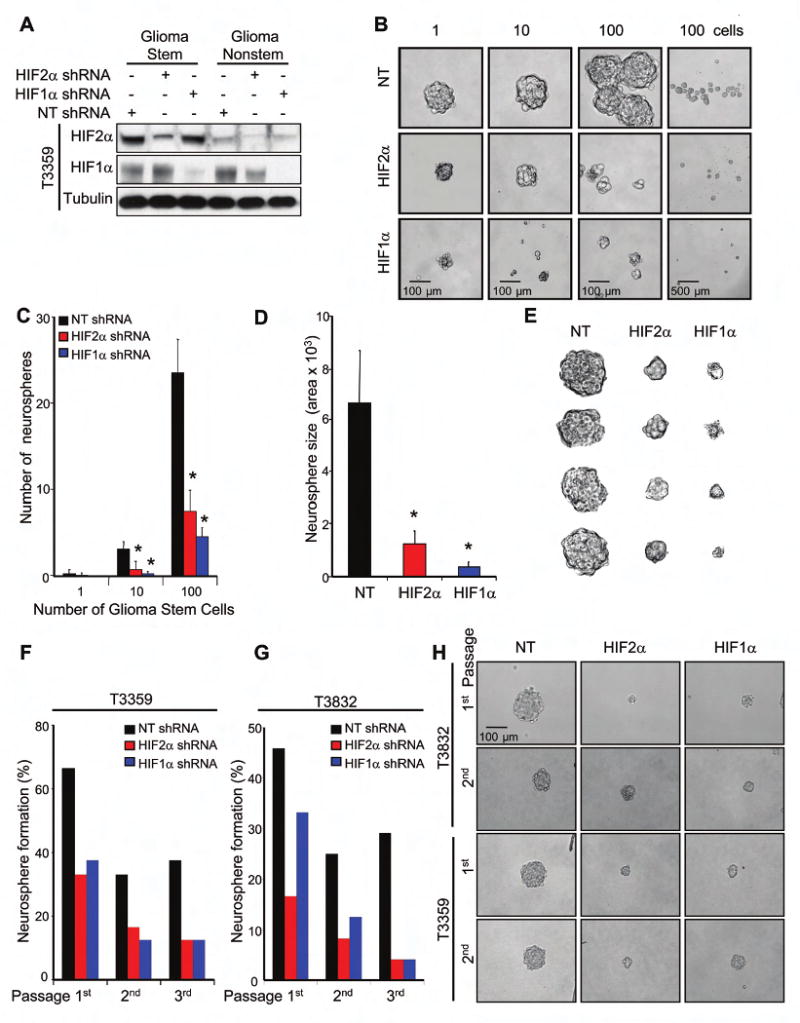
(A) Specific knockdown of HIF1α and HIF2α protein using shRNA. (B-E) 1, 10, or 100 lentiviral infected GSCs isolated from T3359 were cultured in 24-well plates. (B) Representative images of spheres are shown. (C) The total number of neurospheres per well is significantly decreased with HIF targeting. (D) Neurosphere size is significantly reduced by targeting HIF expression. (E) Representative image of neurospheres of neurosphere size in D. (F-G) Targeting HIFs in T3359 or T3832 stem cells decreases neurosphere formation in sequential passages. (H) Representative images of neurospheres formed in sequential passage neurosphere formation assays. *, P < 0.001
Glioma Stem Cells Preferentially Express HIF2α Protein under both Normoxic and Hypoxic Conditions
We interrogated the impact of transcriptional upregulation ofHIF2A mRNA in GSCs on HIF2α protein levels. Although HIF1β and HIF3α levels did not differ between glioblastoma stem and nonstem cells under normoxia and hypoxia conditions (data not shown), total HIF2α protein expression was consistently higher in GSCs than in matched nonstem cancer cells (Figures 3A-J, S7D/E) or normal neural progenitors (Figures 3K-M). HIF2α was highly expressed in GSCs treated with a chemical hypoxia-mimetic (Figures 3A-G, K, L) or grown in a hypoxia chamber under oxygen concentrations ranging from 0.2 to 5% (Figures 3H-J, M). In contrast, HIF1α expression was only increased by more severe hypoxic conditions induced by the chemical mimetic or ≤1% O2 (Figures 3, S7D/E), which is consistent with a previous report that HIF2α (but not HIF1α) accumulates under physiological oxygen levels present in solid tumors (Holmquist-Mengelbier et al., 2006). When oxygen levels were sufficient for HIF1α induction, HIF1α was often expressed at higher levels in nonstem cancer cells than matched GSCs (Figures 3, S7D/E). Of note, the induction of HIF2α and HIF1α expression was dependent on new protein synthesis and regulated by proteosomal degradation (Figures S7D/E) without cell type specific differences in VHL expression (data not shown). These data suggest that GSCs preferentially express HIF2α protein under both normoxic and hypoxic conditions to provide cancer stem cells a survival and growth advantage by activating downstream genes even in modest hypoxia conditions.
Figure 3. Hypoxia Potently Induced HIF2α Protein Expression in Glioma Stem Cells.
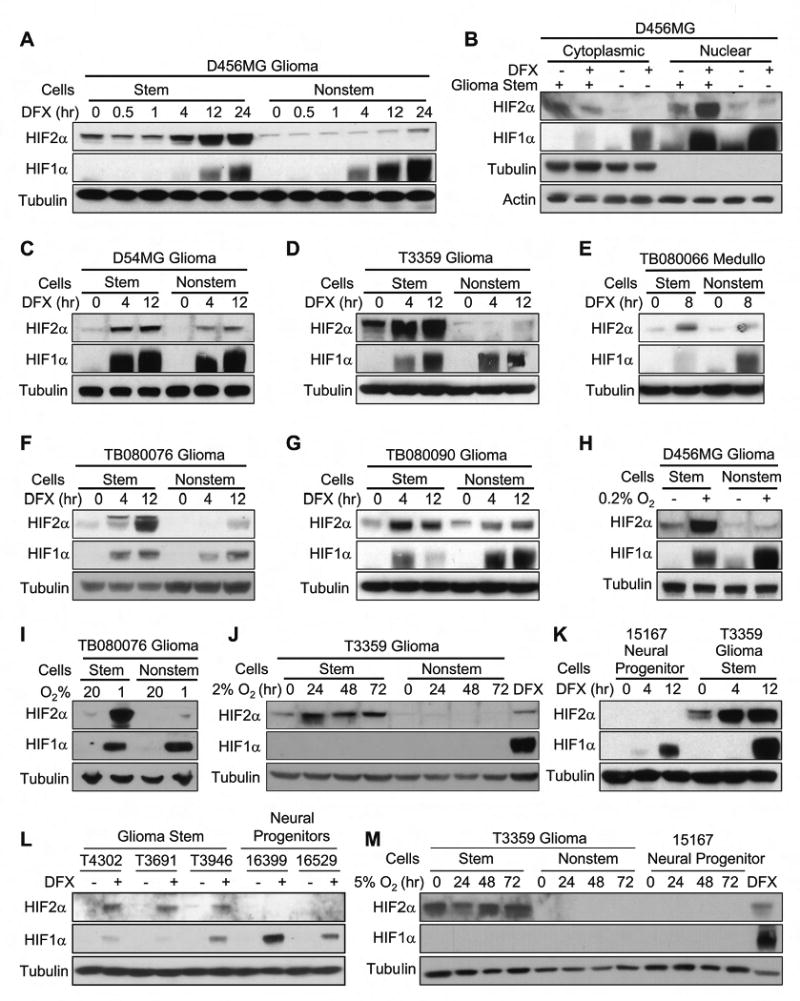
(A-G) Glioblastoma stem and nonstem cells isolated from multiple samples were treated with DFX to mimic hypoxia for the indicated times. Nuclear and cytoplasmic fractions (B) or total cell lysate (A, C, E-G) were analyzed. (H) Cells isolated from D456MG were cultured under 20% or 0.2% oxygen for 24 hr and analyzed by immunoblotting. (I) Cells isolated from TB080076 were cultured under normoxia (20% oxygen) or hypoxia (1% oxygen) for 24 hr and analyzed by immunoblotting. (J) Cells isolated from T3359 were cultured under more modest hypoxia (2% oxygen) and total cell lysates were analyzed. (K) T3359 GSCs and CD133+ normal neural progenitors were treated with 100 μM DFX and total cell lysates were analyzed. (L) GSCs (T4302, T3691, and T3946) and the normal human neural progenitors (16399 and 16529) were treated with DFX and analyzed by immunoblotting. (M) Higher HIF2α protein expression is maintained with a relatively physiological level of oxygen in GSCs. Cells were cultured under physiological level of oxygen (5% oxygen) for the indicated times and total cell lysates collected. In (J) and (M), and DFX treated samples were used as a positive control.
We further examined whether HIF2α upregulation in GSCs generally occurs as a stem cell phenotype. Similar to the mRNA data indicating minimal HIF2A in human neural progenitors (Figures 2A, S5A, S6A), we found that HIF2α was almost undetectable in normal human neural progenitor cells resulting in consistent over-expression of HIF2α in the GSC population (Figures 3K-M). In contrast, HIF1α accumulated in both human neural progenitors and GSCs under hypoxia (Figures 3K/L). Taken together, these data indicate that HIF1α upregulation is a shared molecular response in both the normal and cancer stem cell compartments while HIF2α induction is restricted to cancer stem cells.
To examine the expression pattern of HIF1α and HIF2α in vivo, we preformed immunohistochemistry (IHC) on paraffin embedded primary human glioblastoma surgical biopsy specimens (Figures 4A, S8, S9; Table S5). In the human tumor sections, HIF1α antibody marked the majority of tumor cells (∼60%) arranged about the regions of necrosis in most samples. In contrast, HIF2a demonstrated more variable and rare staining, predominately located immediately around regions of necrosis, where it was expressed by 1 to 10% of cells. HIF2α expression was also frequently observed around proliferating blood vessels where 1% to 10% of tumor cells were stained. Of note, previous studies suggest that the perivascular region is enriched for brain tumor stem cells (Bao et al, 2006b; Calabrese et al., 2007; Christensen et al, 2008). Consistent with these results, we found that CD133 and another potential brain tumor stem cell marker, Olig2 (Ligon et al, 2007), were expressed by 1-10% of tumor cells adjacent to blood vessels (Figures 4A, S8A). We therefore performed immunofluorescence studies on frozen primary human tumor samples to determine if HIF2α and CD133 co-localized in vivo. Indeed, we found that most tumor cells that expressed HIF2α co-expressed CD133, although not all CD133 positive cells expressed HIF2α (Figures 4B, S10). FACS analysis of glioma stem and nonstem populations confirmed the co-expression of CD133 and HIF2α (Figures 4C/D). Together, our data suggest HIF2α is a molecular immunophenotype specific for glioblastoma tumor stem cells and not a general stem cell phenotype.
Figure 4. HIF2α Co-Expressed with Cancer Stem Cell Markers in Human Glioblastoma Biopsy Specimens.
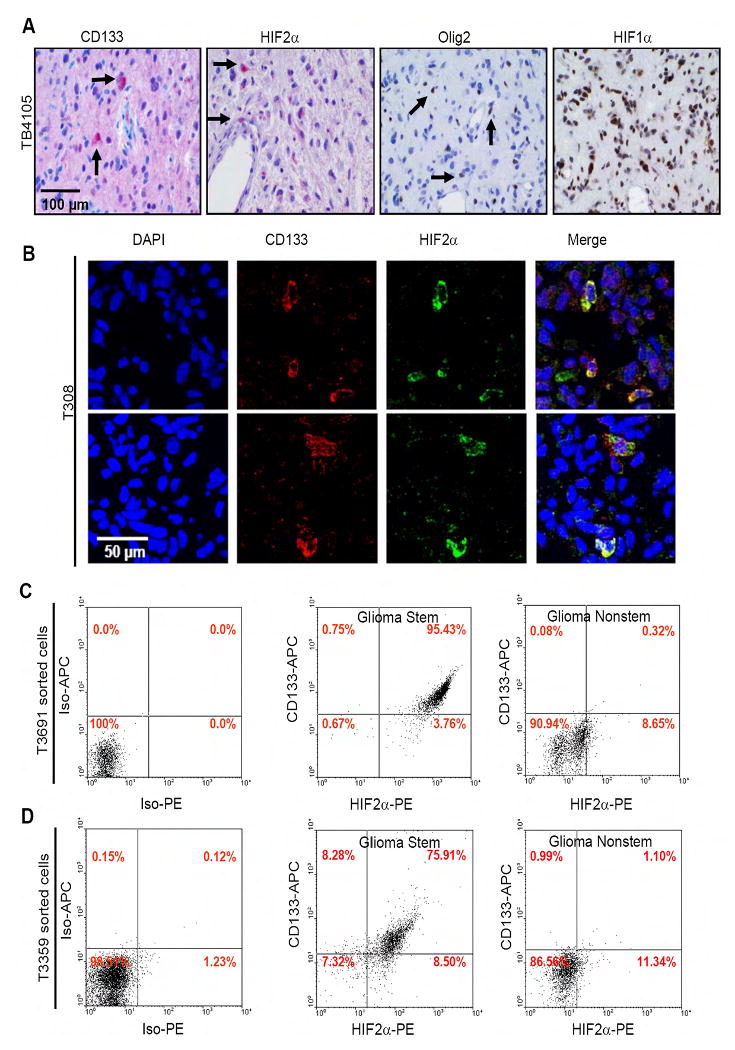
(A) Restricted pattern of HIF2α and stem cell marker expression in human brain tumor patient specimens. (B) Immunofluorescence of cells in human brain tumor patient specimens demonstrates co-localization of CD133 and HIF2α. (C-D) Cells expressing the cancer stem cell marker CD133 also express HIF2α. Glioma stem and nonstem cells isolated from T3691 (C) or T3359 (D) were analyzed for CD133 and HIF2α expression via FACS using anti-CD133-APC and anti-HIF2α-PE.
HIFs Are Required for Glioma Stem Cell Growth and Survival
As HIF2α and HIF1α regulation differs between glioblastoma stem and nonstem cells, we examined the requirement for HIFs in the tumor subpopulations and cancer stem cell biology using a lentiviral shRNA based system. We achieved knockdown efficiency of ∼70-95% for both HIF1α and HIF2α at the mRNA level (Figure S11A/B), although the efficiency of HIF1α knockdown was consistently greater than that of HIF2α at the protein level (Figures 5A, 6B, 7B, S15A). As in previous reports (Keith and Simon, 2007; Holmquist-Mengelbier et al., 2006), HIF2α knockdown was selectively associated with reduced mRNA levels of Glut1 and SerpinB9 while targeting HIF1α significantly decreased PGK1 mRNA (Figure S11). These data demonstrate the ability to specifically target HIF2α and HIF1α with resulting distinct molecular effects.
Figure 6. HIF Knockdown Reduced Glioma Stem Cell Growth Due to Elevated Apoptosis.
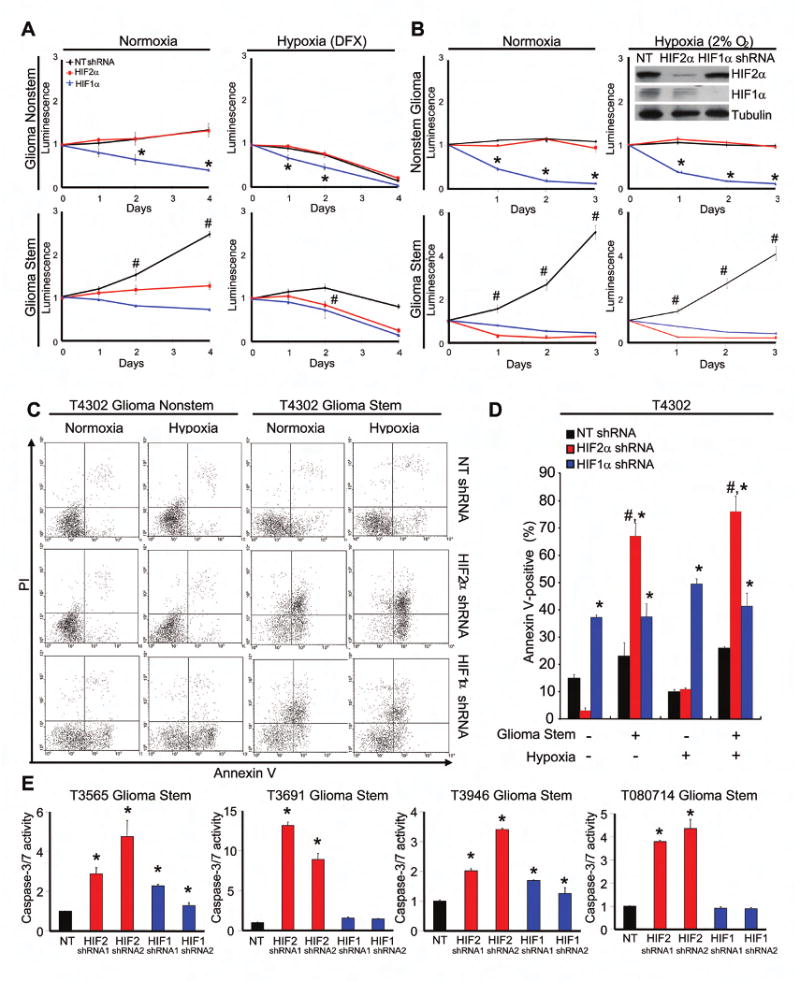
(A) Targeting HIF2α in glioblastoma stem, but not nonstem, cells decreases growth. Cell titers were determined using the CellTiter-Glo Luminescent Cell Viability Assay kit. *, P < 0.05 with ANOVA comparison of HIF1α shRNA to either NT shRNA or HIF2α shRNA. #, P <0.01 with ANOVA comparison of NT shRNA to either HIF1α or HIF2α shRNA. (B) Cells were plated and quantified as in A but cultured in 20% or 2% oxygen as indicated. Inset shows effective knockdown with HIF2α and HIF1α shRNAs. (C-D) Targeting HIFs leads to increased apoptosis in GSCs as determined by the Annexin V staining. *, P < 0.05 with ANOVA comparison to nontargeting shRNA of the same cell type and hypoxia treatment. #, P <0.01 with ANOVA comparison of HIF2α shRNA treated GSCs to HIF2α shRNA treated nonstem cells with identical oxygen treatment. (E) Targeting HIFs results in increased caspase-3/7 activity in GSCs. *, P < 0.05
Figure 7. HIF Knockdown Decreased Glioma Stem Cell Mediated Angiogenesis.
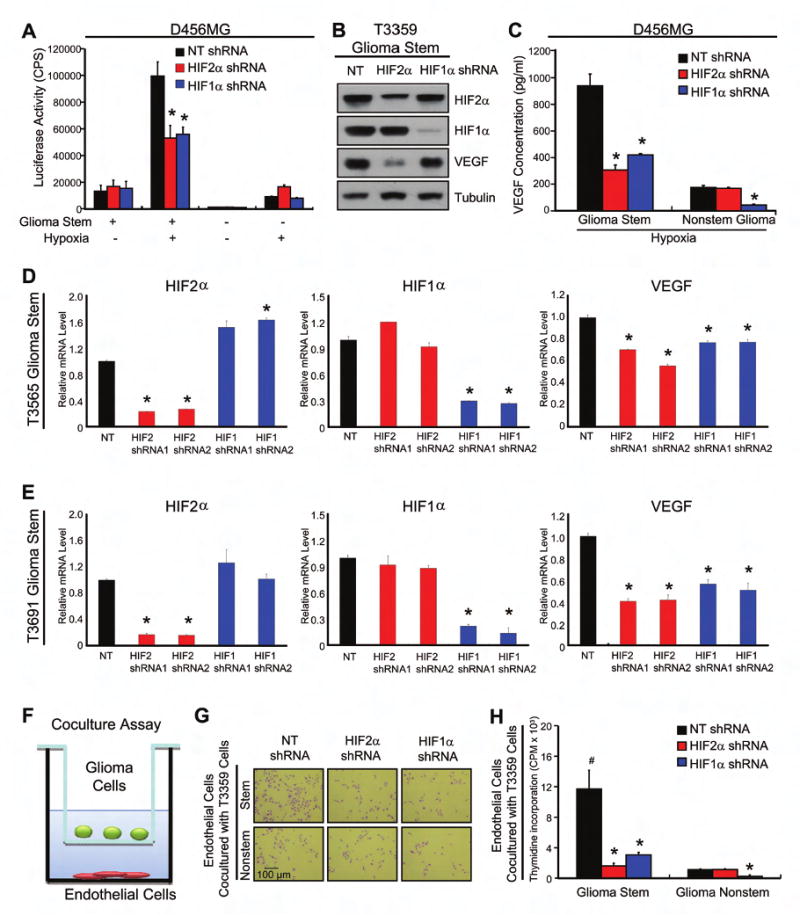
(A) HIF2α knockdown prevents hypoxia induced activation of the VEGF Promoter. *, P < 0.001 with ANOVA comparison of HIF2α or HIF1α shRNA treated stem cells to nontargeting shRNA with hypoxia. (B) HIF knockdown reduces VEGF protein expression in GSCs. (C) HIF2α knockdown reduces VEGF expression in stem, but not nonstem, glioblastoma cells. *, P < 0.01 (D-E) HIF knockdown reduces VEGF mRNA level in GSCs. *, P < 0.01 (F-H) HIF2α knockdown in GSCs reduced cancer cell-mediated endothelial cell proliferation. (F) Representative diagram of the coculture assay. (G) Representative images of cocultured HMVEC cells after cells fixed with 4% PFA and then stained with toluidine blue. (H) HMVEC proliferation was measured through [3H]-thymidine incorporation. *, P < 0.001 by ANOVA with comparison of HIF2α or HIF1α shRNA infected glioma cells to corresponding nontargeting shRNA infected cells. #, P < 0.001 by ANOVA comparison of nontargeting shRNA infected GSCs to nonstem nontargeting control cells.
To determine the biological consequences of HIF knockdown, we first assessed neurosphere formation in GSCs (Figure 5) as we did not observe neurosphere formation in the nonstem cells (data not shown) similar to our prior report (Bao et al, 2006a). HIF knockdown impaired neurosphere formation not only in primary assays (Figures 5B-E, S12) but also in secondary and tertiary passages (Figures 5F-H), indicating the HIFs are required for proliferation of GSCs in vitro. Consistent with this notion, levels of the HIFs appear to also be coordinately regulated through the differentiation status. Growth factor withdrawal induces differentiation and is associated with a decrease in HIF2α protein levels (Figure S13).
Even though a minority of GSCs with HIF1α or HIF2α knockdown retained neurosphere formation potential, the size of the resultant neurospheres was significantly reduced (Figure 5), suggesting the HIFs are required for GSC proliferation or survival. We therefore determined the growth of GSCs and nonstem cells under normoxia or hypoxia when HIF1α or HIF2α expression was targeted by lentiviral transduced shRNA (Figures 6A/B). The requirement for HIF2α in cell growth was restricted to GSCs as no effect of HIF2α shRNA was observed in matched nonstem cancer cells (Figure 6A/B). In contrast, HIF1α knockdown resulted in reduced cell growth in both glioblastoma stem and nonstem cells (Figure 6A/B).
Consistent with the cell growth data, we found that targeting HIFs with lentiviral shRNA resulted in decreased cell survival. Loss of HIF2α in GSCs consistently resulted in an induction of apoptosis determined with Annexin V staining (Figures 6C/D, S14, S15) and caspase activation (Figures 6E, S15C). No requirement for HIF2α was detected in nonstem glioma cells, even under hypoxia (Figures 6C/D, S14). In contrast, HIF1α contributed to the survival of both glioma stem and nonstem cells, but HIF1α shRNA was sometimes less acutely toxic than HIF2α shRNA (Figures 6C-E, S15). The elevation in apoptosis due to loss of HIF expression in GSCs was consistent with an increase in the percentage of cells in the sub-G0 and G1 phases of the cell cycle and a decrease in cycling and G2 phase cells (Figure S16). Together these data demonstrate requirements for both HIF1α and HIF2α in GSC biology with a specific requirement for HIF2α in the GSC, but not nonstem, tumor subpopulation for growth and survival. Due to differences in the efficiency of the shRNA constructs (HIF1α knockdown was consistently more efficient than HIF2α knockdown), it is not possible to absolutely determine the relative importance of the HIFs in GSCs.
HIF2α Is Required for VEGF Expression in Glioma Stem Cells but Not Nonstem Cells whereas HIF1α Is Required in Both Tumor Subpopulations
In addition to their role in tumor initiation, our prior data demonstrate cancer stem cells promote tumor maintenance by enhancing angiogenesis via elevated VEGF (Bao et al, 2006b). As VEGF is a known HIF target gene (Kaur et al, 2005), we determined whether HIF2α and HIF1α are required for glioblastoma stem and nonstem cell VEGF expression. Knockdown of HIF2α or HIF1α in GSCs under hypoxia significantly reduced VEGF promoter activity (Figure 7A), mRNA level (Figures 7D/E, S11), and intracellular (Figure 7B) and secreted (Figure 7C) VEGF protein levels. In matched nonstem cancer cells, there was no requirement for HIF2α in VEGF transcription or protein production (Figures 7A/C). These results strongly suggest that HIF1α is required in both glioblastoma stem and nonstem cells for the induction of VEGF expression by transcriptionally regulating the VEGF promoter, whereas there is a specific requirement for HIF2α for VEGF production in GSCs.
As VEGF can support brain tumor angiogenesis through regulation of endothelial cell proliferation and survival (Jain et al, 2007; Plate et al., 1992), we examined if knockdown of HIFs in glioblastoma stem and nonstem cells could significantly impact endothelial cell growth (Figures 7F-H). We performed a coculture experiment, in which glioblastoma cells were cultured in an upper chamber while human microvascular endothelial cells (HMVEC) were planted in the lower wells (Figure 7F). These two chambers were separated by a permeable membrane with 0.4 μm pores, which prevented physical contact between glioblastoma cells and endothelial cells but allowed transfer of secreted factors. Consistent with our previous report (Bao et al, 2006b), GSCs significantly increased endothelial cell numbers and proliferation in comparison to nonstem cancer cells, as determined by direct cell number counting (Figure 7G and data not shown) and [3H]-thymidine incorporation assay on HMVEC (Figure 7H). Knockdown of either HIF2α or HIF1α reduced the paracrine effects of GSCs on endothelial cells, but endothelial cell growth supported by nonstem glioblastoma cells was only affected by targeting HIF1α (Figure 7H). These data are consistent with the observed differences in HIF requirements for VEGF expression in the tumor subpopulations, and suggest a specific role for HIF2α in GSC-mediated angiogenesis by affecting endothelial cell growth.
Targeting HIFs in Glioma Stem Cells Decreases Tumorigenic Capacity and Increases the Survival of Mice Bearing Intracranial Xenografts
Considering the in vitro requirements for HIF2α and HIF1α in GSC proliferation, survival, and VEGF production, we determined the impact of HIF knockdown on GSC tumorigenic capability in vivo (Figures 8A-G) When GSCs transduced with nontargeting control shRNA or shRNA targeting HIF2α or HIF1α were intracranially implanted into immunocompromised mice, we observed a significant decrease in tumor formation and an increase in the survival of tumor bearing mice when HIF1α or HIF2α were targeted (Figures 8A-G). We further found that targeting HIFs can reduce the tumorigenic potential of GSCs in an in vivo limiting dilution assay (Figure 8G). As knockdown of HIF2α increased the survival of tumor bearing mice as well as or significantly more than HIF1α (Figures 8A-G), but was usually targeted less efficiently at the protein level (Figure 5A, 6B, 7B, S16A), our data may underestimate the importance of HIF2α for the in vivo propagation of GSCs. In fact, tumors arising from unselected HIF2α knockdown cells (Figures 8B-E) expressed HIF2α indicating that these tumors likely originated from unsuccessfully targeted cells (Figure S17). When GSCs underwent puromycin marker selection to confirm successful infection, HIF knockdown cells failed to form any tumors even after six months (Figure 8F).
Figure 8. HIF Knockdown Suppressed Cancer Stem Cell Mediated Tumor Growth.
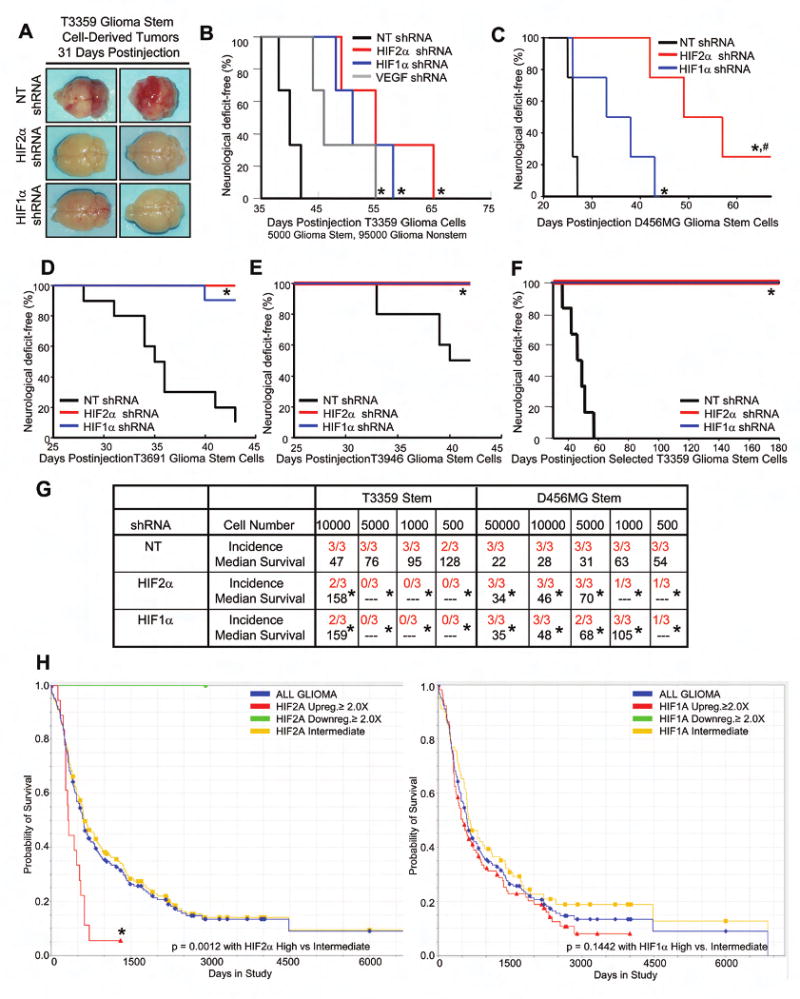
(A) Gross histology demonstrates highly vascular tumors in glioblastoma stem derived tumors from cells infected with nontargeting shRNA but not HIF targeting shRNAs. (B) Targeting VEGF or HIFs within the cancer stem cell subpopulation increases survival. 5000 infected GSCs were mixed with 95000 matched uninfected nonstem cells and injected into the mice brains. *, P < 0.03. (C-E) Targeting HIFs in the cancer stem cell subpopulation decreases tumorigenesis. *, P < 0.05. (F) Tumors do not form from GSCs selected for the incorporation of a puromycin marker associated with HIF targeting shRNA. *, P < 0.03. (G) In vivo limiting dilution assay demonstrates GSCs are less tumorigenic when HIFs are targeted. *, P < 0.03. (H) HIF2α but not HIF1α mRNA level correlates with patient survival (the Rembrandt database of the National Cancer Institute). There was only one patient with more than two fold HIF2A downregulation. No patients with HIF1A downregulation were observed. Analysis of the NCI TCGA database yields similar results (data not shown).
As brain tumor stem cells usually only account for a small percentage of bulk tumor cells in our studies, we inquired as to whether targeting only the GSC population could impact bulk tumor growth. We therefore employed an in vivo mixing experiment in which we prospectively segregated cancer stem cell enriched and depleted tumor populations, genetically manipulated the stem cell population, and xenotransplanted a mixture of stem and nonstem cancer cell populations at a 1:20 ratio (i.e. 5% of the total cancer cells were cancer stem cells similar to the fraction in human glioblastoma specimens) (Figure 8B). As expected, tumor cell mixtures with GSCs transduced with nontargeting shRNA control rapidly formed tumors with a histopathology consistent with a glioblastoma when implanted intracranially into immunocompromised mice. In contrast, tumor cell mixtures that included GSCs transduced with either HIF1α or HIF2α shRNA display impaired tumor formation potential indicating that targeting HIFs only in CSCs could have therapeutic benefit (Figure 8B and data not shown). Targeting HIFs likely impairs tumor growth through several mechanisms as many genes are regulated by HIFs as demonstrated above, including regulators of survival. One downstream HIF target that may be important in vivo is VEGF. We found that targeting VEGF exclusively in the GSC population in our cell mixing experiments can increase the survival of tumor bearing mice and decrease tumor angiogenesis, suggesting that reducing VEGF production and thus angiogenesis could be one of the potential mechanisms by which targeting HIFs in GSCs decreased tumorigenesis in vivo (Figure 8B). Together, our data demonstrate that HIFs are required to maintain the tumorigenic potential of GSCs and that targeting HIF2α may be a cancer stem cell directed therapy.
Elevated HIF2α Expression Is Associated with Poor Survival of Glioma Patients
To investigate whether targeting HIF2α may have a therapeutic benefit for the glioma patient population, we utilized the REMBRANDT (Repository of Molecular Brain Neoplasia Data) database of the National Cancer Institute (http://caintegrator-info.nci.nih.gov/rembrant). We analyzed the data to determine the survival of glioma patients with intermediate, low, or high expression of HIF2A or HIF1A (Figure 8H). We found a significant decrease in the probability of survival with elevated HIF2A expression, with no significant difference in survival with elevated HIF1A expression. As this gene expression database measures mRNA levels, and HIF2A but not HIF1A is regulated by hypoxia at the transcriptional level, the survival information contained in HIF2A levels may be both a surrogate for the presence of hypoxia as well as quantification of GSCs. These data demonstrate HIFs differentially affect patient outcome and strongly support a specific and important role for HIF2α in gliomas.
Discussion
Glioblastomas are among the most lethal of cancers with current therapies providing only palliation. While the successful cancer cures require eliminating all tumor cells, cancer stem cells may represent particular therapeutic challenges. The direct characterization of cancer stem cells may yield therapeutic targets that are not evident by whole tumor analyses. For example, we recently demonstrated that L1CAM, a cell adhesion molecule, was preferentially expressed in brain tumor stem cells and was essential to tumor initiation (Bao et al, 2008). Paramount in the development of cancer stem cell targeting agents must be the recognition that previously unrecognized toxicities may occur if a molecular pathway is shared with normal stem cells. We have therefore sought to identify molecular contributors involved in cancer stem cells without significant expression in the organ specific progenitor compartment, specifically neural progenitors. Based on these criteria, HIF2α appears to be an attractive target as it is specifically expressed by brain tumor stem cells but not neural progenitor cells, whereas HIF1α is shared by these cellular populations. Indeed, HIF1α is essential in neural development (Tomita et al, 2003) whereas animals with the targeted disruption of HIF2 display defects in other organ systems (Compernolle et al, 2002).
Hypoxia is a well recognized tumor microenvironmental condition that is linked to poor patient outcome and resistance to therapies (Teicher, 1994; Liang, 1996; Semenza, 2004; Chi et al., 2006; Vaupel and Mayer, 2007; Sathornsumetee et al., 2008). Cellular responses to hypoxia are frequently regulated by the HIFs leading to the attempted development of anti-HIF therapies, with limited success to date. Because of our prior work that identified cancer stem cells as a contributor to tumor angiogenesis, we interrogated the HIFs and other hypoxia target genes in brain tumor stem cells. As we expected, all cancer cells responded to acute hypoxia through the increase of HIF1α protein (Figures 3A-H, 3J). Although these conditions have been widely used in hypoxia studies, some reports suggest that the level of oxygenation may fluctuate and more modest restrictions in oxygen availability may more closely represent actual intratumoral conditions (Inoue and Ohnuma, 1989; Kimura et al., 1996; Cardenas-Navia et al, 2004). A recent report suggested that unlike HIF1α, which is only stabilized under acute hypoxic conditions, HIF2α may accumulate under modest hypoxia or even normal physiological oxygen levels (Holmquist-Mengelbier et al., 2006). Indeed, we found under 2-5% oxygen levels that HIF2α is the dominant hypoxia-inducible factor present in the cancer stem cell population (Figures 3I-L) and that HIF2α is expressed at wide range of oxygen levels. This indicates that HIF2α may provide cancer stem cells a growth advantage by activating downstream genes even without hypoxia stimulation in vitro and in vivo. Our immunohistochemical analysis of glioblastoma surgical specimens revealed that a significant fraction of HIF2α positive cells are located adjacent to blood vessels (Figures 4, S3). Therefore, it will be of great interest to determine whether HIF2α functions differentially under various oxygen tensions during tumorigenesis in vivo. It is also notable that the role of HIF2α was likely to be underestimated in previous cancer studies with cell lines or bulk tumor populations as cancer stem cells frequently account for only a restricted fraction of the overall tumor (less than 10% of tumor cells).
Prospective identification of cancer stem cells has been challenging, and the relationship of cancer stem cells to normal stem cells is controversial. In fact, the terminology used to describe the stem cell-like tumor population remains unresolved. Some researchers advocate a description based on the functional assays used to define these cells (i.e. tumor propagation), but others highlight the phenotypic similarities to normal stem cells. We have defined GSCs functionally as current methods for cancer stem cell enrichment from solid cancers remain imperfect. However, we utilized the term cancer stem cell as we note their self renewal and differentiation potentials., Cultures enriched for cancer stem cells with currently known cancer stem cell markers remain heterogeneous, as not every isolated cell is capable of self renewal or tumor propagation. These data suggest that additional cell surface markers or intracellular molecules contribute to the cancer stem cell phenotype. Our data suggest that HIF2α identifies a subpopulation of CD133 positive cells. The vast majority of HIF2α positive cells express CD133, but HIF2α and CD133 do not overlap exclusively: not all HIF2α positive cells are CD133 positive, and not all CD133 positive cells are HIF2α positive. Targeting HIF2α did not uniformly kill all CD133 positive cells suggesting a heterogenous dependence on HIF2α in this cancer stem cell population. The role of HIF2α in tumors which are not driven by CD133 expression (Beier et al, 2007; Zheng et al., 2007; Wang et al., 2008) is still unresolved, but we did not observe HIF2α expression in a rat glioma cell line in which CD133 negative cells were reported to be tumorigenic (Zheng et al, 2007). We also cannot complete the functional studies required to define cancer stem cells with HIF2α due to its intracellular localization. Our cancer stem cell cultures therefore remain heterogeneous for HIF2α expression. HIF2α does appear to localize with cancer stem cell markers in vitro and in vivo, suggesting that HIF2α positive cells are enriched in a cancer stem cell phenotype. Together, our results suggest that HIF2α may mark a subpopulation of cancer stem cells essential for tumor growth.
The HIFs function through the transcriptional regulation of a number of important gene products. Besides VEGF, the expression of Oct4, Glut1, and SerpinB9 genes was induced by HIF2α in our studies. Oct4 is a core regulator in stem cell self-renew and differentiation (Pan et al., 2002; Wang et al, 2006) and very recently validated as a cancer stem cell target (Hu et al., 2008). The glucose transporter Glut1 is frequently up-regulated in cancer cells to facilitate their accelerated metabolism (Macheda et al, 2005; Younes et al, 1995). The proteinase inhibitor SerpinB9 may prevent cytotoxic T cell mediated apoptosis of target cells (Trapani and Sutton, 2003) and can directly inhibit caspases (Young et al, 2000). Indeed, SerpinB9 is up-regulated in some melanoma and leukemia patients and its upregulation predicts poor outcome in high grade melanoma patients (van Houdt et al., 2005). These reports suggest that HIF2α mediated upregulation of Oct4, Glut1, and SerpinB9 may provide cancer stem cells with advantages in metabolism, proliferation, survival, and escape from immune surveilance.
Normal stem cells reside within highly defined anatomical niches that provide important cues to maintain stem cells in undifferentiated states or promote the acquisition of a more differentiated state. Recent studies suggest that cancer stem cells may also be harbored in specific niches (Gilbertson and Rich, 2007), but many aspects of the cancer stem cell niche are unknown. Our analysis of surgical glioblastoma biopsy specimens suggests that there may be at least two areas enriched for cancer stem cells. We observed GSCs around blood vessels, consistent with prior reports of a perivascular niche for normal stem cells (Tan et al., 2008; Yoshida et al., 2007) and GSCs (Calabrese et al, 2007), However, we also observed GSCs around regions of necrosis, which are hypoxic, suggesting that there may be more than one GSC niche. These results may parallel the hematopoietic stem cell location in the bone marrow, in which these cells are located around the endosteum and vascular sinusoids (Kiel and Morrison, 2008). The regulation of the bone marrow niche is an area of active investigation but it is notable that the bone marrow is maintained at a relatively low oxygen tension relative to the systemic circulation (Parmar et al., 2007). Hypoxia regulates many aspects of tumor biology, contributing to tumor cell proliferation, resistance to anti-neoplastic agents, angiogenic drive, and metastasis/invasion (Pouyssegur et al, 2006). These pro-tumorigenic effects of hypoxia may be due, at least in part, to the promotion of a stem cell-like phenotype in cancer cells in a solid tumor. Hypoxia creates cellular stresses that negatively regulate cell proliferation and survival, but hypoxia is also able to promote normal stem cell maintenance and block differentiation (Ezashi et al., 2005; Keith and Simon, 2007). Together, these data indicate hypoxia may be a functional component of a cancer stem cell niche (Gilbertson and Rich, 2007; Keith and Simon, 2007). Difficultly in reconciling the localization of cancer stem cells to both hypoxic regions and areas around tumor vasculature is resolved with the understanding that angiogenic vasculature is poorly functional and often associated with regions of hypoxia (Jain et al., 2007). In addition, HIF2α is expressed by the cancer stem cells at oxygen concentrations that approximate normal in vivo oxygen levels (2-5% in Figure 3). Thus, cancer stem cells may support the development and maintenance of their own niche by producing angiogenic factors to support blood vessel formation and tumor growth while still being maintained by hypoxia in adjacent regions. However, it remains possible that there are distinct subpopulations of cancer stem cells which are exclusively associated with hypoxic or perivascular regions and may be defined by further elucidation of cancer stem cell markers and molecular profiles.
The dependence of cancer stem cells on a hypoxic and perivascular niche offers potential therapeutic strategies based on vascular targeting. As anti-angiogenic therapies continue to be developed for many cancers, including glioblastomas, efficacy can be improved by increasing our understanding of the molecular mechanisms by which these agents function. We previously demonstrated that the VEGF neutralizing antibody bevacizumab (Avastin) specifically inhibits the pro-angiogenic effects of GSCs (Bao et al., 2006b), suggesting that anti-VEGF therapies may disrupt the stem cell niche (Calabrese et al., 2007; Gilbertson and Rich, 2007). We now demonstrate that the HIFs, key regulators of VEGF expression and angiogenic drive, promote stem cell maintenance and VEGF expression. Consistent with HIF1α's recognition as a molecular cancer target, we determined that HIF1α is required for the proliferation, survival, and angiogenesis of both the cancer stem cells and nonstem cancer cells. However, we have defined a unique requirement for HIF2α in the cancer stem cell subpopulation. Notably, HIF2A mRNA is significantly transcriptionally up-regulated under normoxia and hypoxia in GSCs in comparison to nonstem cancer cells, whereas HIF1α protein is usually higher under hypoxia in nonstem cancer cells. We found that targeting HIF2α in GSCs is as effective as or more effective in vivo than targeting HIF1α, suggesting that targeting HIF1α without recognizing the contribution of HIF2α to hypoxia responses overlooks an important potential compensatory mechanism. It is important to note that comparing the efficacy of targeting HIF1α and HIF2α cannot be directly compared in our studies as the efficiency of knockdown was significantly different (HIF1α was more efficiently targeted). HIF2α may have additional advantages as a target because the lack of expression in neural progenitor cells as well as its documented role in activating the myc pathway (another stem cell pathway) in contrast to HIF1α (Gordan et al., 2007). Future studies will be directed towards defining the downstream molecular mechanisms beyond caspase activation and VEGF expression by which the HIFs regulate cancer stem cell survival and tumor growth. Additional studies will be devoted towards defining the upstream mechanisms that regulate HIFs in cancer stem cells.
Our results have direct clinical relevance as we have recently determined that hypoxic markers, including HIF2α, provide useful biomarkers for predicting patient survival from treatment initiation in a trial of the anti-VEGF antibody bevacizumab in combination with irinotecan (Sathornsumetee et al., 2008). Using this malignant glioma patient cohort, we now find that the expression of HIF2α in tumor specimens collected at diagnosis can predict patient survival from the time of diagnosis. This conclusion is supported by another independent glioblastoma database from National Cancer Institute, which also suggests that patients with HIF2α upregulation have significantly shorter survival in comparison to those with lower HIF2α expression (Figure S6C). Thus, our data supports the development of HIF2α directed therapies and demonstrates differential molecular responses to hypoxia in the cancer stem cell subpopulation.
Experimental Procedures
Isolation of Glioma Stem Cells, Nonstem Glioma Cells, and Normal Neural Progenitors
Matched cultures enriched or depleted for GSCs were isolated from primary human brain tumor patient specimens or human glioblastoma xenografts as previously described in accordance with a Duke University Institutional Review Board approved protocol concurrent with national regulatory standards with patients signing informed consent (Bao et al, 2006a; Bao et al., 2006b). Briefly, tumors were disaggregated by Papain Dissociation System and filtered by 70μm cell strainer according to the manufacturer's instructions. Cells were then cultured in stem cell culture medium supplemented as detailed below for at least four hr to recover surface antigens. Cells were then labelled with APC- or PE-conjugated CD133 antibody, and sorted by fluorescence-activated cell sorting (FACS). Alternatively, cells were separated by magnetic sorting column using microbead-conjugated CD133 antibodies. CD133 positive cells were designated as GSCs whereas CD133 negative cells utilized as nonstem glioma cells. Normal human neural progenitors were obtained from Lonza and the use of these materials is considered exempt as human subjects by the Duke Institutional Review Board (See Supplemental Experimental Procedures for more details).
Tissue Culture and Hypoxia Induction
GSCs were cultured in Neurobasal media with B27 without Vitamin A (Invitrogen), bFGF (10 ng/ml) and EGF (10 ng/ml). After trypsinizing, nonstem tumor cells were cultured overnight in 10% serum DMEM to allow cell attachment and survival. Then, DMEM medium was removed and the cells cultured in supplemental Neuralbasal medium in order for experiments to be performed in identical medias. In order to induce hypoxia, cells were cultured in hypoxia chambers (Sheldon Manufacturing for 0.2% O2, Sanyo for 1%, 2% and 5%). Alternatively, cells were treated by 100 or 200 μM hypoxia-mimic chemical deferrioxamine mesylate (DFX, Sigma).
Lentiviral Mediated shRNA Targeting
Lentiviral shRNA clones (Mission RNAi) targeting HIF1α, HIF2α VEGF and nontargeting control sequences were obtained from Sigma. Lentiviruses were produced in 293FT cells with packing mix (ViraPower Lentiviral Expression Systems, Invitrogen) according to the manufacturer's instruction. Efficiency of different lentiviral shRNA clones in cells was determined by Western blot analysis and Real Time PCR.
In Vivo Tumor Formation Assays
Intracranial or subcutaneous transplantation of GSCs into nude mice was performed as described in accordance with a Duke University Institutional Animal Care and Use Committee approved protocol concurrent with national regulatory standards (Bao et al., 2006a). Briefly, 72 hr after lentiviral infection, cells were counted and certain number cells were implanted into the right frontal lobes of athymic BALB/c nu/nu mice. In some cases, 48 hr after infection, 1 μg/ml puromycin was applied to select infected cells for 48 hr before counting. Mice were maintained up to 25 weeks or until the development of neurological symptoms. Brains of euthanized mice were collected, fixed in 4% Paraformaldehyde (PFA), and paraffin embedded.
Statistical Analysis
Descriptive statistics were generated for all quantitative data with presentation of means ± standard error. Significance was tested by one-way ANOVA using the SAS Enterprise Guide 3.0 (Cary, NC) or GraphPad InStat 3.0 software (San Diego, CA). For in vivo studies, Kaplan Meier curves and log-rank analysis were performed using MedCalc software (Belgium).
Supplementary Material
Acknowledgments
Financial support was provided by the Childhood Brain Tumor Foundation, the Pediatric Brain Tumor Foundation of the United States, Accelerate Brain Cancer Cure, Alexander and Margaret Stewart Trust, Brain Tumor Society, Goldhirsh Foundation, Duke Comprehensive Cancer Center Stem Cell Initiative Grant (J.R.), NIH grants NS047409, NS054276, and CA116659 (J.R.). J.R. is a Damon Runyon-Lilly Clinical Investigator supported by the Damon Runyon Cancer Research Foundation and a Sidney Kimmel Foundation for Cancer Research Scholar. We thank Z. Su, Y. H. Sun, S. Keir, D. Satterfield, L. Ehinger and J. Faison for technical assistance; M. Cook and B. Harvat for assistance with flow cytometry. We are also grateful to R. Wechsler-Reya for helpful discussions.
Footnotes
SUPPLEMENTAL DATA
The Supplemental Data include Supplemental Experimental Procedures, five tables and seventeen figures and can be found with this article online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bao S, Wu Q, Li Z, Sathornsumetee S, Wang H, McLendon RE, Hjelmeland AB, Rich JN. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 2008;68:6043–6048. doi: 10.1158/0008-5472.CAN-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006a;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006b;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics molecular profiles. Cancer Research. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- Blazek ER, Foutch JL, Maki G. Daoy medulloblastoma cells that express CD133 are radioresistant relative to CD133- cells, and the CD133+ sector is enlarged by hypoxia. Int J Radiat Oncol Biol Phys. 2007;67:1–5. doi: 10.1016/j.ijrobp.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Cardenas-Navia LI, Yu D, Braun RD, Brizel DM, Secomb TW, Dewhirst MW. Tumor-dependent kinetics of partial pressure of oxygen fluctuations during air and oxygen breathing. Cancer Res. 2004;64:6010–6017. doi: 10.1158/0008-5472.CAN-03-0947. [DOI] [PubMed] [Google Scholar]
- Chi JT, Wang Z, Nuyten DS, Rodriguez EH, Schaner ME, Salim A, Wang Y, Kristensen GB, Helland A, Borresen-Dale AL, et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 2006;3:e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Schroder HD, Kristensen BW. CD133 identifies perivascular niches in grade II-IV astrocytomas. J Neurooncol. 2008 doi: 10.1007/s11060-008-9648-8. Epub. [DOI] [PubMed] [Google Scholar]
- Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest. 2003;112:126–135. doi: 10.1172/JCI17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Fandrey J, Bichet S, Wartenberg M, Marti HH, Bauer C, Wenger RH, Acker H. Oxygen supply and oxygen-dependent gene expression in differentiating embryonic stem cells. Proc Natl Acad Sci USA. 1996;93:2867–2872. doi: 10.1073/pnas.93.7.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson RJ, Rich JN. Making a tumour's bed: GSCs and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambardzumyan D, Squatrito M, Holland EC. Radiation resistance and stem-like cells in brain tumors. Cancer Cell. 2006;10:454–456. doi: 10.1016/j.ccr.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol Cell Biol. 2006;26:3514–3526. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Liu S, Breiter DR, Wang F, Tang Y, Sun S. Octamer 4 small interfering RNA results in cancer stem cell-like cell apoptosis. Cancer Res. 2008;68:6533–6540. doi: 10.1158/0008-5472.CAN-07-6642. [DOI] [PubMed] [Google Scholar]
- Inoue S, Ohnuma T. Effects of physiological oxygen environment on drug-induced cell lethality of multicellular tumor spheroids from human lung cancer. Sel Cancer Ther. 1989;5:13–22. doi: 10.1089/sct.1989.5.13. [DOI] [PubMed] [Google Scholar]
- Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol. 2005;7:134–153. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- Kimura H, Braun RD, Ong ET, Hsu R, Secomb TW, Papahadjopoulos D, Hong K, Dewhirst MW. Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Res. 1996;56:5522–5528. [PubMed] [Google Scholar]
- Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- Liang BC. Effects of hypoxia on drug resistance phenotype and genotype in human glioma cell lines. J Neurooncol. 1996;29:149–155. doi: 10.1007/BF00182138. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Huillard E, Mehta S, Kesari S, Liu H, Alberta JA, Bachoo RM, Kane M, Louis DN, Depinho RA, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503–517. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cellular Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- Pan GJ, Chang ZY, Scholer HR, Pei D. Stem cell pluripotency and transcription factor Oct4. Cell Res. 2002;12:321–329. doi: 10.1038/sj.cr.7290134. [DOI] [PubMed] [Google Scholar]
- Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Platet N, Liu SY, Atifi ME, Oliver L, Vallette FM, Berger F, Wion D. Influence of oxygen tension on CD133 phenotype in human glioma cell cultures. Cancer Lett. 2007;258:286–290. doi: 10.1016/j.canlet.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Sathornsumetee S, Cao Y, Marcello JE, Herndon JE, 2nd, McLendon RE, Desjardins A, Friedman HS, Dewhirst MW, Vredenburgh JJ, Rich JN. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26:271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Intratumoral hypoxia, radiation resistance, and HIF-1. Cancer Cell. 2004;5:405–406. doi: 10.1016/s1535-6108(04)00118-7. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White Fat Progenitor Cells Reside in the Adipose Vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev. 1994;13:139–168. doi: 10.1007/BF00689633. [DOI] [PubMed] [Google Scholar]
- Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Tomita S, Ueno M, Sakamoto M, Kitahama Y, Ueki M, Maekawa N, Sakamoto H, Gassmann M, Kageyama R, Ueda N, et al. Defective brain development in mice lacking the Hif-1alpha gene in neural cells. Mol Cell Biol. 2003;23:6739–6749. doi: 10.1128/MCB.23.19.6739-6749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani JA, Sutton VR. Granzyme B: pro-apoptotic, antiviral and antitumor functions. Curr Opin Immuol. 2003;15:533–543. doi: 10.1016/s0952-7915(03)00107-9. [DOI] [PubMed] [Google Scholar]
- van Houdt IS, Oudejans JJ, van den Eertwegh AJ, Baars A, Vos W, Bladergroen BA, Rimoldi D, Muris JJ, Hooijberg E, Gundy CM, et al. Expression of the apoptosis inhibitor protease inhibitor 9 predicts clinical outcome in vaccinated patients with stage III and IV melanoma. Clin Cancer Res. 2005;11:6400–6407. doi: 10.1158/1078-0432.CCR-05-0306. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993;82:3610–3615. [PubMed] [Google Scholar]
- Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- Wang J, Sakariassen PØ, Tsinkalovsky O, Immervoll H, Bøe SO, Svendsen A, Prestegarden L, Røsland G, Thorsen F, Stuhr L, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- Wulf GG, Wang RY, Kuehnle I, Weidner D, Marini F, Brenner MK, Andreeff M, Goodell MA. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98:1166–1173. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nabeshima Y. A Vasculature-ssociated Niche for Undifferentiated Spermatogonia in the Mouse Testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- Younes M, Brown RW, Mody DR, Fernandez L, Laucirica R. GLUT1 expression in human breast carcinoma: correlation with known prognostic markers. Anticancer Res. 1995;15:2895–2898. [PubMed] [Google Scholar]
- Young JL, Sukhova GK, Foster D, Kisiel W, Libby P, Schonbeck U. The serpin proteinase inhibitor 9 is an endogenous inhibitor of interleukin 1beta-converting enzyme (caspase-1) activity in human vascular smooth muscle cells. J Exp Med. 2000;191:1535–1544. doi: 10.1084/jem.191.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Shen G, Yang X, Liu W. Most C6 cells are cancer stem cells: evidence from clonal and population analyses. Cancer Res. 2007;67:3691–3697. doi: 10.1158/0008-5472.CAN-06-3912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


