Abstract
Background/Aims
To compare the intraocular pressure (IOP)-lowering efficacy of travoprost 0.004%/timolol 0.5% in fixed combination with the unfixed combination of latanoprost 0.005% and timolol 0.5% in open-angle glaucoma or ocular hypertension patients with IOP levels below 18 mmHg on the unfixed combination of latanoprost 0.005% and timolol 0.5%.
Methods
Following a 30-day open-label run-in with latanoprost QD PM and timolol QD AM, subjects with intraocular pressure below 18 mmHg were randomized to continue concomitant latanoprost QD PM and timolol QD AM or switch to travoprost 0.004%/timolol 0.5% QD AM and vehicle QD PM in masked fashion and were followed for 3 months. The primary efficacy endpoint was mean IOP reduction from baseline.
Results
There were no clinically relevant or statistically significant differences in mean IOP, mean IOP change from baseline, or percentage IOP change from baseline between the two treatment groups. Between-group differences in mean IOP were within ±0.3 mmHg at all time points (p ≥ 0.384), and between-group differences in mean IOP change from baseline were within ±0.4 mmHg at all time points. Overall, 88% of patients whose IOP was less than 18 mmHg on the unfixed combination of latanoprost and timolol remained well controlled on the same regimen in the masked portion of the study, compared with 92% who remained well controlled after switching to travoprost/timolol.
Conclusion
Travoprost 0.004%/timolol 0.5% administered once daily and concomitant administration of timolol 0.5% and latanoprost 0.005% produce similar maintenance of IOP-lowering effect in patients who were previously well controlled on concomitant administration of latanoprost and timolol. Patients who are well controlled on latanoprost and timolol concomitant therapy can be switched to once-daily therapy with travoprost 0.004%/timolol 0.5% with no expected compromise in the safety and efficacy of their treatment.
Keywords: travoprost, timolol, glaucoma, intraocular pressure, fixed combination
Introduction
Elevated intraocular pressure (IOP) is the only known modifiable risk factor for the development and progression of glaucoma. Reduction of IOP has been shown to prevent both the development (Kass et al 2002) and the progression (Collaborative Normal-Tension Glaucoma Study Group 1998; Heijl et al 2002) of glaucoma. In the European Union (EU) and elsewhere, the prostaglandin analogue class of IOP-lowering drugs has become the most commonly used first-line drug class for the treatment of elevated IOP in patients with open-angle glaucoma or ocular hypertension (Schwartz and Budenz 2004). Many patients will require more than one medication to achieve IOP targets (Kass et al 2002), and beta-blockers are commonly used as adjunctive therapy to prostaglandin analogues in patients requiring a multi-drug regimen.
Numerous manufacturers have developed fixed combinations of prostaglandin analogues and timolol 0.5%. The travoprost 0.004%/timolol 0.5% fixed combination (Alcon, Ft. Worth) has been shown in clinical trials to be equally safe and efficacious as concomitant therapy with timolol in the morning and travoprost at night (Schuman et al 2005), and to have greater efficacy than either travoprost or timolol monotherapy (Schuman et al 2005; Barnebey et al 2005). The purpose of this study was to compare the safety and efficacy of the fixed combination travoprost 0.004%/timolol 0.5% versus therapy with the unfixed combination of latanoprost 0.005% (Xalatan®, Pfizer, New York, NY) and timolol 0.5%, each dosed once daily (QD), in eyes with IOP less than 18 mmHg on unfixed latanoprost and timolol therapy.
Subjects and methods
This was a prospective, multicenter, double-masked, active-controlled, parallel-group, randomized clinical trial conducted at 20 sites in the United States. The study was reviewed and approved by the appropriate institutional review board at all participating sites, all participating subjects provided written informed consent to participate, and the study was conducted in full compliance with all tenets of the Declaration of Helsinki.
Subjects
Eligible subjects were adults 18 years of age or older diagnosed with open-angle glaucoma (including pigmentary or pseudoexfoliation glaucoma) or ocular hypertension. Subjects must have used both latanoprost 0.005% once daily and timolol 0.5% either once or twice daily in both eyes for a minimum of 30 days before the screening visit, with an IOP measurement below 18 mmHg at the screening visit. (All IOP values in this study represent the mean of two consecutive measurements if within 4 mmHg; otherwise a third measurement was obtained and the value was calculated as the mean of the two measurements closest to each other or of all three if equally spaced.) Subjects were excluded if they met any of the following criteria: females of childbearing potential who were pregnant, planned to become pregnant, were breastfeeding, or were not using a highly effective method of birth control; history of recurrent severe ocular inflammatory disease, clinically significant or progressive retinal disease, severe ocular pathology precluding the use of an ocular prostaglandin, or ocular anomaly preventing accurate applanation tonometry; history of ocular trauma or intraocular surgery within the past 6 months; or ocular infection or inflammation or ocular laser surgery within the past 3 months. In addition, subjects were excluded if they had best-corrected visual acuity worse than 0.6 logMAR in either eye; extremely narrow or closed iridocorneal angles; cup-to-disc ratio greater than 0.8; central visual field loss; the need for additional IOP-lowering therapy or any form of glucocorticoid therapy during the study period; or a history of cardiac, pulmonary, hepatic or renal disease precluding the use of a topical beta-blocker.
Methods
Medical and ocular history were obtained, and visual acuity, slit-lamp examination, Goldmann tonometry, gonioscopy, automated perimetry, and dilated fundus examination were performed at a Screening visit to establish eligibility. Eligible subjects with IOP below 18 mmHg following at least 30 days of treatment with latanoprost 0.005% (QD PM) and timolol 0.5% (either QD AM or BID) continued to use latanoprost QD PM and timolol 0.5% QD AM in both eyes for an additional 30 day open-label run-in and then returned for an Eligibility visit. At this visit, Goldmann applanation was performed at 8 AM, 10 AM, 4 PM, and 8 PM (with timolol 0.5% being instilled 15 minutes following the 8 AM measurement). Subjects with mean IOP below 18 mmHg in both eyes at all time points were randomized in a 1:1 ratio to receive either the fixed combination of travoprost 0.004%/timolol 0.5% once daily in both eyes at 8 AM, or to receive timolol 0.5% at 8 AM and latanoprost 0.005% at 8 PM in both eyes. All subjects were given two masked bottles of study medication, one labeled “Morning” and one labeled “Evening”. Subjects randomized to the travoprost/timolol treatment group were given “Morning” bottles containing the fixed combination of travoprost 0.004%/timolol 0.5% and “Evening” bottles containing vehicle. Subjects randomized to the concomitant latanoprost and timolol treatment group were given “Morning” bottles containing timolol 0.5% and “Evening” bottles containing latanoprost 0.005%. Subjects were instructed to instill one drop from the “Morning” bottle at 8 AM and one drop from the “Evening” bottle at 8 PM every day. Subjects were then seen at Weeks 2 and 6 and Month 3 after randomization.
At the Week 2 visit, Goldmann tonometry was performed at 8 AM before instilling the “Morning” medication. The “Morning” dose was instilled 15 minutes later, and subjects were instructed to continue dosing from the “Morning” and “Evening” bottles. The Week 6 visit was identical to the Week 2 visit. At the Month 3 visit, Goldmann tonometry was performed at 8 AM (with “Morning” dosing 15 minutes later), 10 AM, 4 PM, and 8 PM. Following the 8 PM IOP measurement, a dilated fundus examination was performed, after which subjects exited the study. Subjects were queried regarding adverse events at all three on-treatment visits.
Statistical analysis
The primary statistical objective of this study was to compare the IOP-lowering efficacy of the fixed combination travoprost 0.004%/timolol 0.5% to the concomitant administration of latanoprost 0.005% and timolol 0.5%. The primary efficacy parameter was mean IOP change from baseline at Weeks 2 and 6 and Month 3. One eye per patient was included in the analysis, selected as follows: the eye with the higher IOP at 8 AM on the eligibility visit, or at 10 AM if equal, or at 4 PM if equal, or at 8 PM if equal, or the right eye if equal. The safety analysis included all subjects who received any study medication; the intent-to-treat analysis included all subjects who completed at least one on-therapy visit; and the per-protocol analysis included all patients in the intent-to-treat analysis who satisfied all protocol criteria.
The primary analysis consisted of a descriptive summary of IOP change from baseline for each treatment group. A paired t-test was used to further describe the change from baseline at each time point. Differences in mean IOP, mean IOP change from baseline, and percent IOP change from baseline between the two treatment groups were evaluated using a two-sample t-test. The experimental hypotheses were that there were no differences between treatment groups at each of the time points. Sample size was calculated before initiation of the study. With 68 evaluable subjects per group, mean IOP change from baseline could be estimated to within ±0.9 mmHg based on the width of a 95% confidence interval, with an assumed standard deviation for IOP change of 3.5 mmHg and using a t-statistic (α = 0.05). Similarly, the difference between treatment groups for mean IOP change could be estimated to within ±1.2 mmHg using a t-statistic (α = 0.05).
Results
Overall, 156 subjects were enrolled in this study and were included in the safety analysis; seven subjects were excluded from the intent-to-treat analysis, leaving 149 evaluable subjects; and 21 were excluded from the per-protocol analysis, leaving 135 evaluable subjects. Efficacy data in this report are based on the intent-to-treat analysis, as there were no significant differences between the intent-to-treat and the per protocol analyses. The mean age of subjects was 66.9 years; 56% were female; and the ethnic make-up was 60% Caucasian, 28% African-American, 9% Hispanic, and 3% Asian. These demographics were not statistically different between treatment groups.
Mean IOPs at baseline and at each on-therapy time point for both treatment groups are given in Table 1. Between-group differences in mean IOP were within ±0.3 mmHg at all baseline and on-therapy time points, and were not statistically significant at any time point.
Table 1.
Mean IOP (mmHg) at all time points for both treatment groups
| Treatment | Baseline
|
Week 2
|
Week 6
|
Month 3
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 8 AM | 10 AM | 4 PM | 8 PM | 8 AM | 8 AM | 8 AM | 10 AM | 4 PM | 8 PM | |
| Travoprost 0.004%/timolol 0.5% | 15.4 | 14.6 | 14.5 | 14.4 | 15.3 | 15.4 | 15.5 | 15.1 | 15.1 | 14.7 |
| Latanoprost 0.005% + timolol 0.5% | 15.4 | 14.7 | 14.6 | 14.7 | 15.4 | 15.6 | 15.5 | 14.8 | 14.9 | 14.8 |
| Difference | −0.0 | −0.1 | −0.1 | −0.3 | −0.1 | −0.3 | 0.1 | 0.3 | 0.2 | −0.1 |
| P-value* | 0.9773 | 0.8606 | 0.8637 | 0.3839 | 0.8573 | 0.5422 | 0.8783 | 0.4497 | 0.6831 | 0.7982 |
P-value from two sample t-test.
Mean IOP values and associated differences have been rounded to one decimal place for presentation.
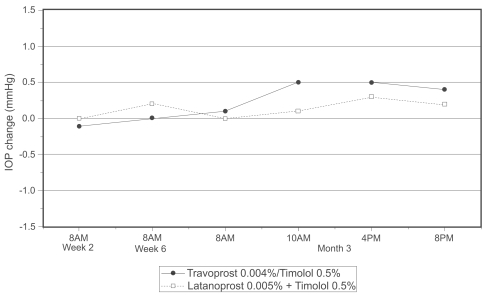
Mean IOP changes from baseline are given in Table 2. Subjects randomized to continue treatment with concomitant latanoprost and timolol demonstrated mean IOP changes within ±0.3 mmHg compared with baseline. Subjects randomized to switch from latanoprost and timolol concomitant therapy to travoprost/timolol fixed combination therapy demonstrated mean IOP changes within ±0.5 mmHg compared with baseline. The between-group differences in mean IOP change were within ±0.4 mmHg at all time points and were not statistically significant at any time point (Figure 1).
Table 2.
Mean IOP change from baseline (mmHg) and mean percent IOP change from baseline
| Treatment | Week 2
|
Week 6
|
Month 3
|
|||
|---|---|---|---|---|---|---|
| 8 AM | 8 AM | 8 AM | 10 AM | 4 PM | 8 PM | |
| Travoprost 0.004%/timolol 0.5% | ||||||
| Mean IOP change | −0.1 | −0.0 | 0.1 | 0.5 | 0.5 | 0.4 |
| Mean % IOP change | 0.0 | 0.3 | 1.2 | 4.6 | 4.5 | 3.4 |
| N | 73 | 73 | 73 | 71 | 71 | 71 |
| Latanoprost 0.005% + timolol 0.5% | ||||||
| Mean IOP change | −0.0 | 0.2 | 0.0 | 0.1 | 0.3 | 0.2 |
| Mean % IOP change | −0.2 | 1.3 | 0.3 | 0.9 | 2.6 | 2.0 |
| N | 75 | 75 | 75 | 73 | 73 | 73 |
Estimates based on descriptive statistics.
Mean IOP values and associated differences have been rounded to one decimal place for presentation.
Figure 1.
Difference in mean IOP change from baseline between travoprost/timolol and latanoprost + timolol groups.
Mean percentage IOP change from baseline was also similar between groups (Table 2). Subjects randomized to the travoprost/timolol fixed combination maintained stable IOP levels when switched from concomitant latanoprost and timolol, with mean percentage IOP changes ≤4.6% across all on-therapy time points. Subjects randomized to continue on concomitant latanoprost and timolol therapy also maintained stable IOP levels, with mean percentage IOP changes ≤2.6% across all on-therapy time points. The between-group differences in mean percentage IOP change were within ±3.7% at all time points and were not statistically significant at any time point.
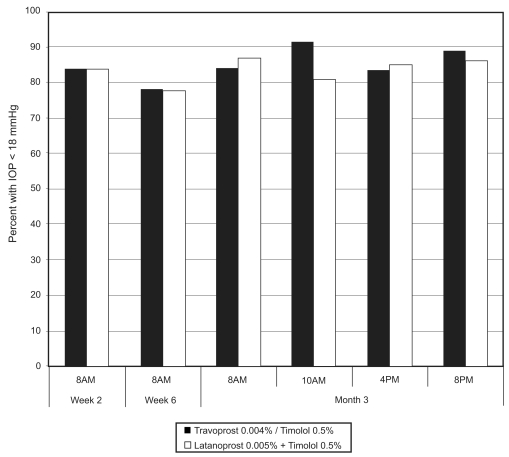
All subjects had mean IOP below 18 mmHg following the 30-day run-in with concomitant latanoprost and timolol therapy, with up to 92% of subjects in the travoprost 0.004%/timolol 0.5% treatment group and up to 88% of subjects in the unfixed latanoprost and timolol treatment group maintaining mean IOP less than 18 mmHg after randomization (Figure 2). The percentages of patients who maintained IOP below 18 mmHg at all on-therapy visits were 59% and 56%, respectively, for the fixed travoprost 0.004%/timolol 0.5% and unfixed latanoprost and timolol treatment groups (p = 0.72).
Figure 2.
Percentage of patients who maintained IOP less than 18 mmHg at each study visit.
Both treatments were well tolerated by subjects; adverse events (AEs) were predominately nonserious, generally mild or moderate in intensity, and no subject discontinued the study due to an AE. AEs were reported by 30.4% (24/79) of the subjects in the travoprost/timolol group and by 40.3% (31/77) of the subjects in the latanoprost and timolol concomitant group. The most frequently reported treatment-related AEs in the travoprost 0.004%/timolol 0.5% group were ocular hyperemia, ocular discomfort, ocular pruritus, and blurred vision (incidence 2.5% each), while the most frequently reported treatment-related AE in the latanoprost and timolol group was ocular hyperemia (incidence 2.6%). There were no clinically relevant differences between the treatment groups in the overall safety population. Five serious adverse events were reported but all were assessed as unrelated to the study drug.
Discussion
The travoprost 0.004%/timolol 0.5% fixed combination dosed once daily in the morning provided similar IOP control to concomitant therapy with timolol 0.5% dosed once daily in the morning and latanoprost 0.005% dosed once daily in the evening. Subjects who were well controlled (mean IOP <18 mmHg) on concomitant latanoprost and timolol maintained comparable IOP control when switched to once-daily treatment with the travoprost/timolol fixed combination. These findings are consistent with prior reports demonstrating similar IOP-lowering efficacy (Netland et al 2001; Parrish et al 2003) and circadian IOP control (Orzalesi et al 2006) between travoprost and latanoprost.
In the present study, the travoprost/timolol fixed combination was dosed in the morning. Several studies have demonstrated that morning versus evening dosing of travoprost provides equivalent mean 24-hour IOP reduction (Denis et al 2006; Konstas et al 2006).
The results of this study support the conclusion that patients with open-angle glaucoma or ocular hypertension controlled on unfixed latanoprost and timolol therapy are likely to be equally well controlled on once-daily travoprost/timolol in fixed combination. Up to 92% of subjects in the travoprost/timolol group and up to 88% of subjects in the unfixed latanoprost and timolol group maintained IOP below 18 mmHg after randomization, with 59% and 56%, respectively, demonstrating IOP below 18 mmHg at all on-therapy visits.
Switching from unfixed latanoprost and timolol therapy to travoprost/timolol fixed combination therapy represents two important regimen changes: a reduction in the number of medication bottles in the regimen, and a reduction in the number of drops per day. A regimen with fewer medications equates to fewer co-payments or lower overall drug costs for patients without prescription drug insurance. Fewer drops per day reduces the complexity of the therapeutic regimen, which is likely to improve adherence (Patel and Spaeth 1995). Fixed combination therapy also reduces the washout effect whereby rapid sequential instillation of multiple eye drop medications reduces absorption of the earlier drops due to washout by the later drops (Chrai et al 1974). Furthermore, administration of two medications in a single drop minimizes exposure of the ocular surface to other drop components, including the preservative benzalkonium chloride, which has been linked to ocular surface changes (de Jong et al 1994), dry eye symptoms ( Pisella et al 2002; Mundorf et al 2003) and chronic subclinical conjunctival inflammation (Broadway et al 1994a; Noecker et al 2004) that may reduce the success of eventual glaucoma filtration surgery (Lavin et al 1990; Broadway et al 1993, 1994b; Baudouin 1996).
The safety of the travoprost/timolol fixed combination has been established in large, multicenter, Phase III clinical trials (Barnebey et al 2005; Hughes et al 2005; Schuman et al 2005). In the present study, the travoprost/timolol fixed combination was found to have comparable safety to the concomitant use of latanoprost and timolol. This is expected given that the nature of side effects between the various prostaglandin agents has been comparable, with only minor differences in the relative frequency of these side effects (Netland et al 2001; Parrish et al 2003).
In conclusion, subjects with open-angle glaucoma or ocular hypertension with IOP <18 mmHg on concomitant therapy with latanoprost 0.005% in the evening and timolol 0.5% in the morning can be safety switched to once-daily dosing with the fixed combination travoprost 0.004%/timolol 0.5% with no statistically significant or clinically relevant change in IOP control and no additional safety risks.
Acknowledgments
This study was funded by Alcon Laboratories, Inc, Ft. Worth, Texas, USA.
Appendix: Study Group
Stanley Berke, Edward Burney, Anastasios Costarides, Douglas Day, Harvey DuBiner, Richard Evans, Kevin Greenidge, J Charles Henry, Alexander Kent, Michael Kottler, Bradley Kwapiszeski, Christopher Lin, Stephen Lin, Jeffrey Lozier, James Peace, Douglas Rhee, Kenneth Sall, Howard Schenker, Mark Weiss.
References
- Barnebey HS, Orengo-Nania S, Flowers BE, et al. The safety and efficacy of travoprost 0.004%/timolol 0.5% fixed combination ophthalmic solution. Am J Ophthalmol. 2005;140:1–7. doi: 10.1016/j.ajo.2005.02.043. [DOI] [PubMed] [Google Scholar]
- Baudouin C. Side effects of antiglaucomatous drugs on the ocular surface. Curr Opin Ophthalmol. 1996;7:80–6. doi: 10.1097/00055735-199604000-00014. [DOI] [PubMed] [Google Scholar]
- Broadway D, Grierson I, Hitchings R. Adverse effects of topical antiglaucomatous medications on the conjunctiva. Br J Ophthalmol. 1993;77:590–6. doi: 10.1136/bjo.77.9.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadway DC, Grierson I, O’Brien C, et al. Adverse effects of topical antiglaucoma medication. I. The conjunctival cell profile. Arch Ophthalmol. 1994;112:1437–45. doi: 10.1001/archopht.1994.01090230051020. [DOI] [PubMed] [Google Scholar]
- Broadway DC, Grierson I, O’Brien C, et al. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112:1446–54. doi: 10.1001/archopht.1994.01090230060021. [DOI] [PubMed] [Google Scholar]
- Chrai SS, Makoid MC, Eriksen SP, et al. Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J Pharm Sci. 1974;63:333–8. doi: 10.1002/jps.2600630304. [DOI] [PubMed] [Google Scholar]
- Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–97. doi: 10.1016/s0002-9394(98)00223-2. Erratum in: Am J Ophthalmol, 1999;127:120. [DOI] [PubMed] [Google Scholar]
- de Jong C, Stolwijk T, Kuppens E, et al. Topical timolol with and without benzalkonium chloride: epithelial permeability and autofluorescence of the cornea in glaucoma. Graefes Arch Clin Exp Ophthalmol. 1994;232:221–4. doi: 10.1007/BF00184009. [DOI] [PubMed] [Google Scholar]
- Denis P, Andrew R, Wells D, et al. A comparison of morning and evening instillation of a combination travoprost 0.004%/timolol 0.5% ophthalmic solution. Eur J Ophthalmol. 2006;16:407–15. [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- Hughes BA, Bacharach J, Craven ER, et al. A three-month, multi-center, double-masked study of the safety and efficacy of travoprost 0.004%/timolol 0.5% ophthalmic solution compared to travoprost 0.004% ophthalmic solution and timolol 0.5% dosed concomitantly in subjects with open angle glaucoma or ocular hypertension. J Glaucoma. 2005;14:392–9. doi: 10.1097/01.ijg.0000176935.08392.14. [DOI] [PubMed] [Google Scholar]
- Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- Konstas AG, Mikropoulos D, Kaltsos K, et al. 24-hour intraocular pressure control obtained with evening- versus morning-dosed travoprost in primary open-angle glaucoma. Ophthalmology. 2006;113:446–50. doi: 10.1016/j.ophtha.2005.10.053. [DOI] [PubMed] [Google Scholar]
- Lavin MJ, Wormald RP, Migdal CS, et al. The influence of prior therapy on the success of trabeculectomy. Arch Ophthalmol. 1990;108:1543–8. doi: 10.1001/archopht.1990.01070130045027. [DOI] [PubMed] [Google Scholar]
- Mundorf T, Wilcox KA, Ousler GW, et al. Evaluation of the comfort of Alphagan P compared with Alphagan in irritated eyes. Adv Ther. 2003;20:329–36. doi: 10.1007/BF02849799. [DOI] [PubMed] [Google Scholar]
- Netland PA, Landry T, Sullivan EK, et al. Travoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2001;132:472–84. doi: 10.1016/s0002-9394(01)01177-1. [DOI] [PubMed] [Google Scholar]
- Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004;23:490–6. doi: 10.1097/01.ico.0000116526.57227.82. [DOI] [PubMed] [Google Scholar]
- Orzalesi N, Rossetti L, Bottoli A, et al. Comparison of the effects of latanoprost, travoprost, and bimatoprost on circadian intraocular pressure in patients with glaucoma or ocular hypertension. Ophthalmology. 2006;113:239–46. doi: 10.1016/j.ophtha.2005.10.045. [DOI] [PubMed] [Google Scholar]
- Parrish RK, Palmberg P, Sheu WP. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003;135:688–703. doi: 10.1016/s0002-9394(03)00098-9. [DOI] [PubMed] [Google Scholar]
- Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg. 1995;26:233–6. [PubMed] [Google Scholar]
- Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86:418–23. doi: 10.1136/bjo.86.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman JS, Katz GJ, Lewis RA, et al. Efficacy and safety of a fixed combination of travoprost 0.004%/timolol 0.5% ophthalmic solution once daily for open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2005;140:242–50. doi: 10.1016/j.ajo.2005.02.058. [DOI] [PubMed] [Google Scholar]
- Schwartz K, Budenz D. Current management of glaucoma. Curr Opin Ophthalmol. 2004;15:119–26. doi: 10.1097/00055735-200404000-00011. [DOI] [PubMed] [Google Scholar]