Abstract
Severe damage to cell repair mechanisms of the limbal region can lead to many disorders such as vascularized conjunctivalization, keratinization, corneal scarring, and corneal opacification, collectively described as limbal stem cell deficiency (LSCD). Limbal stem cell deficiency may occur as a result of depletion of stem cells or destruction of their stromal niche. In such cases, apart from conventional corneal transplantation, limbal stem cell transplantation would be needed to restore vision. Limbal stem cells may be replenished by autologous limbal transplants from the healthy fellow eye in unilateral cases, and allografts from living related donors or cadaveric donors in bilateral cases. The induction of iatrogenic LSCD and its sequelae in donor eyes have motivated researchers to cultivate sheets of limbal epithelium ex vivo, from small fragments of donor tissue for the purpose of ocular surface reconstruction.
Keywords: cultivated limbal transplantation, stem cell deficiency
Introduction
The integrity of the self-renewing corneal epithelium relies on the existence of stem cells, which are located in the limbal basal layer (Sun and Green 1977). These limbal stem cells are supported by a unique stromal microenvironment: the stem cell niche (Watt and Hogan 2000). Destructive loss of limbal stem cells or dysfunction of their stromal niche causes what is clinically referred to as limbal stem cell deficiency, characterized by conjunctivalization of the cornea, vascularization, chronic inflammation, and persistent epithelial defects (Dua et al 1994). Severe ocular surface disease resulting from LSCD as in chemical injury, Stevens-Johnson syndrome (SJS) and ocular cicatricial pemphigoid are devastating conditions that represent a major clinical challenge. Conventional corneal transplants alone in these conditions are destined to fail. Ocular surface reconstruction by means of amniotic membrane transplantation and limbal transplantation has been effective to some extent. More recently cultivated limbal stem cell transplantation, which is the focus of this review, has been shown to be a promising treatment modality in the management of severe limbal stem cell deficiency (Pellegrini et al 1997; Koizumi et al 2001b; Sangwan et al 2006).
Limbal stem cells
Stem cells are defined by their capacity of unlimited or prolonged self-renewal that can produce at least one type of highly differentiated progeny (Grueterich et al 2003). The stem cells of the corneal epithelium are located in the limbal basal layer and are the ultimate source of corneal epithelial renewal (Sun and Green 1977). Cotsarelis and colleagues (1989) confirmed the presence of a small subpopulation of slow-cycling limbal basal stem cells that had a significant reserve capacity and proliferative response to wounding.
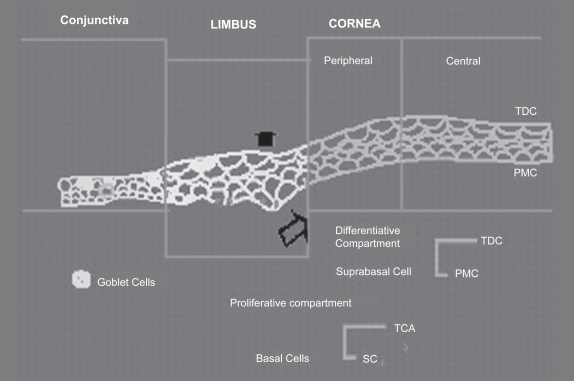
Limbal stem cells have a long life span with a slow cell cycle with low mitotic activity under the normal steady state. Limbal stem cells are supported by a unique stromal microenvironment called the stem cell niche, which consists of certain extracellular matrix components, cell membrane associated molecules, and cytokine dialogues (Watt and Hogan 2000). They may be activated on demand for tissue regeneration to increase their own population or to differentiate into transient amplifying cells (TAC), which are located in the corneal basal epithelium (Figure 1). On the contrary, TAC have a limited proliferating capacity with a short life span and are rapid cycling (Lehrer et al 1998).
Figure 1.
Schematic diagram explaining the steps involved in proliferation and differentiation of limbal stem cells.
Abbreviations: PMC, post-mitotic cells; SC, stem cells; TAC, transient amplifying cells; TDC, Terminally differentiated cells.
The limbal basal epithelial cells express the keratin pair K5/K14, like most of the undifferentiated cells of the stratified epithelia but are devoid of the cornea-specific keratin pair K3/K12 (Schermer et al 1986).
Limbal stem cell deficiency
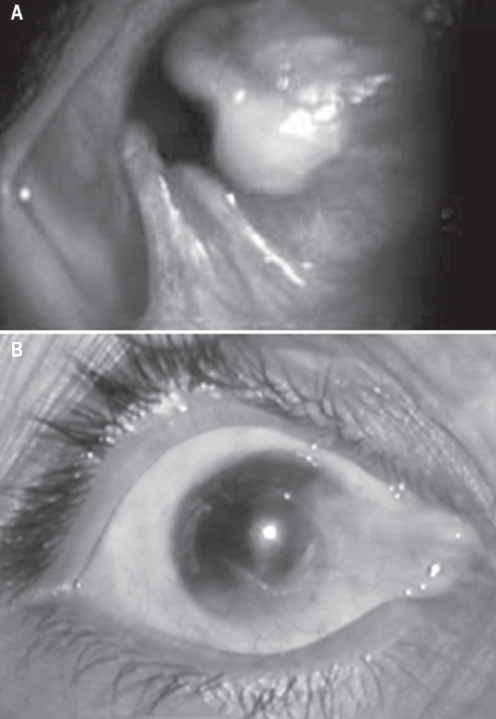
Limbal stem cells or the niche in which they reside can be compromised by a variety of insults leading to what is clinically known as limbal stem cell deficiency (LSCD). LSCD is characterized by conjunctivalization of the cornea (conjunctival epithelial ingrowth), vascularization, chronic inflammation, and poor epithelial integrity that manifests as irregular corneal surface, recurrent erosion, persistent epithelial defects and fibrous ingrowth (Figure 2) (Anderson et al 2001).
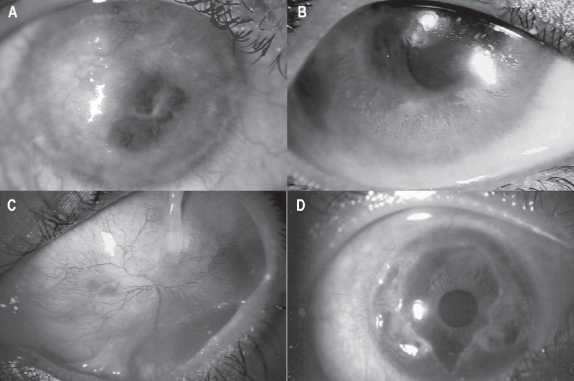
Figure 2.
Slit lamp photo showing causes of limbal stem cell deficiency (LSCD). A: Corneal pannus due to vernal keratoconjunctivitis (VKC). B: Dysfunction of limbal stem cell in a patient with VKC. C: Total stem cell deficiency with conjunctivalisation and multiple symblephara in a patient with chemical injury. D: Peripheral corneal destruction due to Mooren’s ulcer which leads to limbal stem cell deficiency over a period of time.
Limbal stem-cell deficiency can be primary, related to an insufficient stromal microenvironment to support stem cell function, such as aniridia, congenital erythrokerato-dermia, keratitis associated with multiple endocrine deficiencies, neurotrophic (neural and ischaemic) keratopathy and chronic limbitis; or secondary (more common) related to external factors that destroy limbal stem cells such as chemical (most common) or thermal injuries, SJS, ocular cicatricial pemphigoid (OCP), multiple surgeries or cryotherapies, contact lens wear, or extensive microbial infection (Potten and Morris 1988; Tseng 1989; Kruse 1994; Pfister 1994; Dua 1995). Corneal stem-cell deficiency can be diffuse (total) or sectoral (partial) (Figure 3). In the latter case conjunctivalisation of the corneal epithelium affects only part of the corneal surface. In some patients, limbal deficiency may be subclinical at the time of the insult, and may eventually progress to an overt stage of limbal deficiency as the stem cell population depletes further, over time (Dua et al 1994). If the conjunctival stem cells (presumed to be in the fornices) are also depleted, which is rare because of the larger area they occupy the ocular surface is eventually keratinised, which sometimes may be seen at the end stage of OCP or SJS (Coster et al 1995).
Figure 3.
Slit lamp photo of total (A) and partial (B) limbal stem cell deficiency due to chemical injury.
Corneal stem cell deficiency can be best confirmed histologically by the use of impression cytology, which can detect goblet cells containing conjunctival epithelium on the corneal surface (Sangwan 2001). Immunohisto-chemically, the absence of keratin CK3, and the presence of mucin in goblet cells, has been shown by monoclonal antibodies (Kenyon et al 1990). Diagnosis of limbal stem cell deficiency is crucial because patients with this abnormality generally are poor candidates for conventional corneal transplantation. Lamellar or penetrating keratoplasty provides only a temporary replacement of the host’s corneal epithelium because the grafted epithelial cells have a limited proliferative capacity and lifespan (Dua et al 2000).
Management of LSCD
Asymptomatic patients with partial and peripheral conjunctivalisation of the corneal surface may not require intervention. Corneal and conjunctival epithelial cell phenotypes have been known to co-exist on the corneal surface for prolonged periods without significant extension of the conjunctivalised area or any transdifferentiation of conjunctival epithelium into cells of corneal phenotype (Coster et al 1995; Dua 1995). If the visual axis or most of the corneal surface is covered with conjunctiva-like epithelium, mechanical debridement of conjunctival epithelium can allow adequate corneal epithelial healing to occur from the remaining intact limbal epithelium (Coster et al 1995; Dua 1995). Scraping is done with a surgical blade under topical anesthesia at the slit lamp. Any tendency of conjunctiva-like epithelium to re-encroach on to corneal surface is prevented by re-scraping (Coster et al 1995). This procedure can be employed to improve visual function and reduce symptoms even when as little as two clock hours of normal limbus and peripheral cornea remain. When visual improvement is the aim of treatment, the objective should be to achieve normal corneal epithelial cover over the visual axis. An attempt to achieve normal epithelial cover for the entire cornea, when only two clock hours or less of limbus are surviving, may stretch the capacity of the remaining limbus and could eventually lead to epithelial breakdown.
Mechanical debridement of conjunctiva-like epithelium and encouraging the denuded area to be resurfaced with corneal epithelial cells is a valid, simple and effective alternative to limbal transplantation in patients with partial limbal stem cell deficiency (see below).
Mechanical debridement can also be used to prevent migration of conjunctival epithelium on to the cornea in acute situations, with partial corneal and limbal epithelial loss. Close observation of patients with thermal, chemical or mechanical epithelial loss involving the limbus, will allow one to detect the advancing conjunctival epithelial sheet and prevent it from extending on to and beyond the limbus (Coster et al 1995). Tseng and colleagues (1998) have successfully used amniotic membrane transplantation (AMT) and mechanical debridement and advocate its use to treat patients with partial stem cell deficiency. Their study showed that with partial or focal limbal stem cell deficiency, AMT improves both the corneal surface and the vision (Tseng et al 1998).
In patients with total limbal stem cell deficiency, limbal auto- or allo-transplantation is indicated for corneal surface reconstruction. This may be combined with or followed by keratoplasty. Several modifications have been described of the technique proposed by Kenyon and Tseng (1989). All these procedures aim to transplant a new source of epithelium for a diseased ocular surface and the removal of the host’s altered corneal epithelium and pannus. After successful transplantation, the host’s cornea (or grafted cornea) will be permanently covered by epithelium from the donor limbus.
Although all techniques used in stem cell transplantation are in principle similar, the source of donor stem cells can vary. Donor tissue can be obtained from the fellow eye (limbal autograft) in cases of unilateral disease, or from a living related donor (usually gives a better tissue match), or from a cadaver donor (limbal allograft) when both eyes are affected. Limbal transplantation procedures also vary depending on the carrier tissue used for the transfer of the limbal stem cells. Carrier tissue is needed in limbal transplants because it is not possible to transfer limbal stem cells alone. Limbal transplant procedures have used either conjunctiva (conjunctival limbal graft) or cornea (keratolimbal graft) as a carrier tissue for limbal stem cells (Dua et al 2000). Tseng and colleagues (1998) used AMT associated with limbal transplantation in cases with total stem cell deficiency. In the past decade some authors have used conjunctival transplantation to treat corneal stem cell deficiency. This practice was supported by the belief that conjunctival epithelium “transdifferentiates” into cornea-like epithelium. However, conjunctival epithelial transdifferentiation (that is, acquisition of morphologic, biochemical, and physiologic transplantation properties of corneal epithelium) does not occur in humans and conjunctival transplantation to reconstruct the corneal surface in stem cell deficiency is inferior to limbal transplantation (Tseng et al 1984). Conjunctival transplantation is, however, useful in other conditions, for example, to reconstruct the conjunctival surface in cases of symblepharon and to treat primary and recurrent pterygia. Keratoepithelioplasty was proposed by Thoft as another alternative to reconstruct the ocular surface in patients with conjunctivalisation of the corneal surface (Dua et al 2000). In this technique, lenticules of peripheral corneal epithelium with superficial stroma, were grafted. A few years later the same author modified the technique to include limbal tissue, acknowledging the importance of stem cell transplantation in these conditions (Dua et al 2000).
Successful limbal transplantation can achieve rapid surface healing, stable ocular surface without recurrent erosions or persistent epithelial defects, regression of corneal vascularisation and restoration of a smooth and optically improved ocular surface, resulting in improved visual acuity and, probably, increased success for subsequent keratoplasty.
In patients with unilateral total stem cell deficiency a limbal autograft transplantation is recommended. Partial removal of the limbus from the fellow eye is believed to be relatively safe, although some cases may have compromised donor surface after partial removal of the limbal zone. The risk of epithelial problems in the donor eye is low when less than four to six clock hours of limbal tissue and a moderate amount of conjunctiva are removed.
When patients have bilateral total ocular surface disease, allograft transplantation becomes necessary. If living relatives are potential donors, an HLA-matched tissue is preferred. When cadaver donor tissue is used, “fresh” eyes are preferred because the success of the procedure depends on the transplantation of healthy limbal stem cells. Whole eyes are convenient because they provide better stabilisation during dissection of the limbal sclerocorneal rim.
In limbal allografts the surface disorder can recur if there is immunological destruction of the transplanted limbal stem cells. A high rate of immune reactions can be expected because of the high immunogenic stimulus of the limbal transplant, related to the relative abundance of Langerhans cells and HLA-DR antigens. They play an important role in the afferent arm of allograft rejection, and effective immunosuppression is considered essential for at least 12 months after surgery when non-HLA matched limbal allografts are used. In some instances permanent systemic immunosuppression may be needed. Oral cyclosporine A is the most commonly used agent (Tseng et al 1995). In addition to oral cyclosporine, Tsubota et al also used topical cyclosporine (0.05%) and high dose intravenous dexamethasone in their patients (Tsubota et al 1995). The use of FK 506 (tacrolimus; Fujisawa Pharmaceutical Co. Ltd., Osaka, Japan), a new immunosuppressive agent from the fermentation broth of Streptomyces tsukabaensis, for immunosuppression in limbal or corneal allografts has been recently reported by Dua and Blanco (1999). Limbal rejection can be suspected with the development of inflammation and/or acute or chronic severe surface abnormalities. Daya and colleagues (1999) and Dua and Blanco (1999) have reported the clinical features of limbal allograft rejection. In their study, the clinical features of limbal allograft rejection varied according to the presentation, whether acute or chronic, of the rejection episode. Acute rejection was associated with intense sector injection at the limbus, edema, and infiltration of the lenticule, punctate keratopathy and epithelial defects. In low-grade rejection there was mild diffuse or perilimbal injection, elevated perilimbal area, punctate epithelial keratopathy and epithelial irregularity (Daya et al 1999).
Cultivated limbal stem cell transplantation
Kenyon and Tseng (1989) proposed the procedure of conjunctival limbal autograft taken from the healthy fellow eye in patients with unilateral total LSCD. The procedure involves the transplantation of two large free grafts, each spanning from 5–7 mm in limbal arc length, removed from a healthy eye (Kenyon and Tseng 1989). Reported complications in donor eyes have included localized haze in a patient with contact lens-induced keratopathy, pseudopterygium, filamentary keratitis, microperforation during surgery, abnormal epithelium, and corneal depression. Such a concern preludes one from obtaining two such grafts from a donor eye with undiagnosed partial LSCD. Ex vivo expansion of limbal epithelial stem cells has been developed to circumvent potential complications related to conjunctival limbal autograft transplantation. The aim of differentiating these epithelial stem cells ex vivo is to help develop an equivalent of the human cornea using tissue culture techniques. Transplantation of cultivated limbal epithelium is currently the most successful alternative to surface reconstruction in patients with unilateral disease and offers a therapeutic chance to patients with severe bilateral disease (Pellegrini et al 1997; Koizumi et al 2001b; Sangwan et al 2006).
The various protocols for cultivation of limbal epithelium differ in the use of intact versus epithelially denuded amniotic membrane, suspension of epithelial cells rather than explants, co-cultivation of 3T3 fibroblast feeder layers, and air-lifting prior to transplantation. Currently no study has been conducted to compare these cultivation variables in order to determine which one is vital in achieving effective expansion of limbal epithelial progenitor cells (Schwab 1999; Grueterich and Tseng 2002a; Grueterich et al 2002b, 2002c; Koizumi et al 2001a, 2002; Meller et al 2002; Espana et al 2003).
Pellegrini and colleagues (1997) first reported successful reconstruction of the ocular surface in LSCD. A 1 mm2 biopsy sample from the healthy fellow eye was treated with trypsin for 3 hrs. The cells were plated on lethally irradiated 3T3-J2 cells and cultured in 5% carbon dioxide in Dulbecco-Vogt Eagle’s and Ham’s F12 media. Grafts were prepared from confluent secondary cultures which were released from the plastic dish by neutral protease Dispase II and either mounted on a petrolatum gauze or a soft contact lens (Pellegrini et al 1997).
The next advancement in this technique was the use of human amniotic membrane as a substrate for in vitro epithelial cell culture. Koizumi and colleagues (2000a) first cultivated rabbit limbal epithelium on human amniotic membrane (HAM) in vitro and then, after transplantation onto rabbit ocular surface, confirmed the viability of the transplanted cultivated epithelium in vivo. Subsequently they showed that denuded HAM was better than intact cellular HAM for corneal epithelial cell culture (Koizumi et al 2000b). They adopted the culture system using epithelially denuded HAM with an underlying layer of lethally irradiated mouse 3T3 fibroblasts. Their results showed that the expanded epithelium resembled a corneal phenotype with respect to the expression of K3 keratin (Koizumi et al 2001a, 2001b).
Grueterich and colleagues (2002b) demonstrated that culturing limbal explants on an intact amniotic membrane without the use of a 3T3 feeder layer resulted in a limbal epithelial phenotype.
Koizumi and colleagues (2002) demonstrated that both cell suspension and explant culture methods produced a healthy epithelial cell layer, with cells from the former being morphologically more superior.
Currently most investigators prefer the explant culture technique (Tsai et al 2000; Grueterich et al 2002c; Koizumi et al 2000c, 2001b; Shimazaki et al 2002). The benefits of using explants are that they are easy to prepare and there is no danger of damaging the corneal epithelium through enzyme treatment.
The general principles of culturing the cells by explant culture technique involve the following steps: harvesting the limbal tissue (from the contralateral healthy eye in case of a unilateral LSCD, or from donor corneas for bilateral LSCD); selecting the appropriate carrier: a sheet of multi-layered epithelium, human amniotic membrane, collagen shields, or contact lens; preparation of human corneal epithelial medium; and explant cultures (Sangwan 2001).
Confirmation of growth can be done by various methods including-direct observation, whole mount stained preparation, histopathology, immunohistochemistry, thymidine incorporation and by flow cytometry using markers for cell cycle. Details of a few of the procedures can be found in an earlier publication (Vemuganti and Balasubramanian 2002).
Although a number of investigators have included various reconstruction techniques through the use of autogenous conjunctiva, mucous membrane grafts, collagen lattices, synthetic implants, and cell-suspension cultures, the most widely accepted universal substratum for explant cultures is the HAM (Grueterich et al 2003). It was recognized and successfully reintroduced by Kim and Tseng (1995) for corneal surface reconstruction in rabbits. Amniotic membrane is the innermost layer of the fetal or placental membrane and consists of an epithelial monolayer, a thick basement membrane, and an avascular stroma. Preserved HAM can be used a biological substrate without viable and active proliferative cells. It is thus nonimmunogenic and therefore does not require immunosupression when used as a graft for transplantation.
Amniotic membrane serving as stem cell niche
The role of the devitalized epithelium is not yet fully understood. It has been shown that native, intact amniotic membrane (AM) epithelium contains higher levels of epidermal growth factor, keratinocyte growth factor, hepatocyte growth factor, and basic fibroblast growth factor compared with epithelially denuded AM. However, Koizumi and colleagues (2000b) indicated that denuded AM promotes better corneal epithelial cell colonization than intact AM does and that corneal cells from the limbal epithelium colonize denuded AM more readily than epithelial cells from the central cornea. Corneal cells colonize intact HAM much less quickly than denuded amnion. Moreover, migrating limbal SC on denuded HAM have a smooth, uniform leading edge compared with the irregular raised edges of sheets grown on intact HAM. Morphologic observations further strengthen the support for epithelium grown on denuded HAM. The basal cells grown on bare amniotic membrane are nicely columnar, and the more superficial cells seem fairly well differentiated into wing cells and surface cells. In contrast slowly growing epithelial cells on intact HAM do not take the appearance of normal corneal epithelium. However, expanded epithelium from limbal explants on intact AM adopts a limbal epithelial phenotype whereas that expanded on epithelially denuded HAM reveals a corneal epithelial phenotype (Grueterich et al 2002b). Wei and colleagues (2007) have further demonstrated that during ex vivo expansion on intact HAM some limbal epithelial progenitor cells migrate onto intact AM whereas some also invade the limbal stroma, very likely undergoing epithelial-mesenchymal transition. The HAM’s basement membrane contains type IV collagen, ln-1, Ln-5, fibronectin, and collagen VII. The collagen IV subchain α2, is identical to that of the conjunctival BM. Ln play an important role in corneal epithelial cell adhesion. Overall the basement membrane facilitates migration of epithelial cells, reinforces adhesion of basal epithelial cells, promotes epithelial differentiation, and prevents epithelial apoptosis, that is, programmed cell death (Lee et al 2000).
The AM stroma also contains a number of growth factors, various antiangiogenic and anti-inflammatory proteins, and natural inhibitors to various proteases (Sato et al 1998). In view of all of the above-mentioned properties of the AM, there is little doubt that it provides an ideal stromal niche desirable for stem cell expansion.
Limbal cell culture: Explant culture technique
Many investigators have adopted the explant culture technique with variations in substrate, media, feeder cells, and airlifting (Koizumi et al 2000c, 2001b; Tsai et al 2000; Grueterich et al 2002c; Shimazaki et al 2002). To elaborately describe the details of each protocol is beyond the scope of this article. Therefore the modifications that the authors have incorporated in their procedure have been described with respect to reports from previous investigators.
Limbal biopsy
After satisfying the Institutional Review Board, informed consent is obtained from the patients or guardians. Limbal biopsy is performed on the healthy contralateral eye or a healthy area of the ipsilateral eye. The procedure includes careful dissection of a 1 × 2 mm2 piece of limbal epithelium with 1 mm into clear corneal stromal tissue at the limbus under strict aseptic conditions. A conjunctival peritomy is made 2–3 mm from the limbus and dissection of the conjunctiva is carried forwards 1 mm beyond the limbal arcade. The harvested tissue should exclude the Tenon’s capsule and should include the palisades of Vogt. Post-operatively topical antibiotic is used four times day for two weeks. Topical steroids are used in tapering doses for 4 to 6 weeks or until complete healing of donor site.
AMG procurement, processing, and preservation
Amniotic membrane is obtained from prospective donors undergoing caesarean section, who are negative for communicable diseases including HIV, hepatitis, and syphilis. Different protocols exist for the processing and storage (Kim and Tseng 1995; Shimazaki et al 1997). According to Kim and Tseng (1995), the amnion is separated from the chorion by blunt dissection, and washed with balanced salt solution containing a cocktail of antibiotics (50 μg/ml penicillin, 50 μg/ml streptomycin, 100 μg/ml of neomycin, as well as 2.5 μg/ml of amphotericin B) under sterile conditions. The separated membranes are cut in different sizes (3 × 4 mm2) and placed on nitrocellulose paper strips with the epithelial side up. Dulbecco Modified Eagles’ Medium/glycerol (1:1) is used for cryopreservation and the tissues are frozen at −80 °C degrees until further use (Kruse et al 2000). Just before use, the amniotic membrane is thawed at 37 °C for 30 min. and placed on a sterile cut glass slide. The amniotic epithelium is removed by digestion with 0.1% trypsin-EDTA at 37 °C, followed by scraping.
Under sterile conditions, the membrane is inspected under the microscope for complete removal of cells. The de-epithelized membranes are then spread on a glass slide (used as culture inserts) in a Petri plate tucking the edges for a uniform surface (Koizumi et al 2000c, 2001b; Sangwan et al 2006; Shimazaki et al 2002).
Tsai and colleagues (2000) and Grueterich and colleagues (2002c) have cultivated explants on intact AM.
Human corneal epithelial cell growth medium
We use a modified human corneal epithelial cell (HCE) culture medium, prepared using 9.7 g/l Modified Eagle Medium (MEM) with addition of 16.2 g/l Ham F12 serum, 0.01mg/l epidermal growth factor, 0.25 mg/l insulin, 0.1 mg/l cholera toxin, and hydrocortisone.
The medium is filtered with 0.22 mm membrane filters using a vacuum pump. This is supplemented with autologous serum or 10% fetal calf serum (FCS) at the time of use.
Explant culture technique
The authors follow a submerged explant culture system without the use of any feeder cell layer or airlifting (Tsai et al 2000; Grueterich et al 2002c; Shimazaki et al 2002; Sangwan et al 2006). Koizumi and colleagues (2000c, 2001b) have used 3T3 feeder cells along with airlifting for epithelial stratification. Limbal tissue procured by biopsy is shredded into 4–6 fragments, and placed on the de-epithelized membranes separately and allowed to settle down by overnight incubation at 37 °C with 5% CO2 and 95% air (Figure 4).
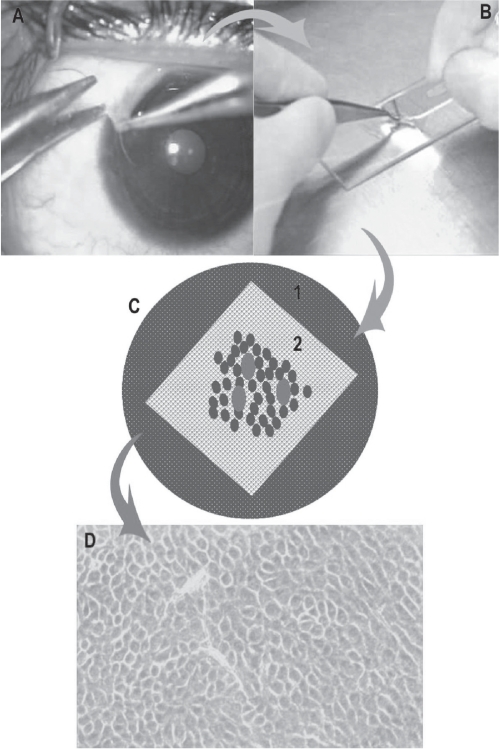
Figure 4.
Schematic diagram showing the steps in cultivation of limbal epithelial stem cells. A: Technique of limbal biopsy (See text for details). B: Processing of tissue in the laboratory and making the explant culture. C: Petridish, glass slide (white) with de-epithelialized human amniotic membrane with explants (red dots) with growing cells around it (blue dots). D: Monolayer of cells (10–14 days old) under phase contrast microscope.
The medium is changed on alternate days for 10–14 days with daily monitoring of cell growth under phase-contrast microscope. In four days polygonal cells could be seen growing from the edges of the explant.
Whole mount preparation
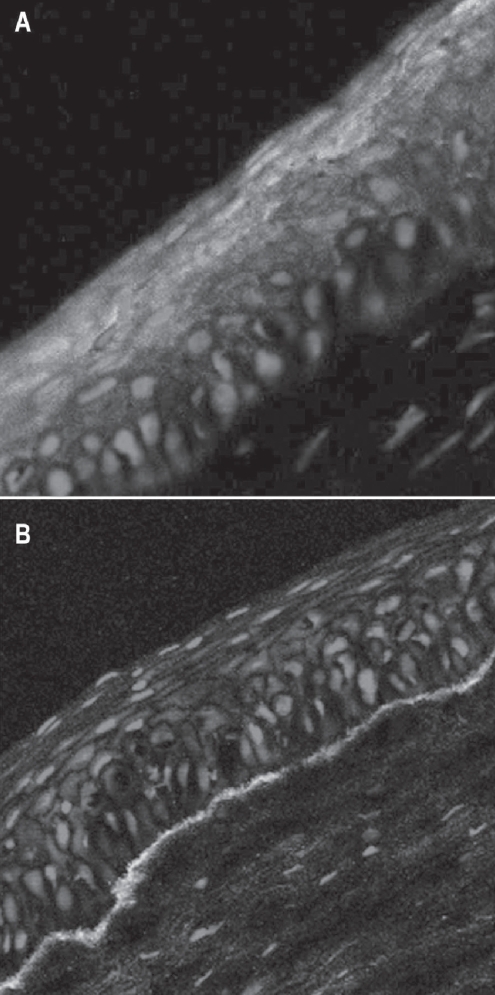
After confirming confluent growth of a monolayer from the explanted tissue over 1–2 weeks, the growth is terminated by replacing the medium with 10% buffered formalin. The whole mount preparation, stained with hematoxylin and eosin, could be seen as bluish rings of stained areas around the original explanted tissue. When observed under the microscope, the cultured cells appear as a monolayer of large polygonal cells with an epithelial appearance. The membrane with the cultured limbal tissue and the cultured cells is fixed in formalin and processed for routine histopathology with paraffin embedding. The sections are cut at 4–5 cm and after deparaffinization, stained with hematoxylin and eosin stain and periodic acid-Schiffs stain. In contrast to the cuboidal epithelium of the normal amniotic membrane the cultured cells form an epithelium of 1–2 layered cells over the amniotic membrane. Immunostaining on the formalin-fixed, paraffin-embedded sections can be done using prediluted antibodies to cytokeratin 3 (K3) and cytokeratin 19 (K19) from DAKO (Copenhagen, Denmark) to confirm the corneal phenotype of the cultured cells (Figure 5) (Vemuganti et al 2004).
Figure 5.
Photomicrograph of cultivated limbal epithelial cells stained for keratin 3 and 12 (A) showing the corneal epithelial phenotype and basement membrane (green) secreted by the growing cells (B).
Cell suspension culture of limbal epithelial cells
Preparation of AM and 3T3 fibroblast cells
As described in detail in previous reports, AMs obtained at the time of caesarean section were washed with sterile phosphate-buffered solution (PBS) containing antibiotics and stored at −80 °C in Dulbecco’s modified Eagle medium and glycerol at a ratio of 1:1. Immediately before use the membrane was thawed, washed 3 times with sterile PBS, and cut into pieces of approximately 2.5 cm2 and denuded.
Confluent 3T3 fibroblasts were inactivated by incubation in 4 μg/ml mitomycin C (MMC) for 2 hours at 37 °C under 5% CO2, and then trypsinized and plated onto plastic dishes (Pellegrini et al 1997; Schwab 1999; Koizumi et al 2000a; Schwab et al 2000).
Cell suspension
The whole limbal ring was cut into two to three peices, and these were incubated at 37 °C for one hour with 1.2 IU dispase. The corneal limbal epithelium including the limbal stem cells were suspended in 3 ml medium, seeded onto three pieces of denuded AM spread on the bottom of the culture inserts, and co-cultured with MMC-inactivated 3T3 fibroblasts. The culture was submerged in the medium for 2 weeks and then exposed to air by lowering the medium level (airlifting) for 2 weeks to promote corneal epithelial differentiation. The culture medium used was Dulbecco’s modified Eagle’s medium and Ham’s F12 (1:1 mixture) (Koizumi et al 2002).
Surgical technique
The surgery is performed 10–14 days after the limbal biopsy. Following strict aseptic precautions, under local or general anesthesia, a drop of 1:1000 epinephrine is instilled into the conjunctival cul de sac to ensure hemostasis. The fibrovascular pannus is dissected off the corneal surface starting 2–3 mm beyond the limbus using a conjunctival spring scissors until the limbus and beyond. A plane of dissection is usually noted in patients who have undergone previous ocular surface reconstruction with AM, facilitating the excision.
Symblepharon release is carried out at the same time and AM is used to reconstruct the ocular surface. Fornix reconstruction is also performed where appropriate.
The AM, with its monolayer of cultured limbal epithelial cells, is then transferred to the ocular surface and anchored in place at the limbus with interrupted 10–0 monofilament nylon sutures placed circumferentially. The knots are trimmed and buried. The peripheral skirt of the AM is anchored to the conjunctiva with 8–0 vicryl sutures. Subconjunctival dexamethasone is given at the end of surgery. Some authors recommend mitomycin C subconjunctivally to prevent recurrence of symblepharon and a bandage contact lens may be inserted.
In some patients with chemical injury, corneal scarring, and who may have undergone prior attempts at surface reconstruction, difficulties may be encountered in suturing the donor corneal tissue to the thin recipient bed. In such cases, cultured LSC transplantation may be combined with either a lamellar keratoplasty or deep anterior lamellar keratoplasty. Preoperative anterior segment interferometry or intraoperative pachymetry after pannus resection may indicate the residual stromal thickness and aid the decision for lamellar keratoplasty (LK) or deep anterior lamellar keratoplasty (DALK).
In cases with anterior to mid stromal scarring and thinning, a lamellar keratoplasty may be done using a crescent blade or an automated keratome. The lamellar donor corneal tissue is sutured to the recipient bed with interrupted 10–0 monofilament nylon sutures and the AM with cultivated limbal epithelium is placed on the entire de-epithelized surface.
Alternatively, a deep anterior lamellar keratoplasty may be performed to provide a smooth interface.
Penetrating keratoplasty (PK) after limbal stem cell transplantation (LSCT)
A penetrating keratoplasty is usually planned 3 months post operatively once the ocular surface has stabilised for visual loss related to corneal scarring in the visual axis. The recipient cornea is trephined in the centre with a disposable trephine and a donor tissue 0.5 mm larger is anchored to the recipient bed with interrupted 10–0 monofilament sutures. A lensectomy, vitrectomy, synechiolysis, and/or intraocular lens implantation may be carried out if deemed necessary. In the immediate post-operative period, it is imperative to watch for signs of epithelial breakdown and allograft rejection. It is worthwhile to perform a primary tarsorrhaphy in such cases.
Eleven of the 19 corneal grafts were successful with a mean follow up of 17.4 months. It is important to note that all these grafts met the criterion for of high risk for corneal graft rejection and none of them were on systemic immunosuppression. The authors attribute these results to a multistaged approach wherein the PK is performed three months post CLSCT as it not only ensures a stable ocular surface but also decreases the risk of corneal graft rejection by controlling inflammation. A second hypothesis is decreased sensitization by decreasing antigen presentation as the cultivated epithelium is devoid of dendritic cells. The early outcome of PK following cultivated limbal epithelial transplantation is very promising with 87% of grafts remaining clear at mean follow up of 8.3 months post PK and 25% developing corneal allograft rejection (Sangwan et al 2005).
Outcome of cultivated LSCT
Pelligrini and colleagues (1997) were the first to show that cultured limbal cells generated cohesive sheets of authentic corneal epithelium, and the autologous cultured corneal epithelium restored the corneal surface of two patients with complete loss of the corneal-limbal epithelium. Long-term follow-up at more than 2 years showed the stability of regenerated corneal epithelium and the striking improvement in patient’s comfort and visual acuity.
Schwab and colleagues (2000) reported a successful outcome, defined as restoration or improvement of vision, along with maintenance of corneal reepithelialization and absence or recurrence of surface disease, obtained in 6 of the 10 patients with autologous procedures and in all 4 of the allogenic procedures (Tsai et al 2000). Follow up ranged 16–19 months with a mean of 13 months. They concluded that both amniotic membrane and corneal epithelial stem cells present within the bio-engineered graft were necessary for successful repair.
Tsai and colleagues (2000) documented complete reepithelialization of the corneal surface occurred within two to four days of transplantation in all six eyes receiving transplants. By one month, the ocular surface was covered with corneal epithelium, and the clarity of the cornea was improved. In five of the six eyes receiving transplants (83%), the mean visual acuity improved from 20/112 to 20/45. In one patient with a chemical burn who had total opacification of the cornea, the acuity improved from the ability to count fingers at 40 cm to 20/200. No patient had recurrent neovascularization or inflammation in the transplanted area during the follow-up period.
Thirteen eyes of 11 patients were studied by Koizumi and colleagues (2001a). These consisted of five eyes with acute SJS, two with chronic SJS, one with an acute chemical injury, two with chronic chemical injuries, two with ocular cicatricial pemphigoid, and one with drug-induced pseudopemphigoid. All of these eyes had total stem cell deficiencies. Corneal limbal epithelium from donor corneas was cultivated for 4 weeks on a denuded AM carrier, with 3T3 fibroblast coculture and air lifting. The cultivated corneal epithelium showed four to five layers of stratification and was well differentiated. After conjunctival tissue removal from the cornea up to 3 mm outside the limbus and subconjunctival tissue treatment with 0.04% mitomycin C, cultivated allocorneal epithelium, including the AM carrier, was transplanted onto the corneal surface up to the limbus. Lamellar keratoplasty, using preserved donor graft without epithelium, was performed simultaneously for five chronic-phase patients showing corneal stromal scarring. Systemic immunosuppression was used to prevent allograft rejection. In all 13 eyes, the entire corneal surface, on which cultivated allocorneal epithelium had been placed, was free from epithelial defects 48 hours after surgery, indicating complete survival of the transplanted corneal epithelium. Visual acuity improved in all eyes after surgery, and 10 of the 13 eyes were restored to good vision (post-operative visual acuity improved two or more lines) 6 months after the operation. During the follow-up period (mean +/− standard deviation, 11.2 +/− 1.3 months), the corneal surfaces were clear, although three eyes experienced epithelial rejection.
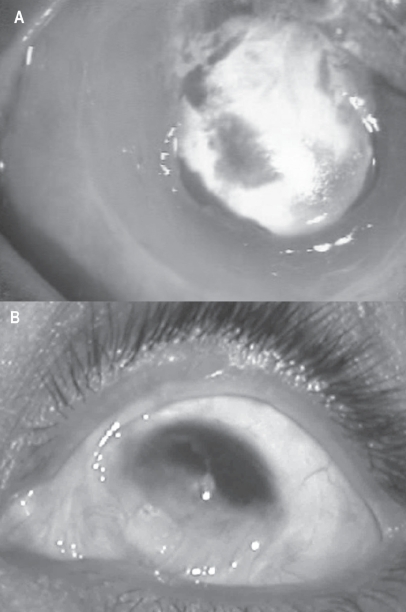
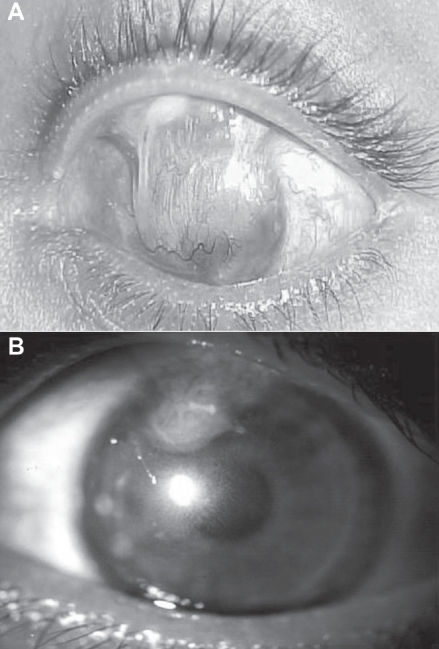
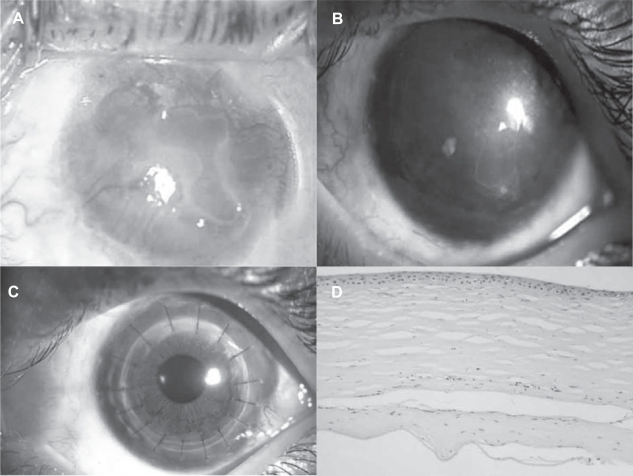
Sangwan and colleagues (2006) have reported the clinical outcome of autologous CLSCT in the largest series of patients with LSCD. The success of the procedure was defined by a stable ocular surface and subjective improvement in the symptoms of the patient with a minimum follow up of 6 weeks (Figure 6). The stability of the ocular surface was assessed clinically, based on absence of recurrent breakdown of the corenal epithelium, increased permeability to fluorescein, or conjunctivalization. Eighty-eight eyes of 86 patients with LSCD underwent autologous cultivated limbal epithelium transplantation, with a mean follow-up of 18.3 months. The etiology of LSCD was alkali burns in 64% patients. Sixty-one eyes had total LSCD (Figures 7A, 7B). Thirty-two of the 88 eyes had undergone amniotic membrane transplantation and 10 eyes had previously undergone limbal transplantation with unfavorable outcome. Nineteen eyes underwent penetrating keratoplasty, of which 11 grafts survived at the final follow-up (Figures 8A, 8B, 8C, 8D). Finally, 57 eyes (73.1%, 95% CI: 63.3–82.9) had a successful outcome with a stable ocular surface without conjunctivalization, 21 eyes (26.9%, 95% CI: 17.1–36.7) were considered failures and 10 patients were lost to follow-up. They propose a novel technique of submerged explant culture wherein denuded HAM is used as a substrate and human corneal epithelial medium for ex vivo cultivation. The 3T3 fibroblast feeder layer or air lifting as described by other researchers is not used. Lastly, a confluent monolayer is transplanted rather than awaiting in vitro stratification.
Figure 6.
Slit lamp photograph of a patient with total limbal stem cell deficiency (A) with multiple symblephara following acid injury. (B) One year after autologus cultivated limbal stem cell transplantation shows clear central cornea and no recurrence of symblephara and a stable ocular surface.
Figure 7.
A. Slit lamp photograph of a patient with total limbal stem cell deficiency with extensive symblephara following alkali injury. B. One year after autologus cultivated limbal stem cell transplantation shows clear central cornea and no recurrence of symblephara and a stable ocular surface.
Figure 8.
A. Slit lamp photograph of a patient with total limbal stem cell deficiency following alkali injury. B. Slit lamp photograph of the cornea showing a persistent scar with stable ocular surface, a year after cultivated LSCT. C. Slit lamp photograph at 6 months after penetrating keratoplasty. D. Photomicrograph of the hematoxylin and eosin-stained section of the excised corneal button showing stratified corneal epithelium subsequent to cultivated LSCT.
Conclusion
The transplantation of cultivated limbal stem cells is a promising new alternative for ocular surface reconstruction in patients with LSCD. The procedure significantly minimizes the risk of iatrogenic LSCD in the donor eye. The results of autologous cultivated limbal stem cell transplants are particularly encouraging and allows for banking of the patient’s cells for repeat procedures. Although much progress has been achieved with constant modifications to the technique of cultivation and transplantation, there is much to be learnt before a successful bioengineered corneal tissue replacement is conceived for this challenging group of patients.
Footnotes
Disclosure
The authors have no proprietary interests in the materials or methods of this article.
References
- Anderson DF, Ellies P, Pires RT, et al. Amniotic membrane in partial limbal stem cell deficiency. Br J Ophthalmol. 2001;85:567–75. doi: 10.1136/bjo.85.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster DL, Aggarwal RK, Williams KA. Surgical management of ocular surface disorders using conjunctival and stem cells allografts. Br J Ophthalmol. 1995;79:977–82. doi: 10.1136/bjo.79.11.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cells. 1989;57:201–9. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Daya SM, Bell RWD, Habib NE, et al. Clinical and pathological findings in keratolimbal allograft rejection. Cornea. 1999;19:443–50. doi: 10.1097/00003226-200007000-00007. [DOI] [PubMed] [Google Scholar]
- Dua HS, Gomes JAP, Singh A. Corneal epithelial wound healing. Br J Ophthalmol. 1994;78:401–8. doi: 10.1136/bjo.78.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua HS. Stem cells of the ocular surface: Scientific principles and clinical applications. Br J Ophthalmol. 1995;79:968–69. doi: 10.1136/bjo.79.11.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua HS, Saini JS, Azuara-Blanco A, et al. Limbal stem cell deficiency: Concept, aetiology, clinical presentation, diagnosis and management. Ind J Ophthalmol. 2000;48(2):83–92. [PubMed] [Google Scholar]
- Dua HS, Blanco AA. Allolimbal transplantation in patients with limbal stem cell deficiency. Br J Opthalmol. 1999;83:414–19. doi: 10.1136/bjo.83.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana EM, Ti SE, Grueterich M, et al. Corneal stromal changes following reconstruction by ex vivo expanded limbal epithelial cells in rabbits with total limbal limbal stem cell deficiency. Br J Ophthalmol. 2003;87:1509–14. doi: 10.1136/bjo.87.12.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueterich M, Tseng SC. Human limbal progenitor cells expanded on intact amniotic membrane ex vivo. Arch Ophthalmol. 2002a;120:783–90. doi: 10.1001/archopht.120.6.783. [DOI] [PubMed] [Google Scholar]
- Grueterich M, Espana E, Tseng SC. Connexin 43 expression and proliferation of human limbal epithelium on intact and denuded amniotic membrane. Invest Ophthalmol Vis Sci. 2002b;43:63–71. [PubMed] [Google Scholar]
- Grueterich M, Espana E, Touhami A, et al. Phenotypic study of a case with successful transplantation of ex vivo expanded human limbal epithelium for unilateral total limbal stem cell deficiency. Ophthalmology. 2002c;109:1547–52. doi: 10.1016/s0161-6420(02)01105-3. [DOI] [PubMed] [Google Scholar]
- Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631–46. doi: 10.1016/j.survophthal.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Kenyon KR, Bulusoglu G, Ziske JD. Clinical pathologic correlations of limbal autograft transplantation. Am J Ophthalmol. 1990;31:1–12. [Google Scholar]
- Kenyon KR, Tseng SCG. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–23. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- Kim JC, Tseng SCG. Transplantation of preserved human amniotic membrane for ocular surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473–84. [PubMed] [Google Scholar]
- Koizumi N, Inatomi T, Quantock AJ, et al. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea. 2000a;19:65–71. doi: 10.1097/00003226-200001000-00013. [DOI] [PubMed] [Google Scholar]
- Koizumi N, Fulwood NJ, Bairaktaris G, et al. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthal Vis Sci. 2000b;41:2506–13. [PubMed] [Google Scholar]
- Koizumi N, Inatomi T, Sotozono C, et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000c;20:173–7. [PubMed] [Google Scholar]
- Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial transplantation for ocular surface reconstruction on acute phase of Stevens-Johnson syndrome. Arch Ophthalmol. 2001a;119:298–300. [PubMed] [Google Scholar]
- Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001b;108:1569–74. doi: 10.1016/s0161-6420(01)00694-7. [DOI] [PubMed] [Google Scholar]
- Koizumi N, Cooper LJ, Fullwood NJ, et al. An evaluation of cultivated corneal limbal epithelial cells, using cell-suspension culture. Invest Ophthalmol Vis Sci. 2002;43:2114–21. [PubMed] [Google Scholar]
- Kruse FE. Stem cells and corneal epithelial regeneration. Eye. 1994;8:170–83. doi: 10.1038/eye.1994.42. [DOI] [PubMed] [Google Scholar]
- Kruse FE, Joussen AM, Rohrschneider K, et al. Cryopreserved human amniotic membrane for ocular surface reconstruction. Graefe’s Arch Clin Exp Ophthalmol. 2000;238:68–75. doi: 10.1007/s004170050012. [DOI] [PubMed] [Google Scholar]
- Lee SB, Li DQ, Tan DTH, et al. Suppression of TGF β signaling in both normal conjunctival fibroblasts and pteryigial body fibroblasts by amniotic membrane. Curr Eye Res. 2000;20:325–334.S. [PubMed] [Google Scholar]
- Lehrer MS, Sun T-T, Lavker RM. Strategies of epithelial repair: modulation of stem cell and transient amplifying cell proliferation. J Cell Sci. 1998;111:2867–75. doi: 10.1242/jcs.111.19.2867. [DOI] [PubMed] [Google Scholar]
- Meller D, Pires RTF, Tseng SCG. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86:463–71. doi: 10.1136/bjo.86.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologus cultivated corneal epithelium. Lancet. 1997;349:990–3. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- Pfister RR. Corneal stem cell disease: Concepts, categorization, and treatment by auto and homotransplantation of limbal stem cells. CLAO J. 1994;20:64–72. [PubMed] [Google Scholar]
- Potten CS, Morris RJ. Epithelial stem cells in vivo. J Cell Sci. 1988;10(Suppl):45–62. doi: 10.1242/jcs.1988.supplement_10.4. [DOI] [PubMed] [Google Scholar]
- Sangwan VS. Limbal stem cells in health and disease. Biosci Rep. 2001;21:385–405. doi: 10.1023/a:1017935624867. [DOI] [PubMed] [Google Scholar]
- Sangwan VS, Matalia HP, Vemuganti GK, et al. Clinical outcome of autologous cultivated limbal epithelium transplantation. Indian J Ophthalmol. 2006;54:29–34. doi: 10.4103/0301-4738.21611. [DOI] [PubMed] [Google Scholar]
- Sangwan VS, Matalia HP, Vemuganti GK, et al. Early results of penetrating keratoplasty after cultivated limbal epithelium transplantation. Arch Ophthalmol. 2005;123:334–40. doi: 10.1001/archopht.123.3.334. [DOI] [PubMed] [Google Scholar]
- Sato H, Shimazaki J, Shinozaki N. Role of growth factors for ocular surface reconstruction after amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 1998;39:S428. [Google Scholar]
- Schermer A, Galvin S, Sun T-T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab IR. Cultured corneal epithelia for ocular surface disease. Trans Am Ophthalmol Soc. 1999;97:891–986. [PMC free article] [PubMed] [Google Scholar]
- Schwab IR, Reyes M, Isseroff M. Successful transplantation of bio-engineered tissue replacements in patients with ocular surface disease. Cornea. 2000;19:421–6. doi: 10.1097/00003226-200007000-00003. [DOI] [PubMed] [Google Scholar]
- Shimazaki J, Yang HY, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997;104:2068–76. doi: 10.1016/s0161-6420(97)30057-8. [DOI] [PubMed] [Google Scholar]
- Shimazaki J, Aiba M, Goto E, et al. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109:1285–90. doi: 10.1016/s0161-6420(02)01089-8. [DOI] [PubMed] [Google Scholar]
- Sun T-T, Green H. Cultured epithelial cells of cornea, conjunctiva and skin: absence of marked intrinsic divergence of their differentiated states. Nature. 1977;269:489–93. doi: 10.1038/269489a0. [DOI] [PubMed] [Google Scholar]
- Tsai RJF, Li L-M, Chen J-K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- Tseng SC, Prabhasawat P, Barton K, et al. Amniotic membrane transplantation with or without limbal allografts for cornea surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431–41. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- Tseng SCG, Hirst LW, Farazdaghi M, et al. Goblet cell density and vascularization during conjunctival transdifferentiation. Invest Ophthalmol Vis Sci. 1984;25:1168–76. [PubMed] [Google Scholar]
- Tseng SCG, Prabhasawat P, Barton K, et al. 1995Amniotic membrane transplantation with or without limbal allografts for severe ocular surface disorders Ophthalmology 1021486–96.9097796 [Google Scholar]
- Tseng SCG. Concept and application of limbal stem cells. Eye. 1989;57:201–9. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Toda I, Saito H, et al. Reconstruction of corneal epithelium by limbal allograft transplantation for severe ocular surface disorders. Ophthalmology. 1995;102:1486–96. doi: 10.1016/s0161-6420(95)30841-x. [DOI] [PubMed] [Google Scholar]
- Vemuganti GK, Balasubramanian D. Heralding the dawn of cultured adult stem cell transplantation. Indian J Biotech. 2002;1:39–49. [Google Scholar]
- Vemuganti GK, Kashyap S, Sangwan VS, et al. Ex vivo potential of cadaveric and fresh limbal tissues to regenerate cultured epithelium. Indian J Ophthalmol. 2004;52:113–20. [PubMed] [Google Scholar]
- Watt FM, Hogan BL. Out of Eden: stem cells and their niche. Science. 2000;287:1427–30. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- Wei L, Hayashida Y, Kuo C, et al. The fate of limbal epithelial progenitor cells during explant culture on intact amniotic membrane. Invest Ophthal Vis Sci. 2007;48:605–13. doi: 10.1167/iovs.06-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]