Abstract
Purpose
To determine patient preference of and ocular discomfort with fixed combination brinzolamide/timolol compared with fixed combination dorzolamide/timolol.
Methods
In a prospective, double-masked, randomized, active-controlled, crossover, multicenter study, patients received 1 drop of brinzolamide/timolol and dorzolamide/timolol in both eyes on consecutive days in random order. Ocular discomfort was rated 1 minute after instillation of each medication, and preference was noted on Day 2. Adverse events, if any, were solicited at each visit.
Results
127 subjects with ocular hypertension or open-angle glaucoma were included in the intent-to-treat analysis. Of the 106 subjects who expressed a drug preference, 79.2% preferred brinzolamide/timolol (p < 0.0001). Ocular discomfort scores were significantly higher with dorzolamide/timolol than brinzolamide/timolol (2.9 vs 1.4, respectively; p < 0.0001). Significantly more patients reported ocular pain and discomfort after dorzolamide/timolol instillation and transient blurred vision after brinzolamide/timolol instillation.
Conclusions
Patients with ocular hypertension or open-angle glaucoma preferred the brinzolamide/timolol fixed combination over the dorzolamide/timolol fixed combination. This is likely due to the greater ocular discomfort associated with dorzolamide/timolol. The differences in preference, discomfort, and adverse events are likely attributable to formulation differences given the similarities of the active ingredients. Stronger patient preference for brinzolamide/timolol may lead to better therapeutic compliance.
Keywords: brinzolamide/timolol, dorzolamide/timolol, patient preference, ocular discomfort, open-angle glaucoma or ocular hypertension
Introduction
Reduction of intraocular pressure (IOP) is the only established therapy for the management of ocular hypertension and open-angle glaucoma. Topical IOP-lowering medications remain the primary treatment option for a majority of patients. A significant number of patients with ocular hypertension or open-angle glaucoma will require more than one medication to achieve adequate control of IOP. Nearly 40% of subjects in the Ocular Hypertension Treatment Study required two or more medications to achieve a 20% reduction in IOP (Kass et al 2002).
Adjunctive therapy involving multiple medication bottles adds complexity compared with single-agent monotherapy, and may introduce a washout effect if co-administered drops are not spaced adequately in time (Fechtner and Realini 2004; Khouri et al 2007). Some of these issues have been identified as barriers to therapeutic compliance (Tsai et al 2003). Fixed combinations of commonly co-administered IOP-lowering agents have been developed to minimize these issues, offering simplification of the dosing regimen and elimination of the washout effect (Fechtner and Realini 2004; Khouri et al 2007).
Tolerability has also been identified as a barrier to compliance (Tsai et al 2003). Ocular comfort is an important aspect of tolerability. Tolerability is an important characteristic of fixed combination therapies. For instance, the most common adverse events associated with dorzolamide (Trusopt®, Merck and Co., Inc., Whitehouse Station, NJ, USA) are ocular burning, stinging, and discomfort, and taste perversion (Trusopt prescribing information 2005); a similar safety profile is observed with the dorzolamide 2%/timolol 0.5% fixed combination (Cosopt®, Merck and Co., Inc., Whitehouse Station, NJ, USA) (Cosopt prescribing information 2006).
Recently, timolol 0.5% and brinzolamide 1% (Azopt®, Alcon Laboratories, Inc., Ft. Worth, TX, USA) have been formulated in a fixed combination (Azarga™, Alcon Laboratories, Inc., Ft. Worth, TX, USA). The most common side effects with brinzolamide are blurred vision and taste perversion; fewer than 5% of patients report ocular discomfort associated with brinzolamide use in clinical trials (Alcon Laboratories, Inc. 2003). In a comparative analysis from 2 prospective clinical trials of brinzolamide and dorzolamide, dorzolamide was associated with significantly more ocular discomfort (p = 0.001), while the brinzolamide group experienced more blurred vision (p < 0.05; Silver 2000).
The goal of this study was to determine patient preference of brinzolamide/timolol compared to dorzolamide/timolol after a single drop of each medication was administered to both eyes.
Methods
This was a prospective, double-masked, randomized, active-controlled, crossover, multi-center study conducted at 10 clinical sites within the United States. The objective of the study was to evaluate the patient preference of a single drop of each medication, brinzolamide 1%/timolol 0.5% ophthalmic suspension and dorzolamide 2%/timolol 0.5% ophthalmic solution, administered to both eyes on 2 consecutive days. The protocol was approved by all relevant Institutional Review Boards, the study was performed in compliance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice, and all participating patients provided written informed consent. The clinical trial registration number for this study was NCT00576342.
Subjects
Patients 18 years of age or older, diagnosed with open-angle glaucoma or ocular hypertension who were on a stable IOP-lowering medication monotherapy regimen for at least 30 days prior to the Screening Visit, were eligible to enroll in the study if their IOP, in the opinion of the investigator, did not present a risk to the patient’s ocular health. Patients were excluded if they met any of the following criteria: best corrected visual acuity was worse than 0.60 logMAR (20/80 Snellen) in either eye; use of contact lenses, topical or systemic corticosteroids, antihistamines, analgesic drugs, or topical cyclosporine within 30 days of the Screening Visit or during the course of the study; use of artificial tears within 3 days of the Screening Visit or during the course of the study; active infectious or noninfectious conjunctivitis, keratitis, scleritis or uveitis in either eye at the Screening Visit; history of intraocular conventional surgery or laser surgery within 90 days of the Screening Visit or during the course of the study; history of or active chronic, recurrent or current severe inflammatory eye disease, clinically significant or progressive retinal disease such as retinal degeneration, diabetic retinopathy, or retinal detachment in either eye; history of severe or serious hypersensitivity to timolol maleate, topical or oral carbonic anhydrase inhibitors, sulfonamide derivatives, or to any components of the study medications; history of severe, unstable or uncontrolled cardiovascular, hepatic, or renal disease (eg, sinus bradycardia, overt cardiac failure, greater than first degree atrioventricular block, cardiogenic shock, clinically relevant angina or uncontrolled hypertension), or bronchial asthma or severe chronic obstructive pulmonary disease that could preclude the safe administration of a topical beta-blocker. In addition, women of childbearing potential (those who were not surgically sterilized or at least 2 years post-menopausal) were not enrolled if any of the following conditions existed: they were pregnant; had a positive result on the urine pregnancy test at the Screening Visit; intended to become pregnant during the study period; were breast-feeding; or were not using highly effective birth control methods.
Procedures
The study was a 3-day, multi-center, double-masked, active-controlled, randomized, crossover design. There were a total of 3 visits on consecutive days in this study: The Screening Visit (Day 0), followed by 2 additional visits (Day 1 and Day 2). The Exit Visit was completed on Day 2, or when the patient discontinued study participation.
After providing informed consent, participating patients underwent a Screening Examination (Day 0) consisting of the following: collection of demographic information, medical history, current and previous medications; administration of a pregnancy test (if applicable); and an eye examination including the assessment of IOP, visual acuity (VA), undilated fundus and slit-lamp examination. Patients who met the inclusion/exclusion criteria were instructed to discontinue their topical IOP-lowering medication at the Screening Visit, for the 3-day duration of the study. Between 12 noon and 8 PM on Day 0, the designated study personnel instilled 1 drop of brinzolamide/timolol in 1 eye and 1 drop of dorzolamide/timolol in the fellow eye in double-masked and randomized fashion, and patients were observed for adverse events. This also allowed patients to experience the range of discomfort that they could expect during the course of the study and more accurately score their perceptions of drop comfort. If both study medications were tolerated, the patient was discharged and asked to return in 1 day at approximately the same time on each of the next 2 days. For the Day 1 and Day 2 Visits, patients were randomized in a 1:1 ratio to receive either brinzolamide/timolol at the Day 1 Visit and dorzolamide/timolol at the Day 2 Visit, or dorzolamide/timolol at the Day 1 Visit and brinzolamide/timolol at the Day 2 Visit. The assigned treatment was administered by designated study site personnel as a single drop in both eyes. At all 3 visits, dosing was done at approximately the same time between 12 noon and 8 PM. Patients completed an Ocular Discomfort Scale (0 “no discomfort” to 9 “substantial discomfort”) approximately 1 minute after the study medication was instilled on the Day 1 and Day 2 Visits. After completion of the Ocular Discomfort Scale on the Day 2 Visit each patient completed a Preference Question for which they checked one of these possible responses: “Prefer 1st Medication”, “No Preference”, or “Prefer 2nd Medication.” Adverse events, defined as any untoward changes (expected or unexpected) in a patient’s ophthalmic and/or medical health that occurred after initiation of study treatment, were obtained at each study visit as solicited or unsolicited comments from the patients and as observations by the investigators (at Day 0, IOP was measured and undilated fundus and slit-lamp examinations were performed). All adverse events were coded using a Medical Dictionary for Regulatory Affairs (version 10.0) and received independent causality assessments from the investigator and the Medical Monitor.
Statistical analysis
The primary statistical objective of this study was to demonstrate that patient preference for brinzolamide/timolol was superior to that of dorzolamide/timolol with respect to ocular comfort using a crossover study design. The primary efficacy parameter was the percentage of patients with a stated preference for either study medication, assessed in the patients who stated an actual preference, using a chi-square test. Primary inference for the test of superiority was based on the intent-to-treat data set. Differences in the ocular discomfort of the two study medications were analyzed as a supportive efficacy measure. An analysis of variance was used to compare the differences in mean ocular discomfort score between the two study medications.
Assuming that up to 13% of patients would express no preference for either study medication, approximately 115 patients were enrolled to ensure at least 100 evaluable patients with a stated study medication preference to provide approximately 90% power to detect a difference between equal preference for either study medication (50% prefer one and 50% prefer the other) and a preference for one or the other of at least 66%. This estimate was based on a 2-sided, 1 sample chi-square test at the α = 0.05 level of significance.
Results
Patient disposition and demographics
A total of 129 patients were enrolled in the study; 63 were randomized to receive brinzolamide/timolol followed by dorzolamide/timolol, and 66 were randomized to the opposite sequence. All 129 patients received at least 1 dose of study medication and were included in the safety analysis. Two patients (1 in each randomization sequence) did not complete the patient preference questionnaire and ocular discomfort assessments and were therefore not evaluable for either the intent-to-treat analysis or the per protocol analysis. An additional 3 patients were excluded from the per protocol analysis due to the use of a disallowed medication at Screening. Per protocol analyses confirmed intent-to-treat analyses.
Demographics of the 129 participating patients are given in Table 1. Patients were predominantly Caucasian (72.9%), 50% were female, and their mean age was 66.2 ± 11.4 years.
Table 1.
Demographics of participating patients (n = 129)
| Mean age (yr) | 66.2 ± 11.4 |
|---|---|
| Gender, n (%) | |
| Female | 65 (50.4) |
| Male | 64 (49.6) |
| Race, n (%) | |
| Caucasian | 94 (72.9) |
| Black or African-American | 23 (17.8) |
| Hispanic | 10 (7.8) |
| Other | 2 (1.6) |
| Diagnosis, n (%) | |
| Open-angle glaucoma | 100 (77.5) |
| Ocular hypertension | 29 (22.5) |
Patient preference and ocular discomfort
Patient preference results are illustrated in Figure 1. Of the 106 patients in the intent-to-treat analysis who expressed a preference, 79.2% (84/106) preferred brinzolamide/timolol over dorzolamide/timolol and 20.8% preferred dorzolamide/timolol over brinzolamide/timolol (p < 0.0001). Only 16.5% (21/127) of patients expressed no preference.
Figure 1.
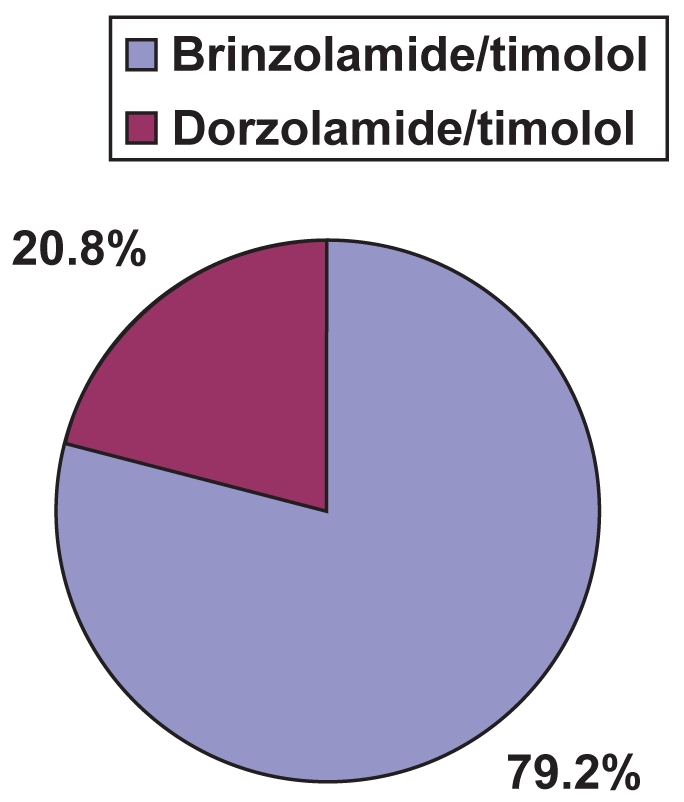
Percentage patient preference among those stating a preference (n = 106; p < 0.0001).
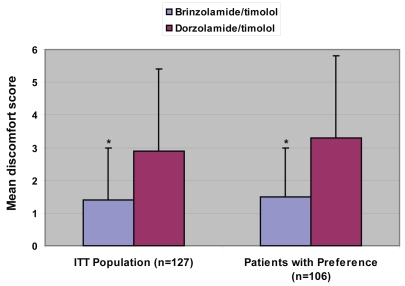
Ocular discomfort results are given in Figure 2. In the overall intent-to-treat analysis, mean discomfort scores were significantly lower for brinzolamide/timolol than for dorzolamide/timolol (1.4 ± 1.6 vs 2.9 ± 2.5, respectively; p < 0.0001). Similarly, among those patients who expressed a preference for one drug over the other, brinzolamide/timolol was significantly more comfortable than dorzolamide/timolol (1.5 ± 1.5 vs 3.3 ± 2.5, respectively; p < 0.0001).
Figure 2.
Ocular discomfort scores.
*p < 0.0001.
ITT = intent-to-treat
Adverse events
Adverse events are summarized in Table 2. Ocular irritation (defined as burning) and eye pain (defined as stinging) were more common with dorzolamide/timolol, and blurred vision was more common with brinzolamide/timolol. In this study, discomfort was mild to moderate in severity in both treatment groups, and no patients left the study due to adverse events.
Table 2.
Summary of adverse events, n (%)
| Brinzolamide/timolol | Dorzolamide/timolol | p value | |
|---|---|---|---|
| Ocular irritation | 7 (5.5) | 22 (17.3) | 0.0029 |
| Ocular pain | 1 (0.8) | 10 (7.9) | 0.0053 |
| Blurred vision | 19 (14.8) | 1 (0.8) | < 0.0001 |
| Other | |||
| Foreign body sensation | 1 (0.8) | 0 (0) | – |
| Increased tearing | 0 (0) | 1 (0.8) | – |
| Taste perversion | 1 (0.8) | 0 (0) | – |
Discussion
In this prospective, randomized, double-masked clinical trial, 4 out of 5 patients with a preference preferred brinzolamide/timolol fixed combination over dorzolamide/timolol fixed combination (79.2% preferred brinzolamide/timolol and 20.8% preferred dorzolamide/timolol).
Because the brinzolamide/timolol fixed combination is formulated as a suspension, it may be more likely to cause transient blurring of vision than the dorzolamide/timolol fixed combination, which is formulated as a solution. In the present study, significantly more patients reported blurred vision after instilling brinzolamide/timolol compared with dorzolamide/timolol. Despite these observations, most patients in this study still preferred brinzolamide/timolol, suggesting that the blurred vision occurring with brinzolamide/timolol was less annoying than the ocular discomfort experienced with dorzolamide/timolol.
One important reason for their preference was ocular comfort. The patients in our study reported significantly lower ocular discomfort scores after instilling brinzolamide/timolol compared to dorzolamide/timolol. In fact, the mean discomfort score for dorzolamide/timolol was more than twice as high as the mean score for brinzolamide/timolol: 2.9 versus 1.4, respectively, in the intent-to-treat analysis. Among the 83.5% of patients who preferred one medication over the other, the difference was 3.3 versus 1.5, respectively. Ocular comfort is a quality that glaucoma patients desire in an IOP-lowering medication: in a willingness-to-pay analysis, Jampel et al (2003) found that nearly 75% of patients would pay more for a comfortable medication than for a drug associated with stinging and burning upon instillation; on average, they would pay approximately 25% more for the comfortable medication.
Significantly more patients in our study reported eye irritation and eye pain as adverse events after instillation of dorzolamide/timolol compared to brinzolamide/timolol. Combined, more than four times more patients reported these adverse events after dorzolamide/timolol compared to brinzolamide/timolol (32 vs 8, respectively; see Table 2). While no subject discontinued participation based on study medication discomfort, this 3-day trial involving motivated and compensated research subjects is unlikely to be representative of a typical clinical patient population using medications chronically and without compensation.
Little is known about the relationship between tolerability and therapeutic compliance. In a systematic classification of the reasons why patients fail to take their glaucoma medications as prescribed, Tsai et al (2003) identified side effects as a potential barrier to compliance. On a representative sample of French patients treated with an IOP-lowering treatment, Nordmann et al (2003) found some associations between burning and stinging, vision-related quality of life and compliance: patients with declared adverse events more often missed instillations. Day et al (2006) reported associations between treatment satisfaction and some adverse events (ocular irritation, conjunctival hyperemia). Lastly, Nordmann et al (2007) found that treatment characteristics are associated with patient preference. Therefore, it is not unreasonable to believe that patients may take a medication less frequently than prescribed if it is associated with significant side effects, including ocular discomfort. This is particularly important when associations between compliance and lack of IOP control have been reported (Konstas et al 2000).
Both of these fixed combinations contain timolol and a topical carbonic anhydrase inhibitor. The differences in ocular discomfort and patient preference observed in this study likely arise from formulation differences, rather than to differences attributable to the drug molecules themselves. The dorzolamide molecule requires an acidic pH to optimize solubility. Thus, both the dorzolamide 2% ophthalmic solution (Merck and Co., Inc. 2005) and the dorzolamide/timolol fixed combination (Merck and Co., Inc. 2006) are formulated with a pH around 5.6. Brinzolamide is adequately soluble at the more physiologic pH of 7.2 (Alcon Laboratories, Inc. 2003). Numerous studies have demonstrated greater comfort and higher patient preference rates for brinzolamide over dorzolamide (Barnebey and Kwok 2000; Silver 2000; Stewart et al 2004; Tsukamoto et al 2005a, b). The difference in pH of the two fixed combinations may explain the difference in comfort reported by the participants.
This study is strengthened by its rigorous prospective, double-masked, randomized, crossover design, and its sample size providing adequate power to detect clinically significant differences in preference. This avoids the subjectiveness of tolerability comparison studies that take place at the time of an unmasked, nonrandomized switch from one medication to another. The crossover design utilized in this study allows for within-patient comparisons rather than between-patient comparisons as necessitated by parallel-group designs, reducing the variability associated with between-groups comparisons. Limitations of this study include the lack of a wash-out period prior to study initiation and the definition of “stable use” of glaucoma medications to be 30 days. While these limitations could introduce bias due to potential side effects of previous glaucoma medications, this bias should be minimized by the fact that both study medications were administered to each patient in random order.
In summary, this study has demonstrated that patients with ocular hypertension or open-angle glaucoma prefer the brinzolamide/timolol fixed combination over the dorzolamide/timolol fixed combination by a margin of nearly 4 to 1. This may be related to the greater ocular discomfort associated with dorzolamide/timolol compared with brinzolamide/timolol. The differences in preference and discomfort are likely attributable to formulation differences given the similarities of the active ingredients. Stronger patient preference for the greater comfort of brinzolamide/timolol may lead to better therapeutic compliance.
Brinzolamide/Timolol Preference Study Group (all USA)
Jason Bacharach, MD, North Bay Associates, Inc., Petaluma, CA. Joseph I Markoff, MD, Philadelphia Eye Associates, Philadelphia, PA. Thomas K Mundorf, MD, Mundorf Eye Center, Charlotte, NC. Kenneth W Olander, MD, PhD, University Eye Surgeons, Maryland, TN. Lee S Peplinsky, OD, FAAO, Kentuckiana Institute for Eye Research, Louisville, KY. Steven H Rauchman, MD, North Valley Eye Medical Group, Inc., Mission Hills, CA. Samuel K Seto, MD, PLLC, Eye Clinic of Edmonds, Edmonds, WA. Sriram Sonty, MD, Midwest Eye Center S.C., Bourbonnais, IL. Carl B Tubbs, MD, Associated Eye Care, Stillwater, MN. Robert D Williams, MD, Taustine Eye Center, Louisville, KY.
Acknowledgments
This study was supported by Alcon Laboratories, Inc. The authors would like to acknowledge Tony Realini, MD and Jennifer Klem, PhD for medical writing contributions, Tonya Smoot, PhD for statistical analyses and Craig Salem, OD for safety analyses.
Footnotes
Disclosures
None of the authors has any conflicts of interest to disclose.
References
- Alcon Laboratories Inc. Azopt (brinzolamide) prescribing information 2003 [Google Scholar]
- Barnebey H, Kwok SY. Patients’ acceptance of a switch from dorzolamide to brinzolamide for the treatment of glaucoma in a clinical practice setting. Clin Ther. 2000;22:1204–12. doi: 10.1016/s0149-2918(00)83063-5. [DOI] [PubMed] [Google Scholar]
- Day DG, Sharpe ED, Atkinson MJ, et al. The clinical validity of the treatment satisfaction survey for intraocular pressure in ocular hypertensive and glaucoma patients. Eye. 2006;20:583–90. doi: 10.1038/sj.eye.6701932. [DOI] [PubMed] [Google Scholar]
- Fechtner RD, Realini T. Fixed combinations of topical glaucoma medications. Curr Opin Ophthalmol. 2004;15:132–5. doi: 10.1097/00055735-200404000-00013. [DOI] [PubMed] [Google Scholar]
- Jampel HD, Schwartz GF, Robin AL, et al. Patient preferences for eye drop characteristics: a willingness-to-pay analysis. Arch Ophthalmol. 2003;121:540–6. doi: 10.1001/archopht.121.4.540. [DOI] [PubMed] [Google Scholar]
- Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- Khouri AS, Realini T, Fechtner RD. Use of fixed-dose combination drugs for the treatment of glaucoma. Drugs Aging. 2007;24:1007–16. doi: 10.2165/00002512-200724120-00004. [DOI] [PubMed] [Google Scholar]
- Konstas AG, Maskaleris G, Gratsonidis S, et al. Compliance and viewpoint of glaucoma patients in Greece. Eye. 2000;14:752–6. doi: 10.1038/eye.2000.197. [DOI] [PubMed] [Google Scholar]
- Nordmann JP, Auzaneau N, Ricard S, et al. Vision related quality of life and topical glaucoma treatment side effects. Health Qual Life Outcomes. 2003;1:75. doi: 10.1186/1477-7525-1-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann JP, Denis P, Vigneux M, et al. Development of the conceptual framework for the Eye-Drop Satisfaction Questionnaire (EDSQ) in glaucoma using a qualitative study. BMC Health Serv Res. 2007;7:124. doi: 10.1186/1472-6963-7-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck and Co Inc. Trusopt (dorzolamide) prescribing information 2005 [Google Scholar]
- Merck and Co Inc. Cosopt (dorzolamide 2%/timolol 0.5% fixed combination) prescribing information 2006 [Google Scholar]
- Silver LH. Ocular comfort of brinzolamide 1.0% ophthalmic suspension compared with dorzolamide 2.0% ophthalmic solution: results from two multicenter comfort studies. Brinzolamide Comfort Study Group. Surv Ophthalmol. 2000;44(Suppl 2):S141–5. doi: 10.1016/s0039-6257(99)00111-3. [DOI] [PubMed] [Google Scholar]
- Stewart WC, Day DG, Stewart JA, et al. Short-term ocular tolerability of dorzolamide 2% and brinzolamide 1% vs placebo in primary open-angle glaucoma and ocular hypertension subjects. Eye. 2004;18:905–10. doi: 10.1038/sj.eye.6701353. [DOI] [PubMed] [Google Scholar]
- Tsai JC, McClure CA, Ramos SE, et al. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003;12:393–8. doi: 10.1097/00061198-200310000-00001. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Noma H, Matsuyama S, et al. The efficacy and safety of topical brinzolamide and dorzolamide when added to the combination therapy of latanoprost and a beta-blocker in patients with glaucoma. J Ocul Pharmacol Ther. 2005a;21:170–3. doi: 10.1089/jop.2005.21.170. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Noma H, Mukai S, et al. The efficacy and ocular discomfort of substituting brinzolamide for dorzolamide in combination therapy with latanoprost, timolol, and dorzolamide. J Ocul Pharmacol Ther. 2005b;21:395–9. doi: 10.1089/jop.2005.21.395. [DOI] [PubMed] [Google Scholar]