Abstract
Objective
To evaluate the efficacy of Systane® Lubricating Eye Drops in improving the symptoms of moderate ocular dryness.
Methods
Fifty subjects with moderate symptoms of ocular dryness were enrolled in this open label study. The mean age of subjects was 57.6 ± 15.4 years. To be eligible, subjects’ tear film break-up time (TFBUT) had to be <10 seconds, and subjects had to have at least one ocular discomfort symptom in addition to dryness. Saline was used for a washout period of 3–5 days. Subjects were re-examined, and those continuing to meet the inclusion criteria were dispensed Systane® and re-examined again after 28 days. At each visit, slitlamp examination was conducted, and ocular discomfort symptoms and TFBUT were evaluated. Subjects rated their overall satisfaction at baseline and on the last visit.
Results
No significant changes in TFBUT or ocular discomfort symptoms were observed after saline use, compared with screening visit. After 28 days of Systane® use there was statistically significant improvement of TFBUT (p = 0.0001) compared with baseline. Subjects experienced significant symptomatic relief for all 6 ocular discomfort symptoms at the endpoint visit.
Conclusion
Systane® effectively relieved the symptoms associated with moderate ocular dryness, with measurable improvement in objective TFBUT, subjective symptoms, and overall satisfaction.
Keywords: Systane, lubricant eye drops, TFBUT, ocular dryness, ocular symptoms
Introduction
The tear film contains a mixture of lipid, aqueous, and mucin layers. It is generally accepted that the tear film in subjects with ocular dryness is unstable and incapable of maintaining the protective qualities that are necessary for its structure and function.
When the tear film is compromised, the corneal and conjunctival epithelium cannot be maintained, and subjects experience the symptoms of discomfort associated with ocular dryness such as burning, stinging, grittiness, foreign body sensation, tearing, ocular fatigue, and dryness (Terry 2001). The mainstay therapy of ocular dryness is tear film correction and improvement with topically administered lubricant eye drops substitutes, which provide mostly a transient relief due to low retention time on the eye surface.
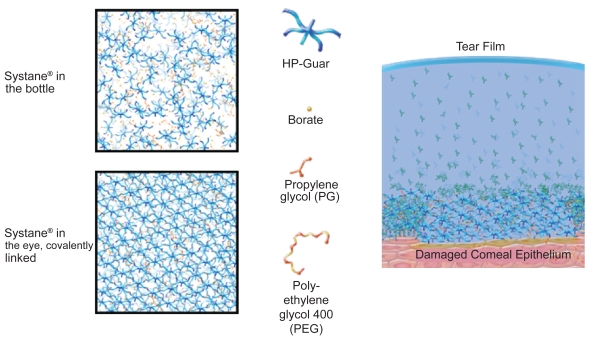
Systane® Lubricating Eye Drops (Alcon Laboratories, Inc., Fort Worth, Texas, USA) contain polyethylene glycol 400 (0.4%) and propylene glycol (0.3%) demulcents with the polymer hydroxypropyl guar (HP-Guar) as a gelling agent, and are preserved with POLYQUAD® (polidronium chloride) 0.001%. In the bottle, Systane® consists of a loosely cross-linked meshwork created by covalent interactions between borate and HP-Guar. When exposed to the higher pH of the ocular surface and tears, which is approximately 7.5 (Yamada et al 1997), HP-Guar forms a reversible cross-link with borate, resulting in a gel-matrix with sheer thinning viscoelastic and bioadhesive properties (Figure 1). The gel-matrix is designed to promote retention of the active demulcents, and, thus, protects the ocular surface (Asgharian et al 2003; Hartstein et al 2005; Gifford et al 2006; Meyer 2006).
Figure 1.
Chemical composition and mechanism of action of Systane® Lubricating Eye Drops.
The purpose of this study was to examine the clinical efficacy of Systane® Lubricating Eye Drops in reducing the subjective symptoms of moderate ocular dryness in a specific patient population.
Methods
Study population
Fifty healthy adults, regardless of gender or race, were enrolled at one research site in Bologna, Italy. The study was approved by an Independent Ethics Committee and was conducted in accordance with the current ethical principals of the Declaration of Helsinki and in accordance with the current legislation on clinical research in Italy. All subjects signed an informed consent form before starting the study. The inclusion/exclusion criteria were designed to exclude subjects with mild symptoms of ocular dryness, and to include subjects with moderate symptoms of ocular dryness (Table 1).
Table 1.
Inclusion and exclusion criteria
| Condition | Criteria |
|---|---|
| Inclusion criteria |
|
| Exclusion criteria |
|
Materials
Sterile saline (0.9% NaCl, Mini-Plasco. B. Braun, Meisungen, Germany) for the washout period. Systane® Lubricating Eye Drops as the test article.
Study design
This was a prospective, open-label, single-center study. The study consisted of 3 office visits carried out in an approximately 5-week period – visit 1 (screening), visit 2 (Day 0/baseline, 3–5 days after visit 1), and visit 3 (Day 28/endpoint, 28 days after visit 2). Each visit was conducted at approximately the same time of day (in the afternoon). Subjects’ tear film break-up time (TFBUT) had to be <10 seconds, and subjects had to have at least 1 ocular discomfort symptom in addition to dryness to be eligible. Saline was used for a run-in period of 3–5 days. At the screening visit, subjects’ demographic information was recorded, along with current/prior use of medications, lubricant eye drops, and ocular pathologies (Table 2). Medications considered necessary to maintaining subjects’ health were used at the discretion of the investigator. Subjects were questioned on how often their eyes felt dry enough for them to want to use lubricant eye drops (none, some, half, most, or all of the time). Furthermore, subjects were asked to complete an “Ocular Discomfort – Severity Questionnaire” grading the severity of their ocular sensations (burning, stinging, grittiness, dryness, foreign body sensation, and itching) on a 4-point scale (Grade: 0 = no discomfort; 1 = mild discomfort; 2 = moderate discomfort; 3 = serious discomfort).
Table 2.
Percentage of subjects’ current/prior medications, lubricating eye drops, and ocular pathologies at screening visit
| Medication or ocular pathology | Number of subjects (%) | |
|---|---|---|
| Ocular treatments | Hyaluronic acid | 16 (32) |
| Eye drops/Hyalistil | 7 (14) | |
| Gental | 4 (8) | |
| Benzalkonium chloride/Celluvisc | 3 (6) | |
| Carbomer/Cellufresh/Hyaluronate/Siccafluid/sodium chloride | 2 (4) | |
| Hylocomod/Lacrinorm/Retinol/Systane/Tetramil | 1 (2) | |
| Ongoing ocular pathology | Cataract | 2 (4) |
| Pinguecola | 1 (2) | |
| Pseudophakia | 1 (2) | |
| Strabismus | 1 (2) | |
| Prior ocular pathology | Cataract surgery | 1 (2) |
| Pseudophakia | 1 (2) | |
| Retinal detachment | 1 (2) |
A thorough slit-lamp biomicroscope examination was conducted to examine and record any abnormalities in the lids/eyelashes, anterior chamber, iris, and lens, as well as to grade the level of conjunctival injections (Grade: 0 = none – normal, no hyperemia; 1 = mild, trace hyperemia; 2 = moderate, diffuse hyperemia; 3 = severe, horizontal banding of hyperemia). TFBUT, which is defined as the time taken from blink until the first dark spots or streaks appear within the fluorescein enhanced tear film (Barabino et al 2004), was measured and recorded (average of 3 measurements) using sodium fluorescein strips (Haag-Streit International). Subjects who met the inclusion criteria of the study were dispensed saline solution as a run-in drop. This served to normalize the study population to an identical regimen of eye drops use, and allowed for washout and minimization of any effect from previous lubricant eye drops/medications use. Subjects were instructed to use the saline drops 4 times daily (qid) in both eyes. In addition, subjects were instructed to administer the saline eye drops at 8:00 AM on the day of their next office visit (Day 0/baseline) and to refrain from administering any other eye drops until after the examination. This allowed for approximately 6 hours between the last administration of eye drops and TFBUT measurements.
After 3–5 days, subjects returned (visit 2: Day 0/baseline) for an eligibility follow-up examination in which the above measurements were repeated. In addition, subjects answered a satisfaction questionnaire. Subjects continuing to satisfy the inclusion criteria were dispensed Systane® Lubricating Eye Drops and were instructed to use 1–2 drops in each eye qid (upon awakening, noon, early evening, and before bedtime). Likewise, subjects were instructed to administer Systane® drops at 8:00 AM on the day of their next office visit (Day 28/endpoint) and to refrain from administering any other eye drops until after the examination. After 28 days (visit 3: Day 28/endpoint), subjects returned for follow-up. The same examination measures and questions of visits 1 and 2 were repeated. In addition, subjects were asked to complete a satisfaction questionnaire. The satisfaction questionnaire included the following statements: “my eyes feel dry in the morning,” “my eyes feel dry at the end of the day,” “my eyes feel comfortable upon instillation of drops,” “my eyes feel refreshed when I use the drops,” “my eyes feel refreshed longer than expected when I use the drops,” and “I frequently forget my symptoms during the use of the drops.” Subjects recorded their answers using a 5-point scale (Scale: 1 = strongly disagree, 2 = disagree, 3 = undecided, 4 = agree, and 5 = strongly agree). Adverse events were also reported on visit 3.
Statistical analysis
The usual descriptive statistics were performed for all variables: average, standard deviation (SD), mean, minimum/maximum value for continuous variables, and absolute/relative frequency of categorical variables. Also, a t test was used with a bilateral significance level of 5%.
Results
Fifty subjects were enrolled and successfully completed all aspects of the study. The mean age of subjects was 57.6 ± 15.4 years (range, 26–85); 40 subjects were female. When asked how often their eyes felt dry enough to want to use lubricant eye drops, none of the subjects responded with “none of the time,” 30 subjects responded “some,” 17 “half,” 2 “most,” and 1 subject responded “all of the time.” When questioned regarding their current use of lubricating eye drops, 44 subjects reported use of lubricating eye drops.
Tear film break-up time
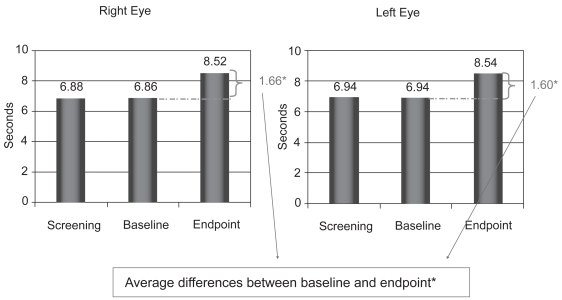
The tear film analysis showed no significant changes in TFBUT between screening and baseline visit (6.8 seconds OD and 6.9 seconds OS). However, a statistically significant increase was observed in the mean sodium fluorescein TFBUT from 6.9 seconds at baseline visit to 8.5 seconds at the endpoint visit (Day 28) in the left eye, and from 6.8 seconds to 8.5 seconds in the right eye (p = 0.0001) (Figure 2).
Figure 2.
Average increase in tear film break-up time (TFBUT) from baseline (Day 0) to endpoint visit (Day 28). *p = 0.0001.
Ocular discomfort symptoms
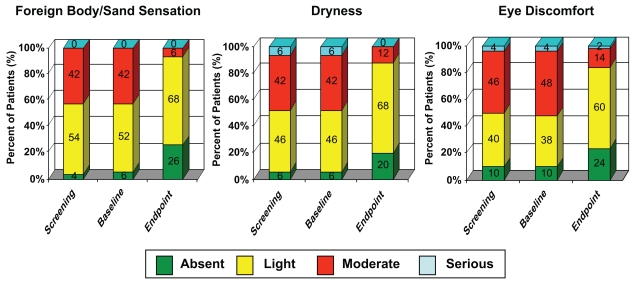
No significant changes in ocular discomfort symptoms were observed between screening and baseline visits. A significant improvement was recorded over the 28-day period of the trial for all reported sensations from the Ocular Discomfort – Severity questionnaire (Figure 3). Burning sensation was reported as “moderate” by 13/50 and “serious” by 1/50 subjects at baseline visit. At the endpoint visit, burning sensation was reported as “moderate” by 4/50 and “serious” by 0/50 subjects. Stinging sensation was reported as “moderate” by 7/50 subjects at baseline visit and by 3/50 subjects at the endpoint visit. These data attest to the ability of Systane® to improve the subjective ocular discomfort symptoms of ocular dryness.
Figure 3.
Percent improvement of patients with ocular discomfort symptoms.
Overall subjects’ satisfaction
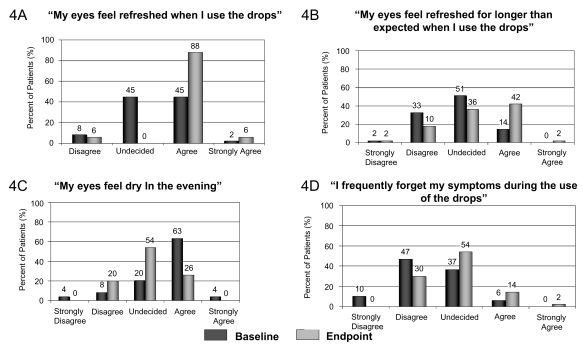
A significant difference was observed with the overall satisfaction questions at endpoint visit. Compared with baseline visit, most subjects agreed with the statements “My eyes feel refreshed when I use the drops” (44/50), “My eyes feel comfortable upon instillation of the drops” (36/50), and “My eyes feel refreshed longer than expected when I use the drops” (21/50). Most subjects responded with “undecided” to the statements “My eyes feel dry at the end of the day” (27/50), “I frequently forget my symptoms during the use of the drops” (27/50), and “My eyes feel dry in the morning” (21/50) (Figure 4).
Figure 4.
Subjects’ satisfaction with the improvement of their subjective symptoms at endpoint visit (Day 28) compared with baseline visit (Day 0).
Discussion
Ocular dryness arises from inadequate lubrication of the ocular surface from a variety of underlying conditions and environmental factors. Though there are many contributing factors, ocular dryness is due predominantly to either aqueous deficiency or excessive evaporation of the tear film. Although new therapeutic strategies based on the pathophysiology of ocular dryness are currently being developed, lubricating eye drops continue to be the mainstay care of ocular dryness. The major goal of these products is to restore ocular surface integrity in an attempt to reduce the symptoms associated with ocular dryness. Systane® Lubricating Eye Drops provide a physical protective barrier that works in conjunction with blinking to help spread the tear film over the eye surface. A protected ocular surface, in fact, will exist as long as the tear film is stable and when the TFBUT matches or exceeds the inter-blink interval (IBI). Conversely, when the TFBUT is less than the IBI, the corneal surface will be unprotected for a period of time between blinks, leading to ocular dryness (Ousler et al 2007).
Systane® works by binding to the hydrophobic exposed areas of the epithelial cells, attaching a protective HP-Guar tear-gel matrix that helps restore the ocular surface and increase TFBUT (Korb et al 2002, 2005). Ubels et al (2004) demonstrated that Systane® provided long-term protection from desiccation of the intact cornea. In Ubels’ study, Systane® allowed the in vivo exposed corneal epithelium to recover the normal barrier function.
The purpose of this current study was to obtain further evidence of the clinical efficacy of Systane® in reducing ocular discomfort symptoms of ocular dryness by increasing TFBUT. This study has demonstrated that the use of Systane® qid over a 28-day period statistically significantly extended TFBUT. Other studies have investigated the ocular surface retention properties of Systane® and its effects on corneal and conjunctival staining as well as TFBUT. Gifford et al conducted a similar study and showed significant improvement in ocular symptoms and TFBUT (Gifford et al 2006). The authors reported that the gelling properties of HP-Guar might be responsible for the stability of the ocular surface through its retention of the demulcents on the ocular surface. Systane® significantly increased TFBUT up to 30 minutes post instillation compared with carboxymethylcellulose 0.5% and polysorbate 80/glycerin (Refresh Tears® and Refresh Endura™, Allergan, Inc., Irvine CA, USA), presumably due to the HP-Guar borate gel-matrix component (Ousler et al 2007). Guillon et al demonstrated that Systane® significantly increased TFBUT at each measurement (30 minutes interval) up to 120 minutes post instillation (Guillon et al 2004). The ocular protection index (OPI) is a measure of the ratio of TFBUT to the IBI. D’Arienzo et al demonstrated that Systane® exhibited a greater extension in TFBUT from baseline at 45, 60, and 90 minutes postdrop instillation than Optive™ during the important sustaining phase, indicating its ability to lubricate and protect the ocular surface for a longer period than Optive. However, both Systane® and Optive™ showed improvements shortly after instillation during the bulking phase (D’Arienzo et al 2007).
The overall results from the Ocular Discomfort – Severity questionnaire showed that the use of Systane® during the course of this study was associated with a significant reduction of ocular discomfort. Two similar studies conducted by Gifford et al (2006) and Harstein et al (2005) asking the same questions over the same duration and using an identical Systane® regimen also showed significant overall reduction of ocular discomfort. Therefore, this current study adds more evidence and weight to the conclusions drawn by Gifford and Hartstein, which state that Systane® is effective in improving ocular discomfort in subjects with moderate symptoms of ocular dryness, one of the primary roles of lubricating eye drops.
The final part of this study examined the subjects’ overall satisfaction with Systane® as lubricant eye drops by looking into subjects’ responses to 6 acceptability elements at the endpoint visit. Gifford et al (2006), Hartstein et al (2005), and Christensen et al (2004) reported the use of similar questions and the receipt of similar responses in their corresponding clinical studies. Subjects were aware of the relief Systane® provided, agreeing more strongly to “My eyes feel refreshed when I use the drops.” The frequency of symptoms was also reduced, with most subjects agreeing, “My eyes feel refreshed longer than I expected.” Some subjects could not answer some of the acceptability questions and reported “uncertain/undecided” as their answer. One possible reason may be the ambiguity of the statements themselves, leaving the patient undecided.
Limitations of this study are that it was an open-label and uncontrolled study that did not control for the placebo effects or compare the results with that of a control group, although the symptoms at the endpoint visit were compared with the symptoms at the baseline visit. Therefore, a future double-masked, randomized, controlled study design with perhaps longer duration to study the long-term effects of Systane® on ocular dryness is warranted.
Conclusion
Systane® Lubricating Eye Drops improved the symptoms of ocular discomfort in subjects with moderate symptoms of ocular dryness. Statistically significant improvement was evident in the extension of TFBUT from Day 0/baseline to Day 28/endpoint. Subjective symptoms also showed significant improvement and overall, subjects were satisfied with the use of Systane®.
Acknowledgments
The study was funded by a grant from Alcon Laboratories, Inc., Italy. The authors thank Heba Costandy, MD, MS, for medical writing contributions.
Footnotes
The data in this paper were first presented at the 9th International Ocular Inflammation Society (IOIS) Annual Meeting, September 17–20, 2007, Paris, France, and the European Association for Vision and Eye Research (EVER) Congress, October 3–6, 2007, Portoroz, Slovenia
Disclosures
The authors have no conflicts of interest to disclose.
References
- Asgharian B, Nolen L, Meadows D, et al. Novel gel-forming ophthalmic polymer system for artificial tear solution [abstract]; Assoc Res Vis Ophthalmol; May 4–9; Ft. Lauderdale, FL, USA. 2003. Abst 2472. [Google Scholar]
- Barabino S, Chen W, Dana MR. Tear film and ocular surface tests in animal models of dry eye: uses and limitations. Exp Eye Res. 2004;79:613–21. doi: 10.1016/j.exer.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Christensen MT, Cohen S, Rinehart J, et al. Clinical evaluation of an HP-Guar gellable lubricant eye drop for the relief of dryness of the eye. US-based study. Curr Eye Res. 2004;28:55–62. doi: 10.1076/ceyr.28.1.55.23495. [DOI] [PubMed] [Google Scholar]
- D’Arienzo P, Ousler GW, III, Schindelar MR. A comparison of two marketed artificial tears in improvement of tear film stability as measured by tear film break-up time (TFBUT) and ocular protection and ocular protection index (OPI). US-based study [poster]. Tear Film and Ocular Surface Society; September 5–8; Taormina, Italy. 2007. [Google Scholar]
- Gifford P, Evans BJW, Morris J. A clinical evaluation of systane. Contact Lens Ant Eye. 2006;29:31–40. doi: 10.1016/j.clae.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Guillon M, Chamberlain P, Marks MG, et al. Effect of eye drop composition characteristics on tear film. US-based study [abstract] Invest Ophthalmol Vis Sci. 2004;45 E-Abst 3878. [Google Scholar]
- Hartstein I, Khwarg S, Przydryga J. An open-label evaluation of HP-Guar gellable lubricant eye drops for the improvement of dry eye signs and symptoms in a moderate dry eye adult population. US-based study. Curr Med Res Opinion. 2005;21:255–60. doi: 10.1185/030079905x26252. [DOI] [PubMed] [Google Scholar]
- Korb DR, Herman JP, Greiner JV, et al. Lid wiper epitheliopathy and dry eye symptoms. US-based study. Eye Contact Lens. 2005;31:2–8. doi: 10.1097/01.icl.0000140910.03095.fa. [DOI] [PubMed] [Google Scholar]
- Korb DR, Greiner JV, Herman JP, et al. Lid-wiper epitheliopathy and dry-eye symptoms in contact lens wearer. US-based study. CLAO J. 2002;28:211–16. doi: 10.1097/01.ICL.0000029344.37847.5A. [DOI] [PubMed] [Google Scholar]
- Meyer AE. Tissue-on-tissue testing of dry eye formulations for reduction of bioadhesion. US-based study. J Adhesion. 2006;82:607–27. [Google Scholar]
- Ousler GW, Michaelson C, Christensen MT. An evaluation of tear film break-up time extension and ocular protection index scores between three marketed lubricant eye drops. US-based study. Cornea. 2007;26:949–52. doi: 10.1097/ICO.0b013e3180de1c38. [DOI] [PubMed] [Google Scholar]
- Terry MA. Dry eye in the elderly. Drugs Aging. 2001;18:101–7. doi: 10.2165/00002512-200118020-00003. [DOI] [PubMed] [Google Scholar]
- Ubels JL, Clousing DP, van Haitsma TA, et al. Pre-clinical investigation of the efficacy of an artificial tear solution containing hudroxypropyl-guar as a gelling agent. US-based study. Curr Eye Res. 2004;28:437–44. doi: 10.1080/02713680490503787. [DOI] [PubMed] [Google Scholar]
- Yamada M, Mochizuki H, Kawai M, et al. Fluorophotometric measurement of pH of human tears in vivo. Curr Eye Res. 1997;16:482–6. doi: 10.1076/ceyr.16.5.482.7050. [DOI] [PubMed] [Google Scholar]