Abstract
Background
Surgical ventricular reconstruction (SVR) is used in conjunction with coronary artery bypass graft surgery (CABG) to improve left ventricular function and clinical outcomes in selected patients with ischemic heart failure. The impact of SVR on quality of life and medical costs is unknown.
Methods
We compared CABG plus SVR with CABG alone in 1000 patients with ischemic heart failure, a large anterior wall scar, and a left ventricular ejection fraction ≤ 0.35. In 991 (99% of eligible), we collected a battery of quality of life (QOL) instruments. The principal, pre-specified QOL measure was the Kansas City Cardiomyopathy Questionnaire (KCCQ), which evaluates the effects of heart failure symptoms on QOL using a scale from 0 to 100 with higher scores indicating better QOL. Structured QOL interviews were conducted at baseline, 4, 12, 24, and 36 months post randomization and were ≥ 92% complete. Cost data were collected on 196 of 200 (98%) patients enrolled in the United States.
Results
Heart-failure-related QOL outcomes did not differ between the two treatment strategies out to 3 years (median KCCQ scores for CABG alone and CABG plus SVR, respectively: baseline 53 versus 54, p=0.53; 3 years 85 versus 84, p=0.89). There were no treatment-related differences in other QOL measures. In the US patients, total index hospitalization costs averaged over $14,500 higher for CABG plus SVR (P=0.004) due primarily to 4.2 extra post-operative high-intensity care days in the hospital.
Conclusions
Addition of SVR to CABG in patients with ischemic heart failure did not improve quality of life but significantly increased health care costs.
Keywords: Heart failure, coronary artery bypass graft surgery, quality of life, cost, surgical ventricular reconstruction
INTRODUCTION
A subset of patients with ischemic cardiomyopathy develop progressive heart failure as a consequence of adverse ventricular remodeling leading to a depressed ejection fraction, a large akinetic region of myocardium, and an abnormal globular shape to the ventricular chamber. Over the past 25 years, cardiac surgeons have developed a novel procedure for excluding or excising the ventricular scar and reshaping the left ventricle to a more normal geometry.1,2 This procedure, known as surgical ventricular reconstruction (SVR), has shown encouraging results in observational studies including significant improvement in heart failure symptoms and quality of life (QOL) relative to pre-surgery status.2-4 However, it remains unclear what incremental clinical and QOL benefits are specifically provided by the SVR, since it is nearly always performed in conjunction with coronary bypass graft surgery (CABG) and in the setting of medical heart failure therapy. In addition, the economic consequences of this procedure have not been previously reported.
Elsewhere we have reported on the primary results of the Surgical Treatment for Ischemic Heart Failure (STICH) trial comparing CABG plus SVR with CABG alone in 1000 patients with ischemic heart failure and a depressed ejection fraction.5 Our hypotheses at the start of this trial were that SVR would improve QOL relative to CABG alone and would be cost effective by conventional criteria. In this article, we provide data on the QOL and cost outcomes, both pre-specified secondary trial endpoints.
METHODS
Patient Population and Clinical Results
The Surgical Treatment for Ischemic Heart Failure (STICH) trial is a National Heart, Lung, and Blood Institute-sponsored program consisting of two international, randomized clinical trials testing two related hypotheses about the use of surgical treatment for ischemic heart failure. The left ventricular reconstruction hypothesis compared CABG plus surgical ventricular reconstruction with CABG alone in 1000 patients with symptomatic heart failure, an anterior wall scar, and a left ventricular ejection fraction ≤ 0.35.6 Rationale, trial design, and complete inclusion and exclusion criteria have been described elsewhere.6 One thousand patients were enrolled into the left ventricular reconstruction hypothesis cohort between September 12, 2002 and January 24, 2006.
As reported elsewhere, SVR had no effect on the primary clinical endpoint of all-cause death or cardiac hospitalization at a median follow-up of 48 months (hazard ratio 0.99, p=0.89).5 The SVR-plus-CABG operation produced a relative decrease of 25% in end systolic volume index compared with a 7% decrease seen in CABG-alone patients.
Quality-of-Life Data Collection
Patients were given a structured QOL interview at baseline (after enrollment but before randomized treatment was performed), 4, 12, 24, and 36 months post-randomization. Baseline interviews were conducted by each site’s coordinators, who had been specially trained to conduct the QOL interviews. Follow-up interviews for patients in the United States and Canada were conducted via telephone by trained interviewers from the Duke Clinical Research Institute’s Outcomes Research Group. Follow-up interviews for patients in all other countries were conducted by each site’s trained coordinators.
We collected baseline QOL data on 991 (99%) of 1000 patients randomized into the STICH trial left ventricular reconstruction hypothesis cohort. From a total of 4509 expected patient contacts, 4136 QOL questionnaires were collected, representing 82% to 99% of patients eligible for this assessment at each follow-up (Figure 1). Patient refusal was 0.8%, and 6.6% of forms collected were incomplete. A short proxy form was collected for incapacitated patients.
Figure 1.
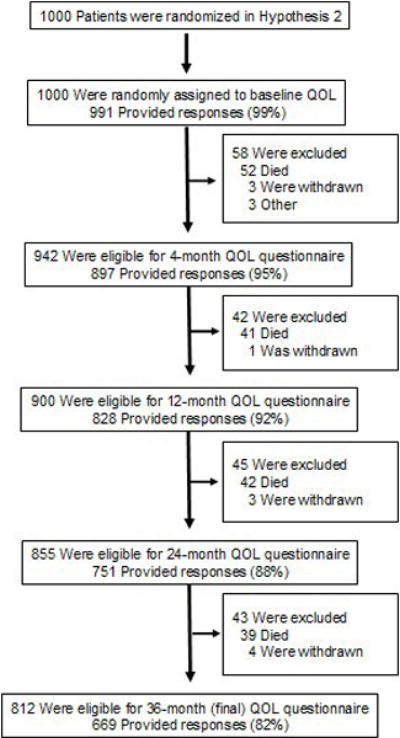
This figure shows the rate of QOL data collection at each point in follow-up and reasons for missing data.
This study was conducted in collaboration with and supported by the NHLBI. This part of the study had no other funding. All patients provided informed consent and study protocol approval was obtained from each site’s institutional review board or ethics committee. The authors designed the study, collected and analyzed the data, wrote all versions of this paper, and are fully responsible for its contents.
Quality of Life Measures
Our principal, pre-specified quality of life measure was the Kansas City Cardiomyopathy Questionnaire (KCCQ) Overall Summary Score. The KCCQ is a 23-item, disease-specific QOL instrument used to measure the effect of heart failure symptoms on functional limitations, social limitations, self efficacy, and patient satisfaction with overall quality of life.7 In addition to the Overall Summary Score, scores can be calculated for six component subscales. KCCQ scores range from 1-100, with higher scores indicating a more favorable status.7 A difference of 5 points or more is considered clinically significant.8
To assess the effects of angina symptoms on QOL, we used three scales from the Seattle Angina Questionnaire (SAQ): anginal frequency, anginal stability, and quality of life.9 The anginal frequency scale assesses the frequency of angina symptoms in the previous 4 weeks. Higher scores reflect lower incidence of anginal symptoms. The anginal stability scale measures changes in angina frequency with a score of 50 representing no change. The quality of life scale measures the effect of angina symptoms on patients’ perceptions of their quality of life, with higher scores being more favorable and a clinically significant difference being 5 points or greater.9
To supplement these condition-specific scales, we collected a brief overall generic measure of heath status (the SF-12) plus five scales (psychological well-being, role physical, role emotional, social function, and vitality) from the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36).10 The SF-12 is scored in two summary scales, a Physical and a Mental Component.11 Both the SF-12 and the SF-36 scales were scored by calculating the raw scores then transforming raw scores to a 0 to 100 score with larger values indicating more favorable health status. The scores were then standardized to a population norm-based score where a value of 50 represents the average value obtained in the US general population in 1998. In brief, z-scores were first computed for each scale for each study subject by subtracting the US mean value from 1998 data for that scale and dividing by the standard deviation from the 1998 US population. The result was then multiplied by 10 and added to 50 to produce a norm-based score with a mean of 50 and a standard deviation of 10. A clinically significant difference for this scoring system has not been established but can be approximated by a ¼ standard deviation or 2.5 points or more.
To assess depressive symptoms, we used the Center for Epidemiologic Stress-Depression Scale (CES-D), a 20-item instrument that assesses if a patient is experiencing mild to moderate depression or has a possibility of major depression.12 CES-D scores range from 0 to 60 with a score ≥ 16 indicating depression.12
The Cardiac Self-Efficacy Questionnaire (CSE) is a 13-item questionnaire designed to measure a patient’s confidence in controlling their disease symptoms and maintaining physical functioning.13 Patient responses are scored on a 0-100 scale with higher scores reflecting higher patient confidence.
The EuroQoL 5-D is a generic instrument consisting of two parts: a five dimension assessment of health status that can be mapped to population utility weights and a self-rating (0-100) of current health-related quality of life.14,15
Resource Use and Data Collection and Analysis
Resource use was collected on the case report form by site coordinators for all patients and included information about the length of the surgery, post-operative time in the ICU, total length of stay, and rates of rehospitalization. We collected index hospitalization cost data from 196 of 200 (98%) patients enrolled in the United States. One patient withdrew immediately, one died pre-surgery and no hospital bill was collected, one refused surgery, and one never had the surgery. Hospital costs were collected from UB 92/04 hospital billing data and converted from charges to costs using department-level correction factors in each hospital’s annual Medicare Cost Report, as described previously.16,17 Physician costs were estimated with a previously derived algorithm using physician-based care identified on the clinical case report form and the medical bills.16,18 Since our analyses took a societal perspective rather than a reimbursement perspective, we assigned costs to each identified unit of physician service rather than employing a global reimbursement rate. Costs were then assigned using the 2008 Medicare Fee Schedule. Costs are reported in 2008 U.S. Dollars.
Statistical Analyses
Descriptive statistics included percentages for discrete variables and medians with interquartile ranges, means with standard deviations, or both for continuous variables. Comparisons were performed according to randomized treatment assignment. The chi-square test was used for discrete variable comparisons, and the Wilcoxon rank-sum variable was used for continuous variables. Reported p-values were not adjusted for multiple comparisons. Along with the difference in mean costs between the two arms, we calculated the difference in median costs using the Hodges-Lehman statistic, and a non-parametric confidence limit for the difference was calculated.
RESULTS
Patient Population and Baseline Characteristics
Baseline characteristics of the two treatment arms were well balanced (data not shown).5 The median age of the study cohort was 62 years (25th and 75th percentiles, 55 years and 69 years). Fifteen percent were female and 9% were minorities. At baseline, 49% of patients had Canadian Class 3 or 4 angina and 49% had New York Heart Association Class 3 or 4 heart failure.
Quality of Life Outcomes
Both treatment groups significantly improved their KCCQ scores in follow-up compared with the pre-operative assessment. The observed improvements in disease-specific health status occurred rapidly and were sustained throughout follow-up (baseline median KCCQ Overall Summary scores = 53 for CABG alone and 54 for CABG plus SVR; 4-month median scores = 79 and 79; 36-month median scores = 85 and 84). However, median KCCQ Overall Summary scores did not differ between the two treatment groups at baseline or any follow-up interval (Table 1). Results in the six KCCQ subscales were consistent with the Overall Summary score comparison.
Table 1.
Kansas City Cardiomyopathy Questionnaire Scores by Intention-to-Treat
| CABG alone | CABG + SVR | P-value | |
|---|---|---|---|
| Kansas City Cardiomyopathy Questionnaire | |||
| Overall Summary | |||
| Baseline | |||
| No. of patients | 496 | 492 | |
| Median (interquartile range) | 53 (36, 70) | 54 (38, 72) | 0.53 |
| Mean ± standard deviation | 54 ± 22 | 54 ± 22 | |
| 4 mo | |||
| No. of patients | 446 | 443 | |
| Median (interquartile range) | 79 (56, 92) | 79 (63, 92) | 0.26 |
| Mean ± standard deviation | 72 ± 24 | 74 ± 23 | |
| 12 mo | |||
| No. of patients | 411 | 416 | |
| Median (interquartile range) | 84 (59, 95) | 82 (66, 94) | 0.76 |
| Mean ± standard deviation | 76 ± 23 | 76 ± 22 | |
| 24 mo | |||
| No. of patients | 368 | 374 | |
| Median (interquartile range) | 84 (60, 95) | 84 (64, 94) | 0.89 |
| Mean ± standard deviation | 75 ± 24 | 77 ± 22 | |
| 36 mo | |||
| No. of patients | 329 | 335 | |
| Median (interquartile range) | 85 (65, 95) | 84 (63, 95) | 0.89 |
| Mean ± standard deviation | 75 ± 25 | 77 ± 22 | |
| Physical Limitation | |||
| Baseline | |||
| No. of patients | 484 | 480 | |
| Median (interquartile range) | 63 (38, 83) | 63 (45, 80) | 0.40 |
| Mean ± standard deviation | 59 ± 26 | 61 ± 25 | |
| 4 mo | |||
| No. of patients | 433 | 432 | |
| Median (interquartile range) | 83 (58, 95) | 83 (64, 96) | 0.13 |
| Mean ± standard deviation | 74 ± 25 | 76 ± 25 | |
| 12 mo | |||
| No. of patients | 402 | 411 | |
| Median (interquartile range) | 84 (65, 100) | 88 (67, 96) | 0.86 |
| Mean ± standard deviation | 77 ± 25 | 78 ± 24 | |
| 24 mo | |||
| No. of patients | 363 | 368 | |
| Median (interquartile range) | 88 (60, 100) | 85 (66, 96) | 0.89 |
| Mean ± standard deviation | 77 ± 25 | 78 ± 23 | |
| 36 mo | |||
| No. of patients | 321 | 324 | |
| Median (interquartile range) | 88 (69, 100) | 88 (67, 96) | 0.87 |
| Mean ± standard deviation | 78 ± 25 | 78 ± 23 | |
| Symptom Stability | |||
| Baseline | |||
| No. of patients | 494 | 492 | |
| Median (interquartile range) | 50 (25, 50) | 50 (50, 50) | 0.38 |
| Mean ± standard deviation | 48 ± 25 | 49 ± 24 | |
| 4 mo | |||
| No. of patients | 436 | 435 | |
| Median (interquartile range) | 50 (50, 75) | 50 (50, 75) | 0.45 |
| Mean ± standard deviation | 57 ± 22 | 58 ± 22 | |
| 12 mo | |||
| No. of patients | 408 | 416 | |
| Median (interquartile range) | 50 (50, 50) | 50 (50, 50) | 0.62 |
| Mean ± standard deviation | 53 ± 16 | 53 ± 19 | |
| 24 mo | |||
| No. of patients | 362 | 372 | |
| Median (interquartile range) | 50 (50, 50) | 50 (50, 50) | 0.82 |
| Mean ± standard deviation | 50 ± 14 | 50 ± 15 | |
| 36 mo | |||
| No. of patients | 328 | 332 | |
| Median (interquartile range) | 50 (50, 50) | 50 (50, 50) | 0.94 |
| Mean ± standard deviation | 49 ± 16 | 50 ± 13 | |
| Symptom Frequency | |||
| Baseline | |||
| No. of patients | 493 | 491 | |
| Median (interquartile range) | 67 (50, 88) | 71 (50, 88) | 0.19 |
| Mean ± standard deviation | 65 ± 26 | 67 ± 26 | |
| 4 mo | |||
| No. of patients | 440 | 434 | |
| Median (interquartile range) | 83 (63, 100) | 88 (67, 100) | 0.07 |
| Mean ± standard deviation | 77 ± 24 | 80 ± 23 | |
| 12 mo | |||
| No. of patients | 409 | 415 | |
| Median (interquartile range) | 90 (67, 100) | 90 (71, 100) | 0.94 |
| Mean ± standard deviation | 80 ± 24 | 80 ± 24 | |
| 24 mo | |||
| No. of patients | 367 | 374 | |
| Median (interquartile range) | 92 (67, 100) | 92 (69, 100) | 0.67 |
| Mean ± standard deviation | 80 ± 24 | 81 ± 24 | |
| 36 mo | |||
| No. of patients | 329 | 334 | |
| Median (interquartile range) | 92 (67, 100) | 92 (69, 100) | 0.44 |
| Mean ± standard deviation | 80 ± 26 | 82 ± 23 | |
| Symptom Burden | |||
| Baseline | |||
| No. of patients | 494 | 492 | |
| Median (interquartile range) | 67 (50, 92) | 75 (50, 83) | 0.23 |
| Mean ± standard deviation | 67 ± 25 | 69 ± 24 | |
| 4 mo | |||
| No. of patients | 441 | 436 | |
| Median (interquartile range) | 83 (67, 100) | 83 (67, 100) | 0.43 |
| Mean ± standard deviation | 79 ± 23 | 81 ± 21 | |
| 12 mo | |||
| No. of patients | 410 | 416 | |
| Median (interquartile range) | 92 (67, 100) | 92 (67, 100) | 0.35 |
| Mean ± standard deviation | 81 ± 23 | 81 ± 23 | |
| 24 mo | |||
| No. of patients | 368 | 373 | |
| Median (interquartile range) | 92 (67, 100) | 92 (67, 100) | 0.90 |
| Mean ± standard deviation | 81 ± 23 | 82 ± 22 | |
| 36 mo | |||
| No. of patients | 329 | 332 | |
| Median (interquartile range) | 92 (67, 100) | 92 (67, 100) | 0.48 |
| Mean ± standard deviation | 81 ± 25 | 83 ± 21 | |
| Total Symptom | |||
| Baseline | |||
| No. of patients | 494 | 492 | |
| Median (interquartile range) | 68 (48, 86) | 71 (52, 88) | 0.18 |
| Mean ± standard deviation | 66 ± 24 | 68 ± 24 | |
| 4 mo | |||
| No. of patients | 441 | 436 | |
| Median (interquartile range) | 84 (65, 100) | 88 (71, 100) | 0.17 |
| Mean ± standard deviation | 78 ± 23 | 81 ± 21 | |
| 12 mo | |||
| No. of patients | 410 | 416 | |
| Median (interquartile range) | 90 (68, 100) | 88 (71, 100) | 0.59 |
| Mean ± standard deviation | 81 ± 23 | 81 ± 23 | |
| 24 mo | |||
| No. of patients | 368 | 374 | |
| Median (interquartile range) | 90 (71, 100) | 90 (71, 100) | 0.78 |
| Mean ± standard deviation | 81 ± 23 | 82 ± 22 | |
| 36 mo | |||
| No. of patients | 329 | 334 | |
| Median (interquartile range) | 90 (70, 100) | 92 (68, 100) | 0.48 |
| Mean ± standard deviation | 80 ± 25 | 82 ± 21 | |
| Quality of Life | |||
| Baseline | |||
| No. of patients | 493 | 490 | |
| Median (interquartile range) | 42 (17, 58) | 33 (25, 58) | 0.70 |
| Mean ± standard deviation | 40 ± 25 | 39 ± 23 | |
| 4 mo | |||
| No. of patients | 441 | 433 | |
| Median (interquartile range) | 75 (50, 92) | 75 (58, 92) | 0.47 |
| Mean ± standard deviation | 68 ± 27 | 70 ± 25 | |
| 12 mo | |||
| No. of patients | 409 | 416 | |
| Median (interquartile range) | 75 (50, 92) | 75 (58, 92) | 0.87 |
| Mean ± standard deviation | 71 ± 27 | 72 ± 25 | |
| 24 mo | |||
| No. of patients | 365 | 373 | |
| Median (interquartile range) | 75 (58, 92) | 75 (58, 92) | 0.84 |
| Mean ± standard deviation | 71 ± 27 | 71 ± 25 | |
| 36 mo | |||
| No. of patients | 328 | 333 | |
| Median (interquartile range) | 75 (58, 92) | 83 (50, 92) | 0.82 |
| Mean ± standard deviation | 71 ± 27 | 72 ± 26 | |
| Social Limitation | |||
| Baseline | |||
| No. of patients | 465 | 467 | |
| Median (interquartile range) | 44 (19, 75) | 44 (25, 75) | 0.63 |
| Mean ± standard deviation | 47 ± 31 | 48 ± 31 | |
| 4 mo | |||
| No. of patients | 424 | 412 | |
| Median (interquartile range) | 75 (50, 94) | 75 (50, 100) | 0.29 |
| Mean ± standard deviation | 69 ± 29 | 71 ± 28 | |
| 12 mo | |||
| No. of patients | 387 | 400 | |
| Median (interquartile range) | 81 (58, 100) | 83 (63, 94) | 0.68 |
| Mean ± standard deviation | 75 ± 27 | 75 ± 26 | |
| 24 mo | |||
| No. of patients | 353 | 356 | |
| Median (interquartile range) | 81 (50, 100) | 86 (56, 100) | 0.25 |
| Mean ± standard deviation | 73 ± 29 | 76 ± 26 | |
| 36 mo | |||
| No. of patients | 318 | 309 | |
| Median (interquartile range) | 83 (58, 100) | 83 (56, 100) | 0.68 |
| Mean ± standard deviation | 73 ± 31 | 75 ± 27 | |
| Clinical Summary | |||
| Baseline | |||
| No. of patients | 496 | 492 | |
| Median (interquartile range) | 65 (46, 83) | 68 (49, 84) | 0.26 |
| Mean ± standard deviation | 63 ± 23 | 65 ± 22 | |
| 4 mo | |||
| No. of patients | 445 | 443 | |
| Median (interquartile range) | 84 (63, 95) | 84 (67, 96) | 0.23 |
| Mean ± standard deviation | 76 ± 23 | 78 ± 22 | |
| 12 mo | |||
| No. of patients | 411 | 416 | |
| Median (interquartile range) | 88 (66, 97) | 86 (70, 96) | 0.66 |
| Mean ± standard deviation | 79 ± 22 | 79 ± 22 | |
| 24 mo | |||
| No. of patients | 368 | 374 | |
| Median (interquartile range) | 88 (65, 97) | 87 (69, 96) | 0.98 |
| Mean ± standard deviation | 79 ± 22 | 80 ± 21 | |
| 36 mo | |||
| No. of patients | 329 | 335 | |
| Median (interquartile range) | 88 (67, 98) | 88 (69, 98) | 0.86 |
| Mean ± standard deviation | 79 ± 24 | 80 ± 21 |
0-100 scale with higher scores representing better functioning
CABG=coronary artery bypass grafting surgery, SVR=surgical ventricular reconstruction.
P-value for continuous variables is based on Wilcoxon rank-sum test.
No treatment differences were seen in the Seattle Angina Questionnaire anginal frequency, anginal stability, or quality of life scales (Appendix Table A).
The SF-12 Physical and Mental Component comparisons, reflecting generic health status, showed a single statistically significant difference for the Mental Component at one single time point that was not consistent with the remaining comparisons (Appendix Table A). A similar single significant difference at the same time point was seen for the SF-36 MHI-5, which shares 2 questions with the SF-12 Mental Component. No treatment differences were seen in the other SF-36 scales.
Depressive symptoms decreased significantly post-operatively in both treatment groups but were not different between each group at any point during follow up (Appendix Table A).
No significant treatment-related differences were found in the 0-100 general health self-rating scale or the EuroQoL 5-D.
Resource Use and Medical Costs in US Patients
Total operative time, need for post-operative PA catheters, intraaortic balloon pumps, and intravenous inotrope therapy were all greater in the CABG-plus-SVR arm (Table 2). The CABG-plus-SVR arm had 4.2 extra post-operative intensive care unit days (p<0.001). Days spent in non-ICU rooms and pre-operative days in the hospital did not differ significantly by treatment. Total length of stay for the CABG-only group was 13.5 ± 13.0 days and for the CABG-plus-SVR group 16.8 ± 12.3 days (p=0.03). Rates of any follow-up all-cause hospitalization from randomization to the latest follow-up were equivalent for the two arms (66.7% for CABG alone and 72.5% for CABG plus SVR, p=0.37).
Table 2.
Index Hospital Costs and Resource Use in US Patients
| CABG | CABG + SVR | P-value | |
|---|---|---|---|
| Resource Use | |||
| Total time in OR*- hours | |||
| No. of patients | 99 | 96 | |
| Median (interquartile range) | 5.4 (4.7, 6.6) | 6.7 (5.7, 7.7) | |
| Mean ± standard deviation | 5.7 ± 1.3 | 6.8 ± 1.5 | <0.001 |
| Post-op time in ICU/CCU*- days | |||
| No. of patients | 99 | 94 | |
| Median (interquartile range) | 2.2 (1.3, 4.0) | 4.8 (2.0, 8.7) | |
| Mean ± standard deviation | 3.4 ± 3.8 | 7.6 ± 11.5 | <0.001 |
| Total ICU time**- days | |||
| No. of patients | 100 | 96 | |
| Median (interquartile range) | 4.5 (2.0, 7.5) | 6.0 (4.0, 12.0) | |
| Mean ± standard deviation | 6.0 ± 9.5 | 9.9 ± 10.6 | 0.0002 |
| Post-op length of stay*- days | |||
| No. of patients | 99 | 95 | |
| Median (interquartile range) | 7.0 (5.0, 10.0) | 9.0 (7.0, 17.0) | |
| Mean ± standard deviation | 9.5 ± 10.5 | 13.4 ± 11.7 | <0.001 |
| Total length of stay**- days | |||
| No. of patients | 100 | 96 | |
| Median (interquartile range) | 11.0 (7.0, 16.0) | 12.5 (8.0, 18.5) | |
| Mean ± standard deviation | 13.5 ± 13.0 | 16.8 ± 12.3 | 0.03 |
| Other Resource Use | |||
| PA Catheter* | |||
| No. of patients | 101 | 98 | |
| Patients receiving PA catheter- % | 17.8 | 27.6 | 0.10 |
| IABP for low CO* | |||
| No. of patients | 101 | 98 | |
| Patients receiving IABP- % | 11.9 | 32.7 | 0.0003 |
| Inotropes for low CO* | |||
| No. of patients | 101 | 98 | |
| Patients receiving inotropes- % | 38.6 | 62.2 | 0.0008 |
| Costs | |||
| Hospital admission** | |||
| No. of patients | 100 | 96 | |
| Median (interquartile range) | $38,858 ($26,508, $57,841) | $49,011 ($33,586, $77,322) | |
| Mean ± standard deviation | $50,939 ± 46,458 | $64,202 ± 49,172 | 0.006 |
| Physician fees** | |||
| No. of patients | 100 | 96 | |
| Median (interquartile range) | $4750 ($3963, $5959) | $6028 ($4837, $7454) | |
| Mean ± standard deviation | $ 5183 ± 2306 | $ 6515 ± 2463 | <0.0001 |
| Total index hospitalization cost** | |||
| No. of patients | 100 | 96 | |
| Median (interquartile range) | $44,760 ($30,481, $63,379) | $54,650 ($38,044, $85,794) | |
| Mean ± standard deviation | $56,122 ± 48,552 | $70,717 ± 51,367 | 0.004 |
Costs are reported in 2008 US Dollars. CO=cardiac output, IABP= intra-aortic balloon pump
The data for these items comes from the clinical case report form.
The data for these items comes from the hospital bills.
Total index hospitalization costs were $14,595 higher for CABG plus SVR (p=0.006) (Table 2 and Figure 2). The median difference in costs was $10,966 (Hodges-Lehman statistic) and the 95% confidence limit was $3677 to $18,218 (p=0.004).
Figure 2.
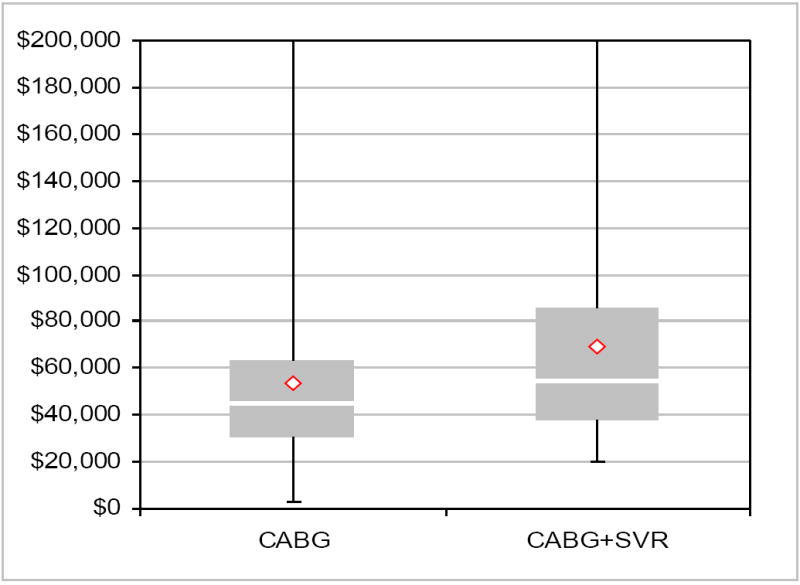
A box and whisker plot of the distribution of total index hospitalization costs for the 196 U.S. patients with cost data. Diamond symbol is mean, central bar is median, top and bottom of box are 75th and 25th percentiles respectively. Error bars represent the minimum and maximum, and the plot truncates the baseline costs at $200,000. Two patients in the CABG-plus-SVR group and one patient in the CABG-only group were above this figure. The plot shows that the entire distribution of costs for the CABG-plus-SVR arm is shifted up (toward higher cost) relative to the CABG-alone arm indicating that the difference between the two arms is not driven by a small proportion of outlier values.
DISCUSSION
The STICH trial provides the first comprehensive clinical, quality of life, and economic randomized trial evaluation of the strategy of adding surgical ventricular reconstruction (SVR) to coronary bypass surgery in patients with advanced ischemic cardiomyopathy. We hypothesized that the benefits of surgically creating a smaller, more normally shaped ventricle would include reduced heart failure symptoms with consequent improved functioning and QOL and reduced need for rehospitalization. We also postulated that if these clinical results were obtained, the incremental cost of the SVR procedure would be judged good value for money based on conventional cost-effectiveness criteria. However, our data do not show any evidence of incremental benefit in health-related QOL by adding SVR to CABG in patients with ischemic heart failure and large anterior scar. Because SVR significantly increases costs, we can confidently conclude that there is no justification for routine performance of this technique in STICH-eligible patients.
One notable feature of our trial results is the substantial and consistent improvement in multiple domains of QOL observed following surgery compared with the pre-operative state. As reported in the primary clinical report from this trial, only 4% of patients had NYHA symptoms class I pre-operatively and 15% had class IV symptoms, while post-operatively 40% of the survivors were class I and 2% were class IV.5 The Kansas City Cardiomyopathy Questionnaire improved over time by about 30 points, corresponding to a large, clinically important treatment effect. For reference, a 5-point change for this scale is regarded as a small but clinically meaningful change, a 10-point change is considered a moderately large change, and changes >20 points are large.8 Notably, all 6 subscales of the KCCQ showed the same patterns of improvement. In addition, other measures of both functioning and well-being showed clinically meaningful improvements relative to the pre-operative state indicating significant increases in psychological well-being, role functioning, social functioning, and self-efficacy with about a 50% reduction in the prevalence of depressive symptoms. Further insights into these changes will be provided by the second randomized trial in the STICH program, which compares medical therapy alone with medical therapy plus CABG in ischemic heart failure patients eligible for CABG.6
Several small observational studies have previously reported on the QOL effects of SVR.3,4 While they observed post-operative improvement in QOL, the small samples and lack of adequate controls made it impossible to discern what role the SVR had in creating the improvement. The 1,198-patient RESTORE registry reported a similar level of improvement in heart failure symptoms to that seen in STICH but was also unable to isolate the contribution of the SVR due to the absence of a control group.2
No prior study of SVR has reported on the incremental costs of the procedure in the U.S. healthcare system. We expected the procedure to be at least modestly more expensive initially, since it required more operative time to perform relative to a CABG alone. Our study also shows that the post-operative course was more complex and required higher intensity, ICU-based care than CABG alone. We cannot discern from our data whether the patient’s clinical course prompted the extra use of PA catheters, balloon pumps, inotropic stimulants, and extra time in the ICU or whether this was chosen out of an abundance of caution by the surgeons, who were not blinded to the treatment assignment.
Caveats relevant to our QOL results are primarily those that pertain to the underlying trial. To the extent that a cohort of SVR-eligible patients exists who were not enrolled in the trial, perhaps because clinicians did not have equipoise regarding their enrollment, our results might not be generalizable to such patients. However, one advantage of a large international clinical trial is that differences in equipoise and decision-making among investigators will often result in enrollment of the same broad group of ischemic heart failure patients that are being considered for these procedures in clinical practices. We did not calculate medical costs for patients enrolled in STICH outside the United States, given the absence of suitable cost weights and the possible effects of differences in practice patterns. While post-operative length of stay differences were smaller in the non-U.S. centers, the CABG-plus-SVR group had a longer length of stay. Although the magnitude of the cost difference between the treatment arms might vary among centers, both in the United States and internationally, performing the SVR procedure clearly increased costs due to a more complex post-operative course and there was no evidence at all of a late decrease in rehospitalization or repeat cardiac procedures that might have provided some offset of the higher initial costs. In economic terms, any procedure that costs more and does not provide some incremental patient outcome benefits is dominated, meaning that the alternative less costly and equally effective treatment would always be preferred.18
In summary, we found no evidence that adding surgical ventricular reconstruction to coronary bypass graft surgery provided any incremental improvements in quality of life out to 3 years following surgery. Since SVR increases the complexity of post-operative care and consequently significantly increases the cost of the procedure over CABG alone, our results do not provide any justification for continued use of this procedure in STICH-eligible patients.
Acknowledgments
The authors appreciate the editorial assistance of Melanie R. Daniels. We are particularly indebted to the coordinators at the STICH sites that collected data for this portion of the STICH research effort and to the 1000 patients who agreed to participate in this clinical trial.
Supported by grant UO1 HL69011 from the National Heart, Lung, and Blood Institute/ National Institutes of Health, Bethesda, Maryland. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Footnotes
DISCLOSURES Dr. John Spertus reports that he holds the copyright for the Kansas City Cardiomyopathy Questionnaire and the Seattle Angina Questionnaire. There are no other conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dor V. Reconstructive left ventricular surgery for post-ischemic akinetic dilatation. Semin Thorac Cardiovasc Surg. 1997;9:139–45. [PubMed] [Google Scholar]
- 2.Athanasuleas CL, Buckberg GD, Stanley AW, Siler W, Dor V, Di DM, Menicanti L, meida de OS, Beyersdorf F, Kron IL, Suma H, Kouchoukos NT, Moore W, McCarthy PM, Oz MC, Fontan F, Scott ML, Accola KA. Surgical ventricular restoration in the treatment of congestive heart failure due to post-infarction ventricular dilation. J Am Coll Cardiol. 2004;44:1439–45. doi: 10.1016/j.jacc.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Sartipy U, Albage A, Lindblom D. Improved health-related quality of life and functional status after surgical ventricular restoration. Ann Thorac Surg. 2007;83:1381–7. doi: 10.1016/j.athoracsur.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 4.Cotrufo M, Romano G, De Santo LS, Della CA, Amarelli C, Cafarella G, Maiello C, Scardone M. Treatment of extensive ischemic cardiomyopathy: quality of life following two different surgical strategies. Eur J Cardiothorac Surg. 2005;27:481–7. doi: 10.1016/j.ejcts.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Jones RH STICH Hypothesis 2 Investigators. Surgical Treatment for Ischemic Heart Failure (STICH) trial evaluation of surgical ventricular reconstruction. 2009 Submitted. [Google Scholar]
- 6.Velazquez EJ, Lee KL, O’Connor CM, Oh JK, Bonow RO, Pohost GM, Feldman AM, Mark DB, Panza JA, Sopko G, Rouleau JL, Jones RH. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007;134:1540–7. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 8.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–15. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–41. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 10.Ware JEJ, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual & Interpretation Guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 11.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 13.Sullivan MD, LaCroix AZ, Russo J, Katon WJ. Self-efficacy and self-reported functional status in coronary heart disease: a six-month prospective study. Psychosom Med. 1998;60:473–8. doi: 10.1097/00006842-199807000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Rosser RM, Stintonen H. The EuroQoL quality of life project. In: Walker SR, Rosser RM, editors. Quality of Life Assessment: Key Issues in the 1990s. Dordrecht: Kluwer: Academic Publishers; 1993. pp. 197–9. [Google Scholar]
- 15.EuroQol--a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 16.Mark DB, Nelson CL, Anstrom KJ, Al-Khatib SM, Tsiatis AA, Cowper PA, Clapp-Channing NE, Davidson-Ray L, Poole JE, Johnson G, Anderson J, Lee KL, Bardy GH. Cost-effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Circulation. 2006;114:135–42. doi: 10.1161/CIRCULATIONAHA.105.581884. [DOI] [PubMed] [Google Scholar]
- 17.Mark DB, Pan W, Clapp-Channing NE, Anstrom KJ, Ross JR, Fox RS, Devlin GP, Martin CE, Adlbrecht C, Cowper PA, Davidson-Ray L, Cohen EA, Lamas GA, Hochman JS. Quality of life after late invasive therapy for occluded arteries. N Engl J Med. 2009;360:774–83. doi: 10.1056/NEJMoa0805151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mark DB, Hlatky MA. Medical economics and the assessment of value in cardiovascular medicine: Part I. Circulation. 2002;106:516–20. doi: 10.1161/01.cir.0000021407.93752.7b. [DOI] [PubMed] [Google Scholar]


