Summary
Current dietary management of long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD; long-chain-(S)-3-hydroxyacyl-CoA: NAD+ oxidoreductase, EC 1.1.1.211) deficiency (LCHADD) is based on avoiding fasting, and minimizing energy production from long-chain fatty acids. We report the effects of various dietary manipulations on plasma and urinary laboratory values in a child with LCHADD. In our patient, a diet restricted to 9% of total energy from long-chain fatty acids and administration of 1.5 g medium-chain triglyceride oil per kg body weight normalized plasma acylcarnitine and lactate levels, but dicarboxylic acid excretion remained approximately ten times normal. Plasma docosahexaenoic acid (DHA, 22: 6n – 3) was consistently low over a 2-year period; DHA deficiency may be related to the development of pigmentary retinopathy seen in this patient population. We also conducted a survey of metabolic physicians who treat children with LCHADD to determine current dietary interventions employed and the effects of these interventions on symptoms of this disease. Survey results indicate that a diet low in long-chain fatty acids, supplemented with medium-chain triclyceride oil, decreased the incidence of hypoketotic hypoglycaemia, and improved hypotonia, hepatomegaly, cardiomyopathy, and lactic acidosis. However, dietary treatment did not appear to effect peripheral neuropathy, pigmentary retinopathy or myoglobinuria.
Current treatment for children with long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD; long-chain-(S)-3-hydroxyacyl-CoA: NAD+ oxidoreductase, EC 1.1.1.211) deficiency (LCHADD) is based on minimizing energy production from long-chain fatty acid β-oxidation. Results from a survey of metabolic physicians regarding the treatment of 19 patients with LCHADD, ages 10 months to 17.5 years, are presented. As with many metabolic diseases, diet modifications are essential in the routine management of this disorder but little has been published regarding the dietary management of LCHADD. We report our experience with dietary manipulations aimed at normalizing plasma and urine laboratory values and maintaining metabolic control in a child with LCHADD.
CLINICAL HISTORY
This 27-week-gestation female was delivered by emergency caesarean section following a pregnancy complicated by maternal HELLP (hypertension, elevated liver enzymes, and low platelets) syndrome. The baby received surfactant replacement therapy but required mechanical ventilation for 11 days owing to respiratory distress and subsequently developed bronchopulmonary dysplasia requiring oxygen, bronchodilator, diuretic, and glucocorticoid therapy through the first 5 months of life. An echocardiogram at 19 days of age found a moderate-sized patent ductus arteriosus, which closed spontaneously. Cardiac examination, including echocardiography, was normal at 6 months of age. Ophthalmological examination at 7 months of age was normal.
A previous female sibling had died at 6 months of age. The pregnancy with that child had also been complicated by maternal HELLP syndrome, and post-mortem examination of the sibling demonstrated diffuse infiltration of the liver with fat. No specific cause of death was determined. Because of the family history and the reported association between maternal HELLP syndrome and fetal LCHAD deficiency (Treem et al 1994), an extensive metabolic evaluation of the proband was performed. Plasma amino acid analysis on day 2 of life while the baby was receiving dextrose-containing i.v. fluids showed elevated tyrosine (456 μmol/L), and 4-hydroxyphenyl-acetic, 4-hydroxyphenyllactic, and 4-hydroxyphenylpyruvic acids were detected in urine by organic acid analysis (GC-MS). No succinylacetone or dicarboxylic acids were detected. These abnormalities resolved by 3 weeks of life, at which time the infant was being fed human breast milk supplemented with breast milk fortifier. Plasma acylcarnitine analysis (Biochemical Genetics/Laboratory Diagnostics, Duke Medical Center, North Carolina) at 2 months of age detected multiple long-chain 3-hydroxyacylcarnitines, suggesting the diagnosis of LCHAD deficiency. LCHAD deficiency was confirmed by enzymatic analysis in cultured skin fibroblasts (Dr Daniel Hale, University of Pennsylvania). Residual LCHAD activity was 5.6 pmol/min per mg protein (normal values 8.4–11.2 pmol/min per mg). Sequence analysis of the mitochondrial trifunctional enzyme α-subunit gene revealed compound heterozygosity for a G-to-C mutation at nucleotide position 1528 in one allele and a C-to-T mutation at position 1132 in the other allele (Sims et al 1995).
Dietary management
With a suspected diagnosis of LCHADD at 2 months of age, an infant formula containing predominately medium-chain triglycerides (MCT) and low in long-chain fatty acids (LCFA) was begun. Owing to poor oral intake, a gastrostomy tube was placed at 4 months of age. Diet at 8 months of age provided 12% of energy as LCFA and 38% of energy as MCT (4.5 g/kg per day). On this regimen, long-chain hydroxylated species continued to be detected in plasma by acylcarnitine analysis and the high intake of MCT induced the urinary excretion of medium-chain dicarboxylic acids.
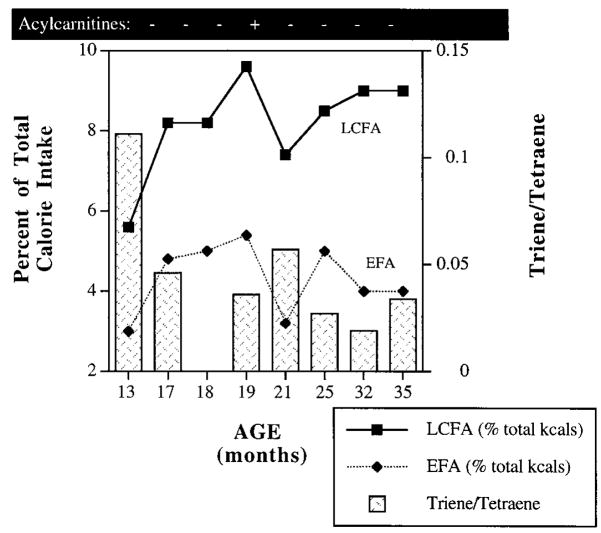
Our goal was to determine a diet prescription to normalize laboratory indices such as plasma acylcarnitines and urinary organic acids as much as possible while maintaining the health and growth of this child. We began with a diet plan that prevented fasting, decreased dietary intake of LCFA, and provided adequate nutrient intake. Fasting was prevented with continuous tube feeding (TF) during the night and bolus feedings every 4 h during the day from age 4 months to 2 years. At 2 years of age, the continuous TF was stopped and cornstarch was added to the patient’s night-time feeding at 2.5 g/kg body weight. The patient tolerated this regimen well with no episodes of hypoglycaemia. To decrease the production of hydroxylated LCFA species, the percentage of energy from LCFA was decreased. Normal plasma acylcarnitine profiles were achieved when LCFA intake remained below 9% of total energy. Slight increases in long-chain acylcarnitines were detected when LCFA intake increased above this level (Figure 1).
Figure 1.
Dietary intake of LCFA and EFA and plasma triene: tetraene ratio in a child with LCHADD. LCFA, long-chain fatty acids; EFA, essential fatty acids. LCFA and EFA intake is expressed as percentage of total energy. Triene: tetraene ratio normal values = 0.02 ± 0.01. Low LCFA and EFA intake is associated with elevated triene: tetraene ratios, indicative of EFA deficiency
MCT supplementation is associated with increased urinary excretion of medium-chain dicarboxylic acids (suberic, sebacic, and adipic acids) which are not normally detected in urine (Baugart et al 1994). To reduce excretion of dicarboxylic acids, MCT intake was gradually decreased. MCT intake was decreased from 2.0 g/kg body weight (18% of energy) at 1 year of age to 1.18 g/kg (12.5% of energy) at 18 months of age, at which time dicarboxylic acids were no longer detected in urine.
Lactic acid measured in venous blood at 13 and 17 months and in arterial blood at 18 months was persistently elevated (> 2 mmol/L) (Figure 2). MCT intake was increased to monitor response in arterial lactate. As MCT intake increased, arterial lactate decreased at both 19 and 21 months of age. A normal venous lactate of 1.5 mmol/L was measured at 31 months of age on 1.5 g/kg MCT or 15% of total energy. At 3 years of age, the patient continues on 1.5 g MCT/kg (15% of energy), blood lactate remains within normal limits, and urinary excretion of suberic, sebacic and adipic acid remain approximately 10 times normal. MCT intake below 1.5 g/kg per day was associated with increased blood lactate concentration in this child.
Figure 2.

Dietary MCT intake and blood lactate concentrations in a child with LCHADD. MCT, medium-chain fatty acids. MCT intake is expressed as g/kg per day. Normal blood lactate ≤2.0 mmol/L. MCT oil intake less than 1.5 g/kg body weight is associated with elevated blood lactate concentrations
Because of the restriction of LCFA, children with LCHADD are at risk for developing essential fatty acid (EFA) deficiency. At 13 months, LCFA comprised only 5.6% of total energy with 3.0% as EFA (Figure 1). At this time, the triene: tetraene ratio (the ratio of plasma eicosatrienoic acid (20: 3n – 9) to arachidonic acid (20: 4n – 6)) was 0.111, indicative of EFA deficiency (normal 0.02 ± 0.01) (Holman, 1960). To increase EFA intake, safflower oil was added and the triene: tetraene ratio improved. However, small increases in LCFA metabolites were measured by plasma acylcarnitine analysis as total LCFA increased above 9% of energy. Currently, the patient follows a diet with 9% of energy from LCFA, 4% as EFA. The triene: tetraene ratio remains between 0.02 and 0.03 and acylcarnitine analysis remains normal.
Plasma concentrations of α-linolenic acid (LNA, C18: 3n – 3) and docosahexaenoic acid (DHA, C22: 6n – 3) are given in Figure 3. Of interest, plasma DHA concentrations remained deficient throughout the 2-year period despite normal concentration of LNA, the precursor for DHA synthesis. Plasma concentration of docosapentaenoic acid (DPA, C22: 5n – 3), another product of LNA elongation, was normal (data not shown).
Figure 3.
Plasma n – 3 fatty acids in a child with LCHADD. Plasma fatty acids are expressed as μg/ml plasma. Normal levels are LNA = 13.29 ± 6.37; DHA = 38.05 ± 18.06. Levels of LNA vary with LCFA intake. DHA levels remain significantly below normal until oral supplementation of DHA was initiated at 35 months. DHA, docosahexaenoic acid; LNA, α-linolenic acid
Oral carnitine was started at age 9 months because plasma free carnitine was low. The treatment of LCHADD with oral carnitine supplementation is controversial, but we have seen no signs of toxicity at a dose of 50 mg/kg.
Since starting diet treatment, this 4-year-old patient had only one episode of hypoglycaemia, vomiting, and myoglobinuria associated with a viral infection at 32 months. She has no signs of delayed development, neuropathy, muscle weakness or cardiomyopathy. Fundoscopic examination has been normal, although visual acuity as measured by visual evoked cortical potential (VECP) analysis is mildly diminished, suggesting early pigmentary retinopathy. Growth is appropriate with height at 10th to 25th centile, and weight at 25th and 50th centile.
SURVEY RESULTS
To determine the effect of treatment on other patients with LCHADD, a survey was sent to 23 physicians who treat this disorder. Fourteen physicians returned a total of 19 surveys. The age of the 19 patients ranged from 10 months to 17.5 years. Average age of onset of symptoms was 8 months (range birth to 29 months). Average age at diagnosis was 25 months (range 1 month to 11 years). For all but two children, the diagnosis of LCHADD was established following one or more episodes of acute metabolic decompensation. In the remaining two patients, the diagnosis of LCHADD was entertained prior to onset of symptoms because of family history. All diagnoses were confirmed by enzyme analysis, and mutational analysis was complete for 10 patients. Of these 10 patients, 9 were either homozygous or heterozygous for the G1528C mutation. Eight of 19 mothers had symptoms of HELLP syndrome during the affected pregnancy.
At the time of the survey, 3 of 19 patients had expired during metabolic crisis. One child died prior to and two died after initiation of LCHADD-specific therapy. Of the 18 patients who received LCHADD-specific therapy, all were treated with a low-fat diet and avoidance of fasting. Table 1 shows the response to treatment for these 18 patients. Sixteen patients received MCT oil supplement. All physicians reported a decrease in frequency of hypoketotic hypoglycaemic episodes once LCHADD-specific therapy was initiated, even in the two children who later died during episodes of severe acidosis. Of the 18 treated patients, 5 had no episodes of metabolic decompensation after LCHADD-specific treatment was begun. In our survey, LCHADD-specific dietary therapy had minimal effect upon the chronic degenerative symptoms of LCHADD such as the development of pigmentary retinopathy and vision loss, the development of peripheral neuropathy, or recurrent episodes of myoglobinuria and muscle pain without hypoglycaemia or acidosis.
Table 1.
Results of a survey of metabolic physicians treating children with LCHADDa
| Symptoms | Number observed/total (% observed) | Mean clinical scoreb (range of scores) |
|---|---|---|
| Metabolic decompensating episodes | 16/18 (89%) | 4.25 (1–5) |
| Hypotonia | 9/16 (56%) | 4.1 (1–5) |
| Developmental delay | 9/17 (53%) | 3.1 (1–5) |
| Failure to thrive | 7/17 (41%) | 4.9 (4–5) |
| Hepatomegaly/hepatic failure | 9/17 (53%) | 4.4 (3–5) |
| Cardiomyopathy | 6/18 (33%) | 4.5 (4–5) |
| Peripheral neuropathy | 6/17 (35%) | 2.2 (1–5) |
| Pigmentary retinopathy | 12/17 (71%) | 1.8 (1–5) |
| Myoglobinuria | 7/18 (39%) | 2.6 (1–5) |
| Lactic acidosis | 6/15 (40%) | 4.7 (4–5) |
| Overall response to treatment | 3.8 (2–5) |
Physicians were asked to identify which symptoms were seen in their patients, what treatment was initiated, and how dietary therapy affected the symptoms observed. Response of specific symptoms to dietary therapy was given a clinical score from 1 to 5 with 1 being deteriorating condition and 5 being reversal of observed symptoms
Clinical score (response to dietary therapy): 1 = deteriorating condition; 2 = no change in clinical condition; 3 = a little improvement; 4 = good improvement; 5 = complete reversal of symptoms
DISCUSSION
The primary goals of nutritional management of LCHADD are to prevent fasting and to decrease dietary intake of LCFA in the diet in order to minimize production of abnormal LCFA metabolites. Plasma acylcarnitine analysis indicated the presence or absence of long-chain 3-hydroxyacylcarnitines. These metabolites were detected in the plasma of this child at 2 months of age, prior to diagnosis, and at 19 months of age when 10% of total energy was from LCFA. Reducing total LCFA intake to below 10% of total energy normalized plasma acylcarnitines.
However, the diet must provide sufficient LCFA to prevent EFA deficiency and adequate energy intake to support normal growth. MCT-oil supplementation has been utilized to increase energy intake from fat yet bypass the enzymatic block in LCFA metabolism. Our experience treating a child with LCHADD and the survey responses suggest better clinical outcome with MCT supplementation, including reduced incidence of metabolic decompensation, an observation reported previously (Brown-Harrison et al 1996; Duran et al 1991).
In our survey, 18 patients with LCHADD were treated with a LCFA-restricted diet; 16 of these were also supplemented with MCT oil. Of the symptoms reported, all appear to improve with treatment except for the chronic problems of retinal degeneration, peripheral neuropathy or recurrent episodes of myoglobinuria. Of the two patients not supplemented with MCT, one died in a metabolic crisis, and the other had the greatest number of metabolic decompensating episodes after beginning dietary treatment.
Increased excretion of omega-oxidation products, suberic, sebacic and adipic acids has been noted in healthy infants fed formulas supplemented with MCT (Baugart et al 1994). In our patient, lactate concentrations increased when MCT intake decreased and total carbohydrate increased. During this period (13–25 months of age), total energy intake (per kg body weight) remained unchanged (100 kcal/kg); only the percentage of energy from carbohydrate and fat varied. Elevated lactate appears to be associated with either excess carbohydrate intake or low MCT intake. LCHADD has been associated with lactic acidosis, and the specific aetiology of lactate accumulation in LCHADD is unproven (Roe and Coates, 1995). In our experience, providing adequate energy as fat, including MCT oil supplementation at 1.5 g/kg or 15% of total energy, is important to maintain normal plasma lactate levels. In the survey, 40% of the patients were found to have episodes of lactic acidosis and response to treatment was favourable (score = 4.7, Table 1).
Plasma DHA content was persistently low in our patient. Although safflower oil supplementation normalized plasma triene to tetraene ratio in our patient, dietary LNA intake remained below recommended amounts (0.05% of energy) (Bjerve et al 1987). Since safflower is a poor source of LNA, we attempted to modify the source of EFA to improve the linoleic acid (C18: 2n – 6) to LNA ratio in the diet. This was difficult to achieve with a diet restricted to less than 9% of energy from LCFA. Despite lower than recommended intake of LNA, plasma concentrations of LNA and DPA in our patient remained within normal limits while DHA deficiency persisted. In preterm infants and animal models that have inadequate dietary LNA intake, low plasma DHA concentrations are associated with concurrent plasma deficiency of all other omega-3 fatty acids (Connor et al 1990; Hoffman and Uauy, 1992). DHA deficiency despite normal LNA concentrations may be caused by limited capability to synthesize DHA from LNA and may not be due to dietary LNA deficiency. Recently, Infante and Huszagh have proposed that DHA synthesis may occur in mitochondria while synthesis of arachidonic acid may occur both in mitochondria and in microsomes (Infante and Huszagh, 1997). We propose that deficiency of LCHAD, a key step in mitochondrial long-chain fatty acid oxidation, interferes with DHA synthesis.
Pigmentary retinopathy is a common complication in patients with LCHADD, but not in patients with other fatty acid β-oxidation defects (Roe and Coates, 1995). Since DHA is found in high concentration in the membrane of retinal photoreceptors (Anderson, 1970), we speculate that DHA deficiency may be a contributing factor in the development of retinal degeneration in LCHADD patients. In the survey, retinal degeneration was one clinical manifestation that continued to deteriorate despite dietary management. We propose that oral supplementation with DHA should be explored to determine whether it could alter the natural history of retinal degeneration in LCHADD.
In summary, many of the metabolic abnormalities in a patient with LCHADD were corrected by adjusting the amount and type of fat intake (Table 2). The dietary treatment of LCHAD deficiency should be individualized for each patient, but these observations offer several guidelines on which to base dietary therapy. The diet should prevent fasting, limiting LCFA to approximately 10% of total energy, and supply additional energy with MCT oil supplementation. For infants and toddlers, a MCT dose of 1–1.5 g/kg or 10–15% of total energy appears appropriate. These children are at risk for EFA deficiency and plasma EFA should be monitored. Despite these measures, plasma DHA levels may continue to be low.
Table 2.
A dietary approach for correcting metabolic abnormalities associated with excess or deficiency of specific dietary fats
| Componenta | Specific nutrient excess | Specific nutrient deficiency |
|---|---|---|
| LCFA or EFA | Plasma long-chain 3-hydroxy acylcarnitines Long-chain 3-hydroxydicarboxylic aciduria |
Plasma essential fatty acid deficiencyb |
| MCT oil | Medium-chain dicarboxylic aciduria | Elevated blood lactate |
| DHA | No known complication | Plasma DHA deficiency |
LCFA, long-chain fatty acids; EFA, essential fatty acids; MCT, medium-chain triglyceride; DHA, docosahexaenoic acid
Plasma EFA deficiency is defined as a triene: tetraene ratio ≥0.03
These results suggest a dietary approach for correcting the abnormal levels of metabolites associated with LCHADD. Further controlled studies are required to determine whether these surrogate laboratory indices correlate with clinical outcomes. In particular, the hypothesis that DHA deficiency plays a role in pigmentary retinopathy and other chronic complications is under study.
References
- Anderson RE. Lipids of ocular tissues IV. A comparison of the phospholipids from the retina of six mammalian species. Eye Exp Res. 1970;10:339–344. doi: 10.1016/s0014-4835(70)80046-x. [DOI] [PubMed] [Google Scholar]
- Baugart S, Pereira GR, Bennett MJ. Excretion of dicarboxylic acids in preterm infants fed medium- or long-chain triglycerides. J Pediatr. 1994;125:509–510. doi: 10.1016/s0022-3476(05)83318-9. [DOI] [PubMed] [Google Scholar]
- Bjerve KS, Mostad IL, Thoresen L. Alpha-linolenic deficiency in patients on long-term gastric-tube feeding: estimation of linolenic acid and long-chain unsaturated n – 3 fatty acid requirement in man. Am J Clin Nutr. 1987;45:66–77. doi: 10.1093/ajcn/45.1.66. [DOI] [PubMed] [Google Scholar]
- Brown-Harrison MC, Nada MA, Sprecher H, et al. Very long chain acyl-CoA dehydrogenase deficiency: successful treatment of acute cardiomyopathy. Biochem Mol Med. 1996;58:59–65. doi: 10.1006/bmme.1996.0033. [DOI] [PubMed] [Google Scholar]
- Connor WE, Neuringer M, Lin DS. Dietary effects on brain fatty acid composition: the reversibility of n – 3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J L ipid Res. 1990;31:237–247. [PubMed] [Google Scholar]
- Duran M, Wanders RJA, de Jager JP, et al. 3-Hydroxydicarboxylic aciduria due to long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency associated with sudden neonatal death: protective effect of medium-chain triglyceride treatment. Eur J Pediatr. 1991;150:190–195. doi: 10.1007/BF01963564. [DOI] [PubMed] [Google Scholar]
- Hoffman DR, Uauy R. Essentiality of dietary n – 3 fatty acids for premature infants: plasma and red blood cell fatty acid composition. L ipids. 1992;27:886–895. doi: 10.1007/BF02535868. [DOI] [PubMed] [Google Scholar]
- Holman RT. The ratio of trienoic: tetraenoic acids in tissue lipids as a measure of essential fatty acid requirement. J Nutr. 1960;70:405–410. doi: 10.1093/jn/70.3.405. [DOI] [PubMed] [Google Scholar]
- Infante JP, Huszagh VA. On the molecular etiology of decreased arachidonic (20: 4n – 6), docosapentaenoic (22: 5n – 3) and docosahexaenoic (22: 6n – 3) acids in Zellweger syndrome and other peroxisomal disorders. Mol Cell Biochem. 1997;168:101–115. doi: 10.1023/a:1006895209833. [DOI] [PubMed] [Google Scholar]
- Roe CR, Coates PM. Mitochondrial fatty acid oxidation disorders. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 1995. pp. 1501–1514. [Google Scholar]
- Treem WR, Rinaldo P, Hale DE, et al. Acute fatty liver of pregnancy and long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. Hepatology. 1994;19:339–345. [PubMed] [Google Scholar]
- Sims HF, Brackett JC, Powell CK, et al. The molecular basis of pediatric long chain 3-hydroxyacyl-CoA dehydrogenase deficiency associated with maternal acute fatty liver of pregnancy. Proc Natl Acad Sci USA. 1995;92:841–845. doi: 10.1073/pnas.92.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]