Abstract
Treatment of many inherited liver enzyme deficiencies requires the removal of toxic intermediate metabolites from the blood of affected individuals. We propose that circulating toxins can be adequately cleared and disease phenotype influenced by enzyme expressed in tissues other than the liver. Phenylalanine hydroxylase (PAH) activity was constitutively expressed in skeletal and cardiac muscle of transgenic mice which carried the PAH cDNA under the transcriptional control of the mouse muscle creatine kinase promoter. Muscle PAH-expressing mice were bred to liver PAH-deficient, hyperphenylalaninemic mice to yield progeny that lack PAH activity in liver but express PAH in muscle. These mice exhibited hyperphenylalaninemia at baseline, but serum phenylalanine levels decreased significantly when the mice were supplemented with tetrahydrobiopterin (BH4), a required cofactor for PAH. This is the first demonstration that a liver-specific enzyme, when expressed in a heterologous tissue and supplied with necessary cofactors, can effectively clear toxic metabolites from the circulation of individuals with inherited enzyme deficiency. This result suggests that gene therapy targeted to heterologous tissues, such as muscle, will be effective in the treatment of selected inborn errors of metabolism.
Keywords: inborn errors of metabolism, phenylketonuria, muscle
Introduction
The pathology associated with inherited inborn errors of metabolism is frequently caused by accumulated intermediate metabolites which are toxic at high concentrations in blood and other tissues. Examples of such metabolites include cholesterol in familial hypercholesterolemia, methylmalonic acid in methylmalonic acidemia, and phenylalanine in phenylketonuria (PKU). Contemporary therapy for these diseases is based upon dietary manipulation or pharmacotherapy to decrease the production of the offending metabolites and has resulted in varying degrees of clinical success. Many investigators are evaluating liver-directed gene therapy as a potential treatment modality for metabolic disorders caused by specific liver enzyme deficiencies, but many currently available gene transfer methods are limited by either inadequate gene expression or short duration.1 Recent results of gene transfer experiments utilizing recombinant adeno-associated virus (rAAV) vectors in rodent liver have been more promising, but the efficacy and stability of rAAV-mediated liver-directed gene transfer is still being evaluated.2,3 As an alternative to liver-directed gene transfer, stable expression of a normally liver-specific enzyme in a heterologous tissue may effectively clear toxic metabolites that accumulate in inborn errors of metabolism. This study was designed to test our hypothesis that heterologous gene expression in skeletal muscle could be an effective therapy for select inborn errors of metabolism.
Our laboratory has pioneered direct injection of naked DNA into skeletal muscle.4 This technique partially corrects dystrophin deficiency in mdx mice, a mouse model of human Duchenne muscular dystrophy.5 Skeletal muscle also has provided a stable platform for the ectopic production and secretion of circulating proteins such as coagulation Factor IX6 and growth hormone.7 Immunization against both viral8 and bacterial9 pathogens has been accomplished by intramuscular injection of naked plasmid DNA containing gene sequences from the microbial pathogen. In this report, we propose an additional application of muscle-directed gene transfer: stable heterologous expression of enzyme protein for the purpose of clearing from the circulation the toxic metabolites that accumulate in inborn errors of metabolism.
We hypothesized that skeletal muscle can be metabolically engineered to express an enzyme of intermediate metabolism that is deficient in a specific inborn error of metabolism, and that, in the presence of necessary cofactors, heterologously expressed enzyme will clear the accumulated toxic metabolite from the circulation allowing the products of the reaction to return to other tissues, such as the liver, for further processing. Skeletal muscle comprises 40% of total body weight in an adult human, is well vascularized, and more easily accessible through percutaneous techniques than is the liver. These features make skeletal muscle an attractive target for gene transfer protocols. To test our hypothesis, we have employed a variety of viral and nonviral DNA transfer techniques to induce phenylalanine hydroxylase (PAH) (EC 1.14.16.1) activity in skeletal muscle of hyperphenylalaninemic Pahenu2 homozygous mice, a well-characterized mouse model for human phenylketonuria.10 Unfortunately, all attempts with contemporary gene transfer methods failed to produce sufficient stable PAH expression in muscle to assess adequately the affect of muscle PAH activity upon the hyperphenylalaninemic phenotype of Pahenu2 mice (unpublished data). Pending the development of more effective muscle-directed DNA transfer methods, we turned to germline modification techniques to produce transgenic mice that constitutively express PAH in muscle. Muscle PAH-expressing mice were bred to Pahenu2 mice, and serum phenylalanine levels were then measured in progeny to determine if PAH protein present in muscle will clear phenylalanine from the circulation of phenylketonuric mice. This experiment directly assessed whether heterologous expression of an enzyme usually confined to the liver would adversely alter muscle function and tested our hypothesis that gene expression in heterologous tissues will be effective therapy for inborn errors of metabolism.
Results
Development of transgenic mice expressing PAH in muscle
We developed transgenic mice which constitutively express PAH in skeletal and cardiac muscle. Mouse liver PAH cDNA (kindly provided by Dr S Woo, Baylor University, Houston, TX, USA) was fused to a 3300 bp DNA sequence from the 5′ regulatory regions of the mouse muscle creatine kinase (MCK) gene (Figure 1). The MCK fragment contains both the promoter and enhancer sequences which drive tissue-specific MCK expression in muscle, and plasmid vectors containing these sequences direct reporter gene expression only in muscle-derived cell lines.11 These same sequences have been shown to drive muscle-specific expression of a reporter gene (chloramphenicol acetyltransferase (CAT)) in transgenic mice.12 Intronic sequences from the rabbit β-globin gene13 were included in the construct to enhance transcription of the PAH cDNA in a transgenic animal. A typical TATA-box sequence and the putative transcription initiation site are located in the 3300 MCK fragment at −38 bp and −7 bp, respectively, relative to the BstEII restriction site. The first translation start site (ATG) is located in the mouse PAH cDNA 53 bp downstream from the 5′ EcoRI site, and a native polyadenylation signal is present in the mouse PAH cDNA downstream from the open reading frame. Transcription of the transgene yields a putative 2.9 kb pre-mRNA that is reduced to 2.3 kb after removal of the β-globin intronic sequence by splicing. Translation yields the 454 amino acid murine PAH monomer. No rabbit β-globin amino acid sequences are present in the final polypeptide.
Figure 1.
MCKβgmPAH transgene. Diagram of the MCKβgmPAH construct employed in the production of transgenic mice. Muscle-specific PAH expression is directed by a 3300 bp sequence containing the enhancer and promoter elements from the mouse muscle creatine kinase (MCK) gene. The putative transcription initiation site (▽) is located at position −7 relative to the BstEII restriction site. The 2 kb mouse PAH cDNA was cloned into the third exon of the rabbit β-globin gene at an EcoRI site. This β-globin gene fragment contained the 3′ end of the second exon beginning at the BamHI site, all of the second intron and third exon. The translation start site (▼) is located in the mPAH cDNA at position +733.
The final 6.7 kb construct was cleaved from the bacterial plasmid backbone and purified for microinjection into nuclei of fertilized eggs according to standard techniques.14 Genomic DNA was isolated from tail biopsies and analyzed for the presence of the transgene by Southern blotting using a portion of the rabbit β-globin sequence as the probe. Of 51 pups, five founder mice (three male, two female) carrying the transgene were detected. Breeding lines were successfully established from four of the founders, and evidence for expression of the transgene was found in offspring of three founder mice.
One of these founder lines, which we designated TgN(MCKPah)15Mes, carried approximately 5–10 copies of the transgene as a tandem repeat. In this mouse line (hereafter referred to as MCKPah), PAH activity was detected in tissue homogenates from liver and kidney (as would normally be expected) but also in both skeletal and cardiac muscle. The specific activity of the enzyme in skeletal muscle from one mouse was approximately one-half of that in liver (43.4 nmol tyrosine produced per milligram protein per hour for muscle versus 112.4 nmol tyrosine per milligram per hour in liver). Given the measured protein content of the tissue homogenates, the total yield of PAH enzyme activity in the two tissues of the transgenic mouse was approximately 2 nmol tyrosine produced per hour per milligram muscle (wet weight) and 14 nmol tyrosine per hour per milligram liver. Production of PAH activity from the transgene was also detected in cardiac muscle homogenate (5.28 nmol per milligram per hour). Native PAH activity in kidney measured 39.1 nmol/mg/h. PAH activity above background levels (0.42 ± 0.37 nmol/mg/h) was not detected in brain, lung, thymus, spleen or testis. The MCKPah mice were healthy, active and showed no signs of impaired muscle function or fertility. In this line, approximately 50% of offspring inherit the transgene indicating no negative selection against transgenic mice. Therefore, ectopic PAH expression in muscle is not detrimental to the health, neurologic function or reproduction of MCKPah mice.
Effect of muscle PAH expression upon hyperphenylalaninemic mice
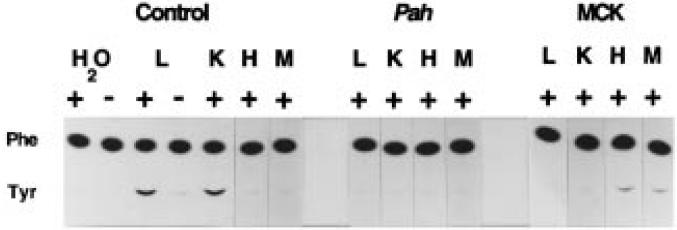
To determine if heterologous expression of PAH in skeletal muscle will clear circulating phenylalanine in hyperphenylalaninemic individuals, we crossed muscle PAH-expressing MCKPah transgenic mice to Pahenu2/Pahenu2 hyperphenylalaninemic mice (which are homozygous for a T to C mutation at nucleotide position 788 of the PAH cDNA).15 The resulting MCKPah transgenic/Pahenu2 heterozygous progeny were then bred to Pahenu2/Pahenu2 mice. In this second generation, 10 mice (designation Tg/Pahenu2) out of 52 progeny were both heterozygous for the MCKβgmPAH transgene and homozygous for the T788C Pahenu2 mutation as verified by mutation-specific PCR analysis. Tg/Pahenu2 mice expressed PAH activity in cardiac and skeletal muscle but not in liver or kidney (Figure 2) confirming that the MCK regulatory sequences direct tissue-specific reporter gene expression in cardiac and skeletal muscle. The lower limit of detection with the radiochemical assay employed in this experiment is 1% of normal liver PAH activity.
Figure 2.
PAH activity in tissues of Tg/Pahenu2 mice. Autoradiogram of thin layer chromatography demonstrating the analysis of PAH activity in liver (L), kidney (K), heart (H) and skeletal muscle (M) of wild-type (Control), Pahenu2/Pahenu2 (Pah) and Tg/Pahenu2 (MCK) mice. C14-labelled phenylalanine (Phe) is incubated with tissue homogenate in the presence (+) or absence (−) of exogenous 6-methyltetrahydropterin. Labelled tyrosine (Tyr) is produced if PAH activity is present. The conversion of phenylalanine to tyrosine in the absence of tissue homogenate is also measured (H2O).
On a standard mouse chow diet (20% protein) and without tetrahydrobiopterin (BH4) supplementation, the serum phenylalanine levels of these mice were significantly elevated (29.9 ± 7.9 mg/dl; n = 10) compared with wild-type C57Bl/6J mice (2.3 ± 0.2 mg/dl, n = 5) and were not significantly different (P = 0.14) from serum phenylalanine levels measured in nontransgenic Pahenu2/Pahenu2 mice (25.6 ± 1.3 mg/dl, n = 5). Also, Tg/Pahenu2 mice exhibited physical characteristics (poor growth, hypopigmentation) identical to those of non-transgenic hyperphenylalaninemic mice. Thus, heterologous PAH expression in skeletal and cardiac muscle alone without BH4 supplementation did not affect the hyperphenylalaninemic phenotype of Pahenu2/Pahenu2 mice.
BH4 supplementation in muscle PAH-expressing hyperphenyalaninemic mice
Tetrahydrobiopterin (BH4) in its reduced state is a required cofactor for PAH activity. BH4 is present in rat skeletal muscle in only small amounts (0.08 μg/g wet weight versus 1.6 μg/g wet weight in the liver).16 Total biopterin was measured in liver and skeletal muscle homogenate from C57Bl/6J mice in our colony by Dr Sheldon Milstien, Bethesda, MD, USA. Murine liver contained 3.0 ± 0.9 pmol biopterin per milligram liver wet weight (mean ± s.d.; n = 4) while skeletal muscle contained only 0.06 ± 0.01 pmol/mg, an amount apparently insufficient to support enough phenylalanine hydroxylation by skeletal muscle to modify serum phenylalanine concentrations or phenotype in Tg/Pahenu2 mice. Tg/Pahenu2 mice and their littermates were supplemented with BH4 using a single dose of 1.0 μmol/g body weight BH4 in 1% ascorbic acid, pH 7, administered by intraperitoneal injection. Blood samples for serum phenylalanine measurement were obtained just before and at 2, 4, 6, 9, 12, 18 and 24 h following the BH4 injection. Serum phenylalanine levels in nonhyperphenylalaninemic mice were not affected by BH4 supplementation regardless of the transgenic status of the mice (data not shown). Six hours after BH4 injection, serum phenylalanine levels in Tg/Pahenu2 mice had decreased an average of 25.7 ± 6.2% (mean ± s.e.; n = 4) but had decreased only 2.3 ± 4.3% in nontransgenic Pahenu2/Pahenu2 mice (n = 3) (P = 0.0015, one-tailed Student's t test) (Figure 3). Serum phenylalanine concentration in a single Tg/Pahenu2 mouse (Figure 3, ‘Best trial’) decreased from 43.7 mg/dl at time 0 to 25.5 mg/dl at 6 h following BH4 injection, a maximal decrease of 42%. Beyond 6 h, serum phenylalanine steadily increased in Tg/Pahenu2 mice reaching pretreatment levels by 24 h after the BH4 injection. Serum phenylalanine levels in nontransgenic Pahenu2/Pahenu2 mice increased above baseline between 10 and 24 h after BH4 injection; we postulate that this increase is due to accelerated protein catabolism associated with repeated handling and anesthesia of the mice. This effect is also seen in nontransgenic Pahenu2/Pahenu2 mice injected only with normal saline and then bled repeatedly for 24 h (data not shown). In summary, data from the single biopterin injection trial demonstrate that heterologous PAH expression in skeletal and cardiac muscle, when supplemented with BH4, can remove phenylalanine from the circulation of hyperphenylalaninemic mice.
Figure 3.
Intraperitoneal BH4 supplementation in muscle PAH-expressing hyperphenylalaninemic mice. Left axis: mean change (%) ± s.e. in serum phenylalanine levels versus time (hrs) in Tg/Pahenu2 ((+) Transgene, solid circles, n = 4) and non-transgenic Pahenu2/Pahenu2 ((−) Transgene, open squares, n = 3) mice supplemented with a single intraperitoneal injection of 1.0 μmol BH4/g body weight. Serum phenylalanine levels were measured repeatedly in each mouse following BH4 injection. Because serum phenylalanine levels at time 0 differed for each mouse, the change in serum phenylalanine from time 0 was calculated at each time-point for each mouse, normalized to the preinjection serum phenylalanine level for that mouse, and expressed as a percentage. Right axis: serum phenylalanine concentration (mg/dl) versus time (h) in a single Tg/Pahenu2 mouse (Best trial, open circles) supplemented with a single intraperitoneal injection of 1.0 μmol BH4/g body weight.
Phenylalanine clearance in Tg/Pahenu2 mice was further enhanced by repeated BH4 injections. In multiple separate trials, Tg/Pahenu2 mice were injected hourly or every 2 h with either reduced BH4 in 1% ascorbic acid, pH 7 or with ascorbic acid alone. In mice receiving BH4, the BH4 dose was either 0.1 or 0.3 μmole BH4/g body weight. Serum phenylalanine levels of mice receiving repeated injections of BH4 decreased from a pretrial average of 31.0 ± 1.6 mg/dl to 12.1 ± 1.8 mg/dl (n = 6, t = 7.94, P < 0.001) by 9 h, a 61% decrease, but increased slightly to 17.5 ± 3.6 mg/dl at 12 h (Figure 4). Serum phenylalanine levels of mice receiving ascorbic acid alone increased from 30.6 ± 2.7 mg/dl to 35.5 ± 3.0 mg/dl (n = 5, t = −1.20, P = 0.26) over the same time period. Repeated handling, anesthesia, injection and phlebotomy of the mice caused weight loss and increasing lethargy in all of the mice regardless of whether BH4 or ascorbic acid alone had been administered. Weight loss and lethargy also occurred in wild-type C57Bl/6J mice treated similarly (data not shown). Most trials were terminated by 12 h because of lethargy in the mice. Two Tg/Pahenu2 mice, one receiving BH4 and the other ascorbic acid, died after 12 h during anesthesia, but the remaining mice recovered spontaneously. Hourly BH4 injections (0.1 μmol BH4/g body weight) were continued for 24 h in a single trial with two Tg/Pahenu2 mice. Serum phenylalanine in one mouse decreased from 34.6 mg/dl to 14.5 mg/dl after 18 h of BH4 injections but rose to 31.4 mg/dl at 24 h coincident with severe lethargy in the mouse. The other mouse appeared healthy throughout the trial, and the serum phenylalanine concentration decreased from 32.1 mg/dl pretrial to 3.1 mg/dl at 24 h (Figure 4). With adequate continual reduced BH4 supply, heterologous expression of PAH in skeletal muscle can effect a persistent decrease of the serum phenylalanine concentrations in phenylketonuric mice to nearly normal levels.
Figure 4.
Repeated intraperitoneal BH4 supplementation in muscle PAH-expressing hyperphenylalaninemic mice. Mean serum phenylalanine levels (mg/dl) ± s.e. versus time (h) of Tg/Pahenu2 mice which received repeated intraperitoneal injections (hourly or every 2 h) of either reduced BH4 in 1% ascorbic acid, pH 7.0 (BH4, open squares; five mice, 12 trials) or 1% ascorbic acid alone (Vit. C, solid diamonds; three mice, seven trials). In a single trial, hourly BH4 injections (0.1 μmol BH4/g body weight) in a Tg/Pahenu2 mouse were associated with a decrease in serum phenylalanine to 3 mg/dl by 18 h (Best trial, open circles).
Discussion
These experiments demonstrate that expression of PAH in skeletal and cardiac muscle in the presence of adequate BH4 can effectively clear phenylalanine from the circulation of hyperphenylalaninemic mice. Phenylalanine clearance in the mice is not dependent upon restoration of PAH activity in liver and can be accomplished via phenylalanine hydroxylation in other tissues. Although skeletal muscle has been used as a platform for the production and secretion of desirable protein products,6,7 this is the first demonstration that a functional enzyme of intermediate metabolism expressed in a heterologous tissue can clear a toxic metabolite from the circulation without causing any apparent adverse effects. The results of our experiments with muscle PAH-expressing transgenic mice prove the principle that expression in a heterologous tissue of a normally liver-specific enzyme can affect the phenotype of an inborn error of metabolism. Until recently, no muscle-directed gene transfer method supplied sufficient stable gene expression to assess directly whether PAH expression in muscle could lower blood phenylalanine levels in hyperphenylalaninemic mice. Recent successes with muscle-directed gene transfer mediated by recombinant adeno-associated virus vectors17,18 or by arterial infusion of plasmid DNA into an extremity19 suggest that stable, physiologically significant gene expression in muscle is achievable; these methods may yield sufficient stable muscle PAH expression to alter the phenotype of phenylketonuric individuals.
In these experiments, the rate of phenylalanine clearance and the average serum phenylalanine measured at various time-points were not significantly different among individual trials regardless of whether the BH4 dose was 0.1 μmol/g body weight hourly, 0.3 μmol/g hourly or 0.3 μmol/g every 2 h (data not shown). This suggests that, once BH4 had been administered to Tg/Pahenu2 mice, the phenylalanine clearance rate may have been limited by factors other than the amount of BH4 in the circulation. Rate limiting factors could include: (1) the amount of PAH protein in muscle of Tg/Pahenu2 mice; (2) the rate of phenylalanine transport from the circulation into PAH-expressing skeletal muscle cells; (3) the rate of BH4 uptake by the muscle; (4) rapid oxidation of BH4 within myocytes; or possibly (5) the rate of tyrosine egress from myocytes. The possibility that PAH protein may be rate limiting can be evaluated by repeating similar experiments in the two remaining MCKβgmPAH-transgenic lines which exhibit lower PAH expression levels in muscle than TgN(MCKPah)15Mes mice. The rate of phenylalanine and tyrosine transport across the sarcolemmal membrane can be measured directly in cultured primary myocytes. It is possible that the rate of BH4 transfer from the circulation into muscle is already maximally saturated at an intraperitoneal dose of 0.1 μmol BH4/gm body weight per hour; further increases in the BH4 dose may not acutely increase the intracellular supply of BH4 in myofibers. Alternatively, the intracellular concentration of reduced BH4 might be limited by the amount of dihydropteridine reductase (DHPR, EC 1.6.99.7) activity in skeletal muscle. DHPR is necessary for maintaining biopterin in its reduced state within cells, and the specific activity of DHPR in rat skeletal muscle is only 0.5 μmol/min/g tissue compared with 8.2 μmol/min/g tissue in rat liver.20 Further investigation of the factors which limit phenylalanine clearance in Tg/Pahenu2 mice is necessary.
We believe that this model will be generalizable to other enzymes, enabling correction of the phenotype of many different inborn errors that are characterized by the accumulation in blood of toxic products of intermediate metabolism. This approach may however be problematic for certain complex metabolic systems. In ornithine trans-carbamoylase (OTC) deficiency for example, the substrate for OTC, carbamyl phosphate, is not detected in blood but is produced from ammonia only locally within the liver. Muscle expression of OTC enzyme would presumably have no effect upon the hyperammonemic phenotype of OTC deficiency. Hyperammonemia associated with other urea cycle defects, such as citrullinemia or argininosuccinicaciduria, in which the substrate for the deficient enzyme is present in the circulation could potentially be treated by enzyme expression in a heterologous tissue. The rate of transport into the target tissue of circulating toxic metabolites could however limit the effectiveness of heterologous gene therapy in select metabolic diseases.
For cofactor-requiring enzyme systems, such as PAH, further improvements in cofactor delivery to the site of heterologous gene expression will be necessary to sustain the desired phenotypic change. PAH, tyrosine hydroxylase, and tryptophan hydroxylase all require BH4 and are normally expressed in tissues which are capable of synthesizing BH4 from GTP. Future studies will identify which elements of the BH4 synthetic pathway are required to produce sufficient BH4 in a heterologous tissue to support hydroxylase activity fully, and these elements could potentially be supplied along with PAH to the target tissue.
Successful implementation of any gene therapy strategy requires an understanding of disease-specific pathophysiology and careful consideration of how these factors will affect the choice of gene transfer technologies. The availability of enzyme substrate and cofactors in the target tissue is an omnipresent issue in the design of any heterologous gene therapy strategy; these concerns will be unique for each different inborn error of metabolism. In the case of PKU and other liver enzymopathies, stable enzyme expression following gene transfer into liver would clearly bypass any problems with substrate or cofactor supply. However, should stable gene expression following liver-directed gene transfer remain elusive, and if problems with cofactor delivery to heterologous tissues are solved, gene therapy for the symptoms of specific liver enzyme deficiencies will not necessarily require the targeting of liver for gene transfer.
Materials and methods
Construction of MCKβgmPAH transgene and development of mice with muscle PAH expression
The mouse liver PAH (mPAH) cDNA21 (kindly provided by Dr S Woo) was subcloned with EcoRI ends into a pBluescript (KS-) (Stratagene, La Jolla, CA, USA)-based vector containing a BamHI–HindIII fragment of the rabbit genomic β-globin gene. This β-globin gene fragment contained the 3′ end of the second exon, all of the second intron and third exon, and an endogenous polyadenylation signal from the β-globin gene.13 The mPAH cDNA was inserted into the third β-globin exon at the EcoRI site. The only transcription start site is located at the 5′ end of the mPAH cDNA as this plasmid lacks the first exon of the rabbit β-globin gene. A 3300 bp XbaI–BstEII fragment from the 5′ regulatory regions of the mouse muscle creatine kinase (MCK) gene containing muscle-specific promoter and enhancer sequences11 was subcloned into XbaI–BamHI sites 5′ to the β-globin sequences using a synthetic BstEII–BamHI linker (Figure 1). The 6.7 kb MCKβgmPAH transgene was cleaved from the bacterial plasmid sequences by XbaI–ClaI digestion and purified by agarose gel electrophoresis, reverse phase column chromatography (Elutip-d column; Schleicher and Schuell, Keene, NH, USA), and ethanol precipitation. The purified DNA was resuspended in 10 mm Tris-HCL, pH 7.4, 0.25 mm EDTA buffer for microinjection into nuclei of F2 C57BL/6J × SJL embryos according to standard techniques for development of transgenic mice.14 Genomic DNA was analyzed for the presence of the MCKβgmPAH transgene by EcoRI or BamHI digestion followed by Southern blotting. A BamHI–EcoRI fragment from the rabbit β-globin gene was 32P-labeled by the random priming method and used as the probe.
Breeding of MCKPah mice to Pahenu2/Pahenu2 mice
Female MCKPah mice were bred to Pahenu2/Pahenu2 males. By Mendelian principles, all progeny were Pahenu2 heterozygotes and 50% inherited the MCKβgmPAH transgene. Female MCKβgmPAH-positive, Pahenu2 heterozygous mice were then bred to Pahenu2/Pahenu2 males to produce progeny (Tg/Pahenu2 mice) which were both homozygous for the Pahenu2 mutation and carried the MCKβgmPAH transgene.
Detection of the Pahenu2 mutation by PCR analysis
The presence of the T788C mutation in the seventh exon of the murine PAH gene eliminates a BbsI restriction site and creates a BsmAI recognition site. To test mice for the T788C mutation, a PCR reaction was designed to amplify from genomic DNA the region of the mouse PAH gene flanking the mutation site. Primers PAH7.30 (5′-GCTGGCTTACTGTCGTCTCG-3′; forward primer) and PAH7.218 (5′-GCAGGCAGTGGATCATGG-3′; reverse primer) were used to amplify a 205 bp fragment from wild-type mouse genomic DNA and genomic DNA from a hyperphenylalaninemic Pahenu2/Pahenu2 mouse. The forward primer corresponds to nucleotides 706–726 of the mouse liver PAH cDNA (GenBank accession No. X51942). The reverse primer corresponds to intronic sequence from 60 to 82 bp downstream from the 3′ end of the seventh exon. The final PCR conditions in 50 μl were 10 mm Tris-HCl, pH 8.3, 50 mm KCl, 2 mm MgCl2, 100 μm each dNTP, and 200 nmol/l of each primer. Each reaction was catalyzed by 0.3 μl Amplitaq DNA polymerase (Perkin-Elmer, Norwalk, CT, USA). Following an initial 2 min at 94°C, the thermocycler was programmed for five cycles of 94°C × 45 s, 60°C × 2 min, 72°C × 2 min, then 30 cycles of 94°C × 45 s, 60°C × 1 min, 72°C × 1 min. The PCR products from wild-type and Pahenu2/Pahenu2 mice were cloned into pCRII (Stratagene, La Jolla, CA, USA) and completely sequenced to confirm the identity of the PCR products and the presence of the T788C mutation in the PCR product from Pahenu2/Pahenu2 mice. The PCR product from wild-type mouse is cleaved by BsmAI into 191 and 14 bp fragments, and BbsI digestion results in 157 and 48 bp fragments. The PCR product from the Pahenu2/Pahenu2 mouse has a T to C transition at nucleotide 53; this mutation is associated with 157, 34 and 14 bp BsmAI fragments and no BbsI cut. The pattern of independent BsmAI and BbsI digestions of this 205 bp PCR product can readily distinguish between Pahenu2 homozygous, Pahenu2 heterozygous and wild-type mice.
Phenotypic characterization of muscle PAH-expressing mice
Serum phenylalanine concentrations were measured in 25 μl serum using a modification of a fluorometric procedure.22
PAH activity was measured in tissue homogenates using a radiochemical technique modified from Ledley, et al.23 The tissues were homogenized in five volumes ice cold 1.15% KCl, 0.005% 2-mercaptoethanol with five strokes of a Pro2000 electric homogenizer (ProScientific, Monroe, CT, USA). Following centrifugation at 1000 g for 15 min at 4°C, supernatant containing 25–500 μg total protein was assayed for PAH activity. In this assay, C14-labeled phenylalanine is hydroxylated to tyrosine by PAH in the presence of the synthetic cofactor, reduced 6-methyl-tetrahydropterin. The final reaction conditions in 100 μl total volume were 0.6 mm cold phenylalanine, 0.3 μCi l-[U-C14]phenylalanine (Amersham, Arlington Heights, IL, USA), 0.15 m KCl, 0.2 m potassium phosphate buffer, pH 6.8, 2 mm dithiothreitol (DTT). The reactions were initiated by adding 2 μl 4.5 mm 6-methyl-tetrahydropterin (Calbiochem, San Diego, CA, USA). Following a 1-h incubation at room temperature, the reactions were boiled and 5 μl each of cold 0.05 m phenylalanine and 0.05 m tyrosine were added. After brief centrifugation, 5 μl from each reaction were spotted on PE SilGel 60 plastic-backed TLC plates (Whatman, Hillsboro, OR, USA). The plates were developed in chloroform:methanol:ammonium hydroxide (55:35:10) for 1 h. The dried TLC plates were placed against radiographic film at room temperature overnight. Any sample with PAH activity gave both phenylalanine and tyrosine spots on the autoradiogram while phenylalanine alone was detected in samples without PAH activity. Following autoradiography, the TLC plates were sprayed with 0.2% ninhydrin in 100% ethanol and dried for several min at 100°C. The now-visible tyrosine and phenylalanine spots were cut out, and the radioactivity in each spot was quantified by scintillation counting. PAH specific activity was calculated from the amount of radioactive tyrosine produced. The positive controls were C57Bl/6J mouse liver homogenate. Negative controls include a reaction with water alone and a duplicate reaction for each sample to which no 6-methyl-tetrahydropterin cofactor was added. Total protein concentration was determined by the bicinchonic acid (BCA) method (Pierce Chemical, Rockford, IL, USA).
Tetrahydrobiopterin administration
Tetrahydrobiopterin (Schircks Laboratories, Jona, Switzerland) was dissolved in 1% ascorbic acid, pH 7 to concentrations of 10–100 mm and administered to mice by intraperitoneal injection using a 27 gauge needle on a 1 ml disposable syringe.
Acknowledgements
This work was funded in part by National Institutes of Health (NIH) grant RO1 DK42709. CO Harding was supported in part by NIH genetics training grant 5T32GM07131–15 and by NIH grant K08 DK02405–02. The authors gratefully acknowledge Professor William Dove and Alexandra Shedlovsky for supplying and assisting with the husbandry of Pahenu2 mice and for critical discussions of this project, J David McDonald for assistance with detection of the Pahenu2 mutation, Savio LC Woo and Randy Eisensmith for the mouse PAH cDNA, Denice Springman for technical assistance with mouse embryo microinjection and implantation, Sheldon Milstien for measurement of tissue biopterin, and Seymour Kaufman for discussions concerning phenylalanine and pterin metabolism.
References
- 1.Bowles N, Woo SLC. Gene therapy for metabolic disorders. Adv Drug Del Rev. 1995;17:293–302. [Google Scholar]
- 2.Koeberl DD, et al. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proc Natl Acad Sci USA. 1997;94:1426–1431. doi: 10.1073/pnas.94.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snyder RO, et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 4.Wolff JA, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 5.Acsadi G, et al. Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature. 1991;352:815–818. doi: 10.1038/352815a0. [DOI] [PubMed] [Google Scholar]
- 6.Dai Y, Roman M, Naviaux RK, Verma IM. Gene therapy via primary myoblasts: long-term expression of factor IX protein following transplantation in vivo. Proc Natl Acad Sci USA. 1992;89:10892–10895. doi: 10.1073/pnas.89.22.10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhawan J, et al. Systemic delivery of human growth hormone by injection of genetically engineered myoblasts. Science. 1991;254:1509–1512. doi: 10.1126/science.1962213. [DOI] [PubMed] [Google Scholar]
- 8.Ulmer JB, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 9.Huygen K, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nature Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 10.Shedlovsky A, McDonald JD, Symula D, Dove WF. Mouse models of human phenylketonuria. Genetics. 1993;134:1205–1210. doi: 10.1093/genetics/134.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaynes JG, et al. The muscle creatine kinase gene is regulated by multiple upstream elements, including a muscle-specific enhancer. Mol Cell Biol. 1988;8:62–70. doi: 10.1128/mcb.8.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson JE, Wold BJ, Hauschka SD. Muscle creatine kinase sequence elements regulating skeletal and cardiac muscle expression in transgenic mice. Mol Cell Biol. 1989;9:3393–3399. doi: 10.1128/mcb.9.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinster RL, et al. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci USA. 1988;85:836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinster R, et al. Factors affecting the efficiency of introducing foreign genes into mice by microinjecting eggs. Proc Natl Acad Sci USA. 1985;82:4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald JD, Charlton CK. Characterization of mutations at the mouse phenylalanine hydroxylase locus. Genomics. 1997;39:402–405. doi: 10.1006/geno.1996.4508. [DOI] [PubMed] [Google Scholar]
- 16.Fukushima T, Nixon JC. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem. 1980;102:176–188. doi: 10.1016/0003-2697(80)90336-x. [DOI] [PubMed] [Google Scholar]
- 17.Herzog RW, et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark KR, Sferra TJ, Johnson PR. Recombinant adeno-associated viral vectors mediate long-term transgene expression in muscle. Hum Gene Ther. 1997;8:659–669. doi: 10.1089/hum.1997.8.6-659. [DOI] [PubMed] [Google Scholar]
- 19.Budker V, et al. The efficient expression of intravascularly delivered DNA in rat muscle. Gene Therapy. 1998;5:272–276. doi: 10.1038/sj.gt.3300572. [DOI] [PubMed] [Google Scholar]
- 20.Craine JE, Hall ES, Kaufman S. The isolation and characterization of dihydropteridine reductase from sheep liver. J Biol Chem. 1972;247:6082–6091. [PubMed] [Google Scholar]
- 21.Ledley FD, Grenett HE, Dunbar BS, Woo SL. Mouse phenylalanine hydroxylase. Homology and divergence from human phenylalanine hydroxylase. Biochem J. 1990;267:399–405. doi: 10.1042/bj2670399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCaman MW, Robins E. Fluorimetric method for the determination of phenylalanine in serum. J Lab Clin Med. 1962;59:885–890. [Google Scholar]
- 23.Ledley FD, Hahn T, Woo SLC. Selection for phenylalanine hydroxylase activity in cells transformed with recombinant retrovirus. Somat Cell Molec Genet. 1987;13:145–154. doi: 10.1007/BF01534694. [DOI] [PubMed] [Google Scholar]