Abstract
The objective of this prospective cohort study was to determine if dietary therapy including docosahexaenoic acid (DHA; C22:6ω-3) supplementation prevents the progression of the severe chorioretinopathy that develops in children with long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) or trifunctional protein (TFP) deficiency. Physical, biochemical, and ophthalmological evaluations, including electroretinogram (ERG) and visual acuity by evoked potential (VEP), were performed at baseline and annually following the initiation of 65–130 mg/day DHA supplementation and continued treatment with a low-fat diet. Fourteen children with LCHAD or TFP deficiency, 1–12 years of age at enrollment, were followed for 2–5 years. Three subjects with TFP β-subunit mutations had normal appearance of the posterior pole of the ocular fundi at enrollment and no changes over the course of the study. Eleven subjects who were homozygote and heterozygote for the common mutation, c.1528G > C, had no change to severe progression of atrophy of the choroid and retina with time. Of these, four subjects had marked to severe chorioretinopathy associated with high levels of plasma hydroxyacylcarnitines and decreased color, night and/or central vision during the study. The plasma level of long-chain 3-hydroxyacylcarnitines, metabolites that accumulate as a result of LCHAD and TFP deficiency, was found to be negatively correlated with maximum ERG amplitude (Rmax) (p=0.0038, R2=0.62). In addition, subjects with sustained low plasma long-chain 3-hydroxyacylcarnitines maintained higher ERG amplitudes with time compared to subjects with chronically high 3-hydroxyacylcarnitines. Visual acuity, as determined with the VEP, appeared to increase with time on DHA supplementation (p=0.051) and there was a trend for a positive correlation with plasma DHA concentrations (p=0.075, R2=0.31). Thus, optimal dietary therapy as indicated by low plasma 3-hydroxyacylcarnitine and high plasma DHA concentrations was associated with retention of retinal function and visual acuity in children with LCHAD or TFP deficiency.
Keywords: Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency, Trifunctional protein deficiency, Hydroxyacylcarnitines, Docosahexaenoic acid, Chorioretinopathy
Introduction
Deficiency of long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD), either isolated or as a part of generalized trifunctional protein (TFP) deficiency, severely impairs mitochondrial long-chain fatty acid (LCFA) β-oxidation. This rare disorder can be caused by mutations in the genes for either the α subunit (OMIM # 600890) or β subunit (OMIM #143450) of the mitochondrial trifunctional protein, but a missense mutation (c.1528G>C) in the α subunit is the most prevalent. The biochemical hallmarks of this disorder are accumulation of long-chain 3-hydroxyacylcarnitines and free fatty acids in plasma and dicarboxylic acids in urine [1,2]. The disease typically manifests with acute episodes of fasting or illness-induced hypoketotic hypoglycemia [3]. Cardiomyopathy, hepatopathy, and rhabdomyolysis are frequent clinical problems during acute exacerbation. These complications can be lethal but appear to be tempered by contemporary dietary therapy [4-6]. Therapy for LCHAD deficiency includes fasting avoidance and consumption of a diet low in long chain fatty acids (LCFA), but supplemented with medium-chain triglycerides (MCT). This approach minimizes LCFA oxidation as evidenced by low plasma long-chain 3-hydroxyacylcarnitine profiles [7].
Patients with LCHAD deficiency frequently develop the long-term complication of a unique, progressive chorioretinopathy leading to significant visual impairment and disability [4,5,8]. The chorioretinopathy associated with LCHAD deficiency is reported to begin with peppery pigment clumping in the macula, which can appear early in life [8]. The disease later progresses to atrophy of the posterior choroid, retinal pigment epithelium (RPE) and retina. Vision is normal early in the course of the disease, but as the retinopathy progresses, patients begin to lose both color and night vision followed by loss of central vision and legal blindness. Thus, both cone- and rod-mediated functions are impaired. The electroretinogram (ERG) also progressively deteriorates during the first decade of life with the advancing retinopathy. Other ocular findings may include progressive myopia and supranuclear cataracts [8].
The etiology of the chorioretinopathy of LCHAD deficiency is unknown, but several authors have implicated toxic effects of accumulating metabolites, such as long-chain 3-hydroxyacylcarnitines or their free fatty acids, or deficiency of the long-chain polyunsaturated fatty acid docosahexaenoic acid (DHA), which has an important role in retinal function [3,9,10]. Our hypotheses are that poor dietary control, as indicated by chronically elevated long-chain 3-hydroxyacylcarnitines, and chronically low plasma DHA are both associated with progressive visual impairment and chorioretinopathy in subjects with LCHAD deficiency. The objective of this prospective cohort study was to determine if optimal dietary therapy and DHA supplementation prevents the progression of chorioretinopathy in children with LCHAD deficiency. Given the small number of patients with this disorder (approximately 100 known cases in the US), a randomized controlled trial was not feasible, and therefore we conducted an open trial of DHA supplementation.
Methods
Subjects
Subjects were ascertained at several metabolic centers in North America and referred to us for study enrollment. The only inclusion criterion was a confirmed diagnosis of LCHAD deficiency; the presence or absence of retinopathy was not assessed prior to enrollment. In each case, the diagnosis of LCHAD deficiency was confirmed by reviewing the medical record for documentation of either the presence of mutations in the TFP genes or by the decrease of LCHAD enzymatic activity as measured in cultured skin fibroblasts. Fourteen subjects with confirmed LCHAD deficiency were enrolled in an open label trial of oral DHA supplementation at two clinical sites (University of Wisconsin–Madison, Madison, WI and Oregon Health and Science University, Portland, OR). Subject characteristics and duration on DHA supplementation are presented in Table 1. Nine cases had previously published mutation analyses [11-16]. The subjects ranged in age from 1 to 12 years at enrollment. All patients were prescribed a diet low in long-chain fat, and 12 were orally supplemented with MCT and carnitine before beginning oral DHA. The Institutional Review Board at both institutions approved the study protocol, and each subject's legal guardian gave written informed consent. Subjects greater than 7 years gave assent to participate.
Table 1.
Characteristics of subjects
| Subject | Gender | Mutations | Age at enrollment (years) |
DHA supplementation |
Residual enzyme activity (nmol/min/mg Protein) |
||
|---|---|---|---|---|---|---|---|
| Dose (mg/day) |
Time supplemented (years) |
LCHADa | Ketothiolasea | ||||
| 1#(15) | F | c.901G > A β-subunit/? | 10 | 130 | 4 | 12.4 | 0.4 |
| 2 | M | c.1528G > C/c.1528G > C | 7 | 130 | 4 | 11.8 | 38.6 |
| 3# | F | c.901G > A β-subunit/? | 5 | 130 | 4 | NA | NA |
| 4(12) | F | c.1528G > C/c.274_278del | 1 | 65 | 4 | 10.2 | 10.7 |
| 5(11) | F | c.1528G > C/c.1528G > C | 1 | 65 | 4 | NA | NA |
| 6(15) | M | β-subunit/β-subunit | 1 | 65 | 4 | ||
| 7(15) | F | c.1528G > C/c.1528G > C | 6 | 130 | 4 | 10.7 | 21.0 |
| 8(12) | F | c.1528G > C /c.274_278del | 1 | 65 | 4 | 0.4 | NA |
| 9f | F | c.1528G > C/c.1528G > C | 3 | 65 | 3 | NA | NA |
| 10f | M | c.1528G > C/c.1528G > C | 4 | 65 | 3 | NA | NA |
| 11 | M | c.1528G > C/? | 9 | 130 | 4 | NA | NA |
| 12(12) | F | c.1528G > C/5 bp del at exon 15/intron boundary |
1 | 65 | 2 | NA | NA |
| 13(12) | F | c.1528G > C/c.1132C > T | 2 | 130 | 5 | NA | NA |
| 14(13) | M | c.1528G > C/c.1678C > T | 5 | 130 | 5 | NA | NA |
Mutations are given as the change in the cDNA (c.) of the α-subunit of the trifunctional protein unless otherwise noted. Superscripts to the subject numbers indicate the literature reference for those subjects with previously published mutation analysis. “?” indicates alleles in which no mutations were identified following the sequencing of exons in both the α and β subunits. Patients have one known mutation and clinical and biochemical evidence of LCHAD/TFP deficiency. “#/f” symbols indicate siblings. MVM=multi-vitamin and mineral supplement; Portagen=infant formula made by Mead Johnson Nutritionals; Alimentum=infant formula made by Ross Products, Abbott Laboratories.
Normal range of LCHAD=43.6–89.9 nmol/min/mg protein; normal range of ketothiolase=16.4–43.0 nmol/min/mg protein.
Protocol
Baseline evaluations included physical, neurological, and ophthalmologic examinations. ERG studies and determination of visual acuity with the swept-spatial-frequency visual evoked cortical potential (sweep VEP) were completed prior to DHA supplementation. Fasting plasma fatty acid analysis, including DHA levels, liver function tests, and plasma acylcarnitines were measured annually. In addition, each subject's dietary intake was recorded for 3 days by their legal guardian and analyzed for nutrient content using commercially available software (Food Processor, ESHA Research, Salem, OR, 1996).
Following baseline exams, all 14 children began oral DHA supplementation while continuing their current diet therapy. DHA was administered as a microencapsulated powder, which is rich in DHA but limited in content of other LCFAs (DHASCO, Martek Biosciences Corporation, Columbia, MD). Sixty-five milligram DHA/day was provided for subjects weighing less than 20 kg, while subjects over 20 kg were prescribed 130 mg DHA/day (Table 1). The dose of DHA was adjusted as subjects grew during the course of the study. Physical, biochemical, and ophthalmologic evaluations were repeated annually. One subject dropped out after 2 years, two subjects have been followed for 3 years, nine subjects followed for 4 years, and two have been followed for 5 years.
Analysis of plasma essential fatty acids (EFA), including DHA, was completed at the Peroxisomal Diseases Laboratory, Kennedy Krieger Institute, Baltimore, MD or the Biochemical Genetics Laboratory, Mayo Clinic, Rochester, MN. Fatty acids were quantified by capillary gas chromatography and expressed as micromoles per liter as previously reported [17,18].
Analyses of plasma acylcarnitines were completed by the Biochemical Genetics Laboratories at Duke University Medical Center, Durham, SC and, the Mayo Clinic, Rochester, MN. Acylcarnitines were quantified by tandem mass spectroscopy and expressed as micromoles per liter [2,19]. Total long-chain hydroxyacylcarnitines were calculated for each sample. Acylcarnitine species included in the total were C14:0-OH, C14:1-OH, C16:0-OH, C18:0-OH, and C18:1-OH.
A clinical ERG protocol that conformed to the standard of the International Society for the Clinical Electrophysiology of Vision was performed at both sites [20-22]. Scotopic testing included rod responses to short (λ < 470 nm, blue) and long wavelength (λ > 600 nm, red) stimuli matched in intensity to produce equal rod-mediated response amplitudes in normal subjects, as well as a bright white flash (mixed rod–cone response). Further testing at OHSU at most time points included a six-intensity stimulus–response series (−3.1, −2.5, −1.8, −0.6, 0.0, and +0.6 log cd s/m2); the resulting b-wave amplitudes were fit with the Naka–Rushton function to derive the parameters Rmax (maximal response amplitude) and log K (log stimulus intensity at half the maximal amplitude, an indicator of retinal sensitivity). Photopic stimuli measuring cone responses included single white flashes presented on a rod-suppressing background (34 cd/m2). Rod and cone responses were analyzed to determine b-wave amplitudes. Younger children were sedated using propofol anesthesia.
Visual acuity was assessed with the sweep VEP method, as described previously [23]. At OHSU, an ENFANT system (Neuroscientific, Morrisville, PA) was used to generate stimuli, record the electrophysiological signals and analyze responses [24,25], and a similar system and methods were used at UW [25,26]. EEG electrodes were placed on the scalp at Oz (active), Cz (reference), and Pz (ground). The infants or children were positioned in front of a video stimulus display (with younger subjects held by a parent), and fixation was monitored by the tester so that electrophysiological signals were processed only when the child was attending to the stimulus. Horizontal grating patterns with 75–80% contrast were contrast-reversed at 7.5 Hz (15 reversals/s). During a single sweep, the spatial frequency was increased progressively (that is, stripe size was decreased) from stripes that were clearly visible to the subject to those below the detection threshold. Data were recorded for a minimum of 10 sweeps, and sweeps meeting signal-to-noise and phase criteria were vector-averaged. The amplitude of the second harmonic (15 Hz component, corresponding to the contrast-reversal frequency) and response phase were determined at each spatial frequency, and the visual acuity threshold was determined by interpolation of the amplitudes for the points above and below threshold. Results were expressed as the acuity threshold (smallest detectable stripe size) in cycles per degree of visual angle. Thirty cycles per degree is the normal adult acuity threshold, equivalent to 20/20 Snellen acuity.
Data analysis
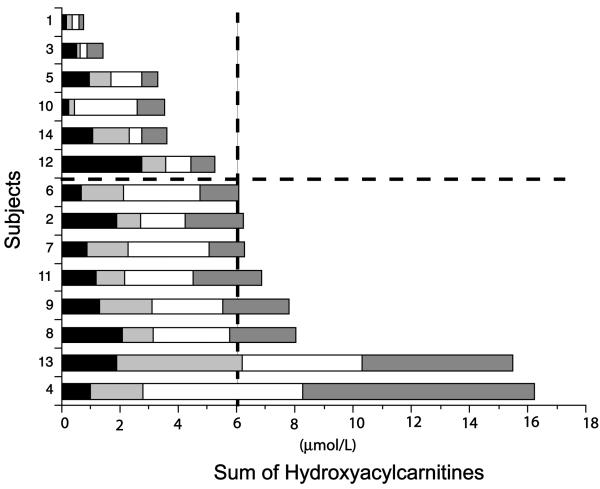
The log of ERG response amplitudes and VEP acuity thresholds was used in statistical analyses. Statistical analyses were completed using SAS (SAS v8.3, SAS Institute, Cary, NC); p ⩽ 0.05 was considered statistically significant. Overall cumulative hydroxyacylcarnitine exposure was estimated by adding the last four annual values for total long-chain hydroxylated acylcarnitines (Fig. 1). For ERG and VEP outcomes, subjects were divided into two groups: above and below the median of cumulative hydroxyacylcarnitine levels (6 μmol/L). Subject 7 whose cumulative hydroxyacylcarnitine level fell at the median was conservatively included in the above median group. Thus, there were six subjects with low cumulative total hydroxyacylcarnitines (6⩽ μmol/L) and eight with high total cumulative hydroxyacylcarnitines (⩾6 μmol/L; Fig. 1).
Fig. 1.
Cumulative hydroxyacylcarnitine levels in 14 children with LCHAD or TFP deficiency measured annually. Each shaded segment represents the total hydroxyacylcarnitine concentration for one blood sample. Subjects were divided into two groups; high and low cumulative hydroxyacylcarnitines as indicated by the dotted lines.
Effect of time and long-chain hydroxyacylcarnitine status
Data were available for 11 subjects through 4 years of DHA supplementation, and therefore these data were used to examine changes over time. Plasma essential fatty acids were analyzed for the effect of time using a single factor repeated measures ANOVA. The effects of long-chain hydroxyacylcarnitine level and time were analyzed using a two-factor repeated measures ANOVA. Post hoc analyses were done using t tests. ERG parameters used for the ANOVA analyses included b-wave amplitudes of the rod response to the scotopic blue flash, mixed rod–cone response to a bright white flash, and cone response to a photopic flash. Data from the ERG intensity–response series (Rmax and log K) were not available for most subjects at baseline.
Multiple linear regression
Backward elimination multiple regression analysis was used to identify independent predictors of ERG and VEP outcome. The most recent ERG and VEP measurements for each subject were used in this analysis, as these were all conducted at the OHSU site. The dependent variables for the ERG included Rmax, log K, and the b-wave amplitudes in response to the scotopic blue flash, bright white flash, and photopic cone flash; for the VEP, the dependent variable was the visual acuity threshold. Cumulative total hydroxyacylcarnitines, the change in plasma DHA concentration from baseline, and age were independent variables entered into the full model. Propofol sedation is known to produce some decrease in ERG amplitudes [27]; however, age and use of sedation were highly correlated, so age was used to represent both sedation use and age of subject.
Results
Retinal appearance and clinical outcomes
Initial and final retinal appearance is described in Table 2. Six subjects had a normal posterior pole appearance at enrollment into the trial; four of these had fine granularity of the RPE in the mid to far periphery observed under sedation. The granularity was not noted in the other two subjects. In our experience, a fine granularity of the RPE in the mid to far periphery precedes the macular pigment clumping previously reported to be the first retinal pigment change observed in children with LCHAD deficiency [8]. The other eight subjects had very mild to moderate pigmentary changes in the posterior pole at enrollment.
Table 2.
Retinal appearance of 14 children with LCHAD deficiency
| Subject | Retinal observations |
|
|---|---|---|
| Initial observation | Final observation | |
| 1 | Fine granularity in mid to far periphery | No change with time |
| 2 | Mottled RPE and choriocapillaris atrophy | Moderate progression of chorioretinopathy |
| 3 | Fine granularity in mid to far periphery | No change with time |
| 4 | Normal posterior pole | Severe hypopigmentation of macula, with severe choriocapillaris atrophy |
| 5 | Fine granularity in mid to far periphery | Mild progression of chorioretinopathy |
| 6 | Fine granularity in mid to far periphery | No change with time |
| 7 | Hypopigmentation of macula | Severe hypopigmentation of macula, with severe choriocapillaris atrophy |
| 8 | Macular pigment clumping | Marked hypopigmentation of macula, with moderate choriocapillaris atrophy |
| 9 | Hyperpigmented fovea with mottled RPE | Mild progression of chorioretinopathy |
| 10 | Hyperpigmented fovea with mottled RPE | Mild progression of chorioretinopathy |
| 11 | Mottled RPE with choriocapillaris atrophy | Marked hypopigmentation of macula, with moderate choriocapillaris atrophy |
| 12 | Normal posterior pole | Moderate progression of chorioretinopathy |
| 13 | Fine granularity in posterior pole and mid-periphery | No change with time |
| 14 | Hyperpigmented macula | Moderate progression of chorioretinopathy |
Retinal appearance from ophthalmologic exam performed by MM or RGW.
Over the course of the study, four subjects had no change in fundus exam, and no decline in ERG response. Three of theses subjects had consistently low hydroxyacylcarnitines. Two subjects had severe progression and two had marked progression of chorioretinopathy with time. Severe or marked progression of retinopathy was associated with decreased night and color vision and declining ERG response. All of these subjects had high cumulative hydroxyacylcarnitines. The remaining subjects had mild to moderate changes in retinal pigments, mild declines in ERG response but no reported change in vision with time.
Seven subjects were hospitalized at least once during the study for illness and/or exercise associated rhabdomyolysis, elevated CPK levels, and dehydration. All were treated with IV fluids and dextrose. During hospitalization, subject 6 suffered acute cardiac arrest and was resuscitated. The subject has physical and mental delays related to hypoxia but no change in retinal pigmentation has been observed. One subject was hospitalized with a broken femur and one for tonsillectomy. All liver function tests were within normal limits; there was no evidence of adverse effects of the DHA supplement. No other complications were reported.
Dietary intake
Subjects consumed 13±7% of total energy as LCFAs (⩾16 carbons in length). Linoleic acid (C18:2n−6) comprised 1.9±1% of total energy and α-linolenic acid (C18:3n−3) comprised 0.3±0.2% of total energy. There was no DHA consumed in the diet; the sole source of DHA was from the supplement. A more detailed analysis of the nutritional intake of 10 of these subjects has been published elsewhere [7].
Plasma essential fatty acids
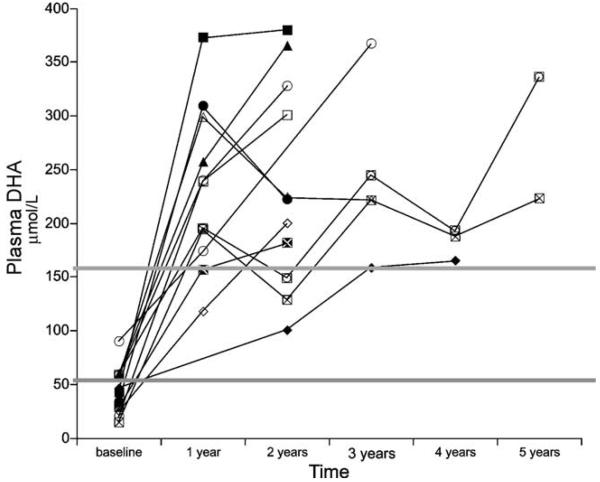
Twelve of 14 subjects had fasting plasma fatty acids measured at baseline. Baseline plasma DHA levels were below the normal range for 7 of these 12 children and in the normal range for the other 5 (Fig. 2). Plasma DHA concentrations increased with oral DHA supplementation in all subjects, but the final plasma DHA concentration was highly variable. This variability is most likely related to subject compliance with the supplementation regimen. Plasma DHA levels significantly increased with time (p=0.007); levels were significantly higher at both year 1 and year 2 than at baseline (p=0.003), but did not differ between years 1 and 2 (p=0.49). However, there were no significant changes in plasma linoleic, α-linolenic or arachidonic acid levels with time (data not shown).
Fig. 2.
Plasma DHA concentrations (μmol/L) in 12 children with LCHAD or TFP deficiency. DHA levels were measured prior to supplementation (baseline) and again annually following the initiation of supplemental DHA (65–130 mg/day). Subjects were followed for 2–5 years. The two gray lines indicate the normal range of DHA concentrations. Plasma DHA significantly increased with time (p=0.007), with levels significantly higher in both years 1 and 2 than at baseline (p=0.003).
ERG outcome
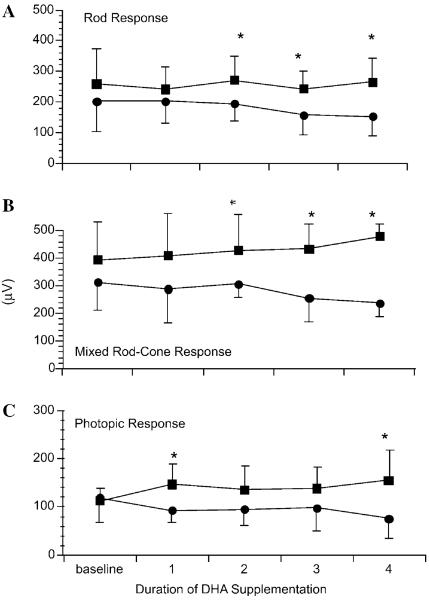
Two of the 14 subjects had missing ERG measurements at some time points and were excluded from the repeated measures analysis. The b-wave amplitudes of the rod response to the scotopic blue flash (p=0.009) and mixed rod–cone response to the bright white flash (p=0.01) were higher in subjects with low cumulative plasma hydroxyacylcarnitines (n=5) compared to subjects with high plasma hydroxyacylcarnitines (n=7; Figs. 3A and B). For b-wave amplitude of the photopic cone response (Fig. 3C), a repeated measures ANOVA showed a significant interaction (p=0.05), and post hoc tests showed a significant difference between the groups at 1 and 4 years (p=0.05).
Fig. 3.
Mean±standard deviation of the mean (SD) ERG amplitudes (μV) in five children with low cumulative plasma hydroxyacylcarnitines (<6 μmol/L) (■) compared to six children with high cumulative plasma hydroxyacylcarnitines (>6 μmol/L) (●) over time. (A) Scotopic blue flash (rod) b-wave amplitude was higher in the group with low hydroxyacylcarnitines (effect of group: p=0.004). (B) Scotopic bright white flash (mixed rod–cone) b-wave amplitude was higher in the group with low hydroxyacylcarnitines (effects of group: p=0.003). (C) Photopic cone flash amplitude did not change in the group with low hydroxyacylcarnitines and decreased in the group with high hydroxyacylcarnitine (effects of group: p=0.08, time, p=0.65, interaction, p=0.05). (*)Indicates individual time points at which the groups were significantly different (Student's t test p < 0.05). Baseline=before DHA supplementation. 1, 2, 3, and 4 years=following 1–4 years of DHA supplementation, respectively.
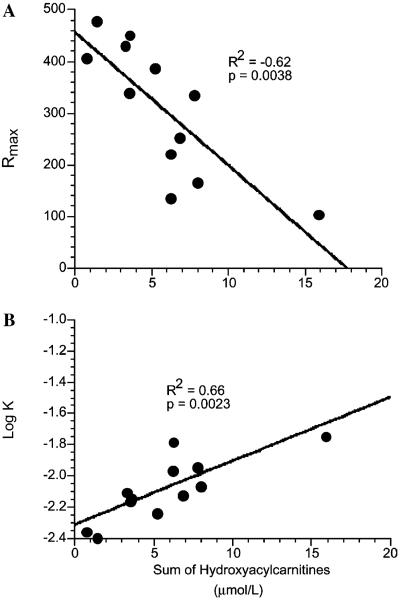
Multiple linear regression analysis (Table 3; n=13) found cumulative hydroxyacylcarnitines to be a significant independent predictor of all ERG parameters tested. Specifically, total hydroxyacylcarnitines predicted 62% of the variability in the final maximal ERG response amplitude (p=0.0038; Fig. 4A) and 66% of the variability in retinal sensitivity (log K, p=0.0023; Fig. 4B). However, neither plasma DHA concentrations nor age were independent factors significantly related to any of the ERG outcomes (n=13). The data suggest that high plasma long-chain 3-hydroxyacylcarnitines were associated with decreased retinal function as measured by ERG.
Table 3.
Multiple linear regression of ERG
| Dependent variable | Significant independent factor | R2 (p value) |
|---|---|---|
| Rmax | Sum of hydroxyacylcarnitines | 0.62 (0.0038) |
| LogK | Sum of hydroxyacylcarnitines | 0.66 (0.0023) |
| Scotopic blue flash (rod response) | Sum of hydroxyacylcarnitines | 0.42 (0.022) |
| Scotopic bright white flash (mixed rod and cone response) | Sum of hydroxyacylcarnitines | 0.69 (0.022) |
| Photopic cone flash | Sum of hydroxyacylcarnitines | 0.78 (0.0049) |
Cumulative sum of hydroxyacylcarnitines, change in plasma DHA and age were entered into the full model. Significant independent factor=factor remaining after nonsignificant factors removed. R2 and p value of the regression equation are given in column 3.
Fig. 4.
(A) There is a negative correlation between cumulative hydroxyacylcarnitine concentration (μmol/L) and the maximal ERG response (Rmax) in 13 children with LCHAD or TFP deficiency (R2=0.62, p=0.004). (B) There is a negative correlation between cumulative hydroxyacylcarnitine concentration (μmol/L) and retinal sensitivity (log K) in 13 children with LCHAD or TFP deficiency (R2=0.66, p=0.002). Low retinal sensitivity=−1.0, high retinal sensitivity=−2.4 log units. Final plasma DHA concentration and age were not significantly related to Rmax, log K, or other ERG outcomes (Table 3).
VEP outcome
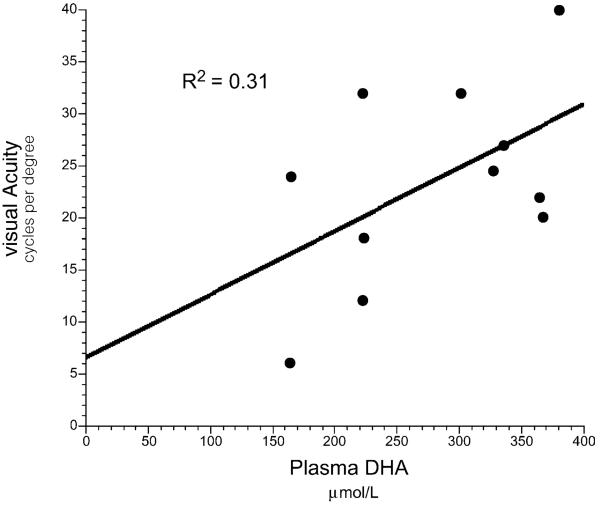
Five of the 14 subjects had missing VEP measurements at some time points and were excluded from the repeated measures analysis. There was no difference in visual acuity as measured by sweep VEP between subjects with low cumulative hydroxyacylcarnitine levels compared to subjects with high cumulative hydroxyacylcarnitine levels. However, there was a significant increase in sweep VEP acuity in subjects during the first 2 years of DHA supplementation (n=9; Table 4; time, p=0.051). In multiple linear regression analysis (n=14), there was a trend toward positive correlation with final plasma DHA (Fig. 5, p=0.075), with this variable predicting 31% of the variability in final sweep VEP acuity, but cumulative hydroxyacylcarnitines and age were not significantly related to VEP outcome. The data suggest that DHA supplementation was associated with a trend toward improved visual acuity as measured by sweep VEP regardless of plasma hydroxyacylcarnitine levels or age.
Table 4.
Visual acuity as determined by visual evoked potential
| Subject | Visual acuity (cycles per degree) |
|||||
|---|---|---|---|---|---|---|
| Time from initiation of DHA supplementation: |
Baseline | 1 year | 2 years | 3 years | 4 years | 5 years |
| 1 | 16 | 23 | 40 | |||
| 2 | 10 | 12 | 12 | |||
| 3 | 11 | 21 | 22 | |||
| 4 | 17.7 | 23.1 | 10 | 24 | ||
| 5 | 6 | 38 | 32 | |||
| 6 | 10 | 18 | ||||
| 7 | 21.9 | 19.4 | 10 | 15 | 20 | |
| 8 | 16.6 | 23.9 | 48 | 32 | ||
| 9 | 10 | 30 | ||||
| 10 | 25 | |||||
| 11 | 19 | 6 | ||||
| 12 | 12.7 | 25.4 | 24.5 | |||
| 13 | 15 | 22 | 24.6 | 18 | ||
| 14 | 24.6 | 28.2 | 22.8 | 19.3 | 27 | |
| p value for time: | 0.051 | |||||
Visual acuity measurements in 14 subjects with LCHAD deficiency over 2–5 years of DHA supplementation. The p value is given for the effect of time over the first 2 years of supplementation. Normal acuity for adults and older children is 30 cpd, equivalent to 20/20 Snellen acuity.
Fig. 5.
There was a trend for a correlation between visual acuity at the most recent sweep VEP (cycles per degree) and the most recent plasma DHA concentration (μmol/L) in 14 children with LCHAD or TFP deficiency (R2=0.310, p=0.075). Cumulative hydroxyacylcarnitine concentration and age were not significantly related to sweep VEP.
Discussion
The etiology of the unique chorioretinopathy in subjects with LCHAD deficiency is unknown, but retinal damage by the accumulation of toxic metabolites of impaired LCFA oxidation has been suggested as a possible mechanism [3,10]. In our cohort, there was a significant negative correlation between plasma long-chain 3-hydroxyacylcarnitine concentration and ERG response. Severe or marked progression of chorioretinopathy on fundus exam was also associated with elevated hydroxyacylcarnitines. Thus, the data from both fundus examination and ERG response suggests that sustained elevations in long-chain 3-hydroxyacylcarnitines are associated with progression of chorioretinopathy and decreased retinal function.
We previously reported that the concentration of plasma hydroxyacylcarnitines is related to the dietary fat intake of children with LCHAD deficiency [7]. Dietary long-chain fat intake was positively correlated and dietary MCT intake was negatively correlated with plasma hydroxyacylcarnitine concentration in 10 patients. Children who consumed 10% or less of total energy from LCFAs and 10% or more of total energy from MCT had significantly lower plasma hydroxyacylcarnitine concentrations. In this cohort, subjects with low cumulative hydroxyacylcarnitines had no change or mild to moderate progression of chorioretinopathy with no report of vision loss. Progression of the retinopathy did occur in some subjects with good metabolic control but it appeared to be at a slower rate than many subjects with high hydroxyacylcarnitines.
Plasma hydroxylated acylcarnitine metabolites are closely correlated to plasma levels of the corresponding free hydroxylated fatty acids [7,28,29]. Others have speculated that the carnitine ester may be detoxifying and the free hydroxylated acid may be toxic but no data to support or refute that speculation has been published. Thus, the association of increased hydroxyacylcarnitine with declining ERG response may be related to increased free hydroxy-fatty acids and not the carnitine ester. Further study to determine the effects of both compounds on retinal cells is needed to answer this question.
Toxic metabolites of LCFA oxidation may be produced systemically and reach the retina through normal circulation. However, recent studies have demonstrated that RPE and photoreceptor cells have LCFA enzymes and are capable of oxidizing long-chain fats [30]. Thus local production of toxic metabolites within the retina is possible. Regardless of the source of toxic metabolites, a high fat diet increases, but MCT supplementation suppresses, LCFA oxidation.
Specific genetic mutations likely affect plasma acylcarnitine levels as well. In our cohort, subjects with β-subunit mutations had much lower plasma long-chain hydroxyacylcarnitines. It is not possible to determine whether genotype or dietary management played the more critical role in lowering plasma acylcarnitines in this data set, because subjects who had β-subunit mutations also had among the lowest dietary LCFA intakes and the highest MCT intakes for all the participants.
DHA (C22:6n−3) supplementation has been shown to elevate previously reduced plasma DHA levels to the normal range and improve visual acuity as measured behaviorally or by VEP in both rhesus monkeys and human infants [31-34]. Increased levels of n−3 fatty acid were also correlated with improved ERG parameters including an increased Rmax and a decreased log K in preterm infants [35], whereas n−3 deficiency produces ERG abnormalities in several animal models [36,37]. We previously demonstrated improved visual acuity in 3 of 4 children with LCHAD deficiency following 1 year of DHA supplementation [9]. Because the VEP measures the function of the entire visual pathway from photoreceptors to primary visual cortex, the precise site within the visual system where supplemental DHA acts to enhance vision is unknown. Retinal photoreceptor and RPE cell membranes have a high concentration, and therefore a presumed high need for DHA [38]. DHA supplementation may enhance retinal function by restoring DHA levels to a sufficient concentration in retinal photoreceptor and RPE cell membranes. Alternately, or in addition, DHA may improve signal transmission from the retina to the occipital cortex. In our current study, increasing effectiveness of supplemental DHA, as indicated by plasma levels, was not associated with improved retinal function as measured by ERG. However, visual acuity significantly improved after 2 years of supplementation, and there was a trend toward a positive correlation of DHA levels with acuity independent of age or plasma hydroxyacylcarnitine levels. The difference in the two outcomes is likely to be due to the predominant, or earlier, effect of LCHAD deficiency on the macula, which is critical for visual acuity but contributes little to the full-field ERG.
The improvement in cone-mediated spatial vision over the course of the study might be attributed to maturation rather than DHA supplementation; however, with this VEP method, acuity increases rapidly during the first 6–8 months to nearly adult levels and shows only a small and gradual increase thereafter [23,39]. The lack of association in our subjects between age and visual acuity is in agreement with these findings and supports the conclusion that the observed acuity improvements were due to DHA supplementation rather than age alone. However, in the absence of an untreated control group, this result is not definitive.
DHA deficiency has been observed in some but not all children with LCHAD deficiency [4,40]. Children with LCHAD deficiency are treated with a very low-fat diet and are thus at an increased risk for developing essential fatty acid deficiency. In this cohort at baseline, over 50% of the subjects had plasma DHA levels below the normal range. Nevertheless, even some of the subjects with normal plasma DHA levels at baseline had visual acuity improvement with DHA supplementation. Maintaining plasma DHA levels in the upper range of normal by providing adequate amounts of the precursor α-linolenic acid (C18:3n−3) is challenging within the constraint of limiting dietary LCFAs to 10% of total energy or less. In addition, at this level of dietary fat restriction, elongation of essential fatty acid precursors might be limited [41]. Given the potential for DHA deficiency, the availability of DHA supplements that are limited in their content of other LCFAs, the safety of these supplements, and the improvement in visual acuity observed in this cohort, we believe that prescribing moderate amounts of DHA (60–100 mg/day) for all children with LCHAD deficiency is advisable.
Our data suggest that the etiology of visual impairment in LCHAD deficiency is multi-factorial. First, retinal function as measured by ERG is strongly inversely correlated with plasma concentrations of long-chain 3-hydroxyacylcarnitines. We hypothesize that long-chain 3-hydroxyacylcarnitines or related metabolites are toxic to RPE and/or photoreceptor cells, but cell culture experiments are needed to test this hypothesis. Secondly, DHA supplementation may improve visual acuity in children with LCHAD deficiency as has been observed in other populations. To prevent or slow the progression of chorioretinopathy and subsequent vision loss, children with LCHAD deficiency should follow a very low-fat diet (10% of total energy or less) supplemented with MCT (10% of energy or more). In addition, moderate amounts of supplemental DHA (60–130 mg/day) may improve visual acuity in children with LCHAD deficiency regardless of age or metabolic control.
Acknowledgments
We thank Dr. Arnold Strauss (Vanderbilt University, Nashville, TN) for providing the mutation analysis results of subjects not available in the medical record, and Melissa Krahmer and Andrea Billingslea for assistance with ERG and acuity testing. We thank Sara Wilson for her assistance with preparation of the manuscript. This research was supported by PHS Grants 5 MO1 RR00334 and RR03186. Post-doctoral training for MB Gillingham was supported by PHS Grant T32GM08796 and by the Doernbecher Foundation. Martek Biosciences Corporation supplied the DHA supplements (DHASCO). R.G.W. and M.N. were supported in part from grants from The Foundation Fighting Blindness, Owings Mills, MD, and Research to Prevent Blindness, New York, NY.
References
- 1.Costa CG, Dorland L, Holwerda U, de Almeida IT, Poll-The BT, Jakobs C, Duran M. Simultaneous analysis of plasma free fatty acids and their 3-hydroxy analogs in fatty acid beta-oxidation disorders. Clin. Chem. 1998;44:463–471. [PubMed] [Google Scholar]
- 2.Van Hove JL, Kahler SG, Feezor MD, Ramakrishna JP, Hart P, Treem WR, Shen JJ, Matern D, Millington DS. Acylcarnitines in plasma and blood spots of patients with long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. J. Inherit. Metab. Dis. 2000;23:571–582. doi: 10.1023/a:1005673828469. [DOI] [PubMed] [Google Scholar]
- 3.den Boer ME, Wanders RJ, Morris AA, Lodewijk IJ, Heymans HS, Wijburg FA. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: clinical presentation and follow-up of 50 patients. Pediatrics. 2002;109:99–104. doi: 10.1542/peds.109.1.99. [DOI] [PubMed] [Google Scholar]
- 4.Gillingham M, Van Calcar SC, Ney DM, Wolff J, Harding CO. Dietary management of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHADD). A case report and survey. J. Inherit. Metab. Dis. 1999;22:123–131. doi: 10.1023/a:1005437616934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saudubray JM, Martin D, de Lonlay P, Touati G, Poggi-Travert F, Bonnet D, Jouvet P, Boutron M, Slama A, Vianey-Saban C, Bonnefont JP, Rabier D, Kamoun P, Brivet M. Recognition and management of fatty acid oxidation defects: a series of 107 patients. J. Inherit. Metab. Dis. 1999;22:488–502. doi: 10.1023/a:1005556207210. [DOI] [PubMed] [Google Scholar]
- 6.Tyni T, Palotie A, Viinikka L, Valanne L, Salo MK, von Dobeln U, Jackson S, Wanders R, Venizelos N, Pihko H. Long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency with the G1528C mutation: clinical presentation of thirteen patients [see comments] J. Pediatr. 1997;130:67–76. doi: 10.1016/s0022-3476(97)70312-3. [DOI] [PubMed] [Google Scholar]
- 7.Gillingham MB, Connor WE, Matern D, Rinaldo P, Burlingame T, Meeuws K, Harding CO. Optimal dietary therapy of long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency. Mol. Genet. Metab. 2003;79:114–123. doi: 10.1016/s1096-7192(03)00073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyni T, Kivela T, Lappi M, Summanen P, Nikoskelainen E, Pihko H. Ophthalmologic findings in long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency caused by the G1528C mutation: a new type of hereditary metabolic chorioretinopathy. Ophthalmology. 1998;105:810–824. doi: 10.1016/S0161-6420(98)95019-9. [DOI] [PubMed] [Google Scholar]
- 9.Harding CO, Gillingham MB, Van Calcar SC, Wolff JA, Verhoeve JN, Mills MD. Docosahexaenoic acid (DHA) and retinal function in children with long chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency. J. Inherit. Met. Dis. 1999;22:276–280. doi: 10.1023/a:1005502626406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrijver-Wieling I, van Rens GH, Wittebol-Post D, Smeitink JA, de Jager JP, de Klerk HB, van Lith GH. Retinal dystrophy in long chain 3-hydroxy-acyl-CoA dehydrogenase deficiency. Br. J. Ophthalmol. 1997;81:291–294. doi: 10.1136/bjo.81.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hintz SR, Matern D, Strauss A, Bennett MJ, Hoyme HE, Schelley S, Kobori J, Colby C, Lehman NL, Enns GM. Early neonatal diagnosis of long-chain 3-hydroxyacyl coenzyme a dehydrogenase and mitochondrial trifunctional protein deficiencies. Mol. Genet. Metab. 2002;75:120–127. doi: 10.1006/mgme.2001.3282. [DOI] [PubMed] [Google Scholar]
- 12.Ibdah JA, Bennett MJ, Rinaldo P, Zhao Y, Gibson B, Sims HF, Strauss AW. A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women. N. Engl. J. Med. 1999;340:1723–1731. doi: 10.1056/NEJM199906033402204. [DOI] [PubMed] [Google Scholar]
- 13.Isaacs JD, Jr., Sims HF, Powell CK, Bennett MJ, Hale DE, Treem WR, Strauss AW. Maternal acute fatty liver of pregnancy associated with fetal trifunctional protein deficiency: molecular characterization of a novel maternal mutant allele. Pediatr. Res. 1996;40:393–398. doi: 10.1203/00006450-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Sims HF, Brackett JC, Powell CK, Treem WR, Hale DE, Bennett MJ, Gibson B, Shapiro S, Strauss AW. The molecular basis of pediatric long chain 3-hydroxyacyl-CoA dehydrogenase deficiency associated with maternal acute fatty liver of pregnancy. Proc. Natl. Acad. Sci. USA. 1995;92:841–845. doi: 10.1073/pnas.92.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spiekerkoetter U, Sun B, Khuchua Z, Bennett MJ, Strauss AW. Molecular and phenotypic heterogeneity in mitochondrial trifunctional protein deficiency due to beta-subunit mutations. Hum. Mutat. 2003;21:598–607. doi: 10.1002/humu.10211. [DOI] [PubMed] [Google Scholar]
- 16.Treem WR, Rinaldo P, Hale DE, Stanley CA, Millington DS, Hyams JS, Jackson S, Turnbull DM. Acute fatty liver of pregnancy and long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. Hepatology. 1994;19:339–345. [PubMed] [Google Scholar]
- 17.Lagerstedt SA, Hinrichs DR, Batt SM, Magera MJ, Rinaldo P, McConnell JP. Quantitative determination of plasma c8-c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol. Genet. Metab. 2001;73:38–45. doi: 10.1006/mgme.2001.3170. [DOI] [PubMed] [Google Scholar]
- 18.Moser AJ, Raymond GV, Moser HW. Plasma and red blood cell fatty acids in peroxisomal disorders. Neurochem. Res. 1999;24:187–197. doi: 10.1023/a:1022549618333. [DOI] [PubMed] [Google Scholar]
- 19.Matern D, Strauss AW, Hillman SL, Mayatepek E, Millington DS, Trefz FK. Diagnosis of mitochondrial trifunctional protein deficiency in a blood spot from the newborn screening card by tandem mass spectrometry and DNA analysis. Pediatr. Res. 1999;46:45–49. doi: 10.1203/00006450-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Marmor MF, Zrenner E. Standard for clinical electroretinography (1994 update) Doc. Ophthalmol. 1995;89:199–210. doi: 10.1007/BF01203373. [DOI] [PubMed] [Google Scholar]
- 21.Weleber RG, Gupta N, Trzupek KM, Wepner MS, Kurz DE, Milam AH. Electroretinographic and clinicopathologic correlations of retinal dysfunction in infantile neuronal ceroid lipofuscinosis (infantile Batten disease) Mol. Genet. Metab. 2004;83:128–137. doi: 10.1016/j.ymgme.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Weleber RG. The effect of age on human cone and rod ganzfeld electroretinograms. Invest. Ophthalmol. Vis. Sci. 1981;20:392–399. [PubMed] [Google Scholar]
- 23.Norcia AM, Tyler CW. Spatial frequency sweep VEP: visual acuity during the first year of life. Vision Res. 1985;25:1399–1408. doi: 10.1016/0042-6989(85)90217-2. [DOI] [PubMed] [Google Scholar]
- 24.Zemon V, Hartmann EE, Gordon J, Prunte-Glowazki A. An electrophysiological technique for assessment of the development of spatial vision. Optomol. Vis. Sci. 1997;74:708–716. doi: 10.1097/00006324-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor DL, Hall R, Adamkin D, Auestad N, Castillo M, Connor WE, Connor SL, Fitzgerald K, Groh-Wargo S, Hartmann EE, Jacobs J, Janowsky J, Lucas A, Margeson D, Mena P, Neuringer M, Nesin M, Singer L, Stephenson T, Szabo J, Zemon V. Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics. 2001;108:359–371. doi: 10.1542/peds.108.2.359. [DOI] [PubMed] [Google Scholar]
- 26.Ver Hoeve JN, Danilov YP, Kim CB, Spear PD. VEP and PERG acuity in anesthetized young adult rhesus monkeys. Vis. Neurosci. 1999;16:607–617. doi: 10.1017/s0952523899164010. [DOI] [PubMed] [Google Scholar]
- 27.Andreasson S, Tornqvist K, Ehinger B. Full-field electroretinograms during general anesthesia in normal children compared to examination with topical anesthesia. Acta Ophthalmol. (Copenh) 1993;71:491–495. doi: 10.1111/j.1755-3768.1993.tb04624.x. [DOI] [PubMed] [Google Scholar]
- 28.Jones PM, Moffitt M, Joseph D, Harthcock PA, Boriack RL, Ibdah JA, Strauss AW, Bennett MJ. Accumulation of free 3-hydroxy fatty acids in the culture media of fibroblasts from patients deficient in long-chain l-3-hydroxyacyl-CoA dehydrogenase: a useful diagnostic aid. Clin. Chem. 2001;47:1190–1194. [PubMed] [Google Scholar]
- 29.Jones PM, Quinn R, Fennessey PV, Tjoa S, Goodman SI, Fiore S, Burlina AB, Rinaldo P, Boriack RL, Bennett MJ. Improved stable isotope dilution-gas chromatography-mass spectrometry method for serum or plasma free 3-hydroxy-fatty acids and its utility for the study of disorders of mitochondrial fatty acid beta-oxidation. Clin. Chem. 2000;46:149–155. [PubMed] [Google Scholar]
- 30.Tyni T, Johnson M, Eaton S, Pourfarzam M, Andrews R, Turnbull DM. Mitochondrial fatty acid beta-oxidation in the retinal pigment epithelium. Pediatr. Res. 2002;52:595–600. doi: 10.1203/00006450-200210000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Neuringer M, Connor WE, Lin DS, Barstad L, Luck S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc. Natl. Acad. Sci. USA. 1986;83:4021–4025. doi: 10.1073/pnas.83.11.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuringer M, Connor WE, Van Petten C, Barstad L. Dietary omega-3 fatty acid deficiency and visual loss in infant rhesus monkeys. J. Clin. Invest. 1984;73:272–276. doi: 10.1172/JCI111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birch EE, Birch D, Uauy R. Visual function and essentiality of a-linolenic acid and docosahexaenoic acid in human infants. In: Yehuda S, Mostofsky DI, editors. Handbook of Essential Fatty Acid Biology: Biochemistry, Physiology, and Behavioral Neurobiology. Humana Press; Totowa, NJ: 1997. pp. 183–199. [Google Scholar]
- 34.SanGiovanni JP, Berkey CS, Dwyer JT, Colditz GA. Dietary essential fatty acids, long-chain polyunsaturated fatty acids, and visual resolution acuity in healthy fullterm infants: a systematic review. Early Hum. Dev. 2000;57:165–188. doi: 10.1016/s0378-3782(00)00050-5. [DOI] [PubMed] [Google Scholar]
- 35.Birch EE, Birch DG, Hoffman DR, Uauy R. Dietary essential fatty acid supply and visual acuity development. Invest. Ophthalmol. Vis. Sci. 1992;33:3242–3253. [PubMed] [Google Scholar]
- 36.Weisinger HS, Vingrys AJ, Abedin L, Sinclair AJ. Effect of diet on the rate of depletion of n-3 fatty acids in the retina of the guinea pig. J. Lipid Res. 1998;39:1274–1279. [PubMed] [Google Scholar]
- 37.Jeffrey BG, Mitchell DC, Gibson RA, Neuringer M. n−3 fatty acid deficiency alters recovery of the rod photoresponse in rhesus monkeys. Invest. Ophthalmol. Vis. Sci. 2002;43:2806–2814. [PubMed] [Google Scholar]
- 38.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 39.Hamer RD, Norcia AM, Tyler CW, Hsu-Winges C. The development of monocular and binocular VEP acuity. Vision Res. 1989;29:397–408. doi: 10.1016/0042-6989(89)90004-7. [DOI] [PubMed] [Google Scholar]
- 40.Lund AM, Dixon MA, Vreken P, Leonard JV, Morris AA. Plasma and erythrocyte fatty acid concentrations in long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. J. Inherit. Metab. Dis. 2003;26:410–412. doi: 10.1023/a:1025175606891. [DOI] [PubMed] [Google Scholar]
- 41.Bistrian BR. Clinical aspects of essential fatty acid metabolism: Jonathan Rhoads Lecture. J. Parenter. Enteral Nutr. 2003;27:168–175. doi: 10.1177/0148607103027003168. [DOI] [PubMed] [Google Scholar]