Abstract
Phenylalanine homeostasis in mammals is primarily controlled by liver phenylalanine hydroxylase (PAH) activity. Inherited PAH deficiency (phenylketonuria or PKU) leads to hyperphenylalaninemia in both mice and humans. A low level of residual liver PAH activity ensures near-normal dietary protein tolerance with normal serum phenylalanine level, but the precise threshold for normal phenylalanine clearance is unknown. We employed hepatocyte transplantation under selective growth conditions to investigate the minimal number of PAH-expressing hepatocytes necessary to prevent hyperphenylalaninemia in mice. Serum phenylalanine levels remained normal in mice exhibiting nearly complete liver repopulation with PAH-deficient hepatocytes (<5% residual wild-type liver PAH activity). Conversely, transplantation of PAH-positive hepatocytes into PAH-deficient Pahenu2 mice, a model of human PKU, yielded a significant decrease in serum phenylalanine (<700 μM) when liver repopulation exceeded approximately 5%. These data suggest that restoration of phenylalanine homeostasis requires PAH activity in only a minority of hepatocytes.
Keywords: phenylketonuria, phenylalanine, hepatocyte transplantation, mouse model
Introduction
Phenylketonuria (PKU) is one of the most common inborn errors of metabolism, with an incidence of approximately 1:16,000 births in North America. The most common cause of PKU is deficiency of phenylalanine hydroxylase (PAH; EC 1.14.16.1) due to recessively inherited mutations in the PAH gene [1]. PAH is a homotetramer expressed primarily in liver, but also in pancreas and kidney, that catalyzes the irreversible hydroxylation of phenylalanine (Phe) to tyrosine (Tyr) [2] and requires the cofactor tetrahydrobiopterin (BH4). Reduction of blood Phe levels through dietary protein restriction prevents the major manifestations of the disease (mental retardation, seizures, and growth failure), but shortcomings in this strategy exist, including lifelong commitment to an unpalatable and expensive diet and persistent mild cognitive deficits in some treated children [3]. Enzyme replacement or substitution, cell transplantation, organ transplantation, and gene therapy are promising alternative approaches to the treatment of inborn errors of metabolism such as PKU. However, a detailed understanding of the physiologic requirements for inducing effective Phe clearance is critical to the successful development of these treatment strategies.
Here, we sought to define the minimal number of PAH-expressing hepatocytes necessary to prevent hyperphenylalaninemia in mice. Published data in mice [4] suggest that total liver PAH activity of approximately 10% wild-type levels is sufficient to maintain normal Phe homeostasis without dietary protein restriction, but it is not known whether PAH activity in a small minority of hepatocytes can maintain normal Phe clearance or whether correction of hyperphenylalaninemia would require PAH activity in the majority of hepatocytes. Our hypothesis was that as few as 5–10% PAH-positive hepatocytes in mouse liver would be sufficient to prevent hyperphenylalaninemia without dietary protein restriction. To examine this hypothesis, we employed hepatocyte-mediated therapeutic liver repopulation to develop a series of mice with <20% PAH-positive hepatocytes and measured the effect upon blood Phe. The success of liver repopulation following hepatocyte transplantation depends upon two factors: an active stimulus for liver regeneration at the time of transplant and a selective growth advantage for donor hepatocytes [5]. For example, wild-type donor hepatocytes have a substantial growth advantage over native cells following transplantation into mice with genetic fumarylacetoacetate hydrolase (FAH) deficiency, a model of human tyrosinemia type I [6]. In this model, FAH deficiency leads to apoptotic loss of native hepatocytes and replacement with FAH-positive donor cells. Without further manipulation, PAH-positive hepatocytes have no selective growth advantage over PAH-deficient cells. However, in the appropriate selective model system, hepatocyte transplantation can still be used as an experimental tool in the investigation of Phe homeostasis. In our transplantation experiments, we employed PAH-deficient Pahenu2 mice, a model for human PKU, and created an advantage for donor hepatocytes by incorporating into recipient animals the growth-selective conditions inherent in genetic FAH deficiency.
Results
Transplantation of PAH-Deficient Hepatocytes into Tyrosinemic Mice
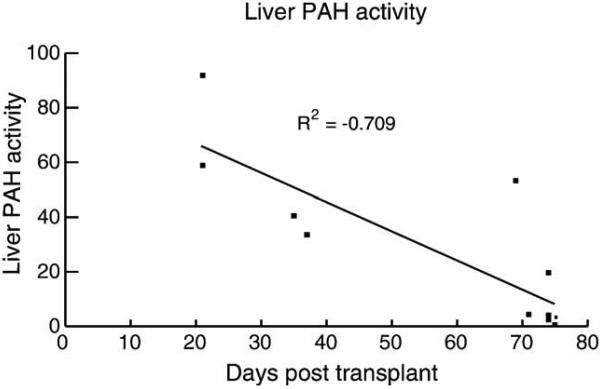
In the absence of a selective growth advantage, transplantation of wild-type primary hepatocytes into PAH-deficient homozygous Pahenu2/Pahenu2 mice has not yielded detectable liver repopulation with PAH-positive donor cells even following partial hepatectomy to stimulate liver regeneration (data not shown). However, wild-type hepatocytes do enjoy a significant growth advantage following transplantation into FahΔexon5 mice [6]. FahΔexon5 mice are homozygous for a targeted deletion of the Fah gene and exhibit complete FAH deficiency, tyrosinemia, progressive liver degeneration, and early death [7]. Oral administration of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC) prevents liver failure and rescues FAH-deficient mice [8]. In the absence of NTBC, transplantation of wild-type hepatocytes into FahΔexon5/FahΔexon5 mice yields nearly complete liver repopulation with FAH-positive donor cells and phenotypic rescue [6]. In our initial experiment, we transplanted PAH-deficient primary hepatocytes from Pahenu2/Pahenu2 mice into tyrosinemic FahΔexon5/FahΔexon5 mice. Our hypothesis was that following liver repopulation with PAH-deficient hepatocytes, hyperphenylalaninemia would occur in animals with fewer than 5–10% residual PAH-positive hepatocytes. Because the donor hepatocytes used in the initial transplants were obtained from Pahenu2 mice on the C57Bl/6J background, transplant recipient 129/Sv-FahΔexon5 mice were also homozygous for a targeted deletion of rag1 [9]; deficiency of cell-mediated immunity in these mice prevented rejection of noncongenic donor hepatocytes. We injected a total of 25 129/Sv-FahΔexon5/FahΔexon5, rag1/rag1 mice intrasplenically with 5–6 × 105 C57Bl/6J-Pahenu2/Pahenu2 hepatocytes. NTBC therapy was discontinued 1 day after transplantation to induce liver repopulation. Thirteen FahΔexon5/FahΔexon5 mice survived off NTBC and were successfully rescued by hepatocyte transplantation. We obtained liver samples at 21–75 days following transplant and analyzed them immunohistologically for the presence of FAH protein or homogenized them for radiometric analysis of PAH activity. A representative liver section stained for FAH protein is displayed in Fig. 1A, demonstrating substantial repopulation of the liver with FAH-positive hepatocytes in a FahΔexon5 mouse 35 days after receiving 6 × 105 donor hepatocytes. The mean liver PAH activity in transplanted mice was 24.8 ± 8.0 nmol Tyr produced/h/mg protein (mean ± SE, n = 13) but ranged from 2.0 to 91.9. The mean liver PAH activity in transplanted mice was significantly lower than that of wild-type mice (92.6 ± 18.6 nmol/h/mg, n = 6; P < 0.001). As expected, the mean liver PAH activity in transplanted mice was inversely correlated with the time between transplant and tissue harvest (Fig. 2), demonstrating that the extent of liver repopulation with FAH-positive/PAH-negative hepatocytes increased with time after transplant. The time course of liver repopulation in this experiment was similar to that previously reported in FahΔexon5 mice [6]. Based upon residual liver PAH activity of 2.0 nmol/h/mg in a single animal, transplant-recipient liver had been nearly completely repopulated with FAH-positive, PAH-negative hepatocytes. That is, very few (<5%) PAH-positive hepatocytes remained in the transplanted liver.
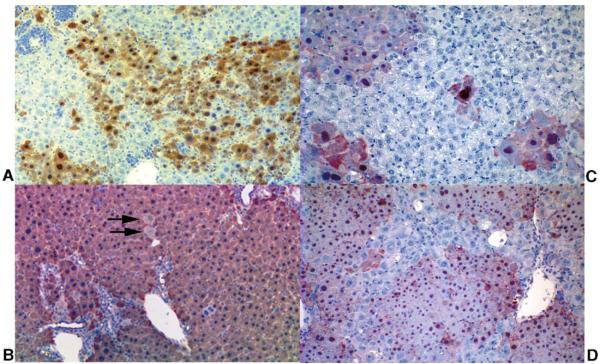
Fig. 1.
FAH immunohistology of FAH-deficient recipient livers following hepatocyte transplantation. (A and B) FahΔexon5/FahΔexon5 liver following transplantation with PAH-deficient Pahenu2/Pahenu2 hepatocytes. (A) Liver exhibiting extensive repopulation with FAH-positive (but PAH-negative) cells 35 days after transplant. (B) Liver from Mouse 2 (Table 1) demonstrating nearly complete repopulation with FAH-positive cells 117 days after hepatocyte transplant and a second round of regeneration induced by partial hepatectomy. A few FAH-deficient hepatocytes remain in the transplant-recipient liver (arrows). (C and D) Pah/Fah liver following transplantation with wild-type FAH-positive, PAH-positive hepatocytes. (C) Liver from Mouse F (Table 2) containing discrete islands of FAH-positive hepatocytes. The repopulation frequency measured 6.3 ± 1.0% by PAH assay. (D) Liver of Mouse A (Table 2) demonstrating more extensive repopulation (20.5 ± 1.8% by PAH assay) with FAH-positive wild-type hepatocytes.
Fig. 2.
Plot of liver PAH activity in transplanted FahΔexon5/FahΔexon5 mice vs time in days after transplant. The extent of repopulation increased and hence PAH activity (nmol Tyr produced/h/mg protein) decreased with time in animals that received PAH-deficient Pahenu2/Pahenu2 hepatocytes.
Despite nearly complete liver repopulation with PAH-negative hepatocytes, serum Phe levels were only minimally elevated. The mean serum Phe level at the time of tissue harvest was 218 ± 30 μM (range 128–506 μM) compared to 130 ± 14.6 μM pretransplant (P = 0.018). The posttransplant serum Phe levels were substantially lower than the levels typical of Pahenu2/Pahenu2 mice (1574 ± 380 μM, n = 11), which completely lack detectable PAH activity in any tissue. Interestingly, there was no correlation between serum phenylalanine levels posttransplant and the extent of liver repopulation (data not shown). Two not mutually exclusive explanations for this finding are that (1) fewer than 5% PAH-positive hepatocytes are necessary to maintain near-normal Phe homeostasis and that (2) PAH activity in kidney and pancreas (organs known to constitutively express PAH in mouse) [10] is able to compensate for nearly complete but isolated liver PAH deficiency in transplanted FahΔexon5 mice.
To achieve more complete liver repopulation, three transplanted mice successfully underwent a second round of liver regeneration and growth selection beginning 67 days after the initial hepatocyte transplant. We reinstated NTBC therapy for 7 days and then performed a partial hepatectomy (removal of 50–80% of the left lobe) on day 74 after the initial transplant. We discontinued NTBC again 1 day after partial hepatectomy. We euthanized the mice for tissue analysis 42 days after partial hepatectomy (117 days following the hepatocyte transplant). Liver PAH activities and serum Phe levels obtained at the time of partial hepatectomy and at euthanasia are presented in Table 1. PAH activity was undetectable in liver homogenate from two animals (Mice 2 and 3) following partial hepatectomy and growth selection. In our hands, the radiometric PAH activity assay will reliably detect conversion of as little as 1% radiolabeled Phe to Tyr yielding an effective lower limit of detection of approximately 1 nmol Tyr/h/mg protein or about 1% of wild-type liver PAH activity. In fact, despite the lack of detectable PAH activity, a few isolated islands of FAH-negative hepatocytes representing native cells were detected by immunohistology (Fig. 1B) in both Mouse 2 and Mouse 3. Still, liver repopulation >99% was achieved in these two animals. Serum Phe levels increased three- to fourfold in these two mice following a second round of liver regeneration and growth selection but even then did not reach the levels typical of Pahenu2/Pahenu2 mice.
TABLE 1.
Effect of partial hepatectomy and repeated growth selection in FahΔexon5/FahΔexon5 mice receiving PAH-deficient Pahenu2/Pahenu2 hepatocyte transplants
| Liver PAH activity in homogenate (nmol/h/mg protein) | ||||
|---|---|---|---|---|
| At partial hepatectomy (left lobe) |
After partial hepatectomy (entire liver) |
Serum phenylalanine (μM) (normal is 100–160 μM) | ||
| Mouse | At partial hepatectomy | After partial hepatectomy | ||
| 1 | 4.2 | 6.4 | 142 | 529 |
| 2 | 3.7 | None detected | 188 | 824 |
| 3 | 4.1 | None detected | 233 | 771 |
At partial hepatectomy means 74 days after hepatocyte transplant, after partial hepatectomy means 117 days after hepatocyte transplant.
Development of Double-Mutant PAH-Deficient/FAH-Deficient Mice
The data obtained above suggested that PAH expression in fewer than 5% hepatocytes is sufficient to normalize blood Phe levels. To test this hypothesis fully, we wanted a hyperphenylalaninemic mouse model with innate growth-selective characteristics. We bred 129/Sv-Pahenu2 and 129/Sv-FahΔexon5 mice to generate progeny homozygous for both the Pahenu2 mutation and the FahΔexon5 deletion (hereafter designated Pah/Fah). During the initial breedings, 156 progeny were produced from 28 Pahenu2/+, FahΔexon5/+ crosses. Of these, 8 mice (5.1%) were Pah/Fah, an incidence that is not significantly different from the expected incidence of 6.25% (χ2 = 0.335, P < 0.001). The mean serum Phe level in Pah/Fah mice was 1693 ± 72.7 μM (mean ± SE, n = 12), which is not different from Phe levels in the parent Pahenu2 line (1574 ± 380 μM, n = 11). Serum Tyr measured 186 ± 10.6 μM, which is elevated above normal (control range 27.3–82.6 μM) but is lower than the Tyr levels typically measured in FahΔexon5/FahΔexon5 mice (1000 ± 140 μM) [8]. PAH deficiency in Pah/Fah mice prevents the conversion of dietary Phe to Tyr. Consequently, Tyr levels were only minimally elevated despite the lack of FAH activity in the mice.
Hepatocyte Transplantation into Pah/Fah Mice
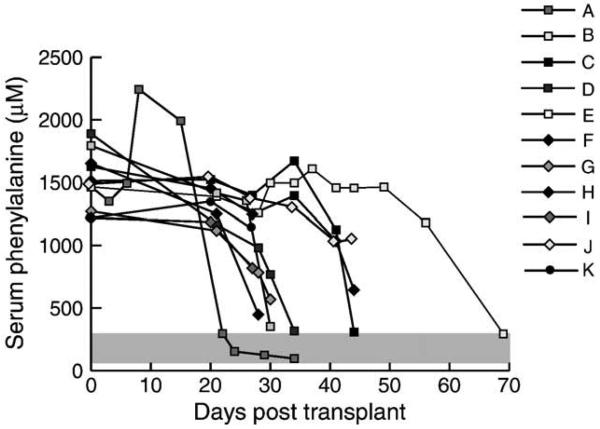
We injected 16 129/Sv-Pah/Fah mice intrasplenically with 3–4 × 105 wild-type 129/Sv hepatocytes expressing 100% normal PAH activity. We measured serum Phe levels weekly thereafter. Ultimately, 11 mice (3 male, 8 female) survived off NTBC and were successfully rescued by hepatocyte transplantation; we then euthanized these animals individually for tissue harvest once serum Phe had decreased to below 1200 μM. The course of serum Phe levels is displayed in Fig. 3, and the specific results are listed in Table 2. The mean time after transplant at which the mice were sacrificed was 36.5 ± 13.1 days (mean ± SE; range 22–69 days). We measured PAH activity either in whole liver homogenate or in single-cell hepatocyte suspension isolated by in situ collagenase perfusion. We calculated the percentage repopulation by comparing the measured liver PAH activity from transplanted mice with that of wild-type animals. Liver PAH activity in transplanted Pah/Fah mice ranged from 1.31 to 63.6 nmol/h/mg and was equivalent to repopulation with 3.2–20% wild-type hepatocytes. The extent of repopulation as estimated by FAH immunohistology varied considerably between individual liver left lobe sections (data not shown) and was not consistently representative of the repopulation frequency measured in the whole of the remaining liver. Still, immunohistochemical examination of transplanted liver did provide visual confirmation of successful liver repopulation by FAH-positive hepatocytes (Figs. 1C and 1D).
Fig. 3.
Serum phenylalanine vs time in Pah/Fah mice after wild-type hepatocyte transplant. Serum phenylalanine levels in Pah/Fah mice are plotted vs days after transplant with wild-type hepatocytes. Serum phenylalanine levels corrected as early as 22 days and as late as 69 days after transplant. Normal range of serum phenylalanine is depicted in the gray-shaded area.
TABLE 2.
Results of wild-type hepatocyte transplantation in Pah/Fah mice
| Mouse | Days posttransplant |
Pretransplant Phe (μM) |
Posttransplant Phe (μM) |
% Wild-type PAH activity (mean ± SE) |
|---|---|---|---|---|
| A | 34 | 1465 | 96 | 20.5 ± 1.8 |
| B | 69 | 1464 | 294 | 8.5 ± 1.5 |
| C | 44 | 1512 | 309 | 9.3 ± 4.1 |
| D | 34 | 1888 | 317 | 6.2 ± 1.5 |
| E | 30 | 1414 | 352 | 4.8 ± 0.9 |
| F | 28 | 1652 | 448 | 6.3 ± 1.0 |
| G | 30 | 1270 | 569 | 5.0 ± 0.6 |
| H | 44 | 1626 | 644 | 7.2 ± 3.2 |
| I | 27 | 1218 | 685 | 6.9 ± 0.4 |
| J | 44 | 1488 | 1053 | 3.2 ± 1.1 |
| K | 27 | 1218 | 1154 | 5.4 ± 3.8 |
| Mean ± SD | 35.7 ± 12.8 | 1474 ± 201 | 538 ± 328 |
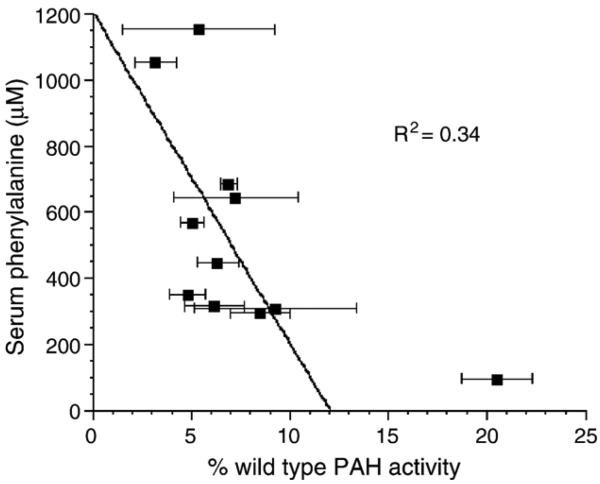
Serum Phe levels decreased after hepatocyte transplantation in all 11 mice (Table 2). The mean serum Phe at euthanasia was 538 ± 328 μM (range 96–1154 μM), a significant decrease from pretransplant serum Phe levels (1474 ± 201 μM; P < 0.001). Posttransplant serum Phe levels are plotted against percentage wild-type liver PAH activity in Fig. 4. There is a trend toward decreasing serum Phe levels as the amount of PAH activity increases from 3 to 9%. However, complete correction of serum Phe to wild-type levels (85–175 μM) was seen only in a single animal with approximately 20% wild-type liver PAH activity. Serum Phe levels are substantially reduced but incompletely corrected in mice with less than 10% liver PAH activity. Still, mice with liver PAH activity of 4.8–9.3% wild-type PAH exhibited on average a 68% reduction in serum Phe (range of serum Phe 316–685 μM) to levels that are generally accepted as adequate treatment in humans with PKU on dietary therapy (Phe < 600 μM).
Fig. 4.
Comparison of serum phenylalanine to extent of liver repopulation in transplanted Pah/Fah mice. Serum Phe levels for transplanted mice at the time of tissue harvest are plotted against % wild-type PAH activity measured in liver homogenate or isolated hepatocytes. Reasoning that Phe clearance would have been completely corrected in the single animal with >10% wild-type PAH activity, regression analysis examining the relationship between serum Phe and liver PAH activity was performed using only mice with <10% wild-type PAH activity (Mice B–K).
Discussion
Understanding the physiologic requirements for inducing effective Phe clearance is critical to the development of successful cell-based or gene-transfer treatments for PKU. The subject of gene therapy for phenylketonuria has recently been reviewed [11]. Published attempts of liver-directed gene therapy for murine PKU have utilized recombinant retrovirus based upon the Moloney murine leukemia virus [12], recombinant adenovirus [4], or adeno-associated virus (AAV) vectors [13-16]. Unfortunately, many of these attempts have been limited to varying degrees by either insufficient or temporary gene expression. Recently, administration of extremely high-titer PAH-expressing recombinant AAV vectors (either AAV serotype 2 vector [15] or AAV2 vector pseudotyped with serotype 5 capsid [14,16]) to Pahenu2 mice has resulted in complete correction of hyperphenylalaninemia in male mice. In the report of Oh et al. [15], portal vein injection of 2 × 1012 recombinant AAV2 vector genomes per mouse yielded liver PAH activity of 17% of normal and a reduction in serum Phe to 360 ± 160 μM by 5 weeks following injection. The percentage of hepatocytes transduced was not reported. Over the next several weeks, the number of vector genomes detectable in liver decreased. At 25 weeks postinjection, mean liver PAH activity had decreased to 9.5% and serum Phe increased to approximately 600 μM. In multiple trials with recombinant AAV vectors, correction of blood Phe levels in treated female Pahenu2 mice has either not occurred or required much higher doses of vector and then was only temporary. These results are probably due to gender-specific differences in efficiency and stability of recombinant AAV-mediated transduction.
Previously, Woo and colleagues infused PAH-expressing recombinant adenovirus into the portal vein of Pahenu2 mice [4]. Serum Phe levels were normalized when PAH activity in liver homogenate was reconstituted to greater than 10% of wild-type activity. Restoration of PAH activity in liver homogenate to 10% of control levels would have been measured in either of two scenarios: correction of cellular PAH activity to ∼10% of control levels in most of the hepatocytes or, alternatively, correction to >10% in only a fraction of hepatocytes in the liver. These two scenarios are not necessarily physiologically equivalent. It is possible that, as the number of PAH-expressing hepatocytes decreases, Phe clearance may become limited by factors other than PAH activity, such as blood flow to PAH-positive hepatocytes, cell membrane Phe transport, or cellular BH4 supply. In fact, flux control analysis in isolated rat hepatocytes suggested that cellular PAH activity and transmembrane Phe transport make equal contributions (that is, they have nearly equal control coefficients of approximately 0.5) to total Phe flux under conditions of basal enzyme activity [17]. Following maximal activation of PAH by glucagon, Phe flux was controlled predominantly by the rate of Phe transport into the hepatocyte. A central question then is whether transduction and PAH expression in a majority of hepatocytes are required for a complete cure, or will enzyme correction in a few hepatocytes accomplish the same goal? Determining the relationship between the number of PAH-expressing hepatocytes, the level of PAH activity, and correction of hyperphenylalaninemia is critical to our understanding of disease pathophysiology and the design of any rational treatment strategy.
Our hypothesis was that repopulation of mouse liver with only 5–10% PAH-expressing wild-type cells would prevent hyperphenylalaninemia. In fact, transplantation of PAH-negative hepatocytes into FahΔexon5 mice suggested that liver repopulation with fewer than 5% hepatocytes with full PAH activity is actually necessary to prevent hyperphenylalaninemia. This interpretation may be complicated by the fact that other tissues of the transplant recipients, namely pancreas and kidney, retain PAH activity. These tissues could make substantial contributions to total body Phe clearance and confound our examination of Phe metabolism in the liver.
Further support of our hypothesis came from wild-type hepatocyte transplantation into PAH-deficient, FAH-deficient mice. Repopulation of Pah/Fah liver with as few as 3.2% wild-type hepatocytes led to a modest reduction in serum Phe levels. Higher levels of liver repopulation yielded substantial reduction of hyperphenyalaninemia, but serum Phe normalized in only a single animal with approximately 20% wild-type liver PAH activity. These data strongly support our hypothesis that PAH expression in a majority of hepatocytes is not required to maintain Phe clearance and that restoration of full PAH activity in >5% of hepatocytes would substantially reduce serum Phe levels in Pahenu2 mice.
The PAH activity in isolated wild-type murine hepatocytes measured in our laboratory is approximately 2.3 × 10−4 nmol Tyr produced/h/cell. Estimating that an adult mouse liver contains approximately 5 × 107 hepatocytes, then a mouse liver with 5% wild-type hepatocytes would be capable of producing 525 nmol Tyr/h or 31,500 nmol Tyr/day from dietary Phe. Adult Pahenu2 mice tolerate approximately 1.8 nmol dietary Phe per day and maintain near-normal serum Phe levels (data not shown). This can be taken as an estimate of the daily requirement for Phe to satisfy anabolic demands. A mouse receiving standard mouse chow (23% protein by weight) consumes approximately 300 nmol Phe per day, the majority of which must be disposed of daily, primarily through hydroxylation by PAH. So, a liver populated with 5% PAH-positive hepatocytes should contain more than sufficient PAH activity to catabolize the typical daily dietary Phe intake of a mouse. In this experiment, we found that 4–9% repopulation yielded substantial but incomplete correction of serum Phe levels. This result suggests that, at this level of repopulation, liver PAH activity is not the only factor important to Phe clearance. As suggested from experiments with isolated rat hepatocytes [17], we propose that the rate of Phe transport into PAH-positive cells greatly influenced Phe clearance and posttransplant serum Phe levels in transplanted mice. Confirmation of this hypothesis will require further study.
The experiments reported here demonstrate that liver repopulation with fewer than 10% PAH-positive hepatocytes significantly lowers serum Phe in mice consuming normal mouse chow. If this result proves applicable to humans as well, then the outlook for liver-directed gene or cell therapy for PKU is optimistic as these results demonstrate that the threshold for phenotypic correction in PAH deficiency is relatively low. Hepatocyte transplantation has been proposed as a possible therapeutic approach for inherited liver enzyme deficiencies [18] such as PKU. Hepatocyte transplantation has been performed and has yielded partial but only temporary effects in a child with Crigler–Najjar syndrome type I [19], an adult with glycogen storage disease type I [20], a 4-year-old girl with infantile Refsum disease [21], and a male infant with ornithine transcarbamylase deficiency [22]. Several other individuals with α1-antitrypsin deficiency have also received hepatocyte transplants [23] but few details of these cases have been published. The therapeutic success of these hepatocyte transplants in humans was likely limited, in most cases, by the lack of a stimulus for liver regeneration at the time of transplant and the lack of any selective growth advantage for donor cells. Immunologic rejection of donor cells in the face of inadequate immunosuppression likely also contributed to the temporary clinical effect of hepatocyte transplant in some cases [22]. The therapeutic threshold for hepatocyte transplantation, that is, the level of liver repopulation necessary to effect a therapeutic benefit, will likely vary among different diseases and will be determined by the number of normal hepatocytes required to correct disease-specific pathophysiology.
The experiments reported here along with previous work by other investigators [5] clearly demonstrate that physiologically relevant liver repopulation requires a selective growth advantage for donor cells over native hepatocytes. This limitation may be illustrated by the following calculation for a model hepatocyte transplant. Assuming that an adult mouse liver contains 1 × 108 hepatocytes, then 5 × 107 hepatocytes will remain following 50% partial hepatectomy (to induce liver regeneration prior to hepatocyte transplant). If 5 × 105 wild-type hepatocytes are transplanted into the recipient and if the cell doubling time is equivalent for both donor and recipient hepatocytes, then one cell cycle will again yield 1 × 108 recipient hepatocytes but only 1 × 106 donor hepatocytes, a repopulation frequency of only 1%. Transplantation of greater numbers of hepatocytes is problematic because of potential portal venous thrombosis. Higher levels of liver repopulation are possible only if the donor hepatocytes have a significant growth advantage over native hepatocytes.
PAH-positive hepatocytes do not enjoy any selective growth advantage over native cells in a PAH-deficient liver; successful hepatocyte transplantation as therapy for human PKU will require the development of a method to provide the necessary growth advantage for donor hepatocytes. Alternatively, liver-directed gene therapy continues to hold promise as a potential therapy for PKU if a gene transfer method can be developed that both is safe and can provide stable PAH expression. Correction of 5–10% of hepatocytes to express 100% wild-type PAH activity per cell would be sufficient to reduce hyperphenylalaninemia substantially.
Materials and Methods
Animal husbandry
Animal care and experimentation were performed in accordance with the guidelines of the Department of Comparative Medicine, Oregon Health & Science University. We utilized the 129/Sv-FahΔexon5 mouse model of fumarylacetoacetate hydrolase (EC 3.7.1.2) deficiency as described [7]. All breeders and FAH-deficient mice were treated with NTBC at a concentration of 7.5 mg/ml in drinking water. NTBC is a reversible competitive inhibitor of p-hydroxyphenylpyruvate dioxidase, an intermediate enzymatic step in Tyr metabolism that is proximal to FAH in the metabolic pathway. The cellular toxin fumarylacetoacetate (FAA) accumulates in FAH deficiency and causes liver failure; administration of NTBC prevents FAA production and rescues FAH-deficient mice. PAH-deficient Pahenu2 mice were homozygous for the same Pah mutation as described in the original BTBR-Pahenu2 strain [24,25] but had been bred and backcrossed onto either the C57Bl/6J or the 129/Sv background. Genotyping for the presence of the FahΔexon5 deletion [7] or the Pahenu2 mutation [26] was performed by PCR analysis of tail biopsy DNA. Blood Tyr levels were measured in Pah/Fah mice by amino acid analyzer [27]. All animals were fed standard mouse chow ad libitum providing approximately 23% of energy as protein.
Hepatocyte transplants
Parenchymal hepatocytes were isolated from donor mice by two-step collagenase perfusion as described [28] except that the collagenase preparation used was Liberase Blendzyme (Roche, Indianapolis, IN, USA), 23 μg/ml. Cell number and viability were determined by trypan blue exclusion in a hemacytometer. The desired number of donor cells was suspended in 100 μl Dulbecco's minimal essential medium supplemented with 10% fetal bovine serum and injected intrasplenically into recipient animals [29]. NTBC therapy of FAH-deficient recipient mice was discontinued following transplantation. The weight of transplanted animals was measured weekly.
Phenotypic characterization of transplanted animals
Serum Phe concentrations were measured weekly in 10 μl serum using a modification of a fluorometric procedure [30]. At the endpoint of the experiment, transplanted mice were euthanized by cardiac exsanguination under inhaled anesthesia. A biopsy of the left hepatic lobe was obtained for immunohistology. For the remaining liver, primary hepatocytes were isolated by collagenase perfusion via the inferior vena cava or portal vein. This procedure allowed biochemical and molecular evaluation of a suspension of primary hepatocytes from the entire liver, eliminated stromal cells, and avoided possible data artifacts secondary to differences in extent of repopulation among different regions of the liver. In some cases, collagenase perfusion was unsuccessful; when that occurred, the whole liver was homogenized for analysis. PAH activity was measured in duplicate or triplicate in hepatocytes or liver homogenate using a radiochemical technique [31] modified as previously described [26]. Total protein was measured using a bicinchoninic acid procedure (Microprotein Assay; Pierce, Rockford, IL, USA). Liver homogenate or primary hepatocytes isolated from wild-type C57Bl/6J mice were used as positive controls. For calculation of percentage liver repopulation, liver PAH activity of transplanted mice was expressed as a percentage of the wild-type PAH activity measured in liver homogenate or isolated hepatocytes in that day's assay.
Acknowledgments
The authors thank David Koeller, K. Michael Gibson, and Melanie Gillingham for critical review of the manuscript. This work was supported by the Oregon Medical Research Foundation (C.O.H.) and National Institutes of Health Grants K08-DK02405 and RO1-DK59371 (C.O.H.) and RO1-DK04252 (M.G.).
Abbreviations
- PKU
phenylketonuria
- Phe
phenylalanine
- PAH
phenylalanine hydroxylase
- Tyr
tyrosine
- FAH
fumarylacetoacetate hydrolase
- NTBC
2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione
REFERENCES
- 1.Scriver CR, Kaufman S. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Valle D, editor. The Metabolic & Molecular Bases of Inherited Disease. McGraw–Hill; New York: 2001. pp. 1667–1724. [Google Scholar]
- 2.Udenfriend S, Cooper JR. The enzymatic conversion of phenylalanine to tyrosine. J. Biol. Chem. 1952;194:503–511. [PubMed] [Google Scholar]
- 3.Azen CG, et al. Intellectual development in 12-year-old children treated for phenylketonuria. Am. J. Dis. Child. 1991;145:35–39. doi: 10.1001/archpedi.1991.02160010037012. [DOI] [PubMed] [Google Scholar]
- 4.Fang B, et al. Gene therapy for phenylketonuria: phenotypic correction in a genetically deficient mouse model by adenovirus-mediated hepatic gene therapy. Gene Ther. 1994;1:247–254. [PubMed] [Google Scholar]
- 5.Laconi E, Laconi S. Principles of hepatocyte repopulation. Semin. Cell Dev. Biol. 2002;13:433–438. doi: 10.1016/s1084952102001313. [DOI] [PubMed] [Google Scholar]
- 6.Overturf K, et al. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat. Genet. 1996;12:266–273. doi: 10.1038/ng0396-266. [DOI] [PubMed] [Google Scholar]
- 7.Grompe M, et al. Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev. 1993;7:2298–2307. doi: 10.1101/gad.7.12a.2298. [DOI] [PubMed] [Google Scholar]
- 8.Al-Dhalimy M, Overturf K, Finegold M, Grompe M. Long-term therapy with NTBC and tyrosine-restricted diet in a murine model of hereditary tyrosinemia type I. Mol. Genet. Metab. 2002;75:38–45. doi: 10.1006/mgme.2001.3266. [DOI] [PubMed] [Google Scholar]
- 9.Mombaerts P, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 10.Tourian A, Goddard J, Puck TT. Phenylalanine hydroxylase activity in mammalian cells. J. Cell. Physiol. 1969;73:159–170. doi: 10.1002/jcp.1040730210. [DOI] [PubMed] [Google Scholar]
- 11.Ding Z, Harding CO, Thöfny B. State-of-the-art 2003 on PKU gene therapy. Mol. Genet. Metab. 2004;81:3–8. doi: 10.1016/j.ymgme.2003.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu T-J, Kay MA, Darlington GJ, Woo SLC. Reconstitution of enzymatic activity in hepatocytes of phenylalanine hydroxylase-deficient mice. Somatic Cell Mol. Genet. 1992;18:89–96. doi: 10.1007/BF01233451. [DOI] [PubMed] [Google Scholar]
- 13.Laipis PJ, et al. Long term reduction of serum phenylalanine levels in a mouse model of PKU by rAAV-mediated gene therapy. Mol. Ther. 2001;3:S293. [Google Scholar]
- 14.Mochizuki S, et al. Long-term correction of hyperphenylalaninemia by AAV-mediated gene transfer leads to behavioral recovery in phenylketonuria mice. Gene Ther. 2004;11:1081–1086. doi: 10.1038/sj.gt.3302262. [DOI] [PubMed] [Google Scholar]
- 15.Oh HJ, Park ES, Kang S, Jo I, Jung SC. Long-term enzymatic and phenotypic correction in the phenylketonuria mouse model by adeno-associated virus vector-mediated gene transfer. Pediatr. Res. 2004;56:278–284. doi: 10.1203/01.PDR.0000132837.29067.0E. [DOI] [PubMed] [Google Scholar]
- 16.Laipis PJ, et al. Correction of maternal phenylketonuria syndrome in the Pahenu2 missense mutant mouse by r-AAV mediated gene therapy. Mol. Ther. 2004;9:S334. [Google Scholar]
- 17.Salter M, Knowles RG, Pogson CI. Transport of the aromatic amino acids into isolated rat liver cells: properties of uptake by two distinct systems. Biochem. J. 1986;233:499–506. doi: 10.1042/bj2330499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Chowdhury JR. Therapeutic potential of hepatocyte transplantation. Semin. Cell Dev. Biol. 2002;13:439–446. doi: 10.1016/s1084952102001325. [DOI] [PubMed] [Google Scholar]
- 19.Fox IJ, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N. Engl. J. Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 20.Muraca M, et al. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317–318. doi: 10.1016/S0140-6736(02)07529-3. [DOI] [PubMed] [Google Scholar]
- 21.Sokal EM, et al. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation. 2003;76:735–738. doi: 10.1097/01.TP.0000077420.81365.53. [DOI] [PubMed] [Google Scholar]
- 22.Horslen SP, et al. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics. 2003;111:1262–1267. doi: 10.1542/peds.111.6.1262. [DOI] [PubMed] [Google Scholar]
- 23.Burlina A. Hepatocyte transplantation for inborn errors of metabolism. J. Inherited Metab. Dis. 2003;26:25. doi: 10.1023/B:BOLI.0000031095.57411.8d. [DOI] [PubMed] [Google Scholar]
- 24.McDonald JD, Bode VC, Dove WF, Shedlovsky A. Pahhph-5: a mouse mutant deficient in phenylalanine hydroxylase. Proc. Natl. Acad. Sci. USA. 1990;87:1965–1967. doi: 10.1073/pnas.87.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald JD, Charlton CK. Characterization of mutations at the mouse phenylalanine hydroxylase locus. Genomics. 1997;39:402–405. doi: 10.1006/geno.1996.4508. [DOI] [PubMed] [Google Scholar]
- 26.Harding CO, Wild K, Chang D, Messing A, Wolff JA. Metabolic engineering as therapy for inborn errors of metabolism—development of mice with phenylalanine hydroxylase expression in muscle. Gene Ther. 1998;5:677–683. doi: 10.1038/sj.gt.3300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slocum RH, Cummings JG. Amino acid analysis of physiological samples. In: Hommes FA, editor. Techniques in Diagnostic Human Biochemical Genetics: a Laboratory Manual. Wiley–Liss; New York: 1991. pp. 87–126. [Google Scholar]
- 28.Grompe M, Jones SN, Loulseged H, Caskey CT. Retroviral-mediated gene transfer of human ornithine transcarbamylase into primary hepatocytes of spf and spf-ash mice. Hum. Gene Ther. 1992;3:35–44. doi: 10.1089/hum.1992.3.1-35. [DOI] [PubMed] [Google Scholar]
- 29.Ponder KP, et al. Mouse hepatocytes migrate to liver parenchyma and function indefinitely after intrasplenic transplantation. Proc. Natl. Acad. Sci. USA. 1991;88:1217–1221. doi: 10.1073/pnas.88.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCaman MW, Robins E. Fluorimetric method for the determination of phenylalanine in serum. J. Lab. Clin. Med. 1962;59:885–890. [Google Scholar]
- 31.Ledley FD, Hahn T, Woo SL. Selection for phenylalanine hydroxylase activity in cells transformed with recombinant retroviruses. Somatic Cell Mol. Genet. 1987;13:145–154. doi: 10.1007/BF01534694. [DOI] [PubMed] [Google Scholar]