Abstract
Phenylketonuria (PKU) caused by phenylalanine hydro-xylase (PAH) deficiency leads to toxic accumulation of phenylalanine (Phe). PAH is predominantly expressed in liver and its activity requires a supply of tetrahydrobiopterin (BH4) cofactor, but we propose that expression of a complete Phe hydroxylating system (PAH plus BH4 synthetic enzymes) in skeletal muscle will lead to therapeutic reduction of blood Phe levels in Pahenu2 mice, a model of human PKU. In order to test this hypothesis, we first developed transgenic Pahenu2 mice that lack liver PAH activity but coexpress, in their skeletal muscle, PAH and guanosine triphosphate cyclohydrolase I (GTPCH). The latter is responsible for the committing enzymatic step in BH4 biosynthesis. Despite sufficient muscle enzyme expression, these mice remained hyperphenylalaninemic, thereby suggesting that expression of additional BH4 synthetic enzymes would be necessary. A recombinant triple-cistronic adeno-associated virus-2 (AAV2) pseudotype 1 vector expressing PAH along with GTPCH and 6-pyruvoyltetrahydrobiopterin synthase (PTPS), the next step in BH4 synthesis, was generated. Injection of this vector into the gastrocnemius muscles of Pahenu2 mice led to stable and long-term reduction of blood Phe and reversal of PKU-associated coat hypopigmentation. We propose that muscle-directed gene therapy will be a viable alternative treatment approach to PKU and other inborn errors of metabolism.
INTRODUCTION
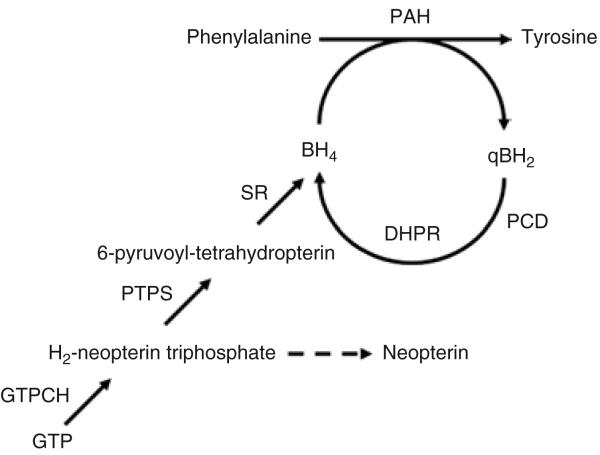
Phenylketonuria (PKU, OMIM 261600) is an autosomal recessive metabolic disease in which the deficiency of the enzyme phenylalanine hydroxylase (PAH, enzyme class 1.14.16.1) leads to systemic accumulation of phenylalanine (Phe) in the body.1,2 The PAH system catalyzes the irreversible hydroxylation of Phe to tyrosine, that occurs primarily in liver and includes the obligatory cofactors tetrahydrobiopterin (BH4) and molecular oxygen. BH4 is synthesized de novo from guanosine triphosphate (GTP) by a three-enzyme pathway involving GTP cyclohydrolase I (GTPCH), 6-pyruvoyltetrahydrobiopterin synthase (PTPS), and sepiapterin reductase, and is regenerated following PAH-mediated Phe hydroxylation by two additional enzymes, pterin-4a-carbinolamine dehydratase and dihydropteridine reductase (see Figure 1).3 The pathology of chronically elevated blood Phe (>360 μmol/l or 6 mg/dl) is associated with mental and growth retardation, micro-cephaly, seizures, and hypopigmentation of skin, hair, and eyes. An important feature regarding therapy of PKU is that PAH deficiency has no direct pathologic effect upon the liver itself; rather, the chronically elevated Phe damages the developing brain through as yet incompletely understood mechanisms.4 Although the underlying pathophysiology of hyperphenylalaninemia remains elusive, the relationship between brain pathology and blood Phe is indisputable, and children with severe PKU can have normal cognitive development when a Phe-restricted diet is initiated during early infancy and blood Phe is maintained at or near normal. Therefore any treatment that successfully reduces blood Phe will ameliorate the PKU phenotype. Contemporary therapy is based upon severe life-long restriction of dietary Phe intake and includes the daily consumption of unpalatable artificial medical foods.5 Noncompliance with the diet is common, especially during adolescence and adulthood. Long-term complications of noncompliance include a leukodystrophy associated with seizures, spasticity and movement disorder, and in women with PKU who are pregnant and noncom-pliant with diet, fetal teratogenesis, the so-called maternal PKU syndrome.6,7 For these reasons, alternative treatment approaches for PKU such as gene therapy continue to be explored.4
Figure 1. The hepatic phenylalanine hydroxylating system.
The tetrahydrobiopterin (BH4) cofactor, which is essential for phenylala-nine hydroxylase (PAH) activity, is endogenously synthesized starting from guanosine triphosphate (GTP) by consecutive action of the three enzymes GTP cyclohydrolase I (GTPCH), 6-pyruvoyltetrahydrobiopterin synthase (PTPS) and sepiapterin reductase (SR), and is maintained by recycling involving two enzymes, pterin-4a-carbinolamine dehydratase (PCD) and dihydropteridine reductase (DHPR).3 Upon limiting PTPS activity, neopterin accumulates in serum by nonenzymatic oxidation and dephosphorylation of the dihydroneopterin triphosphate intermediate (see also text).
Recently we reported long-term correction of hyperphenylalaninemia in the PKU mouse model, C57Bl/6-Pahenu2, after liver-directed gene transfer with a recombinant adeno-associated virus (rAAV) pseudotype 8 vector.8,9 However, questions of expression stability, treatment toxicity, potential for insertional mutagenesis, and safety of the invasive techniques required for accessing human liver remain unanswered in this liver-directed approach. Skeletal muscle has been successfully exploited for expressing foreign transgenes because of its several tissue-specific characteristics, including large total mass, high degree of vascularization, stable environment for protein expression by persistent postmitotic nuclei, and easy accessibility.10 Skeletal muscle has therefore been a gene therapy target, not only in models of muscle disease, but also for immunization and production of secreted therapeutic proteins such as clotting factors or hormones. In this study, we report the long-term correction of hyperphenylalaninemia in C57Bl/6-Pahenu2 mice by ectopic expression of a complete Phe hydroxylating system in skeletal muscle. This was accomplished through intramuscular injection of a triple-cistronic AAV pseudotype 1 vector directing the expression of murine PAH along with the BH4-biosynthetic enzymes GTPCH and PTPS. This effective gene therapy approach that can potentially clear the blood of Phe in skeletal muscle in an animal model, may be explored as a clinically applicable method in PKU patients.
RESULTS
Expression of PAH and GTPCH in muscle of PKU mice corrects hyperphenylalaninemia but requires administration of BH4
It has previously been demonstrated that expression of PAH in skeletal muscle of germline-modified Pahenu2 mice leads to significant reduction in blood Phe levels, if sufficient BH4 cofactor is delivered exogenously.11 An in vitro cell culture study with human primary keratinocytes indicated that cells expressing both PAH and GTPCH were capable of metabolizing Phe.12 Although abundant in liver, BH4 is a limiting cofactor for hepatic PAH activity,13,14 and only minor amounts of BH4 are found in skeletal muscle (Table 1). In a comparison of all three BH4-biosynthetic enzyme activities in muscle to those in liver, skeletal muscle had <1% of GTPCH and only 28% of PTPS activity (Table 1), whereas significant sepiapterin reductase was present (not shown). We therefore constructed a transgene vector containing the murine PAH complementary DNA (cDNA) under the transcriptional control of the muscle creatine kinase promoter, followed by an internal ribosome entry site (IRES) and the human GTPCH cDNA, and employed this construct to produce transgenic mice by germ-line modification. Transgenic mice that expressed both PAH and GTPCH in skeletal muscle were crossed to Pahenu2 mice and bred to homozygosity. The resulting progeny, Tg/Pahenu2 mice, lacked PAH activity in the liver (< 2% of wild type) but expressed PAH and GTPCH in skeletal muscle (Table 1). However, despite substantial activity of PAH and GTPCH in muscle, Tg/Pahenu2 mice remained hyperphenylalaninemic, with serum Phe levels (2077 ± 327 μmol/l) not different from levels typical of nontransgenic homozygous C57Bl/6-Pahenu2 mice (serum Phe = 2107 ± 38 μmol/l; normal serum Phe < 100 μmol/l; Figure 2). An analysis of muscle pterin content revealed substantial neopterin (9.05 ± 0.90 pmol/ mg protein) which is the oxidized and dephosphorylated product of the GTPCH reaction (see Figure 1). In contrast, neopterin was undetectable in skeletal muscle of nontransgenic wild-type mice. In liver of Tg/Pahenu2 mice, dihydroneopterin triphosphate, synthesized de novo by native GTPCH, is rapidly processed further to BH4 through the activities of PTPS and sepiapterin reductase.3 Liver neopterin content in Tg/Pahenu2 mice was therefore appropriately low (0.61 ± 0.32 pmol/mg). Without PTPS activity in skeletal muscle of Tg/Pahenu2 mice, dihydroneopterin triphosphate cannot be processed to BH4. We therefore concluded that the amount of BH4 formed in skeletal muscle of Tg/Pahenu2 mice was insufficient to support physiologically significant Phe hydroxylation. This hypothesis was confirmed by administering BH4 by intraperito-neal injection to Tg/Pahenu2 mice and observing transient normalization of serum Phe levels (Figure 2). The results suggested that muscle PAH activity in Tg/Pahenu2 mice was sufficient to correct hyperphenylalaninemia but that the endogenous muscle BH4 con-tent, despite the presence of GTPCH activity, was insufficient to sustain Phe hydroxylation. Previous experiments with cultured skin fibroblasts have also demonstrated the need for PTPS activity along with PAH and GTPCH to sustain Phe hydroxylation in vitro.15 We therefore reasoned that PTPS activity would also be needed in skeletal muscle in order to synthesize sufficient BH4 and support Phe hydroxylation.
Table 1.
Enzymatic activities of PAH, GTPCH, and PTPS, and BH4 content in liver and muscle tissues of transgenic and AAV2/1-treated PKU mice in comparison with controls
| Liver | Musclea | |||||||
|---|---|---|---|---|---|---|---|---|
| Mouse treatment | PAH (mU/mg) |
GTPCH (μU/mg) |
PTPS (μU/mg) |
BH4 (pmol/mg) |
PAH (mU/mg) |
GTPCH (μU/mg) |
PTPS (μU/mg) |
BH4 (pmol/mg) |
| Wild type (untreated) | 3.5 ± 0.64 | 2.41 ± 0.27 | 8.17 ± 0.74 | 26.64 ± 2.63 | <0.02 | <0.02 | 2.29 ± 1.26 | 1.78 ± 1.08 |
| PKU (Pahenu2 untreated) | <0.02 | 3.33 ± 0.14 | 5.56 ± 0.27 | 43.77 ± 3.38 | <0.02 | <0.02 | 1.12 ± 0.07 | 3.76 ± 0.23 |
| Tg-MCK-PAH-GTPCH (in Pahenu2 background) |
<1%b | 0.98 ± 0.04 | n.d. | 41.12 ± 3.12 | 9–19%b | 0.32 ± 0.03 | n.d. | 1.27 ± 0.36c (21.89 ± 5.19)d |
| PKU + AAV2/1-PKU3 (high)e (untreated foreleg) |
0.15 ± 0.02 (n.a.) |
2.44 ± 0.01 (n.a.) |
7.45 ± 2.85 (n.a.) |
30.69 ± 1.58 (n.a.) |
0.04 ± 0.02 (<0.02) |
0.07 ± 0.01 (0.05 ± 0.01) |
6.68 ± 1.58 (1.10 ± 0.08) |
5.124 ± 0.120 (2.30 ± 1.33) |
| PKU+AAV2/1-PKU3 (high)f (untreated foreleg) |
0.45 ± 0.16 (n.a.) |
0.43 ± 0.13 (n.a.) |
15.31 ± 1.21 (n.a.) |
17.71 ± 1.32 (n.a.) |
0.07 ± 0.05 (<0.02) |
0.03 ± 0.01 (<0.01) |
25.96 ± 4.11 (1.19 ± 0.16) |
1.33 ± 0.65 (1.71 ± 0.11) |
| PKU + AAV2/1-PKU3 (high)g | 0.04 ± 0.01 | n.d. | n.d. | n.d. | 0.05 ± 0.02 | n.d. | n.d. | n.d. |
| PKU+AAV2/1-PKU3 (low)e (untreated foreleg) |
<0.02 (n.a.) |
0.42 ± 0.06 (n.a.) |
13.01 ± 4.69 (n.a.) |
61.70 ± 8.67 (n.a.) |
<0.02 (<0.02) | 0.05 ± 0.01 (<0.01) |
14.17 ± 2.46 (0.84 ± 0.11) |
2.87 ± 0.80 (2.21 ± 0.06) |
| PKU + AAV2/1-PKU4 (high)h (untreated foreleg) |
<0.02 (n.a.) |
1.36 ± 0.08 (n.a.) |
6.87 ± 0.78 (n.a.) |
30.29 ± 0.89 (n.a.) |
0.11 ± 0.02 (<0.02) |
0.22 ± 0.07 (<0.01) |
1.40 ± 0.36 (2.74 ± 0.06) |
5.85 ± 0.46 (4.24 ± 0.93) |
| PKU + AAV2/1-PKU4 (low)i (untreated foreleg) |
<0.02 (n.a.) |
1.72 ± 0.05 (n.a.) |
6.46 ± 0.45 (n.a.) |
48.83 ± 0.84 (n.a.) |
0.09 ± 0.02 (<0.02) |
0.08 ± 0.02 (<0.01) |
1.37 ± 0.71 (0.93 ± 0.06) |
5.56 ± 2.21 (1.93 ± 0.42) |
Abbreviations: AAV, adeno-associated virus; BH4, tetrahydrobiopterin; GTPCH, guanosine triphosphate cyclohydrolase I; high, high vector dose with 3.5 × 1012 vector particles; low, low vector dose with 1.5 × 1012 vector particles; MCK, muscle creatine kinase; n.d., not determined; n.a., not applicable; PAH, phenylalanine hydroxylase; PKU, phenylketonuria; PTPS, 6-pyruvoyltetrahydrobiopterin synthase.
The mice were killed at different time points after administration of recombinant AAV2/1 vectors.
In mice injected with AAV vector, these data reflect the enzyme and BH4content in treated hindeg; values from untreated foreleg muscle are given within parentheses.
Liver and muscle PAH activity of Tg-MCK-PAH-GTPCH mice measured in the laboratory of C.H. and expressed relative to the specific PAH activity in wild-type C57B1/6 liver homogenate (n = 2).
Untreated.
Twenty-four hours after intraperitoneal injection of BH4 (see text and Figure 2).
Twelve hours after intraperitoneal injection of BH4 (see text and Figure 2).
Eight weeks after viral injection.
Twenty-eight weeks after viral injection.
Seventy weeks after viral injection.
Ten weeks after viral injection.
Twenty-two weeks after viral injection.
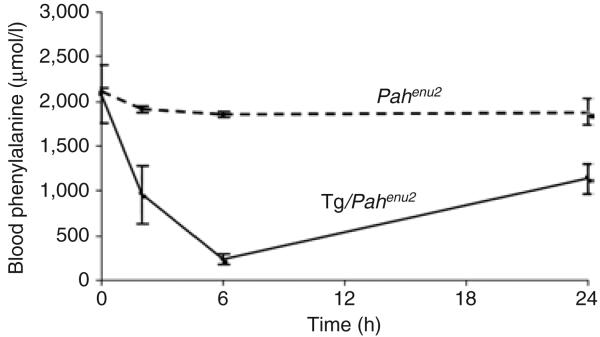
Figure 2. Effect of tetrahydrobiopterin (BH4) supplementation in Tg/Pahenu2 mice expressing phenylalanine hydroxylase (PAH) and GTP cyclohydrolase I in skeletal muscle.
Mean blood values for phenylalanine (Phe) versus time (h) in Pahenu2 PKU mice (broken line; n = 2) and Tg/Pahenu2 mice (solid line; n = 4) after a single intraperitoneal injection of BH4 (1.0 μmol BH4/g body weight in 1% ascorbic acid). Differences in plasma phe concentration following injection of either BH4 or vehicle were compared using a repeated measures analysis of variance. Plasma Phe decreased with time (P = 0.0014) after the injection in all the mice, but the animals that had received BH4 showed a significantly greater decrease in serum phenylalanine than the controls did (P = 0.0048).
Coordinate expression of PAH along with the BH4-biosynthetic genes GTPCH and PTPS from an rAAV2 pseudotype 1 (AAV2/1) vector
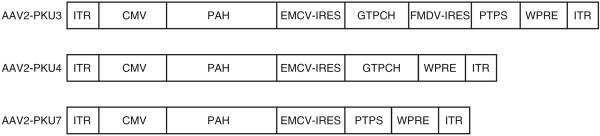
The rAAV2 vectors AAV2-PKU3, AAV2-PKU4, and AAV2-PKU7 were constructed so as to simultaneously express the murine cDNAs for PAH-GTPCH-PTPS, PAH-GTPCH, and PAH-PTPS, respectively, by incorporation of IRES between open reading frames (Figure 3). In these constructs we used the universal cytomegalovirus promoter. Purified AAV2 pseudotype 1 particles were first tested for enzyme activities in vitro after infection of 293T cells (see Table 2). At a multiplicity of infection of 1 × 106, the viral vectors expressed functional genes, the enzyme activities from vector AAV2/1-PKU4 being higher than those from AAV2/1-PKU3. This is most probably because of the smaller size of AAV2/1-PKU4, or the presence of the third gene in AAV2/1-PKU3 influencing the expression levels of the first two genes. In any case, we as well as others16 have observed that in vitro results with AAV transduction do not necessarily predict the relative levels of gene expression in vivo (see later text).
Figure 3. Physical maps of the recombinant adeno-associated virus (rAAV) vectors used.
The two constructs, AAV2-PKU3 and AAV2-PKU4, were used for gene transfer experiments containing the cytomegalovirus promoter (CMV). Transgenes were flanked by inverted terminal repeats (ITRs). The woodchuck post-transcriptional regulatory element (WPRE) sequence was included to enhance gene expression. Two different internal ribosome entry sites (IRESs), derived from the encephalomyocarditis virus (EMCV-IRES) or from the foot and mouth disease virus (FMDV-IRES), were employed to coexpress pyruvoyltetrahydrobiopterin synthase (PTPS) and/or GTP cyclohydrolase I (GTPCH) under the same promoter. PAH, phenylalanine hydroxylase; PKU, phenylketonuria.
Table 2.
In vitro enzyme activities of PAH, GTPCH, and PTPS in AAV-infected 293T cells
| PAH (mU/mg protein) |
GTPCH (μU/mg protein) |
PTPS (μU/mg protein) |
|
|---|---|---|---|
| 293T cells (control) | <0.02 | <0.01 | <1.0 |
| AAV2/1-PKU3 | 0.19 ± 0.01 | 3.17 ± 0.11 | 16.97 ± 3.44 |
| AAV2/1-PKU4 | 4.69 ± 0.34 | 170.27 ± 3.72 | <1.0 |
Abbreviations: AAV, adeno-associated virus; GTPCH, guanosine triphosphate cyclohydrolase I; PAH, phenylalanine hydroxylase; PKU, phenylketonuria; PTPS, 6-pyruvoyltetrahydrobiopterin synthase.
The cells were infected with the different AAV vectors at a multiplicity of infection (MOI) of 106, and enzyme activities were determined 48 hours after treatment.
Direct intramuscular injection of a triple-cistronic AAV2/1 vector corrects hyperphenylalaninemia in C57BL/6-pahenu2 mice
Several authors have confirmed the efficiency of AAV-directed gene transfer to muscle in mice. This approach yields high levels of transduction and stable gene expression, particularly when a capsid from serotype 1 is used.16-22 We therefore generated AAV2/1 particles using our recombinant vectors (shown in Figure 3), and injected these into the gastrocnemius muscle in both hindlegs of PKU mice. Blood Phe levels were monitored periodically for several weeks. As shown in Figure 4a, with a single dose (split into two injections, one for each leg) of 3.5 × 1012 of AAV2/1-PKU3 vector genomes expressing PAH, GTPCH, and PTPS, the blood Phe levels decreased from 1755 ± 224 μmol/l to therapeutic values of 96 ± 28 μmol/l (normal <100 μmol/l) within 1 month after vector administration, and this effect persisted for at least 35 weeks. A gender difference was not observed (three male mice and three female mice were used in this experiment). In some of the mice, the Phe concentrations remained at the therapeutically desirable level of < 360 μmol/l for at least 70 weeks (maximal length of experiment; some mice were killed earlier than 70 weeks for analysis). The normalization of Phe level was accompanied by a complete phenotypic change from brown hair to black by 10 weeks (Figure 5d), and this phenotype was maintained during the rest of the experimental period. At about half the dose of AAV2/1-PKU3(1.5 × 1012 vector genomes), only a marginal decrease in Phe levels was observed 4 weeks after vector administration, from 1,820 ± 250 μmol/l to 1,213 ± 182 μmol/l (see Figure 4a). Although the levels of blood Phe in these animals did not reach therapeutic levels, they remained at a uniform level during the 16 weeks of the experimental period. In contrast to AAV2/1-PKU3-vector therapy containing all three genes, high-dose (3.5 × 1012 vector genomes) or low-dose (1.5 × 1012) administration of AAV2/1-PKU4 expressing only the PAH and GTPCH genes did not lead to therapeutic levels of blood Phe in PKU animals (Figure 4b). With high doses of AAV2/1-PKU4, the Phe level decreased by only approximately one-fourth, from 1,662 ± 229 μmol/l before treatment to 1,257 ± 217 μmol/l 2 weeks after treatment, and this level was maintained during the 15 weeks of experiment. Similarly, after the low-dose treatment, the Phe level decreased from 1,889 ± 314 μmol/l before treatment to 1,462 ± 147 μmol/l 2 weeks after treatment, and remained at this level during the 29 weeks of the experimental period. As an additional control, injection of AAV2/1-PKU7 expressing the PAH and PTPS genes in PKU mice also did not lead to therapeutic levels of blood Phe after either high- or low-dose administration [from 2,201 ± 101 μmol/l before injection to 1,221 ± 10 μmol/l after 4 weeks in the high-dose group (n = 3 mice), and from 2,343 ± 16 μmol/l before injection to 1,610 ± 54 μmol/l after 4 weeks in the low-dose group (n = 2 mice)]. These results, together with the results from transgenic mice (Figure 2) further supported our assumption that PTPS is a limiting factor for BH4 biosynthesis in muscle. Nevertheless, a small decrease in blood Phe was also observed after each of the doses of AAV2/1-PKU4 (Figure 4b). This could be because of limited production, or presence of BH4 cofactor in muscle tissue; alternatively, it could be because of hepatic vector leakage, in view of the fact that we did detect AAV2 genomes in the livers of treated mice (Table 3; see later text). However, the contribution of hepatic viral leakage to the fall in blood Phe might be very limited, given that cytomegalovirus promoter-driven expression is often silenced in the liver.23-26 Evidence of a transient hepatic, i.e., viral, PAH expression is also reflected in the PAH activity observed in individual PKU animals treated with high doses of AAV2/1-PKU3 vectors at 8, 28, and 70 weeks of therapeutic treatment, at which time points we measured 0.15 ± 0.02 mU/mg, 0.45 ± 0.16 mU/mg, and 0.05 ± 0.01 mU/mg, respectively (see Table 1).
Figure 4. Kinetic changes of blood phenylalanine (Phe) concentration in phenylketonuria (PKU) mice after intramuscular adeno-associated virus-2 pseudotype 1 (AAV2/1) vector administration.
Two doses of vectors (a) AAV2/1-PKU3 or (b) AAV2/1-PKU4 were injected into the gastrocnemius muscles of both hindlegs of PKU animals, high dose (high) having 3.5 × 1012 vector particles, and low dose (low) having 1.5 × 1012 vector particles. Methods: Blood was collected onto Guthrie filter cards from tail veins before and after AAV2/1 vector administration into hindleg muscles. Phe concentrations were measured using tandem mass spectrometry. Each time point represents Phe values from at least three animals (male and female). The broken line indicates the Phe level target for dietary treatment in humans with PKU (≤ 360 μmol/l). Blood Phe levels at various time points after AAV2/1 injection were compared with preinjection blood Phe levels, using a Student's t-test. Significant differences found for the high- and low-dose AAV2/1-PKU3-treated animals in a were marked with asterisks (P <0.05). In b, the Phe concentration after treatment, as compared to t = 0, had P >0.05, i.e., no significant changes.
Figure 5. Characterization of phenylalanine hydroxylase (PAH) expression, detection of anti-PAH antibody in serum, and phenotypic reversion of phenylketonuria (PKU) mice after recombinant adeno-associated virus (recombinant AAV) administration.

(a) Detection of Pah-mRNA by reverse transcriptase PCR in different muscles and liver from animals receiving the different treatments. The upper lanes (arrow) contain the expected 78 base pair (bp) Pah transcript, the bottom lanes (arrow head), the 80 bp Gapdh control transcript. The abbreviations wt, PKU3, and PKU4 stand for wild type, AAV2/1-PKU3, and AAV2/1-PKU4, respectively. The dosage was the same as shown in Figure 4 (high-dose treatment 3.5 × 1012 vector particles, low-dose treatment 1.5 × 1012 vector particles). (b) Immunodetection of PAH by western blotting. Each lane contains 20μg of tissue lysates from animals treated with the different vectors. Note that the PKU mouse Bl/6-Pahenu2 expresses mutant PAH protein in liver and the PAH antibody does not distinguish between wild-type and mutant PAH. The arrow indicates the PAH protein (∼51kd.) Abbreviations as in a; M, marker proteins, from top to bottom, 250, 150, 100, 75, 50, 37, 25, 20, and 15kd. (c) For detection of anti-PAH antibody, blood was collected at the indicated time points using the retro-orbital technique. Five hundred nanograms of recombinant PAH protein was probed with serum diluted from 1:100, 1:1,000, 1:10,000, and plotted as absorbance values measured at 492nm. The antibody for positive control was a rabbit anti-PAH. (d) This picture, showing phenotypic reversion of a PKU mouse, was taken ∼10 weeks after gene transfer with AAV2/1-PKU3 (high dose), in comparison with the untreated PKU mouse and a wild-type control. (e) Muscle anti-PAH immunohistology. Immunohistologic staining for PAH protein in untreated Bl/6-Pahenu2 muscle and Bl/6-Pahenu muscle 24 weeks after AAV2/1-PKU3 injection. Fresh frozen cross–sections of gastrocnemius muscles were stained for mouse PAH (green) using a polyclonal rabbit anti-mouse PAH serum and a fluoresceinated goat anti-rabbit immunoglobulin G secondary antibody; for the ubiquitous muscle structural protein desmin (red) using a monoclonal antidesmin antibody and Texas Red–conjugated secondary antibody; and for nuclei using 4′,6-diamidino-2-phenylindole (blue). ×400 Original magnification.
Table 3.
Transduction rate (genome copies per cell) of recombinant AAV2/1 vectors after intramuscular injection
| AAV2/1 vector (dose; time after injection) |
Foreleg muscle |
Hindleg muscle |
Brain | Kidney | Heart | Lung | Spleen | Liver |
|---|---|---|---|---|---|---|---|---|
| AAV2/1-PKU3 (high; 28 weeks) | Negative | 107.00 ± 22.07 | Negative | 5.20 ± 0.26 | 4.60 ± 0.17 | Negative | Negative | 97.67 ± 19.60 |
| AAV2/1-PKU3 (high; 8 weeks) | Negative | 20.00 ± 1.73 | Negative | 5.68 ± 3.23 | 12.71 ± 12.19 | Negative | Negative | 431.83 ± 118.87 |
| AAV2/1-PKU3 (low; 8 weeks) | Negative | 139.33 ± 45.58 | Negative | 3.57 ± 0.40 | 7.10 ± 1.15 | 2.10 ± 0.10 | Negative | 6.48 ± 1.63 |
| AAV2/1-PKU4 (high; 10 weeks) | Negative | 361.20 ± 112.38 | Negative | 2.20 ± 0.20 | 2.33 ± 0.23 | Negative | 2.05 ± 0.14 | 113.00 ± 17.00 |
| AAV2/1-PKU4 (low; 22 weeks) | Negative | 128.17 ± 27.86 | Negative | 2.33 ± 0.24 | 1.83 ± 0.15 | Negative | 1.57 ± 0.23 | 50.67 ± 2.31 |
Abbreviations: AAV, adeno-associated virus; high, high vector dose with 3.5 × 1012 vector particles; low, low vector dose with 1.5 × 1012 vector particles; PKU, phenylketonuria.
From each tissue, 100 ng of isolated genomic DNA was used as a template for quantitative PCR analysis in order to determine vector copy numbers. Primers and probe for the woodchuck post-transcriptional regulatory element sequence were designed with Primer Express (Applied Biosystems). Dilutions of the AAV2/1 vector plasmids were used for generating standard curves.
Molecular characterization of PKU mice expressing the PAH system in muscle after triple-cistronic AAV2/1 gene therapy
AAV2/1-treated mice were eventually killed in order to analyze PAH expression and BH4 content in muscle and other tissues. Because we found virus transduction primarily in hindleg muscles, as expected, but to some extent also in liver, we analyzed these tissues in comparison to foreleg muscles in more detail (see also Table 3). In general, muscle tissue from the hindlegs, where the rAAV2/1 virus had been injected, contained moderately higher BH4 levels than nontreated foreleg muscles from the same animals (Table 1). In contrast, liver BH4 content was not altered after AAV2/1 injection. Liver BH4 content increased in untreated Pahenu2 and Tg/Pahenu2 mice (43.77 ± 3.38 pmol/mg and 41.12. ± 3.12 pmol/mg, respectively) in comparison with wild-type mice (26.64 ± 2.63 pmol/mg). This result reflects the known physiologic response of cofactor biosynthesis to hyperphenylalaninemia.27 Further, we could not detect any neopterin, the product of GTPCH activity, in muscle or any other tissues of AAV2/1-treated animals, thereby indicating that all the neopterin produced had been further converted into BH4. As in the case of BH4 content, the analysis of enzyme activities for PAH and for the BH4-synthesizing enzymes GTPCH and PTPS in vector-treated mice revealed considerable variation but consistent elevation in hindleg muscle tissue where the virus had been injected. Reverse transcriptase PCR and western blotting analyses, and anti-PAH immunohistology (Figure 5a, b and e) were performed in order to further characterize muscle PAH expression. Pah-mRNA and PAH protein were found in all of the virus-treated muscles (quadriceps and gastrocnemius), but not in the untreated foreleg muscle. In parallel, western blotting indicated relatively strong hindleg-muscle PAH expression in all the treated mice, the lowest level of expression being associated with low-dose AAV2/1-PKU3 vector. In order to determine whether PAH protein level correlated with Pah-mRNA level, a quantitative reverse transcriptase PCR method was used. We found the lowest expression of Pah-mRNA in AAV2-PKU-3/1-treated mice that had received the lower vector doses (defined as 1). Relative to this lowest expression level, the mice treated with high doses of AAV2/1-PKU3 had a 3.6-fold higher expression of Pah-mRNA, mice treated with high doses of AAV2/1-PKU4 had a 32-fold higher expression, and mice treated with low doses of AAV2/1-PKU4 had an 18-fold expression increase. That is, the levels of Pah-mRNA transgene expression corresponded approximately with the amount of PAH protein detected by western analysis (Figure 5b). Immunohistologic examination of hindlimb muscle using polyclonal sera raised against murine PAH protein demonstrated diffuse sarcoplasmic distribution of PAH protein in AAV2/1-PKU3-treated mice (Figure 5e) and in AAV2/1-PKU4-treated mice (data not shown). No PAH staining was detected in untreated C57/Bl6-Pahenu2 muscle.
In order to characterize the bio-distribution of viral vectors after muscle injection, the transduction rate profile was compared in different tissues among different animals sacrificed at different time points (Table 3). We found that virus-treated muscle tissue had the highest vector genome copy numbers per cell, and that there was no detectable viral vector in brain or foreleg muscles. Low numbers of vector genomes were found in the kidney and heart. In the case of lung and spleen, some of the animals had a few detectable vector genomes, whereas some did not. We also found considerable numbers of vector genomes in liver, ranging from 7 to 432 vector genome copies per cell. Leakage of AAV2/1 vectors from treated muscle to liver could have occurred because of the large injection volume used for introducing the vector into the hindlegs. As mentioned earlier, we cannot absolutely exclude the possibility that vector-mediated PAH expression in liver contributed to Phe clearance in AAV2/1-treated animals. However, although similar amounts of vector genomes were detected in livers of mice treated with either AAV2/1-PKU3 or AAV2/1-PKU4 vectors, the serum Phe reverted completely to normal levels only in the mice that had received the AAV2/1-PKU3 vector. This result suggests that the amount of putative liver PAH expression in AAV2/1-PKU4-treated mice was insufficient to completely correct hyperphenylalaninemia and that coordinate expression of PAH, GTPCH, and PTPS in skeletal muscle is responsible for improved Phe clearance in Pahenu2 mice treated with AAV2/1-PKU3.
Because PAH is not expressed in muscle of the wild-type mouse, an immune response against ectopic expression of PAH protein might be induced in virally transduced animals. However, as depicted in Figure 5c, we did not detect any anti-PAH antibody in the sera of treated animals at different time points as shown by the direct enzyme linked immunosorbent assay method. Likewise, histologic examination did not reveal any lymphocytic infiltration into AAV2/1-treated muscle.
Analysis of rAAV2-vector genomes in the offspring of treated PKU mice
Because vector application at an early stage in life bears the potential risk of germline transmission, we have also tested for germline transmission after AAV2/1-mediated gene delivery into muscle tissue. For this purpose, we crossed several of the treated PKU male mice with wild-type females and tested 20 offspring mice for the presence of vector sequences, in genomic DNA isolated from tail biopsies. Saturating the amplification to test specifically for the presence of WPRE-DNA did not result in any positive signal (not shown). This result is in agreement with other studies that also showed no evidence for germline transmission after AAV2-mediated gene delivery.28
DISCUSSION
In this study we showed efficient and persistent clearance of serum Phe after stable expression of a complete Phe hydroxylation system, including coordinate expression of PAH activity and BH4-biosynthetic enzymes, in skeletal muscle of hyperphenylalaninemic Pahenu2 mice. The ease of vector administration in this approach in comparison to liver-8,9,29-31 or skin-targeted12 gene delivery makes this novel strategy an attractive potential therapy for PKU. Muscle-targeted heterologous gene expression is not a new strategy. For example, production of factor IX in muscle as potential therapy for hemophilia has been previously reported.32,33 Further, expression of the hepatic bilirubin glucuronosyl transferase gene UGT1A1 in skeletal muscle of rats was shown to correct hyperbilirubinemia, thereby documenting the principle of using muscle to clear a circulating toxic metabolite.34,35 However, to our knowledge, data relating to simultaneous expression of multiple genes following muscle-directed gene transfer have not so far been published. The approach presented here could also provide a treatment strategy for other inherited or acquired diseases that might require expression of multiple gene products.
Intramuscular expression of PAH, GTPCH, and PTPS activities together were required for correction of hyperphenylalaninemia in Pahenu2 mice. The PAH and GTPCH activities as measured in hindleg muscles of AAV2/1 vector-treated mice were low in comparison with those in wild-type liver although transcript and protein levels were high. In contrast, PTPS activity in transduced muscle tissue was found to be expressed with even slightly higher specific activities than in liver (Table 1). Ectopic expression of genes in muscle could lead to functional changes, i.e., post-translational modification may differ from other cell types.10 However, post-transcriptional modification is not required for the activity of PAH36 or GTPCH.3 Hepatic GTPCH activity is highly regulated by the GTPCH feedback regulatory protein; this protein, to our knowledge, is not expressed in skeletal muscle and should not limit the function of muscle GTPCH expressed from AAV2/1 vector.37 Further, in all vector-treated animals, an analysis of muscle pterin content revealed only fully formed BH4 and no neopterin, thereby suggesting that the level of endogenous muscle PTPS activity did not limit BH4 synthesis. We can therefore conclude, on the basis of the therapeutic efficacy demonstrated in this mouse model, that the coordinated expression of PAH, GTPCH, and PTPS in skeletal muscle are necessary and sufficient to decrease systemically high levels of blood Phe.
Immune response against transgene products is one of the major limitations for success in therapeutic gene transfer.21,38-40 In the treatment presented in this study, as well as in liver-directed gene transfer experiments with immune-competent PKU mice,9 we did not detect anti-PAH antibody in the sera of treated animals. This was not surprising, because the PAH, as well as the GTPCH and PTPS, were the murine versions and are cytoplasmic proteins, which are unlikely to present as extracellular antigens.41 Also, because the majority of human PKU cases are caused by missense mutations that retain production of PAH protein,2 the immune response against the wild-type PAH transgene product represents only a minor concern for future gene therapy for PKU, although we have not excluded the possibility of immune response against the viral capsid proteins. Immunity against repeated administration of AAV vectors, where it is required for life-long therapy, is a serious hurdle for application in humans.42 Because the use of the C57BL/6 mouse strain might obscure the immune situation,43 further studies on immune response to rAAV vector administration are required, using other mouse strains and higher organisms such as non-human primates, before human trials can be considered.44
Because symptoms of PKU in hyperphenylalaninemia patients appear early in life, treatment is recommended to be initiated early so as to prevent primarily irreversible brain damage; ideally, treatment should be initiated before 7 days age.4 Because of the rapid proliferation of hepatocytes in newborn liver that dilute and degrade AAV vectors,45 liver-directed gene therapy for PKU may not lead to a permanent cure in newborns.19 In contrast, muscle has been reported to be the most stably transduced tissue at all stages of development, after systemic delivery of AAV2 pseudotype 8 vector in neonatal mice.19,46 Therefore muscle-directed gene therapy may be an attractive treatment approach for human newborns with PKU.
In summary, we report stable correction of hyperphenylalaninemia in a mouse model of human PKU after intramuscular injection of an rAAV2/1 vector expressing a complete Phe hydroxylation system. The relative ease, noninvasiveness, and effectiveness of this muscle-directed gene transfer method make this an attractive potential therapy for PKU and for allied inborn errors of metabolism, and therefore deserves further study.
MATERIALS AND METHODS
Animal husbandry
All animal experiments were carried out in accordance with the guidelines of the Department of Comparative Medicine, Oregon Health and Science University (Portland, OR; transgenic mouse experiments) or of the State Veterinary Office of Zürich (Zürich, Switzerland); and Swiss law on animal protection (AAV2/1 experiments). PAH-deficient C57Bl/6-Pahenu2 mice were homozygous for the same Pah mutation as described in the original BTBR-Pahenu2 strain, but had been bred and backcrossed on to the C57/Bl6 background to facilitate ease of breeding.9 Genotyping was done in accordance with a described protocol.9 All the mice were maintained on standard mouse chow. Blood was collected from tail veins on Guthrie filter cards and Phe concentration was determined using tandem mass spectrometry.
Generation of transgenic animals Tg/Pahenu2 expressing PAH-GTPCH in skeletal muscle
Tg/Pahenu2 mice were produced and characterized in a manner analogous to our earlier described approach.11 The constructed transgene contained the murine PAH-cDNA and human GTPCH cDNA separated by an IRES from encephalomyocarditis virus (EMCV). Muscle-specific expression of the two cDNAs was driven by the 3,300 base pair promoter and enhancer sequences from the mouse muscle creatine kinase gene.47 The transgene was introduced into mouse embryos using standard techniques. The progeny were screened for the presence of the transgene using Southern blotting. A founder line carrying a single copy of the transgene was found to transmit the transgene at the expected frequency and without any untoward effects upon animal health or reproductive fitness. The founder line was bred to Pahenu2 mice to yield progeny (Tg/Pahenu2 mice) that were homozygous for the Pahenu2 mutation and therefore lacked liver PAH activity though they carried the transgene and expressed both murine PAH and human GTPCH in skeletal muscle.
Construction of transgene and AAV2 vectors
Plasmid pUC18 was used as the backbone to generate polycistronic expression cassettes. First, individual mouse cDNAs coding for PAH, GTPCH, and PTPS, as well as IRES sequences from EMCV and FMDV (foot and mouth disease virus) were amplified using PCR with BamHI at the 5′-site and BglII/EcoRI at the 3′-site and subcloned into pUC18 opened with BamHI/EcoRI (for details of IRES elements, see ref. 15). A single NotI site was inserted 5′ to the PAH-cDNA for later cloning into AAV vector plasmid, and a “Flag”-tag sequence was added at the start of PTPS (amino acid sequence DYKDDDDK encoded by the synthetic sequence 5′-GACTACAAGGACGACGATGACAAG-3′). Subsequently, the different fragments were assembled into polycistronic expression cassettes using the compatible overhangs from restriction sites BamHI and BglII. Plasmid pHSY114 contained PAH-EMCV(IRES)-GTPCH and plasmid pHSY116 contained PAH-EMCV(IRES)-GTPCH-FMDVIRES)-Flag-PTPS. The fragments from pHSY114 or pHSY116 were excised by NotI/BglII (filled-in) and cloned into the AAV vector plasmid psub-CMV-WPRE, digested with NotI/PmlI, to result in plasmids AAV2-PKU4 or AAV2-PKU3, respectively. In order to generate AAV2-PKU3, expressing PAH-EMCV(IRES)-Flag-PTPS, plasmid AAV2-PKU3 was digested with NheI/MluI, and both fragments were gel-extracted and purified. The fragment containing the PAH-EMCV(IRES)-GTPCHFMDVIRES)-Flag-PTPS was further excised with XmaI, and the fragment containing the PAH-EMCV(IRES) was isolated. The cDNA coding for the Flag-PTPS was PCR-amplified with XmaI at the 5′-site and MluI at the 3′-site. Subsequently, the three different fragments were assembled using the compatible overhangs from restriction sites NheI, XmaI, and MluI to generate plasmid AAV2-PKU7 (see also Figure 3). The plasmid constructs were confirmed using DNA sequence analysis.
rAAV2 vector pseudotyped with capsids from type 1 were produced in an adenovirus-free system in 293T cells using the triple transient transfection protocol with the AAV2 vector plasmids, an adenovirus helper plasmid (provided by Hansruedi Büeler, University of Kentucky, Lexington, KY), and the AAV serotype 1 packaging plasmid p5E18RXCI (provided by James M Wilson, University of Pennsylvania, Philadelphia, PA). Recombinant virus vectors were purified with two rounds of CsCl gradient centrifugation and concentrated with Amicon Ultra filters (Millipore, Schwerzenback, Switzerland). The physical particle titers of the vectors were determined by TaqMan analysis with primers and probes designed for the woodchuck post-transcriptional regulatory element (WPRE) sequence: forward primer, 5′-CCGTTGTCAGGCAACGTG-3′; reverse primer, 5′-AGCTGACAGGTGGTGGCAAT-3′; probe, 5′-FAM-TGCTGACGCAACCCCCACTGGT-TAMRA-3′. A standard curve was generated by dilution of vector plasmids. Transgene expression from AAV2/1 vectors was evaluated in vitro by infecting cultured 293T cells with recombinant vectors. 293T cells were maintained in standard Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics. Transduction of rAAV2/1 was carried out at a multiplicity of infection of 1 × 106 in 6-well plates, and 48 hours after infection cells were collected and analyzed for enzyme activities.
Administration of rAAV2/1 vectors to Pahenu2 mice
Young adult (8–10 weeks old) hyperphenylalaninemic Pahenu2 mice were selected for muscle injection. Using sterile techniques, the skin of the hindlegs was incised to expose the gastrocnemius muscles. AAV2/1 vectors were injected intramuscularly using a 30 gauge needle at a maximal volume of 100 μl.
Molecular analyses
The number of AAV2 vector genomes per cell from different tissues was determined using quantitative TaqMan-PCR analysis with primers and probes corresponding to the WPRE sequence and designed with Primer Express from Applied Biosystems (Zug, Switzerland): forward primer: 5′-CCGTTGTCAGGCAACGTG-3′, reverse primer: 5′-AGCTGACAGGTGGTGGCAAT-3′, probe: 5′-FAM-TGCTGACGCAACCCCCACTGG-TAMRA-3′. Genomic DNA from different mouse tissues (liver, heart, brain, kidney, lung, muscle, and spleen) was isolated using the DNeasy kit (Qiagen, Hombrechtikon, Switzerland) following the instructions provided. For analysis, 100ng of genomic DNA was used as a template. Dilutions of the AAV2 vector plasmid were used for generating a standard curve for determination of vector genome copies. PCR was carried out at the standard condition for ABI Prism 7700, and the data were analyzed using Sequence Detection System (Applied Biosystems, Zug, Switzerland).
In order to determine relative Pah-mRNA expression, 20–30 mg of mouse liver tissue was used for RNA isolation in accordance with the manufacturer's protocol (QIAmp RNA Blood Mini Kit; Qiagen, Hombrechtikon, Switzerland). Random primed cDNA was prepared from 1μg of total RNA, using the Reverse Transcription System from Promega Catalys (Wallisellen, Switzerland). Quantitative PCR was performed using TaqMan technology and an ABI Prism 7700 sequence detector, and the TaqMan Universal PCR Master Mix (Applied Biosystems, Zug, Switzerland). Primers and probe were designed to amplify Pah messenger RNA (mRNA) as follows: forward primer: 5′-CCGAGAGTTTCAATGATGCCA-3′; reverse primer: 5′-TCATAGCGAA CGGAGAAGGG-3′; probe: 5′-CTTTTGCTGCCACAATCCCCCG-3′ (5′-FAM and 3′-TAMRA-labeled). Standard primers and probe of GAPDH (Applied Biosystems, Zug, Switzerland) were used as internal controls.
PAH enzyme assay and western blot analysis, as well as BH4 and neopterin determination, were performed as described.9, 48
Muscle immunohistology
Muscle samples were flash frozen in isopentane on dry ice and kept at −80 °C until sectioned. Eight-micrometer frozen sections were fixed in methanol, rehydrated, and washed three times in phosphate-buffered saline, pH 7.4. The sections were incubated at 4 °C overnight in a blocking buffer containing Triton X-100, bovine serum albumin, and 1% goat serum. The slides were again washed three times in phosphate-buffered saline and then incubated overnight at 4 °C in blocking buffer containing a 1:200 dilution of polyclonal rabbit anti-mouse PAH antisera (Mirus Bio, Madison, WI) to detect the presence of PAH protein, and a 1:400 dilution of mouse anti-human desmin polyclonal sera (BD Biosciences, Allschwill, Switzerland) to detect the subsarcolemmal protein desmin as a positive control. After being washed, the slides were incubated sequentially with a 1:300 dilution of fluorescein-labeled donkey anti-rabbit immunoglobulin G antisera (Pierce, Socochim, Lausanne, Switzerland) and a 1:200 dilution of Texas Red–labeled goat anti-mouse immunoglobulin G antisera (Pierce, Socochim, Lausanne, Switzerland) for 1 hour each, counterstained with 4,6-diamidino-2-phenylindole in mounting media (Invitrogen, Basel, Switzerland), and imaged on a Leica fluorescence microscope.
Detection of anti-PAH antibody in sera of AAV2/1 treated mice
Serum samples were collected by retro-orbital venipuncture of anesthetized animals. Direct enzyme linked immunosorbent assay was employed for measuring the anti-PAH antibody in accordance with published methods.9 Briefly, 500 ng recombinant mouse PAH protein was coated overnight at 4 °C in phosphate-buffered saline buffer in micro titer plates, and blocked with 0.25% bovine serum albumin-0.05% Tween-20 blocking solution. After being washed, the serum samples were diluted from 1:100 to 1:10,000 in the blocking solution, and 100 μl was added to the wells and incubated for 1 hour at room temperature. A volume of 100 μl horseradish peroxidase–conjugated anti-mouse immunoglobulin G (1:10,000; Sigma, Buchs, Switzerland) was added into the wells and incubated for 30 minutes at room temperature. After extensive washing, 100 μl o-phenylenediamine dihydrochloride substrate (Sigma, Buchs, Switzerland) was added and incubated for 10 minutes. The reactions were stopped by adding 100 μl 4.5 N H2SO4. Absorbance values were measured at 492 nm using a microplate reader. As a positive control, polyclonal rabbit anti-PAH antibody was used as primary antibody and anti-rabbit antibody was used as second antibody for detection.
ACKNOWLEDGMENTS
We are grateful to Nenad Blau and Anna Lauber (University Children's, Hospital Zürich, Zürich, Switzerland) for helpful discussions, Walter Leimbacher, Sandra Holbein, Heidi Voser, and Lucja Kierat (University Children's Hospital Zürich, Zürich, Switzerland), for excellent technical assistance, the mass spectrometry group (Heinz Troxler, University Children's Hospital Zürich, Zürich, Switzerland) of our unit for Phe measurements, Hansruedi Büeler (University of Kentucky, Lexington, KY) for providing material and advice to generate AAV2/1 particles, Aurora Martinez (University of Bergen, Bergen Norway) for purified PAH protein, James M Wilson (University of Pennsylvania, Philadelphia, PA) and the University of Pennsylvania Vector Core of Department of Medicine for the trans plasmid p5E18RXCI for AAV2/1 production, the Institute for Animal Science at the University of Zürich-Irchel for its cooperation, and Felix H Sennhauser (University Children's Hospital Zürich, Zürich, Switzerland) for support. This work was supported by grants to B.T. from the Swiss National Science, the Anna Müller Grocholsky, and the Hartmann Müller Foundations. C.O.H. was supported by National Institutes of Health grant R01 DK/HD59371.
REFERENCES
- 1.Scriver CR, Kaufman S. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th edn. McGraw-Hill; New York: 2001. pp. 1667–1724. [Google Scholar]
- 2.Scriver CR, Hurtubise M, Konecki D, Phommarinh M, Prevost L, Erlandsen H, et al. PAHdb 2003: what a locus-specific knowledgebase can do. Hum Mutat. 2003;21:333–344. doi: 10.1002/humu.10200. [DOI] [PubMed] [Google Scholar]
- 3.Thöny B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347:1–16. [PMC free article] [PubMed] [Google Scholar]
- 4.National Institutes of Health Consensus Development Panel National Institutes of Health Consensus Development Conference Statement: phenylketonuria: screening and management; Pediatrics; October 16–18, 2000; 2001. pp. 972–982. [DOI] [PubMed] [Google Scholar]
- 5.Merrick J, Aspler S, Schwarz G. Phenylalanine-restricted diet should be life long. A case report on long-term follow-up of an adolescent with untreated phenylketonuria. Int J Adolesc Med Health. 2003;15:165–168. doi: 10.1515/ijamh.2003.15.2.165. [DOI] [PubMed] [Google Scholar]
- 6.Levy HL, Ghavami M. Maternal phenylketonuria: a metabolic teratogen. Teratology. 1996;53:176–184. doi: 10.1002/(SICI)1096-9926(199603)53:3<176::AID-TERA5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Guttler F, Azen C, Guldberg P, Romstad A, Hanley WB, Levy HL, et al. Impact of the phenylalanine hydroxylase gene on maternal phenylketonuria outcome. Pediatrics. 2003;112:1530–1533. [PubMed] [Google Scholar]
- 8.Harding CO, Gillingham MB, Hamman K, Clark H, Goebel-Daghighi E, Bird A, et al. Complete correction of hyperphenylalaninemia following liver-directed, recombinant AAV2/8 vector-mediated gene therapy in murine phenylketonuria. Gene Ther. 2006;13:457–462. doi: 10.1038/sj.gt.3302678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding Z, Georgiev P, Thöny B. Administration-route and gender-independent long-term therapeutic correction of phenylketonuria (PKU) in a mouse model by recombinant adeno-associated virus 8 pseudotyped vector-mediated gene transfer. Gene Ther. 2006;13:587–593. doi: 10.1038/sj.gt.3302684. [DOI] [PubMed] [Google Scholar]
- 10.Lu QL, Bou-Gharios G, Partridge TA. Non-viral gene delivery in skeletal muscle: a protein factory. Gene Ther. 2003;10:131–142. doi: 10.1038/sj.gt.3301874. [DOI] [PubMed] [Google Scholar]
- 11.Harding CO, Wild K, Chang D, Messing A, Wolff JA. Metabolic engineering as therapy for inborn errors of metabolism—development of mice with phenylalanine hydroxylase expression in muscle. Gene Ther. 1998;5:677–683. doi: 10.1038/sj.gt.3300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen R, Kolvraa S, Blaese RM, Jensen TG. Development of a skin-based metabolic sink for phenylalanine by overexpression of phenylalanine hydroxylase and GTP cyclohydrolase in primary human keratinocytes. Gene Ther. 2000;7:1971–1978. doi: 10.1038/sj.gt.3301337. [DOI] [PubMed] [Google Scholar]
- 13.Thöny B, Ding Z, Martinez A. Tetrahydrobiopterin protects phenylalanine hydroxylase activity in vivo: implications for tetrahydrobiopterin-responsive hyperphenylalaninemia. FEBS Lett. 2004;577:507–511. doi: 10.1016/j.febslet.2004.10.056. [DOI] [PubMed] [Google Scholar]
- 14.Scavelli R, Ding Z, Blau N, Haavik J, Martinez A, Thöny B. Stimulation of hepatic phenylalanine hydroxylase activity but not Pah-mRNA expression upon oral loading of tetrahydrobiopterin in normal mice. Mol Genet Metab. 2005;86(suppl 1):S153–S155. doi: 10.1016/j.ymgme.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Laufs S, Kim SH, Kim S, Blau N, Thöny B. Reconstitution of a metabolic pathway with triple-cistronic IRES-containing retroviral vectors for correction of tetrahydrobiopterin deficiency. J Gene Med. 2000;2:22–31. doi: 10.1002/(SICI)1521-2254(200001/02)2:1<22::AID-JGM86>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Arruda VR, Schuettrumpf J, Herzog RW, Nichols TC, Robinson N, Lotfi Y, et al. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood. 2004;103:85–92. doi: 10.1182/blood-2003-05-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ, Walsh CE. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- 18.Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 20.Louboutin JP, Wang L, Wilson JM. Gene transfer into skeletal muscle using novel AAV serotypes. J Gene Med. 2005;7:442–451. doi: 10.1002/jgm.686. [DOI] [PubMed] [Google Scholar]
- 21.Carter BJ. Adeno-associated virus vectors in clinical trials. Hum Gene Ther. 2005;16:541–550. doi: 10.1089/hum.2005.16.541. [DOI] [PubMed] [Google Scholar]
- 22.Fisher KJ, Jooss K, Alston J, Yang Y, Haecker SE, High K, et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 23.Guo ZS, Wang LH, Eisensmith RC, Woo SL. Evaluation of promoter strength for hepatic gene expression in vivo following adenovirus-mediated gene transfer. Gene Ther. 1996;3:802–810. [PubMed] [Google Scholar]
- 24.Loser P, Jennings GS, Strauss M, Sandig V. Reactivation of the previously silenced cytomegalovirus major immediate-early promoter in the mouse liver: involvement of NFκB. J Virol. 1998;72:180–190. doi: 10.1128/jvi.72.1.180-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakai H, Herzog RW, Hagstrom JN, Walter J, Kung SH, Yang EY, et al. Adeno-associated viral vector-mediated gene transfer of human blood coagulation factor IX into mouse liver. Blood. 1998;91:4600–4607. [PubMed] [Google Scholar]
- 26.Bilbao R, Reay DP, Li J, Xiao X, Clemens PR. Patterns of gene expression from in utero delivery of adenoviral-associated vector serotype 1. Hum Gene Ther. 2005;16:678–684. doi: 10.1089/hum.2005.16.678. [DOI] [PubMed] [Google Scholar]
- 27.Harada T, Kagamiyama H, Hatakeyama K. Feedback regulation mechanism for the control of GTP cyclohydrolase I activity. Science. 1993;260:1507–1510. doi: 10.1126/science.8502995. [DOI] [PubMed] [Google Scholar]
- 28.Jakob M, Muhle C, Park J, Weiss S, Waddington S, Schneider H. No evidence for germ-line transmission following prenatal and early postnatal AAV-mediated gene delivery. J Gene Med. 2005;7:630–637. doi: 10.1002/jgm.718. [DOI] [PubMed] [Google Scholar]
- 29.Mochizuki S, Mizukami H, Ogura T, Kure S, Ichinohe A, Kojima K, et al. Long-term correction of hyperphenylalaninemia by AAV-mediated gene transfer leads to behavioral recovery in phenylketonuria mice. Gene Ther. 2004;11:1081–1086. doi: 10.1038/sj.gt.3302262. [DOI] [PubMed] [Google Scholar]
- 30.Oh HJ, Park ES, Kang S, Jo I, Jung SC. Long-term enzymatic and phenotypic correction in the phenylketonuria mouse model by adeno-associated virus vector-mediated gene transfer. Pediatr Res. 2004;56:278–284. doi: 10.1203/01.PDR.0000132837.29067.0E. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Woo SL. Complete and persistent phenotypic correction of phenylketonuria in mice by site-specific genome integration of murine phenylalanine hydroxylase cDNA. Proc Natl Acad Sci USA. 2005;102:15581–15586. doi: 10.1073/pnas.0503877102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Herzog RW, Hagstrom JN, Kung SH, Tai SJ, Wilson JM, Fisher KJ, et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 34.Danko I, Jia Z, Zhang G. Nonviral gene transfer into liver and muscle for treatment of hyperbilirubinemia in the gunn rat. Hum Gene Ther. 2004;15:1279–1286. doi: 10.1089/hum.2004.15.1279. [DOI] [PubMed] [Google Scholar]
- 35.Jia Z, Danko I. Long-term correction of hyperbilirubinemia in the Gunn rat by repeated intravenous delivery of naked plasmid DNA into muscle. Mol Ther. 2005;12:860–866. doi: 10.1016/j.ymthe.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Ledley FD, Grenett HE, Woo SL. Biochemical characterization of recombinant human phenylalanine hydroxylase produced in Escherichia coli. J Biol Chem. 1987;262:2228–2233. [PubMed] [Google Scholar]
- 37.Milstien S, Jaffe H, Kowlessur D, Bonner TI. Purification and cloning of the GTP cyclohydrolase I feedback regulatory protein, GFRP. J Biol Chem. 1996;271:19743–19751. doi: 10.1074/jbc.271.33.19743. [DOI] [PubMed] [Google Scholar]
- 38.Flotte TR. Immune responses to recombinant adeno-associated virus vectors: putting preclinical findings into perspective. Hum Gene Ther. 2004;15:716–717. doi: 10.1089/1043034041361190. [DOI] [PubMed] [Google Scholar]
- 39.Bessis N, GarciaCozar FJ, Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11(suppl 1):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 40.Hensley SE, Amalfitano A. Toll-like receptors impact on safety and efficacy of gene transfer vectors. Mol Ther. 2007;15:1417–1412. doi: 10.1038/sj.mt.6300217. [DOI] [PubMed] [Google Scholar]
- 41.Sun JY, Anand-Jawa V, Chatterjee S, Wong KK. Immune responses to adeno-associated virus and its recombinant vectors. Gene Ther. 2003;10:964–976. doi: 10.1038/sj.gt.3302039. [DOI] [PubMed] [Google Scholar]
- 42.Wang CH, Liu DW, Tsao YP, Xiao X, Chen SL. Can genes transduced by adeno-associated virus vectors elicit or evade an immune response? Arch Virol. 2004;149:1–15. doi: 10.1007/s00705-003-0241-3. [DOI] [PubMed] [Google Scholar]
- 43.Waddington SN, Buckley SM, Nivsarkar M, Jezzard S, Schneider H, Dahse T, et al. In utero gene transfer of human factor IX to fetal mice can induce postnatal tolerance of the exogenous clotting factor. Blood. 2003;101:1359–1366. doi: 10.1182/blood-2002-03-0779. [DOI] [PubMed] [Google Scholar]
- 44.Yuasa K, Yoshimura M, Urasawa N, Ohshima S, Howell JM, Nakamura A, et al. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther. 2007;14:1249–1260. doi: 10.1038/sj.gt.3302984. [DOI] [PubMed] [Google Scholar]
- 45.Carter BJ. Adeno-associated virus and the development of adeno-associated virus vectors: a historical perspective. Mol Ther. 2004;10:981–989. doi: 10.1016/j.ymthe.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Paterna JC, Feldon J, Bueler H. Transduction profiles of recombinant adeno-associated virus vectors derived from serotypes 2 and 5 in the nigrostriatal system of rats. J Virol. 2004;78:6808–6817. doi: 10.1128/JVI.78.13.6808-6817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaynes JB, Johnson JE, Buskin JN, Gartside CL, Hauschka SD. The muscle creatine kinase gene is regulated by multiple upstream elements, including a muscle-specific enhancer. Mol Cell Biol. 1988;8:62–70. doi: 10.1128/mcb.8.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niederwieser A, Staudenmann W, Wetzel E. High-performance liquid chromatography with column switching for the analysis of biogenic amine metabolites and pterins. J Chromatogr. 1984;290:237–246. doi: 10.1016/s0021-9673(01)93579-4. [DOI] [PubMed] [Google Scholar]