Abstract
Phenylketonuria (PKU) is one of the most common inborn errors of metabolism with an annual incidence of approximately 1:16,000 live births in North America. Contemporary therapy relies upon lifelong dietary protein restriction and supplementation with phenylalanine-free medical foods. This therapy is expensive and unpalatable; dietary compliance is difficult to maintain throughout life. Non-adherence to the diet is associated with learning disabilities, adult-onset neurodegenerative disease, and maternal PKU syndrome. The fervent dream of many individuals with PKU is a more permanent cure for this disease. This paper will review ongoing efforts to develop viable cell-directed therapies, in particular cell transplantation and gene therapy, for the treatment of PKU.
Keywords: cell transplantation, gene therapy, phenylalanine, phenylketonuria
Baby James died of congenital heart disease. At least, that was the proximate cause of his death. Even if his heart had formed normally, his survival would have been complicated by severe physical and mental disabilities, outcomes associated with the impaired growth and development of his prenatal brain. The aching grief his young mother suffers is compounded manifold by the knowledge that her elevated blood phenylalanine levels caused this complex embryopathy and by the guilt she feels for not controlling her diet during the pregnancy. Although detected by newborn screening and treated from birth, her dietary control of phenylketonuria (PKU) deteriorated during adolescence and led to shortened attention span, poor reasoning skills, and a tendency to impulsive behaviors. The pregnancy was unplanned, prenatal care late, and dietary compliance during first trimester ‘morning sickness’ impossible. By the time she had recognized that she was pregnant, the damage from maternal hyperphenylalaninemia, one of the most potent teratogens known, was done.
Early detection and treatment of PKU embodies the most successful paradigm of newborn screening. Contemporary dietary therapy prevents the major manifestations of the disorder including severe mental retardation, seizures, microcephaly and growth failure (1). However, the diet is complicated, unpalatable and ideally should be maintained throughout life (2). Non-compliance during adolescence and adulthood is common. Elevated phenylalanine (Phe) levels in adults are often associated with attention problems, mood instability, and poor job performance. Chronically elevated Phe may cause a progressive neurodegenerative disorder, affecting white matter that leads to seizures and gait disturbance (3). Finally, as illustrated by the tragic story above, untreated maternal hyperphenylalaninemia is the only teratogen guaranteed to cause birth defects, which include microcephaly, mental retardation, and congenital heart disease (4). The search continues for a permanent cure that would avoid these complications. This paper will review ongoing efforts to develop gene- and cell-based therapies for the permanent treatment of PKU.
The physiology of phenylalanine and PKU
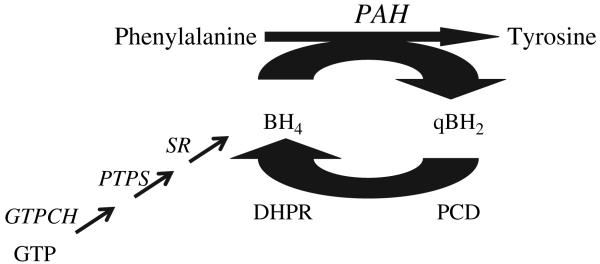
The most common cause of human PKU is deficiency of phenylalanine hydroxylase (PAH; EC 1.14.16.1) due to recessively inherited mutations in the PAH gene. The molecular genetics and patho-physiology of PAH deficiency have been thoroughly reviewed (5). For the sake of brevity, only the key physiologic elements necessary to understand treatment approaches to PKU will be presented here. PAH catalyzes the irreversible hydroxylation of phenylalanine to tyrosine in the phenylalanine degradation pathway (Fig. 1). During the course of phenylalanine hydroxylation, the required pterin cofactor tetrahydrobiopterin (BH4) is oxidized to quinonoid dihydrobiopterin (qBH2). Fully reduced BH4 is regenerated through the activity of two enzymes, pterin-4a-carbinolamine dehydratase (PCD) and dihydropteridine reductase (DHPR) (6). BH4 is also supplied through de novo synthesis from guanosine triphosphate. The presence of PCD and DHPR activities along with adequate BH4 supply are necessary to sustain PAH-mediated phenylalanine hydroxylation.
Fig. 1.
Phenylalanine hydroxylation. Phenylalanine hydroxylase (PAH) catalyzes the irreversible hydroxylation of phenylalanine to tyrosine. PAH is an iron-containing homotetramer that requires the unconjugated pterin cofactor, tetrahydrobiopterin (BH4), and molecular oxygen as cofactors. In the course of the hydroxylation reaction, BH4 is oxidized to quinonoid BH4. Adequate BH4 supply is maintained by reclamation of qBH2 through the catalytic activities of pterin-4a-carbinolamine dehydratase (PCD) and dihydropteridine reductase (DHPR) or by de novo synthesis from guanosine triphosphate (GTP) (6). BH4 synthesis is accomplished through three enzymatic reactions catalyzed sequentially by GTP cyclohydrolase (GTPCH), 6-pyruvoyl-tetrahydropterin synthase (PTPS), and sepiapterin reductase (SR).
Mammals are incapable of synthesizing phenylalanine; phenylalanine is an essential amino acid in the diet. However, in adult humans consuming a typical diet (2–3 g protein/kg/day), only about 10% of dietary phenylalanine is required to supply anabolic needs. The remaining 90% of dietary phenylalanine is hydroxylated to tyrosine. In the absence of PAH activity, limited phenylalanine disposal is provided through urinary excretion of intact phenylalanine or of phenylpyruvic acid, the product of phenylalanine deamination. The detection of phenylpyruvate (a phenylketone) in urine led to the initial description of this disease by the Norwegian physician Dr Asbjorn Fölling in 1934 (7). Dr Fölling named this syndrome imbecillitas phenylpyrouvica in recognition of the association between urinary phenylpyruvate excretion and mental retardation.
Today, population-based screening for PKU [beginning with the widespread implementation of the bacterial inhibition assay developed by Dr Robert Guthrie (8)], diagnostic confirmation following an initial positive screening test, and ongoing monitoring of therapy adherence are based upon measurement of blood phenylalanine concentration. Phenylalanine itself, accumulating to extremely high levels, is primarily responsible for the phenotype associated with untreated PKU. Accumulation of phenylalanine in blood (normally less than 150 μM but often over 2000 μM in untreated patients) severely interferes with growth and maturation of the developing brain, although the exact molecular mechanism by which this occurs has yet to be elucidated. Over 50 years of experience with PKU treatment based upon the restriction of dietary phenylalanine intake have firmly shown the link between clinical outcome and blood phenylalanine levels (1).
Potential approaches to PKU therapy
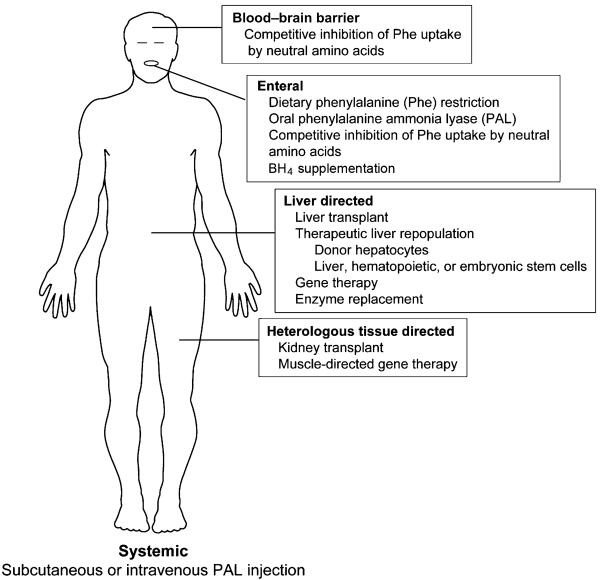
The most important lesson learned from the study of PKU pathogenesis is that reduction of body phenylalanine content, as reflected through the blood phenylalanine level, by any therapeutic approach will prevent the major disease manifestations of PKU. Although the proximate cause of PKU is deficiency of PAH in liver, there is no direct pathologic effect upon the liver. Therefore, restoration of liver PAH activity is not obligatory for the successful therapy of PKU. Given these facts, a considerable list of potential therapeutic approaches can be readily generated through a creative thought experiment (Fig. 2). This list includes currently accepted treatment strategies, potential therapies currently under investigation in laboratories worldwide, and wild conjecture by the author. The imaginative reader could potentially conceive of further methods to lower blood phenylalanine.
Fig. 2.
Potential therapeutic approaches for phenylketonuria.
These various therapeutic approaches can be categorized by site of action or target organ (Fig. 2). The categories include enteral, systemic, liver-directed, and heterologous tissue-directed approaches. The most widely employed contemporary therapy, restriction of dietary phenylalanine intake, is an example of an enteral approach. Alternatively, phenylalanine ammonia-lyase (PAL), a phenylalanine metabolizing enzyme from the yeast Rhodosporidium toruloides, placed in the intestinal lumen can act both to predigest dietary phenylalanine and to metabolize free phenylalanine that enters the intestine from endogenous bound pools (9). Neutral amino acid mixtures ingested orally may compete with phenylalanine for the neutral amino acid transporter on the intestinal enterocyte and reduce phenylalanine absorption (10). Neutral amino acids also compete for a similar amino acid transporter at the blood–brain barrier; raising the blood levels of neutral amino acids other than phenylalanine can partially block the uptake of phenylalanine into the brain and decrease the central nervous system effects of hyperphenylalaninemia (11). For a subset of PKU patients, daily oral administration of sapropterin dihydrochloride (Kuvan™, Bio-Marin Corp., Novato, CA), a synthetic form of BH4, can lower blood phenylalanine levels without altering dietary phenylalanine intake (12). BH4-responsive individuals likely carry PAH mutations that yield residual PAH enzyme activity (13), and BH4 treatment appears to correct defects in the stability or kinetics of residual mutant PAH peptide (14).
In a systemic approach, repetitive injection of recombinantly produced PAL (either subcutaneously or intravenously) might lead to clearance of phenylalanine from the circulation if the recombinant protein can be protected from immunologic attack (15). In the heterologous tissue approach, tissues other than liver such as skeletal muscle might be engineered to ectopically metabolize phenylalanine (16). In fact, successful treatment of hyperphenylalaninemic Pahenu2 mice following intramuscular injection of a recombinant adeno-associated virus (rAAV) vector that coexpresses PAH and the first two enzymatic steps in the BH4 synthetic pathway has recently been reported (17). Muscle does not contain measurable BH4; successful muscle-directed gene therapy for PKU requires both PAH expression and BH4 synthesis in the target tissue. Finally, PAH activity is normally expressed in human kidney; would a kidney transplant from a non-PKU donor restore phenylalanine clearance?
Given its key position in the control of phenylalanine homeostasis, the liver continues to be an obvious target for potential permanent treatment of PKU. Liver transplant has been shown to be curative in a child with PKU who had developed unrelated cryptogenic cirrhosis (18). Rather than whole organ transplant, therapeutic liver repopulation with donor-derived wild-type hepatocytes is another attractive option. The efficacy of whole liver transplant and hepatocyte transplantation as therapies for PKU will be limited by the lifelong need for immunosuppression following transplantation. Alternatively, liver repopulation with hepatocytes derived from either embryonic stem cells or genetically modified autologous adult stem cells might avoid the need for chronic immunosuppression. To avoid cell transplantation altogether, modified PAH enzyme produced recombinantly could be infused intravenously and targeted to the liver (19). Finally, several laboratories have explored a variety of gene transfer methods in attempts to supply the PAH cDNA and restore PAH expression in liver. The remainder of this paper will focus on the topics of therapeutic liver repopulation and liver-directed gene therapy for PKU.
Pahenu2 mice: a model of human PKU
Research on potential novel therapies for PKU has benefited greatly from the availability of an animal model, the Pahenu2 mouse, that accurately recapitulates the human disease. This mouse model was the product of a random chemical (N-ethyl-N-nitrosourea) mutagenesis project undertaken in the laboratory of Dr. William Dove at the University of Wisconsin-Madison (20). One of three PAH-deficient mice lines to result from this effort, the Pahenu2 mouse carries a T to C transition at nucleotide position 788 of the mouse Pah cDNA (21). This missense mutation replaces a conserved phenylalanine in the catalytic site with a serine and completely inactivates the PAH protein. Pah messenger RNA and PAH protein are detectable in liver by Northern and Western blotting, respectively (22), but no PAH enzyme activity is detected using a radiometric enzyme assay in any organ of animals that are homozygous for the Pahenu2 mutation. Homozygous animals are hyperphenylalaninemic, hypopigmented, mildly growth retarded (23), and cognitively impaired (24) relative to wild-type or heterozygous mice. Affected females are fertile, but in a phenomenon that is quite similar to human maternal PKU, many of the offspring have structural defects including congenital heart lesions (25). The Pahenu2 mouse is an outstanding model for human PKU.
Principles of therapeutic liver repopulation
Liver is unique among solid organs in its capability to completely regenerate itself after injury. Therapeutic liver repopulation takes advantage of this regenerative potential and aims to replace diseased liver with fully functional hepatocytes. In progenitor independent liver repopulation, residual healthy native hepatocytes proliferate to replace liver cells lost through surgical manipulation, toxic insult, or disease. For example, surgical partial hepatectomy generates a stimulus for the remaining hepatocytes to divide and replace that portion of the liver that was removed. No external source of new cells is required. In progenitor-dependent liver repopulation, replication and division of the native hepatocytes is impaired and an alternate source of proliferation-competent cells is required to effect successful liver repopulation. For example, radiation to the liver causes cell death but also interferes with DNA replication and blunts the regenerative potential of residual hepatocytes that happened to survive the initial radiation event. A new source of hepatocyte progenitors is required for liver repopulation. Potential sources include fully differentiated hepatocytes transplanted from a healthy donor, pluripotent liver or hematopoietic stem cells, or even embryonic stem cells. In the context of therapeutic liver repopulation for PKU, the goal is to replace PAH-deficient hepatocytes of the affected individual with PAH-positive cells from some other source.
For successful progenitor-dependent therapeutic liver repopulation to occur, two requirements must be met. First, there must be an active stimulus for liver regeneration during the time that progenitor cells are present in the liver. Second, the donor cells must enjoy a selective growth advantage over native hepatocytes (26). Single cell suspensions of hepatocytes, isolated in situ from donor liver by collagenase perfusion, may be injected intrasplenically and will migrate to the liver of the recipient. However, in the absence of a regenerative stimulus, the donor cells will not proliferate. Injection of 2 × 105 donor hepatocytes into the spleen of a healthy wild-type mouse without any growth stimulus will yield at best 0.5–1.0% repopulation of the liver with donor cells (27). This degree of repopulation could yield a positive phenotypic change in a few disorders (such as the hemophilias) where production of even a small amount of the missing gene product is curative but is likely insufficient for most inborn errors of metabolism.
Several approaches to provide a regenerative stimulus at the time of cell transplantation are available. Following subtotal hepatectomy, the remaining liver tissue quickly begins to proliferate and to replace the excised portion of the liver. During this brief window, transplanted donor cells will also proliferate. However, if the rate of cell division for donor cells is similar to that of native hepatocytes, then the proportion of donor cells present, once liver regeneration has been completed, will only be marginally increased. Successful repopulation of the liver with a physiologically significant proportion of donor cells necessitates that the donor cells enjoy a selective growth advantage over native cells. Extensive repopulation can be achieved only if the regenerative capacity of the native liver is completely suppressed and if proliferation is permitted of donor cells alone. A selective growth advantage for wild-type donor cells likely occurs naturally in several disease states such as tyrosinemia type 1 or glycogen storage disease (28) or it can be induced using several exogenous methods such as radiation to the liver or administration of pharmaceuticals that impair DNA replication (alkaloids or mitomycin C) (26).
Hepatocyte transplant experiments with the FahΔexon5 mouse, a model of human tyrosinemia type 1, exemplify the potential for therapeutic liver repopulation. Both human and murine tyrosinemia type 1 are caused by deficiency of fumarylacetoacetyl-CoA hydrolase (FAH), an enzyme in the intermediate metabolism of tyrosine. FahΔexon5 mice are homozygous for a targeted deletion of the Fah gene. Cellular accumulation of the substrate fumarylacetoacetic acid leads to hepatocyte dysfunction and ultimately liver failure. Liver disease can be prevented through administration of the drug 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), which blocks tyrosine metabolism upstream from FAH and prevents accumulation of fumarylacetoacetic acid. The FahΔexon5 phenotype is completely prevented through addition of NTBC to the drinking water of the animals (29). FAH-sufficient wild-type hepatocytes transplanted into FahΔexon5 mice enjoy a tremendous selective growth advantage over FAH-deficient hepatocytes once NTBC treatment is discontinued. Ongoing apoptosis of FAH-negative hepatocytes provides a stimulus for liver regeneration as well. Two months following hepatocyte transplant, more than 90% of liver is repopulated with FAH-expressing hepatocytes (30).
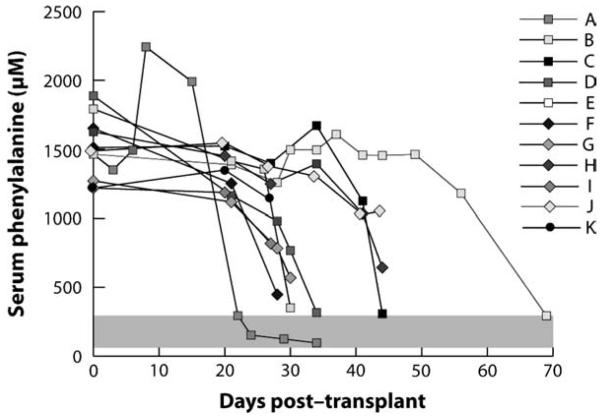
In PKU, PAH deficiency does not disturb hepatocyte metabolism, and PAH-sufficient hepatocytes do not enjoy any growth advantage over PAH-deficient cells following transplantation. To investigate the potential of therapeutic liver repopulation as a treatment for PKU, we have incorporated the inherent selective advantage of tyrosinemia into our experiments (31). First, we bred Pahenu2 mice with FahΔexon5 mice to develop animals that are homozygous for both mutations (hereinafter referred to as PAH/FAH mice) and are therefore both hyperphenylalaninemic and tyrosinemic. These mice are healthy while treated with NTBC but develop liver failure if NTBC is discontinued. We transplanted wild-type hepatocytes from congenic donors into PAH/FAH mice and withdrew NTBC therapy. Serum phenylala-nine levels corrected completely within a few weeks of transplantation (Fig. 3). Upon tissue harvest, we determined that complete correction occurred if greater than 10% repopulation with wild-type hepatocytes had been achieved. Serum phenylalanine levels decreased but not completely to the normal range in animals with 5–10% repopulation. The total amount of PAH activity measured in a liver with 5% repopulation should have been more than sufficient to completely metabolize the daily amount of dietary phenylalanine consumed by the animals, yet serum phenylala-nine levels were incompletely corrected. This result suggests that in animals with less than 10% repopulation, some factor other than total PAH activity, such as phenylalanine transport across the cell membrane of PAH-positive hepatocytes, limits total body phenylalanine clearance. Experiments to further explore this limitation are in progress. The take-home message is that therapeutic liver repopulation could be a viable approach to the permanent treatment of PKU if a selective growth advantage could be achieved for donor hepatocytes.
Fig. 3.
Effect of therapeutic liver repopulation upon serum phenylalanine in phenylalanine hydroxylase/fumarylacetoacetyl-CoA hydrolase deficient (PAH/FAH) mice. Serum phenylalanine levels in 12 PAH/FAH mice plotted vs days after transplant with wild-type hepatocytes. Normal range of serum phenylalanine is depicted in the gray-shaded area. Reproduced with permission from Hamman et al. (31).
The application of therapeutic liver repopulation to human subjects is dependent upon a source of wild-type human hepatocytes and currently limited by the scarcity of donor organs. Additionally, transplantation from an unrelated donor will require ongoing immunosuppression. A potential alternative source of donor cells may be to coax embryonic stem cells or adult stem cells derived from select tissues to develop into healthy hepatocytes. For instance, under the correct selective growth conditions, hematopoietic stem cell transplantation can lead to therapeutic liver repopulation and complete correction of murine tyrosinemia (32). In the future, it may be possible to extract a stem cell population from the patient with PKU, permanently transduce these cells ex vivo to provide a copy of the normal PAH cDNA, reimplant the transduced cells into the patient, and allow them to repopulate the liver. Obviously, many technological hurdles remain before this therapeutic approach will be feasible for PKU or other allied inborn errors of metabolism.
Gene therapy
Restoration of PAH enzyme activity by gene transfer into liver of phenylketonuric individuals would mimic the natural state and has been the goal of many investigators (33, 34). Published attempts of liver-directed gene therapy for murine PKU have utilized a full spectrum of viral and non-viral DNA-based gene transfer methodologies. Experiments with recombinant viral vectors have included trials with retroviral vector based upon the Moloney murine leukemia virus (35), recombinant adenovirus (36), or several different sero-types of rAAV vectors (37-43). DNA-mediated gene transfer methods avoid the inflammatory response and immune stimuli associated with recombinant viral vectors but generally suffer from lower rates of cell transduction. In the author's laboratory, transient liver PAH activity and reduction of blood phenylalanine levels in Pahenu2 mice has been achieved following hydrodynamic delivery (44) of a non-integrating plasmid DNA vector through the portal vein (unpublished results). More recently, sustained partial correction of blood phenylalanine has been achieved following repeated hydrodynamic administration of a PAH-expressing plasmid DNA that is capable of permanently integrating its expression cassette into the host liver genome (45-47). In these experiments, vector integration was achieved by incorporating an integrase system from bacteriophage into the vector. Transient liver expression of the integrase enzyme at the time of plasmid vector injection yielded integration of vector DNA into the host genome at specific sites whose nucleotide sequence was similar to sites in bacterial DNA that are the natural target of the integrase enzyme.
Most published attempts at gene therapy for PKU have utilized recombinant viral vectors. The success of many of these attempts has been limited to varying degrees by either insufficient or temporary gene expression. Investigations in the laboratory of Dr Savio Woo (36) with administration of recombinant adenovirus vector showed that complete correction of serum phenylalanine was achieved if liver PAH activity had been reconstituted to greater than 10% of wild-type activity. Unfortunately, liver PAH expression diminished by 2–3 weeks following vector injection because of florid immune-mediated rejection of adenovirus-transduced hepatocytes. Such an immune response has not been seen in mice following administration of rAAV vectors, and liver PAH expression has been much longer lasting. However, in early rAAV experiments, extremely high doses of rAAV serotype 2 were necessary to effect a reduction in blood phenylalanine (37), and the therapy was less effective in female mice. Pseudo-typing of rAAV vectors with capsid from other AAV serotypes has led to improved tissue tropism; vectors pseudotyped with AAV5 or AAV8 capsid are particularly effective for liver transduction.
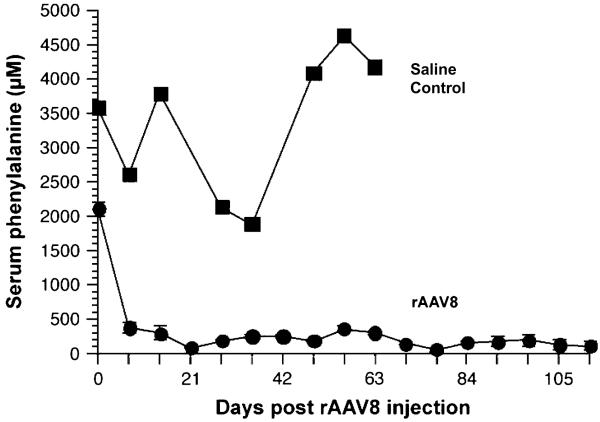
Successful correction of murine hyperphenylalaninemia using recombinant AAV8 (rAAV8) vectors has been achieved in several laboratories (42, 43). In the author's experience, administration of 5 × 1011 rAAV8 vector genomes through portal vein injection has led to complete normalization of blood phenylalanine levels in Pahenu2 mice by 1 week following vector administration (Fig. 4). No adverse effects of rAAV8 administration have been seen. About 8–17 weeks after vector injection, mean liver PAH activity of the treated mice was 11.5% of wild-type liver PAH levels. On average, 14.5 copies of the vector genome were detected per haploid genome in liver DNA from treated mice. The rAAV8 genomes remained predominantly episomal as large molecular weight concatemers. Phenotypic effects of rAAV8 administration included reversal of hyperphenylalaninemia-induced hypopigmentation in the mice. Similar results have been achieved following tail vein injection of rAAV8 vector but with a 10-fold higher vector dose (43).
Fig. 4.
Serum phenylalanine levels of Pahenu2 mice following recombinant adeno-associated virus (rAAV)8 vector administration. Five Pahenu2/Pahenu2 mice received 5 × 1011 vector genomes of phenylalanine hydroxylase (PAH)-expressing rAAV8 through portal vein injection, and serum phenylalanine levels were measured weekly thereafter. A single Pahenu2/Pahenu2 mouse received a saline injection as a control. The data are expressed as mean serum phenylala-nine (μM) ± 1 SD vs time in days post-vector injection. Reproduced with permission from Harding et al. (42).
Further, yet unpublished experiments in the author's laboratory have documented dose dependence with administration of at least 1011 vector genomes necessary to achieve a therapeutic effect and a gradual loss of therapeutic effect over time in animals receiving a dose less than 1012 vector genomes. In mice receiving these lower vector doses, phenylalanine levels begin to rise at about 6 months after treatment; this finding correlates with a loss of both vector genomes and PAH activity in the liver. The mechanism for this late vector loss and methods for its possible prevention are under investigation. The vector cannot be readministered to a treated mouse due to the development of anti-vector capsid-neutralizing antibodies following the initial vector injection, but these antibodies do not typically interfere with expression of the transgene in liver. Immune-mediated destruction of transgene-expressing hepatocytes following rAAV vector administration has not been previously reported in any animal model. However, immune-mediated elimination of rAAV2-transduced hepatocytes was responsible for the loss of coagulation factor IX expression in a human hemophilia A trial (48).
Wild-type AAV is capable of integrating its genome into a specific site on human chromosome 19. Recombinant AAV vectors integrate only rarely and remain primarily episomal. However, integration when it does occur appears to happen in actively transcribed regions of the genome (49) and possible insertional mutagenesis is a concern. Although we have not witnessed the long-term development of any liver tumors in rAAV8-treated mice, insertion of vector genome into actively transcribed microRNA genes in a mouse model of human mucopolysaccharidosis type VI has been associated with the development of hepatic carcinoma following administration of rAAV2 vector (50). Clearly, issues of immunogenicity and potential insertional mutagenesis must be resolved before clinical use of rAAV vectors for liver-directed gene therapy will become widespread. Although these shortcomings must be acknowledged, liver-directed rAAV8-mediated gene transfer has been to date the most effective gene therapy approach in murine PKU.
Conclusions
Dietary intervention continues to be the mainstay of contemporary therapy for PKU and allied inborn errors of metabolism. However, to both patients and clinicians in the field, the limitations and complications of this therapeutic approach are clear. Several imaginative and novel therapeutic strategies for PKU are currently under investigation in several laboratories and clinics internationally. Although much work remains before safe, clinically efficacious methods will be available, cell-directed therapies continue to offer great promise as potential permanent cures for PKU.
Acknowledgements
C. O. H. has been supported by National Institutes of Health grants R01 DK059371, R21 HL78850, and R41 DK075168. The author is indebted to Dr Melanie Gillingham for her critical review of the manuscript and to the many families with PKU who continue to inspire his work.
References
- 1.Azen CG, Koch R, Friedman EG, et al. Intellectual development in 12-year-old children treated for phenylketonuria. Am J Dis Child. 1991;145:35–39. doi: 10.1001/archpedi.1991.02160010037012. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health Consensus Development Conference Statement Phenylketonuria: screening and management; Pediatrics; October 16–18, 2000; 2001. pp. 972–982. [DOI] [PubMed] [Google Scholar]
- 3.Thompson AJ, Smith I, Brenton D, et al. Neurological deterioration in young adults with phenylketonuria. Lancet. 1990;336:602–605. doi: 10.1016/0140-6736(90)93401-a. [DOI] [PubMed] [Google Scholar]
- 4.Platt LD, Koch R, Hanley WB, et al. The international study of pregnancy outcome in women with maternal phenylketonuria: report of a 12-year study. Am J Obstet Gynecol. 2000;182:326–333. doi: 10.1016/s0002-9378(00)70219-5. [DOI] [PubMed] [Google Scholar]
- 5.Donlon J, Levy H, Scriver CR. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. Chapter 77. Online. In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis S, Ballabio A, et al., editors. The metabolic & molecular bases of inherited disease. McGraw-Hill; New York, NY: 2008. [Google Scholar]
- 6.Thöny B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347(Pt 1):1–16. [PMC free article] [PubMed] [Google Scholar]
- 7.Fölling A. Über aussheidung von phenylbrenztraubensaure in den harn als stoffwechselanomalie in Verbindung mit Imbezillitat. Hoppe-Seylers Z Physiol Chem. 1934;277:169. [Google Scholar]
- 8.Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–343. [PubMed] [Google Scholar]
- 9.Sarkissian CN, Shao Z, Blain F, et al. A different approach to treatment of phenylketonuria: phenylalanine degradation with recombinant phenylalanine ammonia lyase. Proc Natl Acad Sci U S A. 1999;96:2339–2344. doi: 10.1073/pnas.96.5.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matalon R, Michals-Matalon K, Bhatia G, et al. Double blind placebo control trial of large neutral amino acids in treatment of PKU: effect on blood phenylalanine. J Inherit Metab Dis. 2007;30:153–158. doi: 10.1007/s10545-007-0556-4. [DOI] [PubMed] [Google Scholar]
- 11.Pietz J, Kreis R, Rupp A, et al. Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J Clin Invest. 1999;103:1169–1178. doi: 10.1172/JCI5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy HL, Milanowski A, Chakrapani A, et al. Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo-controlled study. Lancet. 2007;370:504–510. doi: 10.1016/S0140-6736(07)61234-3. [DOI] [PubMed] [Google Scholar]
- 13.Zurfluh MR, Zschocke J, Lindner M, et al. Molecular genetics of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Hum Mutat. 2008;29:167–175. doi: 10.1002/humu.20637. [DOI] [PubMed] [Google Scholar]
- 14.Erlandsen H, Pey AL, Gamez A, et al. Correction of kinetic and stability defects by tetrahydrobiopterin in phenylketonuria patients with certain phenylalanine hydroxylase mutations. Proc Natl Acad Sci U S A. 2004;101:16903–16908. doi: 10.1073/pnas.0407256101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkissian CN, Gamez A. Phenylalanine ammonia lyase, enzyme substitution therapy for phenylketonuria, where are we now? Mol Genet Metab. 2005;86(Suppl 1):S22–S26. doi: 10.1016/j.ymgme.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Harding CO, Wild K, Chang D, et al. Metabolic engineering as therapy for inborn errors of metabolism–development of mice with phenylalanine hydroxylase expression in muscle. Gene Ther. 1998;5:677–683. doi: 10.1038/sj.gt.3300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Z, Harding CO, Rebuffat A, et al. Correction of murine PKU following AAV-mediated intramuscular expression of a complete phenylalanine hydroxylating system. Mol Ther. 2008;16:673–681. doi: 10.1038/mt.2008.17. doi:10.1038/mt.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vajro P, Strisciuglio P, Houssin D, et al. Correction of phenylketonuria after liver transplantation in a child with cirrhosis. N Engl J Med. 1993;329:363. doi: 10.1056/NEJM199307293290517. [DOI] [PubMed] [Google Scholar]
- 19.Eavri R, Lorberboum-Galski H. A novel approach for enzyme replacement therapy. The use of phenylalanine hydroxylase-based fusion proteins for the treatment of phenylketonuria. J Biol Chem. 2007;282:23402–23409. doi: 10.1074/jbc.M703367200. [DOI] [PubMed] [Google Scholar]
- 20.McDonald JD, Bode VC, Dove WF, et al. Pahhph-5: a mouse mutant deficient in phenylalanine hydroxylase. Proc Natl Acad Sci U S A. 1990;87:1965–1967. doi: 10.1073/pnas.87.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald JD, Charlton CK. Characterization of mutations at the mouse phenylalanine hydroxylase locus. Genomics. 1997;39:402–405. doi: 10.1006/geno.1996.4508. [DOI] [PubMed] [Google Scholar]
- 22.Sarkissian CN, Boulais DM, McDonald JD, et al. A heteroallelic mutant mouse model: a new orthologue for human hyperphenylalaninemia. Mol Genet Metab. 2000;69:188–194. doi: 10.1006/mgme.2000.2974. [DOI] [PubMed] [Google Scholar]
- 23.McDonald JD. Postnatal growth in a mouse genetic model of classical phenylketonuria. Contemp Top Lab Anim Sci. 2000;39:54–56. [PubMed] [Google Scholar]
- 24.Zagreda L, Goodman J, Druin DP, et al. Cognitive deficits in a genetic mouse model of the most common biochemical cause of human mental retardation. J Neurosci. 1999;19:6175–6182. doi: 10.1523/JNEUROSCI.19-14-06175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald JD, Dyer CA, Gailis L, et al. Cardiovascular defects among the progeny of mouse phenylketonuria females. Pediatr Res. 1997;42:103–107. doi: 10.1203/00006450-199707000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Laconi E, Laconi S. Principles of hepatocyte repopulation. Semin Cell Dev Biol. 2002;13:433–438. doi: 10.1016/s1084952102001313. [DOI] [PubMed] [Google Scholar]
- 27.Ponder KP, Gupta S, Leland F, et al. Mouse hepatocytes migrate to liver parenchyma and function indefinitely after intrasplenic transplantation. Proc Natl Acad Sci U S A. 1991;88:1217–1221. doi: 10.1073/pnas.88.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muraca M, Gerunda G, Neri D, et al. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317–318. doi: 10.1016/S0140-6736(02)07529-3. [DOI] [PubMed] [Google Scholar]
- 29.Grompe M, Lindstedt S, al-Dhalimy M, et al. Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1995;10:453–460. doi: 10.1038/ng0895-453. [DOI] [PubMed] [Google Scholar]
- 30.Overturf K, Al-Dhalimy M, Tanguay R, et al. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1996;12:266–273. doi: 10.1038/ng0396-266. [DOI] [PubMed] [Google Scholar]
- 31.Hamman K, Clark H, Montini E, et al. Low therapeutic threshold for hepatocyte replacement in murine phenylketonuria. Mol Ther. 2005;12:337–344. doi: 10.1016/j.ymthe.2005.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 33.Eisensmith RC, Woo SL. Gene therapy for phenylketonuria. Eur J Pediatr. 1996;155(Suppl 1):S16–S19. doi: 10.1007/pl00014237. [DOI] [PubMed] [Google Scholar]
- 34.Ding Z, Harding CO, Thony B. State-of-the-art 2003 on PKU gene therapy. Mol Genet Metab. 2004;81:3–8. doi: 10.1016/j.ymgme.2003.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu T-J, Kay MA, Darlington GJ, et al. Reconstitution of enzymatic activity in hepatocytes of phenylalanine hydroxylase-deficient mice. Somat Cell Mol Genet. 1992;18:89–96. doi: 10.1007/BF01233451. [DOI] [PubMed] [Google Scholar]
- 36.Fang B, Eisensmith RC, Li XHC, et al. Gene therapy for phenylketonuria: phenotypic correction in a genetically deficient mouse model by adenovirus-mediated hepatic gene therapy. Gene Ther. 1994;1:247–254. [PubMed] [Google Scholar]
- 37.Laipis PJ, Reyes L, Embury JE, et al. Long term reduction of serum phenylalanine levels in a mouse model of PKU by rAAV-mediated gene therapy. Mol Ther. 2001;3:S293. [Google Scholar]
- 38.Laipis PJ, Charron CE, Ross K, et al. Long-term correction of phenylketonuria in an animal model by recombinant AAV-based gene therapy. J Inherit Metab Dis. 2002;25:615–616. [Google Scholar]
- 39.Laipis PJ, Charron CE, Embury JE, et al. Correction of maternal phenylketonuria syndrome in the Pahenu2 missense mutant mouse by r-AAV mediated gene therapy. Mol Ther. 2004;9:S334. [Google Scholar]
- 40.Mochizuki S, Mizukami H, Ogura T, et al. Long-term correction of hyperphenylalaninemia by AAV-mediated gene transfer leads to behavioral recovery in phenylketonuria mice. Gene Ther. 2004;11:1081–1086. doi: 10.1038/sj.gt.3302262. [DOI] [PubMed] [Google Scholar]
- 41.Oh HJ, Park ES, Kang S, et al. Long-term enzymatic and phenotypic correction in the phenylketonuria mouse model by adeno-associated virus vector-mediated gene transfer. Pediatr Res. 2004;56:278–284. doi: 10.1203/01.PDR.0000132837.29067.0E. [DOI] [PubMed] [Google Scholar]
- 42.Harding CO, Gillingham MB, Hamman K, et al. Complete correction of hyperphenylalaninemia following liver-directed, recombinant AAV2/8 vector-mediated gene therapy in murine phenylketonuria. Gene Ther. 2006;13:457–462. doi: 10.1038/sj.gt.3302678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding Z, Georgiev P, Thony B. Administration-route and gender-independent long-term therapeutic correction of phenylketonuria (PKU) in a mouse model by recombinant adeno-associated virus 8 pseudotyped vector-mediated gene transfer. Gene Ther. 2006;13:587–593. doi: 10.1038/sj.gt.3302684. [DOI] [PubMed] [Google Scholar]
- 44.Budker V, Zhang G, Knechtle S, et al. Naked DNA delivered intraportally expresses efficiently in hepatocytes. Gene Ther. 1996;3:593–598. [PubMed] [Google Scholar]
- 45.Chen L, Woo SL. Complete and persistent phenotypic correction of phenylketonuria in mice by site-specific genome integration of murine phenylalanine hydroxylase cDNA. Proc Natl Acad Sci U S A. 2005;102:15581–15586. doi: 10.1073/pnas.0503877102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Chen L, Thung SN, Woo SL. Metabolic basis of sexual dimorphism in PKU mice after genome-targeted PAH gene therapy. Mol Ther. 2007;15:1079–1085. doi: 10.1038/sj.mt.6300137. [DOI] [PubMed] [Google Scholar]
- 47.Chen L, Woo SL. Correction in female PKU mice by repeated administration of mPAH cDNA using phiBT1 integration system. Mol Ther. 2007;15:1789–1795. doi: 10.1038/sj.mt.6300257. [DOI] [PubMed] [Google Scholar]
- 48.High K, Manno C, Sabatino D, et al. Immune responses to AAV and to factor IX in a phase I study of AAV-mediated, liver-directed gene transfer for hemophilia B. Mol Ther. 2004;9:S383–S384. [Google Scholar]
- 49.Nakai H, Montini E, Fuess S, et al. AAV serotype 2 vectors preferentially integrate into active genes in mice. Nat Genet. 2003;34:297–302. doi: 10.1038/ng1179. [DOI] [PubMed] [Google Scholar]
- 50.Donsante A, Miller DG, Li Y, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]