Abstract
Rationale
An endocannabinoid signaling system has not been identified in hamsters.
Objective
We examined the existence of an endocannabinioid signaling system in Syrian hamsters using neuroanatomical, biochemical and behavioral pharmacological approaches.
Method
The distribution of cannabinoid receptors was mapped and membrane fatty-acid amide hydrolase (FAAH) activity and levels of fatty-acid amides were measured in hamster brain. The impact of cannabinoid CB1 receptor blockade and inhibition of FAAH was evaluated in the elevated plus maze, rota-rod test and models of unconditioned and conditioned social defeat.
Results
A characteristic heterogeneous distribution of cannabinoid receptors was detected in hamster brain using [3H]CP55,940 binding and autoradiography. The FAAH inhibitor URB597 inhibited FAAH activity (IC50 = 12.8 nM) and elevated levels of fatty-acid amides (N-palmitoyl ethanolamine (PEA) and N-oleoyl ethanolamine (OEA)) in hamster brain. Anandamide levels were not reliably altered. The cannabinoid agonist WIN55,212-2 (1– 10 mg/kg i.p.) induced CB1-mediated motor ataxia. Blockade of CB1 with rimonabant (5 mg/kg i.p.) induced anxiogenic-like behavior in the elevated plus maze. URB597 (0.1–0.3 mg/kg i.p.) induced CB1-mediated anxiolytic-like effects in elevated plus maze, similar to the benzodiazepine diazepam (2 mg/kg i.p.). Diazepam (2–6 mg/kg i.p.) suppressed the expression, but not the acquisition, of conditioned defeat. By contrast, neither URB597 (0.3–3.0 mg/kg i.p.) nor rimonabant (5 mg/kg i.p.) altered unconditioned or conditioned social defeat or rota-rod performance.
Conclusions
Endocannabinoids engage functional CB1 receptors in hamster brain to suppress anxiety-like behavior and undergo enzymatic hydrolysis catalyzed by FAAH. Our results further suggest that neither unconditioned nor conditioned social defeat in the Syrian hamster is dependent upon cannabinoid CB1 receptor activation.
Keywords: Cannabinoids, conditioned defeat, social defeat, elevated plus maze, anxiety, FAAH
Introduction
The localization of cannabinoid receptors in the limbic system of the brain (Herkenham et al. 1991) suggests that endocannabinoids, endogenous cannabis-like compounds, serve naturally to modulate emotional processing. Anandamide is the best characterized of the endocannabinoids isolated so far. Anandamide is synthesized on demand by activity-dependent cleavage of membrane phospholipid precursors (for review see Piomelli 2003). Anandamide is deactivated by intracellular hydrolysis catalyzed by fatty acid amide hydrolase (FAAH) ( Cravatt et al.1996, 2001; Desarnaud et al. 1995; Hillard and Jarrahian 2005). Inhibition of FAAH elevates brain anandamide levels and suppresses anxiety-related behaviors in rats and mice (Bortolato et al. 2007; Fegley et al. 2005; Kathuria et al. 2003; Moreira et al. 2008). By contrast, pharmacological blockade of cannabinoid CB1 receptors induces anxiogenic-like behaviors in the elevated-plus maze (Arevalo et al. 2001; Patel and Hillard 2006; see also Griebel et al. 2005) and two compartment black and white box model of anxiety (Akinshola et al. 1999). Mice lacking CB1 receptors are prone to anxiety-like and depression-like behaviors (Haller et al. 2002, 2004a, b; Maccarrone et al. 2002). Endocannabinoids control extinction of aversive memories (Marsicano et al. 2002) and modulate stress-related responses (Hohmann et al. 2005; Kathuria et al. 2003; Patel and Hillard 2006; Suplita et al. 2008; Valverde et al. 2000) in both rats and mice. An association between heavy cannabis use and anxiety and affective disorders has been described in humans (Degenhardt et al. 2001; Walfish et al. 1990). Thus, endocannabinoids may play a pivotal role in the nervous system modulating emotional responses.
The objective of the present study was to investigate the existence of an endocannabinoid signaling system in the Syrian hamster (Mesocricetus auratus) and characterize its functional roles. We examined the presence of key components of the endocannabinoid signaling system in brains of male Syrian hamsters using neuroanatomical, biochemical and pharmacological approaches. First, we used in vitro receptor binding and quantitative autoradiography to map the distribution of cannabinoid receptors in hamster brain. Second, we demonstrated that FAAH, the predominant enzyme that controls anandamide deactivation in other rodent species, plays a similar role in regulating brain levels of fatty-acid amides in Syrian hamsters. Third, we demonstrated that hamsters receiving the cannabinoid agonist WIN55,212-2 but not the FAAH inhibitor URB597 (Kathuria et al. 2003) exhibit cardinal signs of cannabinoid CB1 receptor activation (Compton et al. 1993) using the rota-rod test of motor ataxia. Fourth, we measured levels of anandamide and other fatty-acid amides in whole hamster brain using high performance liquid chromatograpy mass spectrometry (HPLC/MS). Finally, we asked whether cannabinoid CB1 receptors and endocannabinoids serve naturally to suppress anxiety-like behavior in hamsters.
We examined the impact of blockade of CB1 receptors and inhibition of FAAH on unconditioned and conditioned responses to aversive or anxiety-provoking situations. Unconditioned aversive behavior was measured by exposing hamsters to an elevated plus maze. Both unconditioned (social defeat) and conditioned (conditioned defeat) submissive/defensive behaviors were assessed in hamsters using a naturalistic model of social anxiety (for review see Young 2002). Submissive/defensive behavior was initially elicited in the presence of a larger dominant hamster and subsequently elicited in previously defeated subjects in the presence of a smaller nonaggressive intruder (Jasnow and Huhman 2001). Defeated hamsters exhibit submissive and defensive behavior, avoid social interaction and display an absence of natural territorial aggression. Defeated hamsters exhibit a pervasive subordinate social status even when they are later exposed to smaller, nonaggressive animals (Jasnow and Huhman 2001). Thus, this latter phenomenon is referred to as conditioned defeat (Potegal et al. 1993; Jasnow and Huhman 2001). Despite the considerable utility of this model for studying both social stress and social anxiety, the neurochemical mechanism underlying both unconditioned and conditioned social defeat remains incompletely understood (see also Jasnow et al. 1999, 2005; Jasnow and Huhman 2001).
We also examined whether endocannabinoids and benzodiazepines would differentially modulate anxiety-like behaviors in hamsters in each model. Diazepam suppresses anxiety-like behavior in the elevated plus maze (Pellow et al. 1985; Yanielli et al. 1996), defensive burying test (Treit et al. 1981) and Vogel conflict test (Moreira et al. 2006). The present studies provide the first evidence that hamster brains contain functional cannabinoid CB1 receptors, as well as FAAH, similar to that observed in non-hibernating rodent species. Moreover, this system serves naturally to modulate anxiety-like behavior in the hamster. Finally, our data suggest that URB597 and diazepam differentially modulate anxiety-like behavior in male Syrian hamsters.
Materials and Method
Subjects
Two hundred and ninety two adult male Syrian hamsters (Mesocricetus auratus) (Harlan, Indianapolis, IN) served as experimental subjects. Three rats were used as positive controls to verify specificity of cannabinoid receptor binding and autoradiography. Unconditioned and conditioned defeat experiments used three groups of hamsters. Larger, aggressive hamsters (120–160g) and smaller experimental hamsters (90–120g) were housed individually. Non-aggressive stimulus hamsters (75–85g and 90–100g) were group housed five per cage to minimize aggressiveness. All other hamsters (90–150 g) were housed individually. Experimental animals were housed in a temperature-controlled colony room on a 14:10 light/dark cycle (Jasnow and Huhman 2001;Huhman et al. 2003) for at least two weeks prior to testing. Lights off occurred at 13:00 h. All procedures and protocols were approved by the University of Georgia Institutional Animal Care and Use Committee.
Chemicals
[2H4]-Anandamide, [2H4]-oleoylethanolamide (OEA), and [2H4]-palmitoylethanolamide (PEA) were prepared by reaction of fatty acyl chlorides (Nu-Chek Prep, Elysian, MN) with a 10-fold molar excess of [2H4]-ethanolamine (Cambridge Isotope Laboratories, Andover, MA) in dichloromethane at 0–4°C for 15 min, with stirring. Products were washed with water, dehydrated over sodium sulphate, filtered, dried under N2 and characterized by LC/MS and 1H nuclear magnetic resonance spectroscopy. Purity was >98% by LC/MS. [3H]CP55,940 and rimonabant (SR141716) were gifts from NIDA. All other chemicals were purchased from commercial sources. URB597 was from Cayman Chemical (Ann Arbor, MI). Diazepam was from Abbott Laboratories (North Chicago, IL). WIN55,212-2 and AM251 were from Sigma Aldrich (St. Louis, MO) and TOCRIS Bioscience (Ellisville, MO), respectively. Rimonabant, AM251 and URB597 were dissolved in a vehicle of emulphor: ethanol: saline (1:1:8). Diazepam was purchased at a concentration of 5 mg/ml in a vehicle of polyethylene glycol:ethanol:saline (4:1:5) and diluted in the same vehicle. Drug or vehicle was delivered in a volume of 1–1.2 ml/kg bodyweight, as required.
Tissue Extractions
Unanesthetized subjects (n=18 hamsters; n=3 rats) were decapitated, and brains were rapidly dissected and snap frozen in isopentane precooled to −30°C. Frozen brains were stored at low temperature (−80°C or −30°C) until use.
Receptor Binding and Autoradiography
Sagittal and coronal sections (14 μM thickness) were cryostat cut and mounted four sections per slide. Rat brains were sectioned at the same levels as the hamster brains and processed concurrently. Cannabinoid receptor binding was performed using [3H]CP55,940 (specific activity 77.5 Ci/mmol; Research Triangle Institute, Research Triangle Park, NC) as described (Herkenham et al. 1991; Hohmann et al. 1999; Hohmann and Herkenham 1998). Nonspecific binding was determined in the presence of 10 μM CP55,940. Binding was performed in cytomailers (3 h at 37° C) in 50 mM Tris-HCl (pH 7.4) containing 5% bovine serum albumin (BSA) and 5 nM [3H]CP55,940. Slides were washed (4 h at 0°C) in the same buffer containing 1% BSA, fixed in 0.5% formalin in 50 mM Tris-HCl (pH 7.4 at 25° C) and dried. Sections were apposed to [3H]-sensitive film (Amersham Hyperfilm, GE Healthcare LifeSciences, Piscataway, NJ) together with [3H] standards (3H microscales, Amersham, Arlington Heights, IL) for 15 weeks. Images were captured using an Epson 1650 scanner. Each image was adjusted identically in Adobe Photoshop using the levels tool only.
Assessment of anandamide hydrolyzing activity
Frozen brains were homogenized in ice-cold phosphate buffered-saline (PBS, 20 mM, 10 vol, pH 7.4) containing 0.32 M sucrose. Homogenates were centrifuged at 800g (15 min) and then at 27,000g (30 min). The 27,000g pellet was suspended in PBS and used for the assay. FAAH activity was measured after incubation ( 30 min at 37°C) in Tris buffer (0.5 ml; 50 mM, pH 7.5) containing fatty acid-free BSA (0.05%), membrane protein (50 μg) and anandamide[ethanolamine-3H] (10,000 dpm, specific activity 20 Ci/mmol; American Radiolabeled Chemicals, St. Louis, MO). Reactions were stopped with chloroform/methanol (1:1, 1 ml). Radioactivity in the aqueous layers was measured by liquid scintillation counting.
Lipid extractions
Frozen brains were weighed and homogenized in methanol (1 ml per 100 mg tissue) containing [2H4]-OEA, [2H4]-PEA, [2H4]-anandamide and [2H8]-2-arachidonoyl-sn-glycerol (2-AG) (Cayman Chemicals, Ann Arbor, MI) as internal standards. Lipids were extracted with chloroform (2 vol) and washed with water (1 vol). Endocannabinoids were fractionated by open-bed silica gel column chromatography (Giuffrida et al. 2000). Briefly, lipids were reconstituted in chloroform and loaded onto small glass columns packed with Silica Gel G (60-Å 230–400 Mesh ASTM; Whatman, Clifton, NJ). Fatty acid ethanolamides and 2-AG were eluted with 9:1 chloroform/methanol (vol/vol). Eluates were dried under N2 and reconstituted in 0.1 ml of chloroform/methanol (1:4, vol/vol) for LC/MS analyses.
LC/MS analyses
Hamsters were decapitated 2 hours following treatment with either URB597 (0.3 mg/kg i.p.) or vehicle (n=6 per group). Whole brains were dissected for use in LC/MS analyses to mimic conditions used for rats by Kathuria et al. (2003). An 1100-LC system coupled to a 1946D-MS detector (Agilent Technologies, Inc., Palo Alto, CA) equipped with an electrospray ionization (ESI) interface was used to measure anandamide, OEA, PEA and 2-AG levels in whole hamster brains. Lipids were separated using a XDB Eclipse C18 column (50 × 4.6 mm i.d., 1.8 μm, Zorbax), eluted with a gradient of methanol in water (from 75% to 85% in 2.5 min and then to 90% in 7.5 min) at a flow rate of 1.0 ml/min. Column temperature was kept at 40°C. MS detection was in the positive ionization mode, capillary voltage was set at 3 kV and fragmentor voltage was varied from 120V. N2 was used as drying gas at a flow rate of 13 liters/min and a temperature of 350°C. Nebulizer pressure was set at 60 PSI. Quantifications were conducted using an isotope-dilution method, monitoring Na+ adducts of the molecular ions ([M+Na]+). The lower limit of detection for each compound was 0.02 pmol. Detection responses were linear in the range 0.5–500 pmol.
Behavioral studies
In all studies, the experimenter was blinded to pharmacological treatments.
Rota-rod Assessment of Motor Ataxia
The rota-rod test was used to verify that hamsters exhibit prototypical signs of cannabinoid CB1 receptor activation in response to a cannabinoid agonist (WIN55,212-2) but not following treatment with the FAAH inhibitor (URB597). A secondary objective was to verify that effects of pharmacological treatments could not be attributed to motor impairment. Rota-rod testing consisted of two separate training sessions (on day 1), reliability testing (on day 2) and assessment of motor ataxia after pharmacological manipulations (on day 2). Hamsters were trained (on day 1) to walk on a rotating drum (rotating at 6 rpm) during three 30-second sessions. Animals that were not able to pass reliability standards (i.e. the ability to walk on a rotating drum on two separate one-minute sessions) did not receive pharmacological treatments. WIN55,212-2 (1, 3 or 10 mg/kg i.p.), URB597 (0.1, 0.3 or 3 mg/kg i.p.), diazepam (2 or 6 mg/kg i.p.), rimonabant (1 or 5 mg/kg i.p.)., WIN55, 212-2 (10 mg/kg i.p.) co-administered with rimonabant (5 mg/kg), 4:1:5 polyethylene glycol:ethanol:saline vehicle or 1:1:8 ethanol:emulphor:saline vehicle was administered intraperitoneally (i.p.) 30 min before testing. Motor ataxia was measured as the mean of two rota-rod latency determinations measured in separate one-minute sessions performed 30 and 35 minutes post-injection.
Elevated Plus-maze Testing
Testing consisted of a single five-minute experimental session following placement of hamsters on a cross-shaped maze elevated 34 cm above the floor. The elevated plus-maze was constructed of wood and consisted of two open arms (each 45 cm long × 9 cm wide), two closed arms with no roof (each 45 cm long × 9 cm wide) and a square-shaped central platform (9 × 9 cm). The walls of the closed arms were 38 cm high. The open arms and external surface of the maze were painted white and the closed arms (internal surface) and central platform were painted black, an arrangement similar to that described previously (Onaivi et al. 1990). The environment was illuminated by 60 watt fluorescent track illumination mounted in the ceiling 264 cm above the floor. Illumination measured 108, 26 and 88 foot candles in open arms, closed arms and central platform, respectively. The open and closed arms were perpendicular to each other so that arms of the same type opposed each other. URB597 (0.1 or 0.3 mg/kg), rimonabant (1 or 5 mg/kg), diazepam (2 mg/kg), URB597 (0.3 mg/kg) coadministered with rimonabant (1 mg/kg i.p.) or vehicle (1:1:8 ethanol:emulphor:saline or polyethylene glycol:ethanol: saline) was administered (i.p.) 30 min prior to testing. Animals were placed on the central platform of the maze facing a closed arm. The total amount of time (in seconds) spent on the open or closed arms and the number of entries made into either the open or the closed arms was recorded. Entry into an arm was only recorded as an entry when all four paws were positioned on the white surface of the open arm. The percentage of total time spent by the animal on open and closed arms as well as the percentage of open and closed arm entries was calculated for each subject. The total amount of time spent on the open and closed arms was typically slightly less than 5 min because the time spent on the central platform is not counted. Experimental sessions were videotaped using a CCD camera system (Polycom viewstation FX) and scored from VHS tapes by a single rater (AM) who was blinded to the experimental conditions.
Assessment of Unconditioned and Conditioned Social Defeat
Hamsters were matched by weight and randomly assigned to drug or vehicle groups. Experimental animals were handled daily prior to testing. All experimental sessions were recorded using a CCD camera system (Polycom viewstation FX) and stored on VHS tape. Behaviors were scored on days 1 and 2 by a single trained rater (AM) who was blinded to all experimental conditions. Random subsets of experimental sessions were rescored from the VHS tapes, yielding similar results.
All behavioral testing was performed within the first 3h of the dark phase. Experimental hamsters received a single i.p. injection of vehicle (4:1:5 polyethylene glycol or 1:1:8 emulphor: ethanol: saline), CB1 antagonists rimonabant (5 or 10 mg/kg) or AM251 (5 mg/kg), the FAAH inhibitor URB597 (0.3 or 3 mg/kg), or diazepam (2 or 6 mg/kg). Pharmacological treatments were performed either 30 minutes before assessments of either unconditioned social defeat (on day 1) or conditioned social defeat (on day 2). Each hamster received a single injection either on day 1 or day 2 (see Fig. 7a, 8a, 9a).
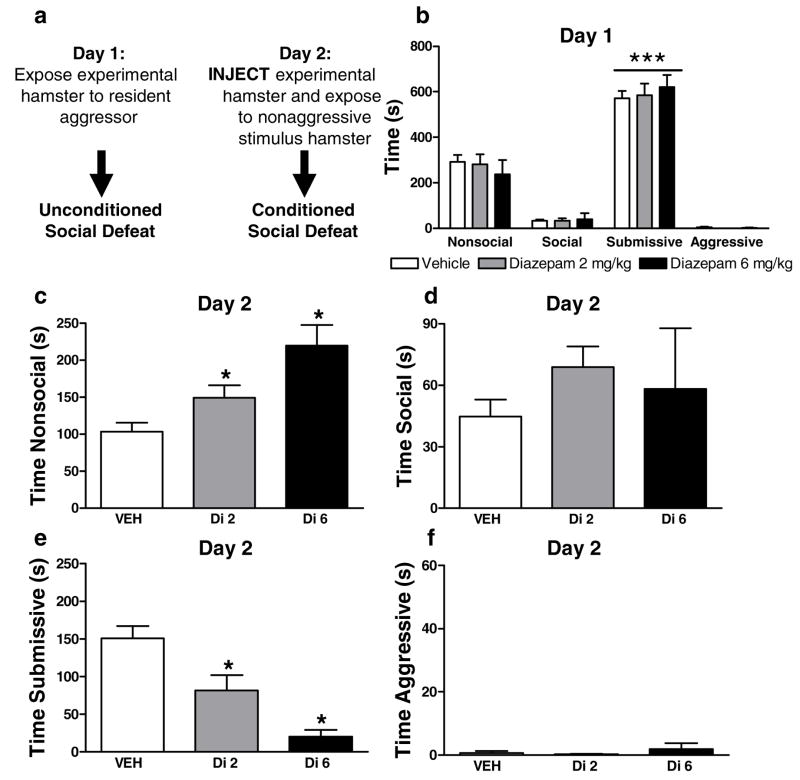
Figure 7.
(a). Timing of pharmacological manipulations relative to assessments of unconditioned and conditioned social defeat. (b) Levels of nonsocial, social, submissive and aggressive behavior on day 1 prior to pharmacological manipulations. Diazepam (Di; 2 and 6 mg/kg i.p.), administered on day 2, (c) increased nonsocial and (e) decreased submissive behavior without altering (d) social or (f) aggressive behavior. ***P<0.001, *P<0.05 versus all comparisons. (n = 4–16 per group)
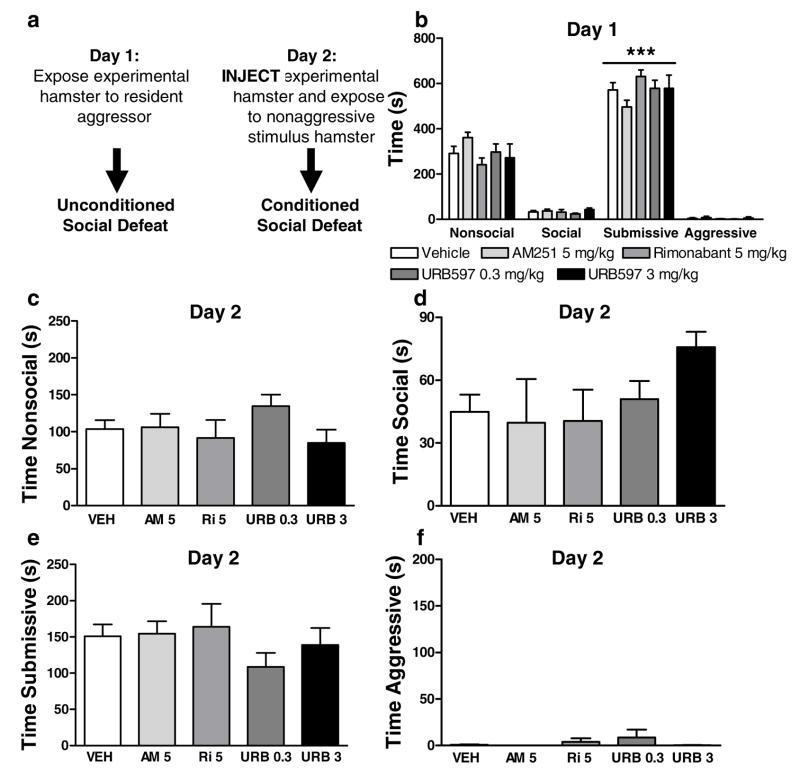
Figure 8.
(a). Timing of pharmacological manipulations relative to assessments of unconditioned and conditioned social defeat. (b) Neither URB597 (0.3 mg/kg i.p.) nor rimonabant (5 mg/kg i.p.) nor AM251 (5 mg/kg i.p.), administered on day 2, altered (c) nonsocial, (d) social, (e) submissive or (e) aggressive behavior in previously defeated hamsters. ***P<0.001 versus other behaviors. (n = 9–13 per group)
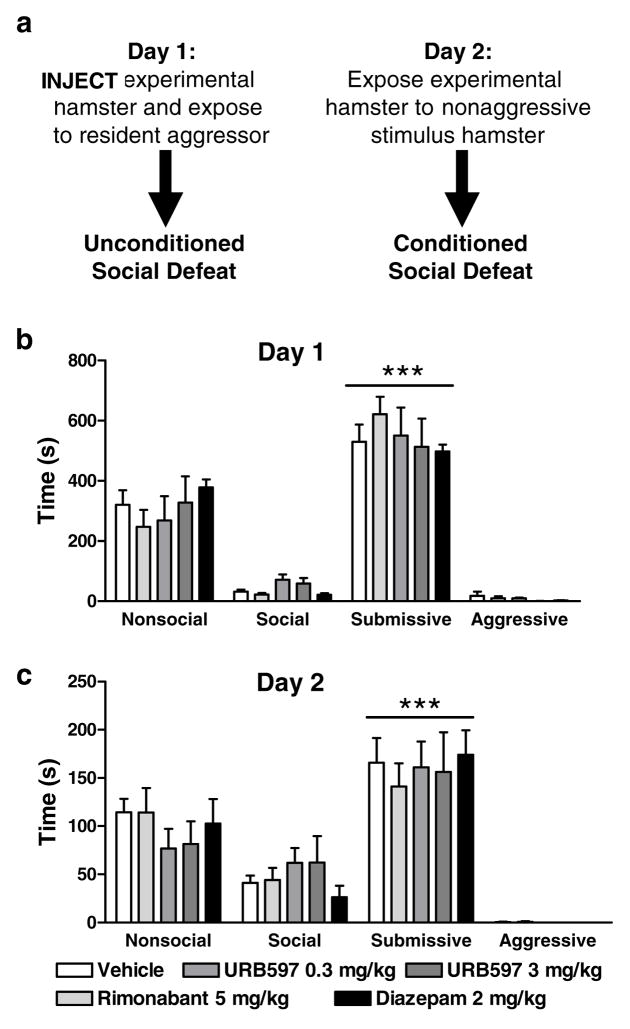
Figure 9.
(a). Timing of pharmacological manipulations relative to assessments of unconditioned and conditioned social defeat. (b) Neither rimonabant (5 mg/kg i.p.), URB597 (0.3 or 3 mg/kg i.p.) or diazepam (2 mg/kg i.p.) altered nonsocial, submissive, social or aggressive behavior in the experimental subjects on day 1. (c) Day 1 pharmacological manipulations (see panel b) did not alter nonsocial, social, submissive or aggressive behavior in previously defeated subjects on day 2. ***P<0.001 versus other behaviors (n = 5–21 per group)
Acquisition of unconditioned social defeat was established by placing an experimental hamster in the home cage of a known resident aggressor hamster for 15 minutes (on day 1). Experimental animals displayed submissive and defensive behavior toward the resident aggressor. Conditioned defeat testing was performed 24 hours after initial acquisition of unconditioned social defeat in all experimental subjects. A non-aggressive intruder was placed in the home cage of the experimental hamster for 5 minutes on day 2. This interval was used to observe an adequate sample of behaviors, and ensure that behaviors remain consistent between tests as described previously (Jasnow and Huhman 2001). Custom software was used to measure total time spent in each of four categories of behavior: 1) non-social behavior (locomotor/exploratory, self-groom, nesting, feeding, pouching, sleeping); 2) social behavior (attend, approach, sniff, investigate, touching nose); 3) submissive/defensive behavior (upright and side defense, tail lift, flee, tooth chatter, full submissive posture, risk assessment, attempted escape from cage, flag); and 4) aggressive behavior (upright and side offense, chase, attack, bite).
Nondefeat control conditions
On day 1, experimental hamsters were placed in the cage of a resident nonaggressive hamster for 15 min in lieu of exposure to a resident aggressor. On day 2, a nonaggressive stimulus hamster was placed in the cage of the nondefeated experimental hamster (determined on day 1) for 5 min. Total time spent in each of four different categories of behavior (non-social, social, submissive/defensive and aggressive behavior) was measured as described above.
Statistical analysis
Data were analyzed by ANOVA and Newman-Keul’s multiple comparison post hoc tests. Planned comparisons were performed using unpaired t-tests, as appropriate. P ≤ 0.05 was considered significant.
Results
Receptor Binding and Autoradiography
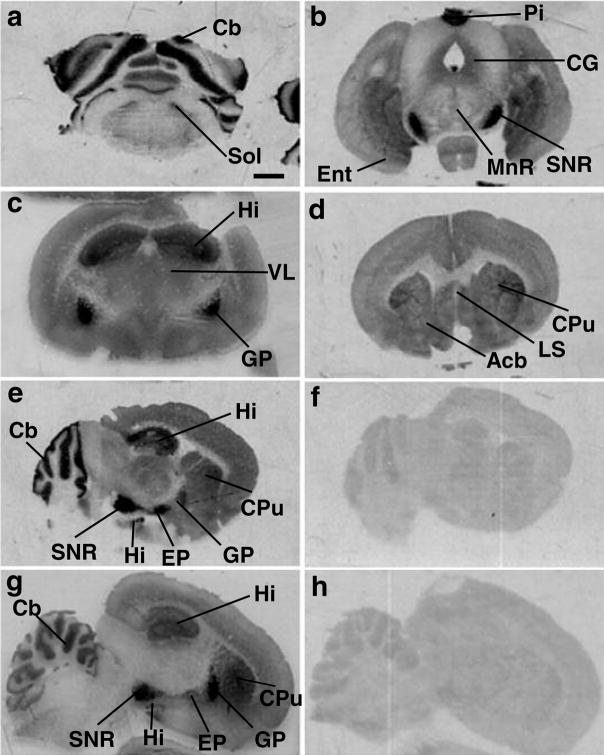
A characteristic heterogeneous pattern of cannabinoid receptor binding sites was observed in hamster brains using [3H]CP55,940 binding and autoradiography (Fig. 1a–f). The distribution of cannabinoid receptor binding sites in hamster brain was qualitatively similar to that observed in rat brain sections processed concurrently (Fig. 1g–h). Dense [3H]CP55,940 binding was detected in the basal ganglia (caudate putamen, globus pallidus, substantia nigra pars reticulata) and other motor structures (e.g. cerebellar molecular layer). Binding was also elevated in limbic structures, including the hippocampal formation and limbic cortical areas (e.g. cingulate cortex). Moderate and low levels of binding were observed in the amygdala and brainstem, respectively.
Figure 1.
Distribution of [3H]CP55,940 binding sites in (a–d) coronal and (e–f) sagittal sections of Syrian hamster brains. (g) Distribution of [3H]CP55,940 binding sites in a sagittal rat brain section that was processed concurrently. Nonspecific binding in (f) hamster and (h) rat brain sections. The scale bar equals 1 mm. See list of abbreviations.
Assessment of anandamide hydrolyzing activity
URB597 inhibited FAAH activity in membranes derived from whole hamster brains with a median inhibitory concentration (IC50) of 12.8 nM (Fig. 2).
Figure 2.
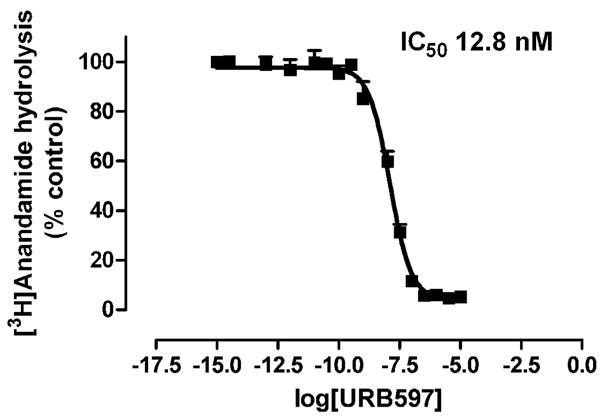
URB597 inhibits anandamide hydrolysis in membranes derived from whole hamster brains.
LC/MS analyses
LC/MS analyses confirmed the presence of the fatty acid ethanolamides anandamide, PEA and OEA as well as the monoacylglycerol 2-AG in lipid extracts derived from whole hamster brains (Table 1). URB597 (0.3 mg/kg i.p.) elevated levels of the fatty-acid amides PEA and OEA in lipid fractions derived from whole hamster brain, but did not reliably alter levels of anandamide (Table 1). As expected, 2-AG levels were not altered by URB597 (Table 1).
Table 1.
URB597 (0.3 mg/kg i.p.) elevates levels of fatty-acid amides in whole hamster brains
| Lipid | Vehicle-treated | URB597-treated |
|---|---|---|
| Anandamide (pmol/g) | 1.677 ± 0.172 | 1.673 ± 0.083 |
| OEA (pmol/g) | 12.877 ± 0.836 | 20.604 ± 4.284* |
| PEA (pmol/g) | 6.317 ± 0.707 | 10.668 ± 1.717* |
| 2-AG (nmol/g) | 4.280 ± 0.417 | 4.107 ± 0.622 |
P ≤ 0.05 versus vehicle treatment (n = 6 per group)
Behavioral experiments
Control conditions
Hamster behavior did not differ between groups receiving either the emulphor: ethanol: saline (1:1:8) or polyethylene glycol: ethanol: saline (4:1:5) vehicle in any study. Therefore, the vehicle groups were pooled into a single control group for all subsequent analyses.
Rota-rod Assessment of Motor Ataxia
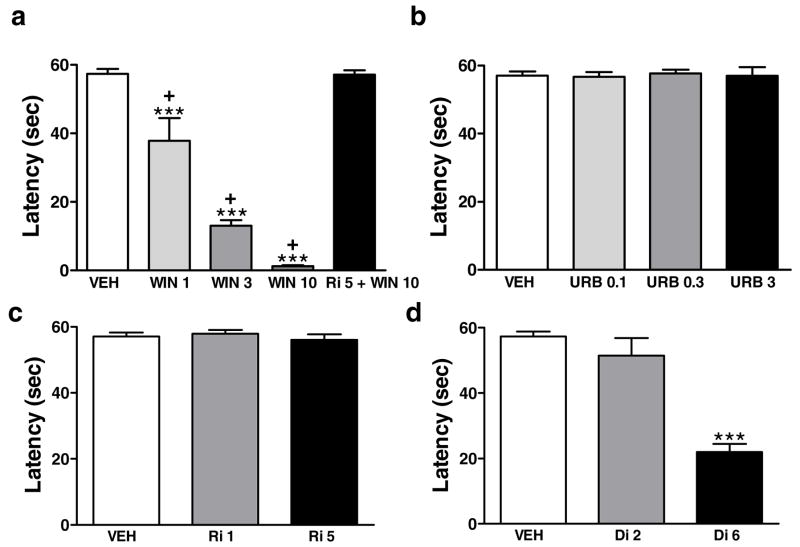
The cannabinoid agonist WIN55,212-2 (1, 3 and 10 mg/kg i.p.) produced CB1-mediated motor ataxia in hamsters [F(4,21)=69.3, P<0.0001] (Fig. 3a). WIN55,212-2 dose-dependently impaired locomotor activity in the rota-rod test relative to vehicle [p < 0.001 for each comparison]. The high dose produced greater impairment of locomotor activity than the middle or low dose [p<0.001 for each comparison]. Rimonabant (5 mg/kg i.p.) blocked the ability of WIN55,212-2 (10 mg/kg i.p.) to induce motor ataxia [p<0.001] (Fig. 3a). By contrast, neither URB597 (0.1, 0.3 or 3 mg/kg i.p.) nor rimonabant (1 or 5 mg/kg i.p.) altered locomotor activity relative to vehicle (Fig. 3b,c). A high dose of diazepam (6 mg/kg i.p.) induced motor ataxia [F(2, 13)=32.2; P<0.001] whereas a low dose (2 mg/kg i.p.) did not alter rota-rod latencies (Fig. 3d).
Figure 3.
Effects of (a) WIN55,212-2 (WIN; 1, 3 and 10 mg/kg i.p.) in the presence and absence of rimonabant (Ri; 5 mg/kg i.p.) in the rota-rod test of motor ataxia. Effects of vehicle (VEH), (b) URB597 (URB; 0.1, 0.3 or 3 mg/kg i.p.), (c) rimonabant (Ri; 1 or 5 mg/kg i.p.), and (d) diazepam (Di; 2 or 6 mg/kg i.p.) on rota-rod latencies. ***P<0.001 versus all other control conditions, +P < 0.05 versus all other WIN55,212-2 doses (ANOVA, Newman-Keuls post hoc test; n = 5–6 per group)
Elevated Plus Maze Testing
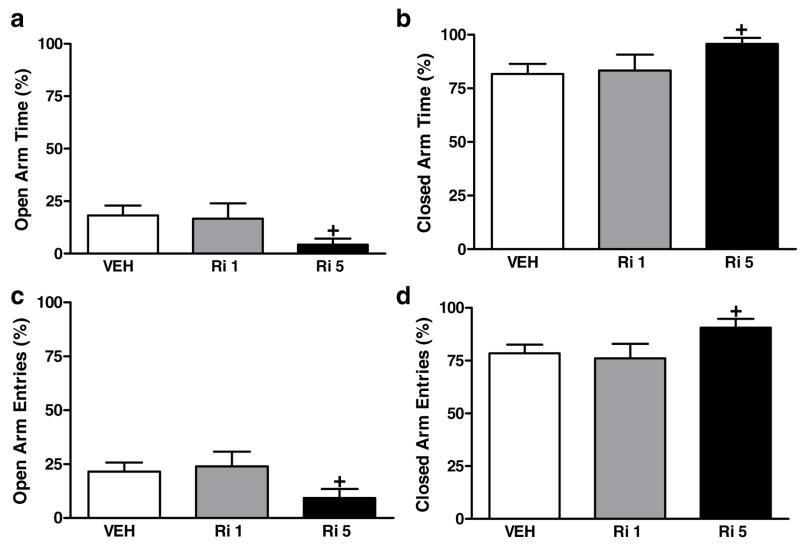
Rimonabant (5 mg/kg i.p.) decreased the percentage of open arm time [p<0.04, planned comparison; Fig. 4a] and increased the percentage of closed arm time [p<0.04, planned comparison; Fig. 4b]. Rimonabant (5 mg/kg i.p.) decreased the percentage of open arm entries [p<0.05, planned comparison; Fig. 4c] and increased the percentage of closed arm entries [p<0.05, planned comparison; Fig. 4d]. A low dose of the antagonist (1 mg/kg i.p.) did not alter any parameter of behavior relative to the control condition (Fig. 4a–d).
Figure 4.
Effects of rimonabant (Ri; 1 or 5 mg/kg i.p.) and vehicle (VEH) on the percentage of (a) open arm time, (b) closed arm time, (c) open arm entries, and (d) closed arm entries in the elevated plus maze. +P<0.05 versus control (planned comparison) (n = 8–22 per group)
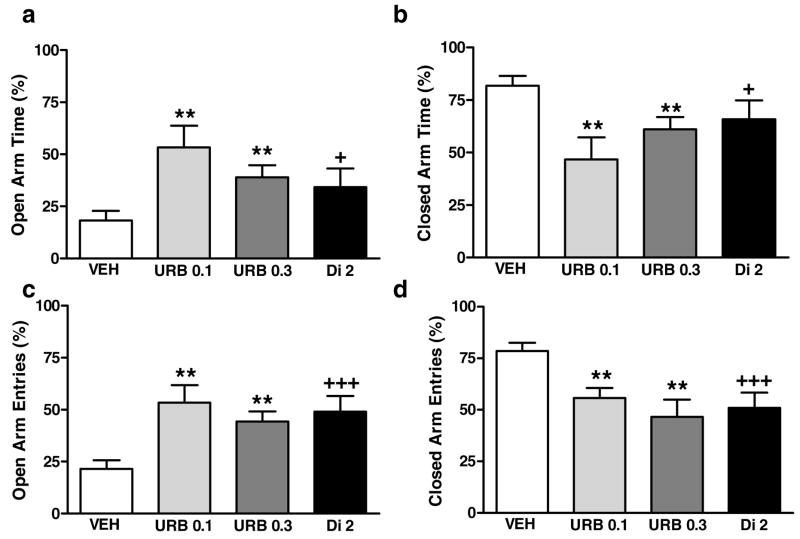
URB597 (0.1 or 0.3 mg/kg i.p.) increased the percentage of open arm time [F(2,53)=6.3, P<0.004, p<0.01 for each comparison; Fig. 5a] and decreased the percentage of closed arm time [F(2, 53) =6.3, P<0.004; p<0.01 for each comparison; Fig. 5b] relative to the control condition. Both doses of URB597 increased the percentage of open arm entries [F(2,53)=8.5, P<0.0007, p<0.01 for each comparison; Fig. 5c] and decreased the percentage of closed arm entries [F(2,53)=8.5, P<0.0007, p<0.01 for each comparison; Fig. 5d] relative to controls. Doses of URB597 did not differ from each other. Effects of URB597 were similar to diazepam (Fig. 5a–d). Diazepam (2 mg/kg i.p.) increased the percentage of open arm time [p<0.05, planned comparison; Fig. 5a] and decreased the percentage of closed arm time [p<0.05, planned comparison; Fig. 5b]. Like URB597, diazepam also increased the percentage of open arm entries [p<0.0007; Fig. 5c] and decreased the percentage of closed arm entries [p<0.0007; Fig. 5d] relative to the control condition.
Figure 5.
Effects of URB597 (URB; 0.1 and 0.3 mg/kg i.p.) and diazepam (Di; 2 mg/kg i.p.) and vehicle (VEH) on the percentage of (a) open arm time, (b) closed arm time, (c) open arm entries and (d) closed arm entries in the elevated plus maze. **P<0.01, *P<0.05 versus control (ANOVA, Newman-Keuls post hoc test), +++P<0.001, +P<0.05 versus control (planned comparison) (n = 8–26 per group)
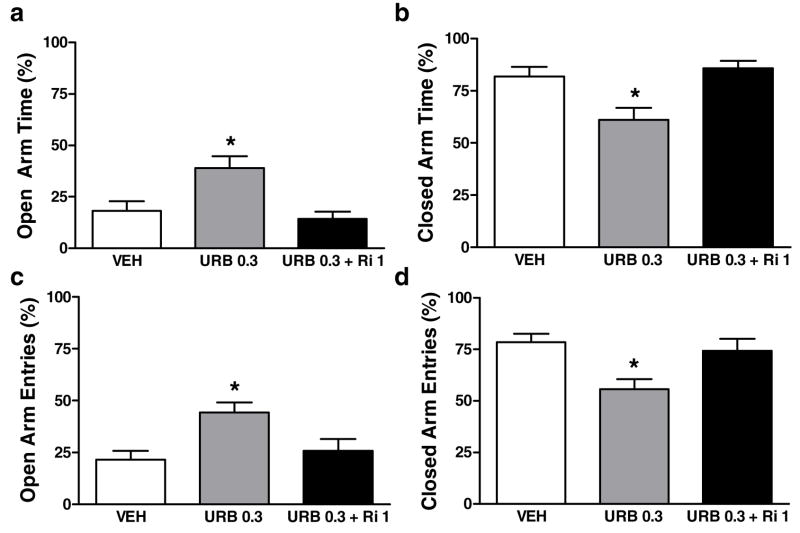
Effects of URB597 were also blocked by rimonabant. The percentage of open arm time was higher [F(2, 56) =6.1, P<0.005] in groups receiving URB597 (0.3 mg/kg i.p.) alone compared to URB597 (0.3 mg/kg i.p.) coadministered with rimonabant (1 mg/kg i.p.; p< 0.05) or the control condition (p < 0.01; Fig. 6a). The percentage of closed arm time was also lower [F(2, 56) =6.1, P<0.005] in groups receiving URB597 (0.3 mg/kg i.p.) alone compared to the antagonist pre-treatment group [p<0.05] or vehicle [p < 0.01, Fig. 6b). URB597 (0.3 mg/kg i.p.) increased the percentage of open arm entries [F(2, 56) =7.0, P<0.003; p < 0.01 versus control; Fig. 6c] and decreased the percentage of closed arm entries [F(2, 56) =7.0, P<0.003; p < 0.01 versus control; Fig. 6d] in a rimonabant-sensitive manner [p< 0.05 for each comparison]. Effects of URB597 (0.3 mg/kg i.p.) coadministered with rimonabant (1 mg/kg i.p.) did not differ from vehicle (Fig. 6a–d) for any dependent measure.
Figure 6.
URB597 (URB; 0.3 mg/kg i.p.) induces a CB1-mediated (a) increase in the percentage of open arm time, (b) decrease in the percentage of closed arm time, (c) increase in the percentage of open arm entries and (d) decrease in the percentage of closed arm entries. Effects were blocked by rimonabant (Ri; 1 mg/kg i.p.). URB597 and vehicle (VEH) data are from Figure 5. *P<0.05 versus all comparisons (ANOVA, Newman-Keuls post hoc test) (n = 11–26 per group)
Unconditioned and Conditioned Defeat
Experimental hamsters exposed to the dominant hamster exhibited a characteristic pattern of unconditioned social defeat on day 1 (P<0.0002 in all studies). Experimental animals exhibited greater submissive/defensive behavior than any other category of behavior (P<0.0002 for each comparison; Fig. 7b, 8b, 9b). On day 1, prior to pharmacological manipulations (Fig. 7a, 8a), nonsocial, social, submissive and aggressive behavior did not differ between groups (Fig. 7b, Fig. 8b). On day 2, experimental subjects exposed to smaller nonaggressive stimulus hamsters exhibited conditioned defeat (Fig. 7e, Fig. 8e, Fig. 9c).
Effects of diazepam on the expression of conditioned defeat
Both doses of diazepam (2 or 6 mg/kg i.p.), administered on day 2, decreased the duration of submissive/defensive behavior [F(2, 22) =9.2, P<0.05, p<0.05 for each comparison] in experimental subjects (Fig. 7e) relative to the control condition. Diazepam increased nonsocial behavior [F(2, 22)=9.8, P<0.05; p<0.05 for each comparison; Fig. 7c) but did not alter aggressive behavior at any dose (Fig. 7f). The low but not the high dose of diazepam produced a trend towards increased social behavior (p=0.07; Fig. 7d).
Effects of URB597 and CB1 receptor blockade on the expression of conditioned defeat
URB597 (0.3 or 3 mg/kg i.p.) or CB1 antagonist (rimonabant or AM251 at 5 mg/kg i.p.) treatments on day 2 (Fig. 8a) failed to alter the expression of conditioned defeat (Fig. 8e). No changes were observed in the duration of any other behavior (Fig. 8c–f). When pharmacological manipulations were performed on day 1 (Fig. 9a), prior to exposure to the dominant hamster, neither diazepam (2 mg/kg i.p.) nor URB597 (0.3 or 3 mg/kg) nor rimonabant (5 mg/kg i.p.) altered either the acquisition of unconditioned social defeat (on day 1; Fig. 9b) or the expression of conditioned defeat (on day 2; Fig. 9c). No other changes in social, nonsocial or aggressive behavior were observed in any study (Fig. 9b,c).
Nondefeat control conditions
Experimental hamsters placed in the cage of a nonaggressive stimulus hamster on day 1 failed to exhibit any signs of submissive/defensive behavior on day 1 (data not shown). Moreover, when the same nondefeated experimental hamsters were subsequently exposed to a nonaggressive intruder on day 2, submissive/defensive behavior was notably absent (data not shown).
Discussion
Localization of key components of an endocannabinoid signaling system to hamster brain
Hamsters contain a functional endocannabinoid signaling system that modulates anxiety-like behavior in a test (the elevated plus-maze) which models the natural fear experienced by rodents toward aviary predators. We documented a characteristic heterogeneous pattern of cannabinoid receptors in hamster brain using [3H]CP55,940 binding and autoradiography. The distribution of cannabinoid receptors was qualitatively similar to that observed in rat brain sections processed concurrently (see also Herkenham et al. 1991). Cannabinoid receptors were dense in motor regions (e.g. basal ganglia and cerebellum) and limbic regions (e.g. hippocampus). We further demonstrated the presence of anandamide and fatty-acid amides that lack affinity for CB1 (OEA and PEA) in hamster brain. Hamsters are a species commonly used in research on seasonality, circadian rhythms and social behavior. Our data establish the feasibility of evaluating possible regulatory changes in cannabinoid receptors and endocannabinoids in these and other species-typical behaviors (e.g. hibernation).
URB597 inhibits FAAH in hamster brain and elevates levels of fatty-acid amides
URB597 potently inhibited FAAH in membranes derived from whole hamster brains in vitro. The IC50 for URB597-induced inhibition of FAAH observed here (12.8 nM) was slightly higher than that reported previously in membranes derived from whole rat brain (4.6 nM; Kathuria et al. 2003) or human liver (Piomelli et al. 2006). Despite the ability of URB597 to robustly inhibit membrane FAAH activity in vitro, in vivo administration of URB597 did not reliably increase anandamide levels at 2 h following treatment. The use of whole brain tissue for LC/MS measurements may have prevented detection of regionally restricted changes in anandamide levels. Consistent with this hypothesis, URB597 elevated levels of fatty-acid amides found in greater abundance than anandamide. It is also possible that non-signaling as well as signaling competent pools of endocannabinoids are measured by LC/MS, masking detection of stimulation-contingent anandamide biosynthesis within discrete brain regions.
Cannabinoid agonists induce CB1-mediated motor impairment in hamsters
The cannabinoid agonist WIN55,212-2 induced prototypic signs of cannabinoid CB1 receptor activation in hamsters. WIN55,212-2 (1, 3 or 10 mg/kg i.p.) dose-dependently impaired the ability of hamsters to walk on a rotating drum. This effect was blocked by rimonabant (5 mg/kg i.p.), which did not alter rota-rod behavior when administered alone. By contrast, the FAAH inhibitor URB597 (0.3 or 3 mg/kg i.p.) failed to alter motor activity in the rotarod test, consistent with previous reports in other rodent species (Kathuria et al. 2003). Thus, FAAH inhibitors, which activate CB1 indirectly by inhibiting anandamide hydrolysis, are not associated with motor impairment that accompanies direct activation of CB1. Diazepam (2 mg/kg i.p.) also failed to impair motor activity in the rota-rod test. Thus, effects of diazepam (2 mg/kg i.p.) in the elevated plus maze and conditioned defeat models are independent of changes in motor behavior.
Bidirectional modulation of anxiety-like behavior following blockade of CB1 and inhibition of FAAH
Rimonabant (5 mg/kg i.p.) increased whereas URB597 (0.1–0.3 mg/kg i.p.) decreased anxiety-like behavior in the elevated plus maze. The high (5 mg/kg i.p.) but not the low (1 mg/kg i.p.) dose of rimonabant decreased the percentage of open arm time and increased the percentage of closed arm time. These observations are consistent with previous observations of anxiogenic-like effects of rimonabant in both rats and mice (Akinshola et al. 1999; Arevalo et al. 2001; see also Haller et al. 2002; Griebel et al. 2005). T he observed increase in the percentage of closed arm entries likely reflects an anxiogenic-like effect induced by blockade of CB1, rather that a change in locomotor activity. In our study, the closed arms of the elevated plus maze were painted black and were dimly illuminated (whereas the open arms were painted white and were brightly illuminated). Moreover, rimonabant did not alter rota-rod latencies at any dose.
Effects of URB597 in hamsters in the elevated plus maze are consistent with previous work documenting anxiolytic-like effects of URB597 in other rodent species (Kathuria et al. 2003; Patel and Hillard 2006; Moreira et al. 2008). The observed effects of URB597 are likely to be mediated by inhibition of anandamide hydrolysis and subsequent activation of CB1 receptors. First, both doses of URB597 produced an identical pattern of effects in the elevated plus maze. Second, rimonabant blocked the complete spectrum of changes induced by URB597 at a dose that was insufficient to alter elevated plus maze behavior. Third, URB597-induced elevations in other fatty acid amides (e.g. PEA and OEA) that do not bind to CB1 cannot account for effects observed here. Fourth, 2-AG levels were not reliably altered by URB597. Increases in levels of other fatty-acid amides that lack affinity for CB1 may also enhance the biological activity of endocannabinoids at CB1 by competing with the same enzymes for hydrolysis (Ben-Shabat et al. 1998).
Differential effects of URB597 and diazepam in the elevated plus maze and conditioned defeat model
Diazepam (2 or 6 mg/kg i.p.) suppressed the expression but not the acquisition of conditioned defeat. Diazepam suppressed submissive/defensive behavior only when it was administered to experimental subjects on day 2 (i.e. after acquisition of unconditioned social defeat) prior to exposure to the nonaggressive stimulus hamster. However, neither blockade of CB1 nor inhibition of FAAH altered conditioned defeat behavior. Moreover, the same pharmacological manipulations, performed on day 1 failed to alter either the acquisition of unconditioned social defeat (on day 1) or the expression of conditioned defeat (on day 2). Thus, diazepam and URB597 may differentially regulate social anxiety.
URB597 suppressed anxiety-like behavior in the elevated plus maze but did not alter submissive/defensive behavior in the social defeat model. Differences in the anxiolytic profile of URB597 in the elevated plus maze and conditioned defeat model may reflect preferential involvement of the hypothalamic-pituitary adrenal (HPA) axis in the defeat model (Jasnow et al. 1999). Consistent with this hypothesis, restraint stress activates the HPA axis and produces concomitant decreases in endocannabinoid levels (Patel et al. 2004, 2005). If similar decreases in endocannabinoid levels are observed in the conditioned defeat model, URB597 may be unable to reinstate anandamide levels. Thus, it is noteworthy that URB597 (0.3 mg/kg i.p.), which inhibits anandamide hydrolysis in rodent brains at the same time point (Fegley et al. 2005), as well as a ten-fold higher dose (3 mg/kg i.p.), both failed to alter conditioned defeat in our study. Endocannabinoids mediate extinction of conditioned fear (Marsicano et al. 2002). Thus, an effect of URB597 could nonetheless be revealed in extinction of conditioned defeat (i.e. under conditions in which a defeated hamster is repeatedly exposed to a nonaggressive intruder).
Unlike URB597, diazepam suppressed anxiety-like behavior in the conditioned defeat model as well as the elevated plus maze. Effects of diazepam (2 mg/kg i.p.) cannot be attributed to motor impairment, as this same dose failed to induce motor ataxia in the rota-rod test. In a previous study, diazepam (6 mg/kg i.p.) increased anticipatory flight in response to a nonaggressive intruder and reduced the number of defensive postures observed in previously defeated subjects (Hebert et al. 1996). Differences in the number of exposures to the aggressive hamsters, the dose and timing of pharmacological manipulations as well as the dependent measure assessed may account for differences between the studies. More work is necessary to determine whether URB597 could enhance effects of diazepam (Naderi et al. 2008) in either the conditioned defeat model or the elevated plus maze.
Benzodiazepines and FAAH inhibitors may differentially modulate anxiety-like behaviors in hamsters. CB1 receptors are localized to terminals of glutamatergic as well as GABAergic neurons (Marsicano and Lutz 1999), where their activation inhibits release of the primary neurotransmitter (Azad et al. 2003). More work is necessary to identify the class or classes of CB1-expressing neurons that mediate effects of rimonabant and URB597 observed here. T he efficacy of URB597 in suppressing anxiety-like behavior in the elevated plus maze in hamsters provides further preclinical support for the therapeutic potential of FAAH inhibitors for treatment of anxiety-related disorders. FAAH inhibitors may be expected to produce a more circumscribed and beneficial spectrum of physiological effects compared to direct CB1 agonists. For example, URB597 decreases anxiety-like behavior in mice and rats (Kathuria et al. 2003; Ognibene et al. 2006; Patel and Hillard 2006) but does not affect reward-related (Ognibene et al. 2006; Scherma et al. 2007) or locomotor behavior. The neuroanatomical substrates mediating the anxiolytic-like effects of URB597 in hamsters remain to be elucidated.
The elevated plus maze has been used extensively in rats and mice as a preclinical screen to predict therapeutic efficacy of putative anxiolytics (Carobrez and Bertoglio 2005; Hogg 1996; Jones et al. 2002; Pellow et al. 1985). The conditioned defeat model has been described as an ethologically relevant model of social anxiety and social stress (Hebert et al. 1996; Young 2002). However, more work is necessary to validate this claim pharmacologically. Hamsters can exhibit dose-dependencies for putative anxiolytics that differ significantly from other rodent species (Kreiskott 1963). Differences in the efficacy of URB597 in the elevated plus maze and the social defeat models may reflect context-specific neurochemical changes that could provide novel insight into therapeutic strategies for treating generalized and social anxiety disorders in humans.
Acknowledgments
Supported by DA021644, DA022702, DA022478 (to AGH and DP). SE was supported by an Achievement Rewards for College Scientists (ARCS) graduate fellowship.
Abbreviations
- Acb
nucleus accumbens
- 2-AG
2-arachidonoylglycerol
- CB1
Cannabinoid receptor subtype 1
- Cb
cerebellum
- CG
central gray
- CPu
caudate putamen
- Ent
entorhinal cortex
- EP
endopeduncular nucleus
- FAAH
fatty acid amide hydrolase
- GP
globus pallidus
- Hi
hippocampus
- HPA
hypothalamic-pituitary adrenal
- LS
lateral septum
- MnR
median raphe nucleus
- OEA
N-oleoyl ethanolamine
- PEA
N-palmitoyl ethanolamine
- Pi
pineal gland
- SNR
substantia nigra pars reticulate
- Sol
solitary n
- VL
ventrolateral thalamic nucleus
Footnotes
Conflict of Interest: DP declares a conflict of interest.
Conflict of interest: Daniele Piomelli is a consultant for Organon Biosciences.
References
- Akinshola BE, Chakrabarti A, Onaivi ES. In-vitro and in-vivo action of cannabinoids. Neurochem Res. 1999;24:1233–40. doi: 10.1023/a:1020968922151. [DOI] [PubMed] [Google Scholar]
- Arevalo C, de Miguel R, Hernandez-Tristan R. Cannabinoid effects on anxiety-related behaviours and hypothalamic neurotransmitters. Pharmacol Biochem Behav. 2001;70:123–31. doi: 10.1016/s0091-3057(01)00578-0. [DOI] [PubMed] [Google Scholar]
- Azad SC, Eder M, Marsicano G, Lutz B, Zieglgansberger W, Rammes G. Activation of the cannabinoid receptor type 1 decreases glutamatergic and GABAergic synaptic transmission in the lateral amygdala of the mouse. Learn Mem. 2003;10:116–28. doi: 10.1101/lm.53303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee MH, Vogel Z, Bisogno T, De Petrocellis L, Di Marzo V, Mechoulam R. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol. 1998;353:23–31. doi: 10.1016/s0014-2999(98)00392-6. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–10. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218–26. [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–6. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–7. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. The relationship between cannabis use, depression and anxiety among Australian adults: findings from the National Survey of Mental Health and Well-Being. Soc Psychiatry Psychiatr Epidemiol. 2001;36:219–27. doi: 10.1007/s001270170052. [DOI] [PubMed] [Google Scholar]
- Desarnaud F, Cadas H, Piomelli D. Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. J Biol Chem. 1995;270:6030–5. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–8. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Rodriguez de Fonseca F, Piomelli D. Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal Biochem. 2000;280:87–93. doi: 10.1006/abio.2000.4509. [DOI] [PubMed] [Google Scholar]
- Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiatry. 2005;57:261–7. doi: 10.1016/j.biopsych.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. The European journal of Neuroscience. 2002;16:1395–8. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Barna I, Freund TF. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci. 2004a;19:1906–12. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol. 2004b;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- Hebert MA, Potegal M, Moore T, Evenson AR, Meyerhoff JL. Diazepam enhances conditioned defeat in hamsters (Mesocricetus auratus) Pharmacol Biochem Behav. 1996;55:405–13. doi: 10.1016/s0091-3057(96)00110-4. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Jarrahian A. Accumulation of anandamide: Evidence for cellular diversity. Neuropharmacology. 2005;48:1072–8. doi: 10.1016/j.neuropharm.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Briley EM, Herkenham M. Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res. 1999;822:17–25. doi: 10.1016/s0006-8993(98)01321-3. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Regulation of cannabinoid and mu opioid receptor binding sites following neonatal capsaicin treatment. Neurosci Lett. 1998;252:13–16. doi: 10.1016/s0304-3940(98)00534-5. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–12. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Banks MC, Owens EC, Huhman KL. Differential effects of two corticotropin-releasing factor antagonists on conditioned defeat in male Syrian hamsters (Mesocricetus auratus) Brain Res. 1999;846:122–8. doi: 10.1016/s0006-8993(99)02007-7. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Huhman KL. Activation of GABA(A) receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Res. 2001;920:142–50. doi: 10.1016/s0006-8993(01)03054-2. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Shi C, Israel JE, Davis M, Huhman KL. Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav Neurosci. 2005;119:1125–30. doi: 10.1037/0735-7044.119.4.1125. [DOI] [PubMed] [Google Scholar]
- Jones N, King SM, Duxon MS. Further evidence for the predictive validity of the unstable elevated exposed plus-maze, a behavioural model of extreme anxiety in rats: differential effects of fluoxetine and chlordiazepoxide. Behav Pharmacol. 2002;13:525–35. doi: 10.1097/00008877-200211000-00002. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nature Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kreiskott H. Zur Verhaltungsforschung im Rahmen der Psychopharmakologie. Medizin und Chemie. 1963;7 [Google Scholar]
- Maccarrone M, Valverde O, Barbaccia ML, Castaänâe A, Maldonado R, Ledent C, Parmentier M, Finazzi-Agráo A. Age-related changes of anandamide metabolism in CB1 cannabinoid receptor knockout mice: correlation with behaviour. Eur J Neurosci. 2002;15:1178–86. doi: 10.1046/j.1460-9568.2002.01957.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–4. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Guimaraes FS. Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1466–71. doi: 10.1016/j.pnpbp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–50. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Naderi N, Haghparast A, Saber-Tehrani A, Rezaii N, Alizadeh AM, Khani A, Motamedi F. Interaction between cannabinoid compounds and diazepam on anxiety-like behaviour of mice. Pharmacol Biochem Behav. 2008;89:64–75. doi: 10.1016/j.pbb.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Ognibene E, Adriani W, Laviola G. Anxiolytic and reward-related properties of URB597, a novel FAAH inhibitor, in CD1 mice. Anxiety Disorders and anxiolytics - Anxiety disorders (basic) 2006:S460. [Google Scholar]
- Onaivi ES, Green MR, Martin BR. Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther. 1990;253:1002–9. [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–11. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–8. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–69. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus–maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–84. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, Dasse O, Monoghan EP, Parrott JA, Putman D. Pharmacological profile of the selective FAAH inhibitor KDS–4103 (URB597) CNS Drug Rev. 2006;12:21–38. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potegal M, Huhman K, Moore T, Meyerhoff J. Conditioned defeat in the Syrian golden hamster (Mesocricetus auratus) Behav Neural Biol. 1993;60:93–102. doi: 10.1016/0163-1047(93)90159-f. [DOI] [PubMed] [Google Scholar]
- Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, Mikics E, Haller J, Yasar S, Tanda G, Goldberg SR. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2007 doi: 10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suplita RL, 2nd, Eisenstein SA, Neely MH, Moise AM, Hohmann AG. Cross-sensitization and cross-tolerance between exogenous cannabinoid antinociception and endocannabinoid-mediated stress-induced analgesia. Neuropharmacology. 2008;54:161–71. doi: 10.1016/j.neuropharm.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Pinel JP, Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacol Biochem Behav. 1981;15:619–26. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- Valverde O, Ledent C, Beslot F, Parmentier M, Roques BP. Reduction of stress-induced analgesia but not of exogenous opioid effects in mice lacking CB1 receptors. Eur J Neurosci. 2000;12:533–9. doi: 10.1046/j.1460-9568.2000.00929.x. [DOI] [PubMed] [Google Scholar]
- Walfish S, Massey R, Krone A. Anxiety and anger among abusers of different substances. Drug Alcohol Depend. 1990;25:253–6. doi: 10.1016/0376-8716(90)90150-d. [DOI] [PubMed] [Google Scholar]
- Yannielli PC, Kanterewicz BI, Cardinali DP. Daily rhythms in spontaneous and diazepam-induced anxiolysis in Syrian hamsters. Pharmacol Biochem Behav. 1996;54:651–6. doi: 10.1016/0091-3057(95)02106-x. [DOI] [PubMed] [Google Scholar]
- Young LJ. The neurobiology of social recognition, approach, and avoidance. Biol Psychiatry. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]