Abstract
Helicobacter infection, one of the most common bacterial infections in man worldwide, is a type 1 carcinogen and the most important risk factor for gastric cancer. Helicobacter pylori bacterial factors, components of the host genetics and immune response, dietary cofactors and decreased acid secretion resulting in bacterial overgrowth are all considered important factors for induction of gastric cancer. Components found in green tea have been shown to inhibit bacterial growth, including the growth of Helicobacter spp. In this study, we assessed the bactericidal and/or bacteriostatic effect of green tea against Helicobacter felis and H. pylori in vitro and evaluated the effects of green tea on the development of Helicobacter-induced gastritis in an animal model. Our data clearly demonstrate profound growth effects of green tea against Helicobacter and, importantly, demonstrate that green tea consumption can prevent gastric mucosal inflammation if ingested prior to exposure to Helicobacter infection. Research in the area of natural food compounds and their effects on various disease states has gained increased acceptance in the past several years. Components within natural remedies such as green tea could be further used for prevention and treatment of Helicobacter-induced gastritis in humans.
Keywords: Helicobacter felis, Helicobacter pylori, Gastric cancer, Green tea, Catechins, Diet
1. Introduction
Helicobacter pylori is one of the most common chronic bacterial infections in man [1–3]. Following the discovery of the linkage between gastric adenocarcinoma and H. pylori infection, antibiotic therapy became an important tool in Helicobacter eradication. However, the wide abuse of antibiotics by the modern world has raised a new problem in medicine, namely antibiotic resistance. Helicobacter pylori resistance to metronidazole has been estimated to approach 60–70% in areas of high antibiotic use, and resistance to macrolides such as clarithromycin is also rising. The search for new chemical compounds with bactericidal or bacteriostatic effects against H. pylori is a challenge for the world’s medical and scientific communities. Different dietary components have been shown to influence the outcome of Helicobacter infection [4–7]. Whilst components of green tea have been suggested to have anti-H. pylori effects in vitro, in vivo correlation is sparse. Interestingly, the combination of the main component of green tea (catechins) and sucralfate has a bactericidal effect on H. pylori infection in Mongolian gerbils [8], and green tea catechins may inhibit the H. pylori urease [9].
Tea is considered one of the most popular beverages in the world, especially in China and Japan. Tea extracts such as catechins inhibit the growth of Staphylococcus aureus, Staphylococcus epidermidis, Vibrio cholerae O1, V. cholerae non-O1, Vibrio parahaemolyticus, Vibrio mimicus, Campylobacter jejuni and Plesiomonas shigelloides in vitro [10] and have antibacterial and bactericidal activity against meticillin-resistant S. aureus (MRSA) in vitro [11]. The importance of green tea and its components has been reviewed by Hamilton-Miller [12]. In the present study, we evaluated the bactericidal and/or bacteriostatic effect of green tea on Helicobacter felis and H. pylori and showed that green tea, in an amount that could be clinically consumed, has bactericidal and bacteriostatic effects in vitro. In vivo studies demonstrated that consumption of green tea when taken before infection prevents gastric mucosal inflammation, and when taken after infection is established diminishes the magnitude of gastritis.
2. Materials and methods
2.1. Animals
Six-week-old male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were housed in microisolator cages under specific pathogen-free conditions, fed standard chow and allowed free access to water and/or green tea as described below. Helicobacter felis and H. pylori bacteria were grown, enumerated and diluted with culture medium and a 500 μL total volume with 5 × 107 colony-forming units (CFU) was given by oral gavage three times at 2-day intervals to establish infection.
2.2. Preparation of green tea extracts
Three grams of dry green tea (‘Chinese green tea’ purchased at a local Chinese store) was ground and suspended in 8 mL of 50% ethanol (equivalent of 37 mg/mL of epigallocatechin gallate). The extract used had a pH of 7.0–7.2. The resulting extract was centrifuged and 25 μL of supernatant was added to 12 sterile papers disks and the ethanol was evaporated in a laminar flow hood. A disk containing no extract and a disk washed with 50% ethanol and dried were used as negative controls.
2.3. Aqueous green tea
Three grams of dry green tea was added to 300 mL of distilled water (after bringing it to the boil and letting it cool down). This formed a solution with a concentration of 1%, which is the amount usually consumed by people. The pH of drinkable green tea was 7.2.
2.4. Helicobacter felis and Helicobacter pylori bacterial cultures
Helicobacter felis (strain 49179) obtained from the American Type Cell Culture (Rockville, MD) and H. pylori Sidney Strain (SSI) (a kind gift from Dr Timothy Wang) were grown as described previously and mice were infected according to a standard protocol [13].
2.5. Bacteriostatic effect
One millilitre of H. pylori or H. felis culture containing 5 × 107 CFU was plated on blood agar plates as described previously [13]. The culture was allowed to adhere to the plate for 3–5 min. After the culture was absorbed, a green tea-embedded disk or a control disk was placed on either side of the bacterial plate using sterile forceps, labelled and plates were incubated for 48 h. All experiments were done in triplicate, repeated once. The zone of inhibition (the distance between the edge of the disk and the edge of the bacterial colony) was measured in millimetres.
2.6. Bactericidal effect
Disks were embedded in a similar fashion onto H. felis and H. pylori bacterial plates that had been grown for 48 h and had a uniform lawn of bacteria. After an additional 48 h in culture, the zone of inhibition was measured in millimetres.
2.7. Green tea inhibits Helicobacter growth in a dose-dependent fashion
One hundred microlitres of H. felis frozen stock (4–6 × 107 CFU) was mixed with aqueous green tea extract (pH 7.0–7.2; 1% sol.) or distilled water at ratios of 1:3, 1:2 or 1:1, seeded on blood agar plates and incubated at 37 °C for 48 h. Plates were scraped from the plates with a sterile razor blade into 1 mL diluents and bacteria were assessed by measurement of the optical density at 600 nm (OD600). OD determination is a more reliable method of determining bacterial numbers than colony counts for H. felis, which due to a combination of clumping and spreading does not give accurate CFU counts as other bacteria do.
2.8. In vivo effects of green tea
Twenty mice were divided randomly into four cohorts of five mice each. The green tea control group was sham-infected and allowed free access to green tea for 8 weeks. No additional water was provided. The green tea/H. felis/green tea group was given green tea 2 weeks before H. felis infection and green tea continued until the end of experiment. The H. felis/green tea group only received green tea beginning immediately after the experimental infection and continued until the end of experiment. The H. felis control group was given free access to water.
2.8.1. Necropsy and histology
Eight weeks after infection with H. felis, mice were euthanised and the stomach was removed, opened longitudinally along the greater curvature and gently rinsed in sterile phosphate-buffered saline. Strips of gastric tissue along the lesser curvature from the squamocolumnar junction through the pylorus were fixed, processed by standard methods and cut into 5 μm sections, stained with haematoxylin and eosin and examined for inflammation and architectural distortion. Gastrointestinal lesion scoring criteria were used as described previously [13].
2.8.2. Quantitative analysis of Helicobacter felis colonisation
A 2 mm × 2 mm piece of gastric mucosa taken at the fundus/antrum border was snap frozen at the time of necropsy and processed as described previously [13]. Standards were made by sequential 10-fold dilutions of purified H. felis DNA producing a range from 500 000 to 5 copies per reaction. This is based on the premise that 2 fg of H. felis chromosomal DNA is equivalent to 1 copy of the H. felis genome. Each sample was analysed in triplicate. Primers sequences for a 225 bp fragment of the flaB gene were as follows: 5′-TTCGATTGGTCCTACAGGCTCAGA-3′; and 5′-TTCTTGTTGATGACA TTGACCAACGCA-3′. The annealing temperature was 55°C.
2.9. Statistical analysis
Helicobacter felis copy numbers are reported as the mean ± 1 standard deviation. Pathology data were compared using Mann–Whitney analysis of non-parametric data and were considered significant at P < 0.05. Student’s t-test was used for statistical analysis of H. felis quantitative polymerase chain reaction (PCR) and for bactericidal and bacteriostatic experiments and was considered significant at P < 0.05.
3. Results and discussion
3.1. Green tea is bacteriostatic against Helicobacter pylori and Helicobacter felis
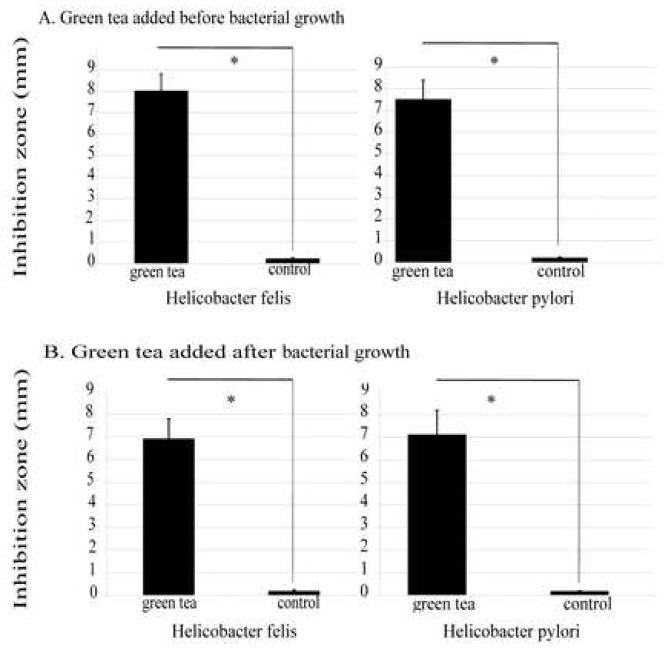
Green tea-embedded disks (pH 7.0) were placed on bacterial plates shortly after bacteria were seeded and liquid was absorbed. Plates were incubated for 48 h. Control disks were embedded with alcohol (vehicle, pH 7.0) only. Green tea had a significant bacteriostatic effect on H. felis and H. pylori (Fig. 1A) (P < 0.05). The difference between the bacteriostatic effect of green tea on H. felis compared with H. pylori was not significant (P > 0.05).
Fig. 1.
Size of the zone of inhibition of Helicobacter felis and Helicobacter pylori with (A) green tea added before bacterial growth (bacteriostatic) and (B) green tea added after bacterial growth (bactericidal). Experiments were repeated three times and results are the mean ± 1 standard deviation of three experiments. * P < 0.05 for green tea vs. control.
3.2. Green tea has a bactericidal effect on actively growing Helicobacter felis and Helicobacter pylori
Bacterial plates were seeded with bacteria and grown for 48 h. Tiny confluent pinpoint colonies of bacteria were visible across the surface. Green tea-embedded disks or control disks (pH 7.0) were placed on the bacterial lawn and incubated for an additional 48 h. The bacterial inhibition zone was measured as the zone of lysis. Dead, but intact, bacteria at the edge of the zone of lysis were not evaluated and therefore the actual zone of killing may be larger than that reported. This would underestimate the desired effect rather than overestimate it. Green tea had a significant bactericidal/lytic effect on H. felis and H. pylori (P < 0.05) (Fig.1B), while the control disks had no effect on bacterial growth. The difference between the bactericidal effects of green tea on H. felis compared with H. pylori was not significant (P > 0.05).
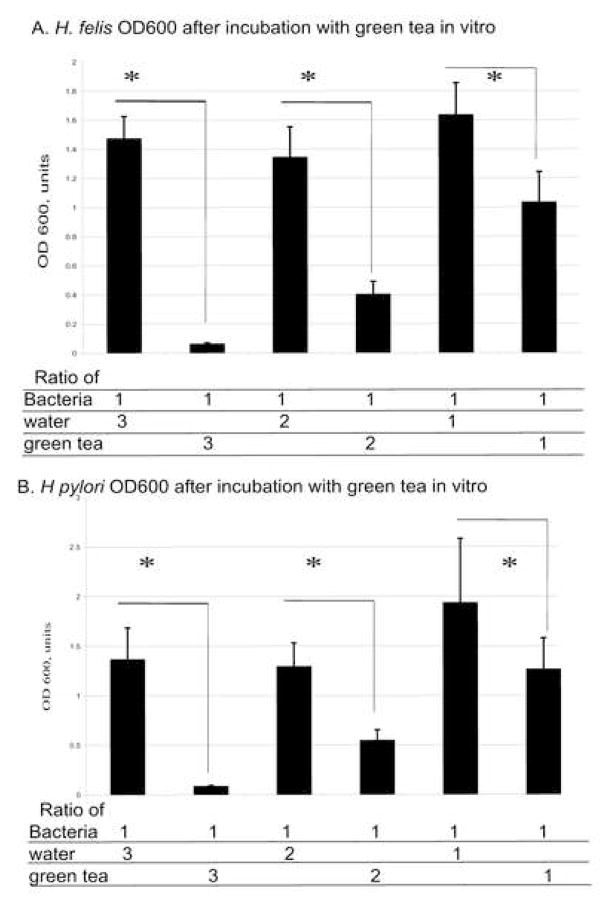
3.3. Green tea has a dose-dependent bactericidal effect on Helicobacter felis and Helicobacter pylori
A series of bacterial suspensions was prepared with various ratios of green tea to bacteria, such that the number of bacteria per plate was kept constant and the concentration of green tea varied. For controls samples, bacteria were incubated with the addition of distilled sterile water in different ratios. The bacterial mixture was plated on blood agar plates and incubated at 37°C for 48 h. The bacteria were then scraped from the plate with a sterile razor blade and quantified by spectrometry at OD600 and plotted against a standard curve for bacterial numbers [14]. The maximum inhibitory effect was obtained at a ratio of 3:1 of green tea to H. felis or H. pylori bacterial suspension (Fig. 2) (P < 0.05). The effect decreased proportionally at ratios of 2:1 and 1:1 of green tea/bacterial suspension for H. felis and H. pylori, respectively (Fig. 2) compared with the water control (P < 0.05). In control samples, the OD600 values did not differ when the ratio of bacteria to water was changed (P > 0.05).
Fig. 2.
Quantitation of (A) Helicobacter felis and (B) Helicobacter pylori suspension cultures. Helicobacter felis or H. pylori was mixed with green tea or distilled water in 1:3, 1:2 or 1:1 ratios. * P < 0.05 for Helicobacter/water vs. Helicobacter/green tea. Experiments were repeated three times and results are reported as the mean ± 1 standard deviation of three experiments. The effect of green tea on H. felis was comparable with the effect on H. pylori at all ratios (P > 0.05). OD600, optical density at 600 nm.
The in vitro inhibition of bacterial growth is perhaps not surprising when one considers the published studies assessing the bactericidal and bacteriostatic effects of green tea on a variety of organisms [10,11]. However, most studies have used highly concentrated extracts or single chemical components, which, while useful for identifying compounds for pharmaceutical manipulation, do not explain the beneficial effects of drinking green tea. Our study demonstrates the beneficial effects of green tea as it would be consumed by the average person.
3.4. Histological evaluation of mice infected with Helicobacter felis and the effect of green tea
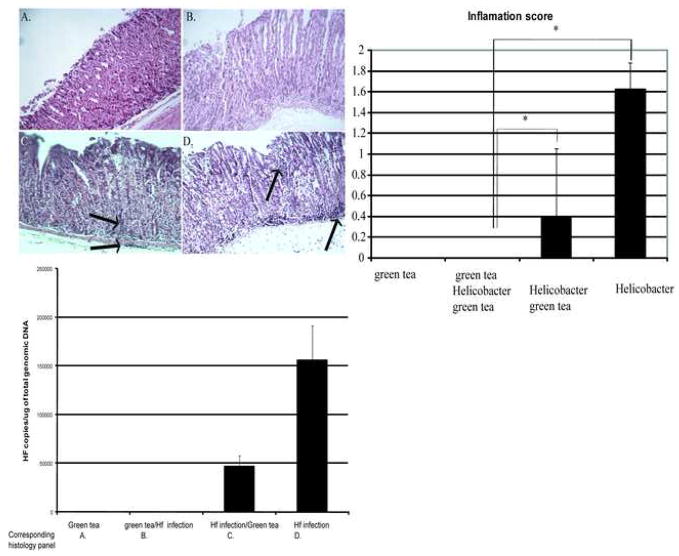
The in vivo effects of an antibacterial compound depend upon several additional factors not tested for in in vitro studies, including: mode of delivery (in this case ingestion); bioavailability; and obtaining optimal concentrations at the necessary site. To determine the clinical effects of green tea on Helicobacter infection, we examined the effects of green tea consumption on gastric inflammation, the major determinant of Helicobacter-related disease. The fundic mucosa at the squamocolumnar junction at the lesser curvature was scored for inflammation using the gastrointestinal lesion scoring criteria [13] in mice drinking green tea only (negative control), mice drinking green tea beginning 2 weeks prior to Helicobacter infection, mice drinking green tea beginning immediately after Helicobacter infection or mice with Helicobacter infection alone (positive control). Mice drinking green tea alone (Fig. 3A) had no inflammation in their stomachs. Mice receiving green tea prior to Helicobacter infection also did not have any inflammation (Fig. 3; P < 0.05). Mice that were infected first with Helicobacter and then received green tea had mild submucosal and intramucosal inflammation (Fig. 3C), which was statistically decreased from the level of inflammation found in the infected positive control group (Fig. 3D; P < 0.05).
Fig. 3.
Histological changes in the stomach of mice infected with Helicobacter felis with or without green tea. (A) Uninfected mice receiving green tea (negative control) showed normal architecture with preserved parietal cells. (B) Mice receiving green tea before (2 weeks) and after H. felis infection showed normal architecture, preserved parietal cells and paucity of inflammatory cells. (C) Mice receiving green tea after H. felis infection showed chronic inflammatory infiltrates in the submucosa and mild loss of parietal cells. (D) Mice infected with H. felis on standard water demonstrate antralisation of the glands and chronic inflammatory infiltrates in the submucosa and within the mucosa between the glands. Haematoxylin and eosin staining, magnification ×400. (Top right) Inflammation score: inflammation, hyperplasia and dysplasia were scored on a 0–4 scale at the squamocolumnar junction at the lesser curvature. * P < 0.05 for experimental groups compared with infected controls. Data are reported as the mean ± 1 standard deviation. (Bottom) Quantitative polymerase chain reaction for H. felis infection. Copy number/μg of total genomic DNA of the stomach. The green tea/Helicobacter/green tea and Helicobacter/green tea groups were compared with infected controls (P < 0.05). Experiments were repeated three times and results are the mean ± 1 standard deviation of three experiments.
3.5. Green tea consumption is associated with a lower Helicobacter felis bacterial load in stomachs of mice drinking green tea before and/or after infection
To determine whether the inflammation scores correlated with alterations in bacterial number, gastric bacteria were quantified in each of the groups. The H. felis bacterial load was determined by real-time PCR using flaB gene-specific primers as described previously [13]. Samples were run in triplicate and the average was used to calculate the number of bacteria per mouse. Mice infected with H. felis receiving only water had the highest bacterial load (Fig. 3, bottom panel). There was a substantial decrease in bacteria in mice that received green tea after infection with H. felis (Fig. 3, bottom panel; P < 0.05), whilst the group of mice that received green tea both before and after infection had no detectable H. felis bacteria and were comparable with non-infected mice that were drinking green tea or water only (Fig. 3, bottom panel), suggesting that green tea may prevent effective colonisation by bacteria. These experimental groups re-create physiologically relevant scenarios, providing the concentration and amount of tea a person might consume rather than using highly concentrated amounts of tea or specific compounds in the tea as reported by others. We tested the scenario of tea consumption prior to infection and the more realistic scenario of consumption after infection, as is more relevant to children becoming infected and adults consuming tea.
Translating in vitro inhibition to clinically relevant in vivo effects on gastric inflammation and decreased bacterial counts has not been shown prior to this study. The greatest impact on decreasing bacterial counts and limiting inflammation is in those mice that received green tea prior to infection. It is interesting to note, however, that even with an established infection green tea decreased the number of bacteria and the inflammatory score. Some epidemiological studies have suggested that green tea offers protection against gastric cancer [15], whilst other studies do not support these findings. It is likely that many factors combine to determine the outcome of infection. Natural inhibitors of bacterial growth and inflammation may offer alternatives to antibiotic therapy for bacterial eradication and may be used as supplements to conventional eradication therapy in populations at high risk for gastric cancer.
Acknowledgments
Funding: National Institutes of Health (NIH).
Footnotes
Ethical approval: Approval was obtained from the Institution Animal Care and Use Committee (IACUC) of the University of Massachusetts Medical School, Worcester, MA, USA.
Competing interests: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 2.Kavermann H, Burns BP, Angermuller K, Odenbreit S, Fischer W, Melchers K, et al. Identification and characterization of Helicobacter pylori genes essential for gastric colonization. J Exp Med. 2003;197:813–22. doi: 10.1084/jem.20021531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham D, Malaty H, Evans D, Evans D, Jr, Klein P, Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991;100:1495–501. doi: 10.1016/0016-5085(91)90644-z. [DOI] [PubMed] [Google Scholar]
- 4.Shibata K, Moriyama M, Fukushima T, Kaetsu A, Miyazaki M, Une H. Green tea consumption and chronic atrophic gastritis: a cross-sectional study in a green tea production village. J Epidemiol. 2000;10:310–6. doi: 10.2188/jea.10.310. [DOI] [PubMed] [Google Scholar]
- 5.Tombola F, Campello S, De Luca L, Ruggiero P, Del Giudice G, Papini E, et al. Plant polyphenols inhibit VacA, a toxin secreted by the gastric pathogen Helicobacter pylori. FEBS Lett. 2003;543:184–9. doi: 10.1016/s0014-5793(03)00443-5. [DOI] [PubMed] [Google Scholar]
- 6.Yanagawa Y, Yamamoto Y, Hara Y, Shimamura T. A combination effect of epigallocatechin gallate, a major compound of green tea catechins, with antibiotics on Helicobacter pylori growth in vitro. Curr Microbiol. 2003;47:244–9. doi: 10.1007/s00284-002-3956-6. [DOI] [PubMed] [Google Scholar]
- 7.Yee YK, Koo MW. Anti-Helicobacter pylori activity of Chinese tea: in vitro study. Aliment Pharmacol Ther. 2000;14:635–8. doi: 10.1046/j.1365-2036.2000.00747.x. [DOI] [PubMed] [Google Scholar]
- 8.Takabayashi F, Harada N, Yamada M, Murohisa B, Oguni I. Inhibitory effect of green tea catechins in combination with sucralfate on Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 2004;39:61–3. doi: 10.1007/s00535-003-1246-0. [DOI] [PubMed] [Google Scholar]
- 9.Matsubara S, Shibata H, Ishikawa F, Yokokura T, Takahashi M, Sugimura T, et al. Suppression of Helicobacter pylori-induced gastritis by green tea extract in Mongolian gerbils. Biochem Biophys Res Commun. 2003;310:715–9. doi: 10.1016/j.bbrc.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 10.Toda M, Okubo S, Ohnishi R, Shimamura T. Antibacterial and bactericidal activities of Japanese green tea [in Japanese] Nippon Saikingaku Zasshi. 1989;44:669–72. doi: 10.3412/jsb.44.669. [DOI] [PubMed] [Google Scholar]
- 11.Toda M, Okubo S, Hara Y, Shimamura T. Antibacterial and bactericidal activities of tea extracts and catechins against methicillin resistant Staphylococcus aureus [in Japanese] Nippon Saikingaku Zasshi. 1991;46:839–45. doi: 10.3412/jsb.46.839. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton-Miller JM. Antimicrobial properties of tea (Camellia sinensis L. ) Antimicrob Agents Chemother. 1995;39:2375–7. doi: 10.1128/aac.39.11.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoicov C, Whary M, Rogers AB, Lee FS, Klucevsek K, Li H, et al. Coinfection modulates inflammatory responses and clinical outcome of Helicobacter felis and Toxoplasma gondii infections. J Immunol. 2004;173:3329–36. doi: 10.4049/jimmunol.173.5.3329. [DOI] [PubMed] [Google Scholar]
- 14.Houghton J, Macera-Bloch L, Harrison L, Korah R. Tumor necrosis factor alpha and interleukin 1 up-regulate gastric mucosal Fas antigen expression in Helicobacter pylori infection. Infect Immun. 2000;68:1189–95. doi: 10.1128/iai.68.3.1189-1195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu G, Hsieh C, Wang L, Yu S, Li X, Jin T. Green-tea consumption and risk of stomach cancer: a population-based case–control study in Shanghai, China. Cancer Causes Control. 1995;6:532–8. doi: 10.1007/BF00054162. [DOI] [PubMed] [Google Scholar]