Abstract
Studies have suggested that cognitive processes such as working memory and temporal control contribute to motor sequence learning. These processes engage overlapping brain regions with sequence learning, but concrete evidence has been lacking. In this study, we determined whether limits in visuospatial working memory capacity and temporal control abilities affect the temporal organization of explicitly acquired motor sequences. Participants performed an explicit sequence learning task, a visuospatial working memory task, and a continuous tapping timing task. We found that visuospatial working memory capacity, but not the CV from the timing task, correlated with the rate of motor sequence learning and the chunking pattern observed in the learned sequence. These results show that individual differences in short-term visuospatial working memory capacity, but not temporal control, predict the temporal structure of explicitly acquired motor sequences.
INTRODUCTION
The ability to learn new action sequences is fundamental for everyday motor behaviors such as typing, playing a musical instrument, or participating in sport. Motor sequence learning is known to be affected by key task parameters such as sequence length (Turcotte et al. 2005; Verwey 2001) and complexity (Howard et al. 2004) and whether the sequence is acquired implicitly or explicitly (Brown and Robertson 2007). The contribution of individual differences in cognitive abilities to motor sequence learning is currently unknown.
There is evidence to suggest that working memory contributes to motor sequence learning. Working memory refers to a cognitive system that involves both active storage and processing to manipulate information for a given task (Baddeley et al. 1986; Miyake and Shah 1999). Variation exists in the number of items that individuals can hold and operate on in working memory (cf. Vogel and Machizawa 2004). Pascual-Leone et al. (1996) reported that transcranial magnetic stimulation (TMS) applied to the contralateral dorsolateral prefrontal cortex (DLPFC), a structure involved in working memory (Jonides et al. 1993), markedly impairs sequence learning. In another TMS study, Robertson et al. (2001) reported that repetitive TMS applied to the DLPFC disrupted sequence learning only when the spatial position of stimuli cued the responses. These authors suggest that the role played by the DLPFC in sequence learning is exclusively related to the processing of spatial cues in working memory. Despite these suggestions, however, concrete evidence that working memory contributes to motor sequence learning is lacking.
Verwey (1996, 2001) has proposed the idea that motor sequence learning engages two components: “buffer loading” and a “dual-processor.” Buffer loading refers to a kind of short-term motor memory (Henry and Rogers 1960; Sternberg et al. 1978) that allows sequence chunks to be programmed in advance of their execution. The dual processor model consists of a cognitive and a motor processor (Verwey 2001). When learning a new sequence, participants have to rely on the cognitive processor to select individual sequence elements one by one, leading to slow execution speeds. Once a sequence is learned, the cognitive processor selects a single representation (i.e., a motor chunk) for the entire sequence while a dedicated motor processor is running in parallel to execute the sequence. Behaviorally, an unevenly distributed temporal pattern between each movement element (i.e., inter-response time) can be observed after repeated practice (Shea et al. 2006; Verwey 1996, 2001). Longer inter-response times between elements represent the divisions of chunks, whereas shorter inter-response times imply strong associations within each chunk.
Previous studies have reported substantial individual differences in motor sequence chunk length (Kennerley et al. 2004; Verwey and Eikelboom 2003). Here, we evaluate whether individual differences in cognitive capacity contribute to this effect. Working memory capacity limitations are known to affect learning of categorization tasks and math problem solving (Beilock and Carr 2005; Decaro et al. 2008). Recent estimates of working memory capacity in the visual and verbal domains is approximately four items (Cowan 2001; Jonides et al. 2008; Luck and Vogel 1997; Vogel and Machizawa 2004) Moreover, during sequence learning, participants are likely to group three or four elements into one chunk (Mckone 1995). Here, we ask whether the rate of motor sequence learning, and the length of motor chunks that participants form, are related to an individual's working memory capacity.
In addition to working memory, temporal control processes may also contribute to the chunking patterns that participants show when learning new motor sequences. Temporal ordering has been considered one of the cognitive components involved in sequence learning (Ashe et al. 2006). Ivry and colleagues have proposed a “common timing mechanism” to explain high correlations among various timing tasks such as perception and motor production (Ivry and Hazeltine 1995). Such superordinate temporal control may act as a building block for a wide range of motor tasks that exhibit temporal structure. Brain imaging studies have shown that the DLPFC, inferior prefrontal cortices, presupplementary motor area (pre-SMA), supplementary motor area (SMA), and cerebellum may act as a general timing network (Maquet et al. 1996; Smith et al. 2003). These areas are also engaged during motor sequence learning (Boyer et al. 2005; Doyon et al. 2002; Kennerley et al. 2004), making it plausible that temporal control processes contribute to motor sequence chunking patterns. Moreover, Kennerley et al. (2004) have shown that rTMS to the pre-SMA disrupts the chunking pattern of acquired motor sequences.
To understand the effects of temporal control on sequence learning, Shin and Ivry (2002, 2003) performed two experiments where the temporal structure, defined by the response-to-stimulus interval, was either correlated or uncorrelated with the spatial sequence. In the correlated condition, the length of the spatial sequence (8 elements) equaled the length of the temporal sequence (8 elements). In the uncorrelated condition, the length of the spatial sequence (8 elements) was longer than the length of the temporal sequence (7 elements). Thus the relationship between the spatial and temporal sequences was not fixed. They found that spatial learning occurred in both experiments, but temporal learning only appeared in the correlated condition. These results suggest that temporal control is integrated with spatial representations in motor sequence learning. Therefore in this study, we also examined whether individual differences in timing control correlate with the temporal chunk patterns exhibited during sequence learning.
We predicted positive correlations between an individual's visuospatial working memory capacity and their temporal control with the rate of motor sequence learning and the length of motor chunks formed during sequence learning. We hypothesized that participants with lower working memory capacity and lower temporal control would show shorter chunk lengths and slower learning, whereas participants with higher working memory capacity and better temporal control would show longer chunk lengths and faster learning. Additionally, we expected that longer chunk lengths would be associated with faster overall sequence performance. Such a pattern would provide support for the idea that working memory and temporal control processes play a role during motor sequence learning.
In addition, Verwey (1996, 2001) has suggested that chunking patterns are represented in an abstract fashion that is not tied to the effector used during training. Previous studies have also shown that structured sequence knowledge is effector independent (Cohen et al. 1990). Thus this study explored whether the chunking pattern developed through learning would be transferable or not. We predicted that acquired chunk patterns would be maintained when participants performed the same sequence with either hand.
It is important to point out that sequence learning can occur either implicitly (i.e., the acquisition of information is not accompanied by conscious awareness of what was learned or the fact that learning occurred; Reber 1993) or explicitly (i.e., participants develop explicit awareness of the sequence structure). This study used an explicit sequence learning paradigm because chunking has been consistently reported during explicit but not implicit learning (Kennerley et al. 2004; Shea et al. 2006; Verwey 1996).
METHODS
Participants
The experimental procedures were approved by the Institutional Review Board of the University of Michigan. Twenty-five right-handed (determined by self-report and the Edinburgh handedness inventory; Oldfield 1971) (mean = 0.92) adults (mean age = 20.9 ± 2.1 yr; 12 males) gave their informed consent and were paid for their participation.
Procedure
Participants performed an explicit sequence learning task (modified from Kennerley et al. 2004), a visuospatial working memory task (modified from Luck and Vogel 1997), and a continuous tapping task (modified from Wing and Kristofferson 1973). For all the tasks, the stimuli were controlled by a PC using custom software written in E-Prime version 1.0 (Psychology Software Tools, Pittsburgh, PA).
SEQUENCE LEARNING TASK.
Participants learned a 12-element sequence of finger movements, cued by colored boxes presented in a spatial array on the computer screen (purple, yellow, green, purple, red, green, red, yellow, green, red, yellow, purple). We selected this sequence because 1) the probability of each element within the sequence was equally distributed; 2) this sequence did not have a fixed grouping pattern (e.g., ABCD); and 3) the sequence does not have runs of three (i.e., triplets) or trills (e.g., ABAB), even when the stimuli were presented continuously in steps 1 and 2. Four visual stimulus boxes were presented on a computer screen with one of the four colors appearing in one of the stimulus boxes. The positions of those four colors were fixed to the four stimulus boxes, i.e., red always appeared at the leftmost; yellow was at the second leftmost, green appeared at the second rightmost box, and purple was at the rightmost position. The colors red, yellow, green, and purple were mapped onto the middle and index fingers of the left hand and the index and middle fingers of the right hand, respectively. There were four learning steps. Step 1: the sequence was presented element by element every 1,000 ms (i.e., each element appeared for 900 ms and then a blank screen appeared for 100 ms) and the participants were instructed to press the corresponding response button as fast as possible. Each complete sequence defined a trial and each block included 10 repetitions of the sequence. There were a total of three blocks of training in step 1. Step 2: the task was the same as in step 1, except that participants had to reach 90% accuracy on 10 consecutive trials for three continuous blocks to move on to the next step. If the accuracy in one of the blocks was <90%, participants had to repeat step 2 (this did not occur for any participants, however). Step 3: during this step, the entire sequence appeared (1 element every 500 ms) before the participants initiated their response. After the presentation of the last element of the sequence, a screen with the instruction “please produce the sequence now” appeared cueing the participant to begin reproducing all 12 of the sequence elements. One trial consisted of the presented sequence and the responses (entire sequence) generated by the participants. There were 10 trials in a block and three blocks in step 3. Again, the participant had to reach 90% accuracy on 10 consecutive trials for the last block of step 3 to progress to the next step. If the accuracy was <90%, participants had to repeat step 3. Step 4: during this step, participants performed the sequence from memory without visual cues at the beginning of each trial. Once the participant could make 30 consecutive memory-guided trials with 90% or higher accuracy (3 blocks with 10 trials each), the sequence learning portion of the study terminated.
Participants performed the transfer task immediately after step 4 of training. For the transfer conditions, participants were asked to generate the sequence from memory using the fingers of one hand (left or right, as opposed to the 2-handed response required during training). Keeping the sequence spatially constant, the colors red, yellow, green, and purple (from leftmost to rightmost) were mapped onto the index, middle, ring, and little fingers of the right hand for the right transfer condition and the little, ring, middle, and index fingers of the left hand for the left transfer condition. Participants performed the two transfer conditions in a counter-balanced fashion.
VISUOSPATIAL WORKING MEMORY TASK.
We slightly modified the visuospatial memory task published by Luck and Vogel (1997) (experiment 1). Participants viewed a sample array (within a 9 × 9-in region) of colored squares (1 × 1 in), followed by a test array. Then, they had to press the “s” key if the two arrays were identical or the “d” key if the two arrays were different. The arrays consisted of 2–10 (array size) colored squares (randomly from 7 colors: red, blue, violet, green, yellow, black, and white). Each color appeared no more than twice for the 8–10 squares conditions. The sample array was presented for 100 ms, followed by a 900-ms blank screen delay, and then a 2,000 ms presentation of the test array. The test array was either the same as the sample array or different in the color of one of the squares. The ratio of same to different arrays was 1:1. For each trial, only one of the colors was changed for the test array. Thus it is possible that the test array contains a color that had not occurred in the sample array on that trial. Therefore this task relied on detection of a change in color and/or location. We used nine different arrays as the stimulus set, each having between 2 and 10 squares. Each array appeared five times in random order.
CONTINUOUS TAPPING TASK.
Participants sat comfortably with their forearm resting on the table. They wore earphones and were instructed to tap the space key on the keyboard using their index finger to coincide with periodic auditory tones in three interval conditions: 500, 1,000, and 1,500 ms. A trial began with the “ready, go” instruction on the computer screen followed by auditory beats. The participants were asked to listen to the beats and synchronize their taps with the tones. After the first response was detected, an additional 12 beats were presented. Following this, the beats were turned off, and the participants were asked to continue tapping as consistently as possible for another 30 intervals (unpaced) until the trial ended. Fifteen trials, presented in three blocks of five trials, were acquired for each interval condition. The order of blocks was randomly presented.
Analysis
SEQUENCE LEARNING.
Across all learning steps (1–4), we labeled the first block of step 1 as block 1, the second block of step 1 as block 2, the third block of step 1 as block 3, the first block of step 2 as block 4, the second block of step 2 as block 5, the third block of step 2 as block 6, the first block of step 3 as block 7, the second block of step 3 as block 8, the third block of step 3 as block 9, the first block of step 4 as block 10, the second block of step 4 as block 11, and the third block of step 4 as block 12.
Early learning steps: steps 1 and 2 (blocks 1–6).
Data from steps 1 and 2 were considered as the early phase of learning. The reaction time (RT) was defined from the appearance of stimulus to the onset of the response. To measure the rate of learning, we calculated the mean RT for every trial and computed the decay constants (b in equation Y = a × Xb̂ + c) within each step (30 trials) using power fitting functions.
Late learning steps: steps 3 and 4 (blocks 7–12).
The data from steps 3 and 4 of learning were used for the chunking analysis, because we were interested in the chunking patterns when participants produced all the sequence elements continuously without the intermittent visual cues. The response time for the first movement in the sequence was defined from the appearance of the go signal to the onset of the first response. Response time for the later sequential elements was calculated between two consecutive responses. Only correct responses were included in the response time analyses.
Definition of chunk points.
As previously reported (Kennerley et al. 2004), the response time for the beginning of each chunk was longer than the response time within the chunks. We used the last block in step 4 (block 12) to predefine the chunking points for individual participants. One-tail paired t-test, from the 3rd to 11th elements of the sequence, were performed to evaluate whether each element was significantly longer than its preceding and succeeding elements (P was preset at the 0.2 level). The 1st, 2nd, and 12th elements were not included in these paired t-test because we assumed that the 1st element was always the beginning of the first chunk and the 2nd and 12th elements were always within the first and last chunks. We also assumed that the shortest possible chunk length was 2 elements and the longest was 10 elements.
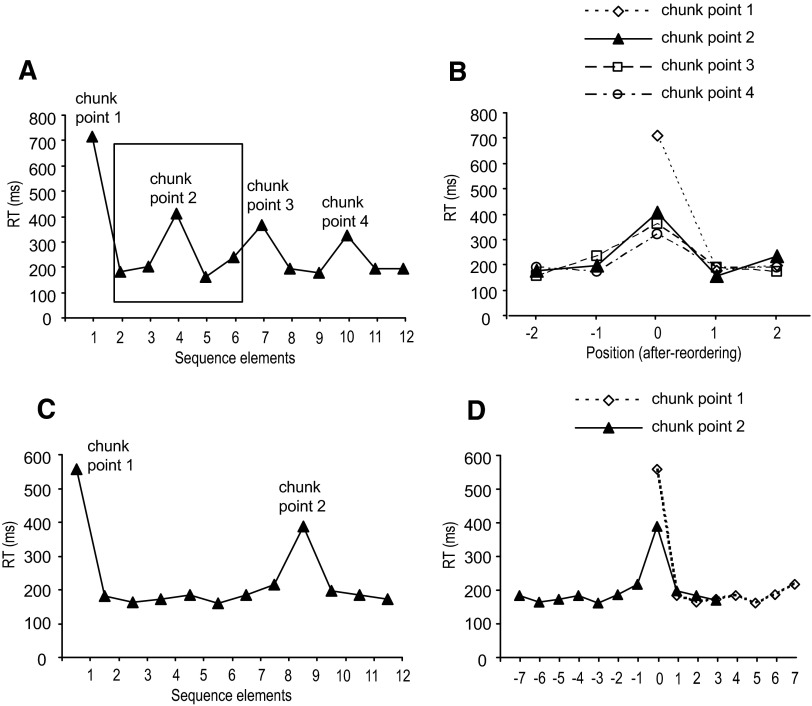
After all the chunk points were defined, we confirmed these individual chunk points at the group level using a reordering procedure based on that presented in Kennerley et al. (2004). The individual data were replotted and aligned to the longest response time for each chunk. All the chunks were treated equally during the analysis. That is, regardless of the length of the chunks, we aligned all the chunk elements to the longest response time at the beginning of each chunk. Figure 1 shows this aligning procedure. The initial analysis identified four chunk points (Fig. 1A). Taking the second chunk point as an example, the fourth element of the sequence was labeled as position 0 because the longest response time for that chunk preceded this element. The third and second elements were labeled as −1 and −2, whereas the fifth and sixth elements were labeled as 1 and 2 for that particular chunk. The same procedure was used for the rest of the chunks for every participant (Fig. 1B). Figure 1C shows the same procedure when the lengths of the chunks were different. These two chunks were weighted equally when all the elements were aligned (Fig. 1D). After reorganizing the chunk points in this manner, we performed a one-way ANOVA on response time. Only when the response time at position 0 was found to be significantly higher than the response times at any other positions did we accept the initially identified chunk points. The mean chunk length was calculated using the 12 (elements) divided by the number of chunks. To evaluate how much time participants spent at the beginning of a chunk compared with the rest of the chunks, the response time ratio between and within chunks was computed using the mean response time for the first element of the chunks divided by the mean response time for the remaining chunk elements.
FIG. 1.
Illustration of the chunking realignment procedure. A: 4 initial chunking points identified for 1 representative participant. B: plots realigned with respect to each chunk point in the sequence. C: 2 initial chunking points with different chunk lengths for another participant. D: plots realigned to each chunk point for the example in C.
Definition of how quickly chunks formed during training.
First, the mean response time for each element of a sequence was computed across every block in steps 3 and 4. Then, the sequence elements for each block were reordered from the longest to the shortest RT. Because the chunk points for each participant were already defined using the above procedure, we matched the reordered elements across blocks. As long as any earlier block showed the same reordered pattern as the last block, we defined that block as the beginning of the developed chunk pattern during training. Taking subject B in Fig. 3A as an example, the reordered block 1 step 4 was elements 1, 6, 2, 9, 5, 7, 4, 8, 3, 12, 10, and 11 from the longest to the shortest RT. The reordered block 2 step 4 was elements 1, 9, 7, 4, 8, 3, 2, 5, 12, 6, 11, and 10. The defined chunks for subject B were elements 1 and 9. Only the first two elements in the reordered block 2 step 4 matched with the final chunks. Thus we concluded that subject B formed their final chunk pattern at the second block in step 4.
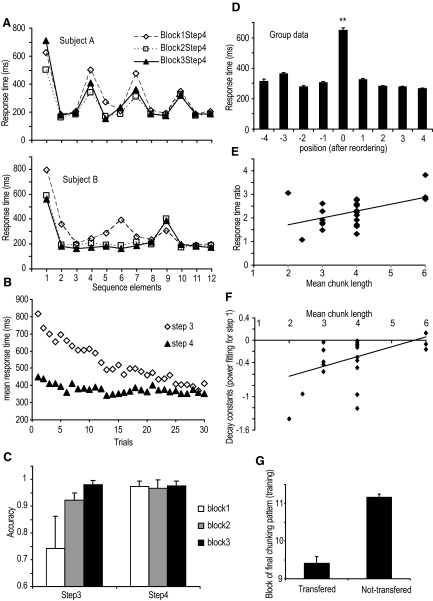
FIG. 3.
A: the response time from the 3 blocks of step 4 of sequence training are depicted for 2 representative participants. B: the mean response time for each trial in steps 3 and 4. C: the mean accuracy for each block in steps 3 and 4. D: group mean response time data (last block of step 4), after replotting with respect to each participant's initially determined chunk points. E: correlation between response time ratio (the mean response time for the 1st element of the chunks divided by the mean response time for the remaining chunk elements) and mean chunk length. F: correlation between the decay constants for step 1 and mean chunk length. G: block at which participants formed their final chunking pattern during training in the transfer and nontransfer groups.
Transfer conditions: determining transfer of chunk patterns.
The mean response times for the first block within each transfer condition were calculated. We used the same reordering and matching procedures as described above. If the reordered elements in either one of the two transfer conditions showed the same pattern as that in the last block (block 12), we deemed that successful transfer of the chunking pattern had occurred.
VISUOSPATIAL WORKING MEMORY TASK.
The formula was K = S × (H − F), where K is the memory capacity, S is the size of the array, H is the observed hit rate, and F is the false alarm rate (Vogel and Machizawa 2004). The K value for each array size was first calculated. Then, the average K across all array sizes was computed to represent the visuospatial memory capacity for each participant.
CONTINUOUS TAPPING TASK.
Any tapping intervals that were shorter or longer than 2 SD of the mean were excluded from the analysis. The CV, a measure to examine the consistency of timing control (Ivry and Hazeltine 1995), was calculated using the SD of the tapping interval divided by the mean and multiplied by 100 to measure the temporal consistency of the movements.
RESULTS
Sequence learning
EARLY LEARNING STEPS: STEPS 1 AND 2 (BLOCKS 1–6).
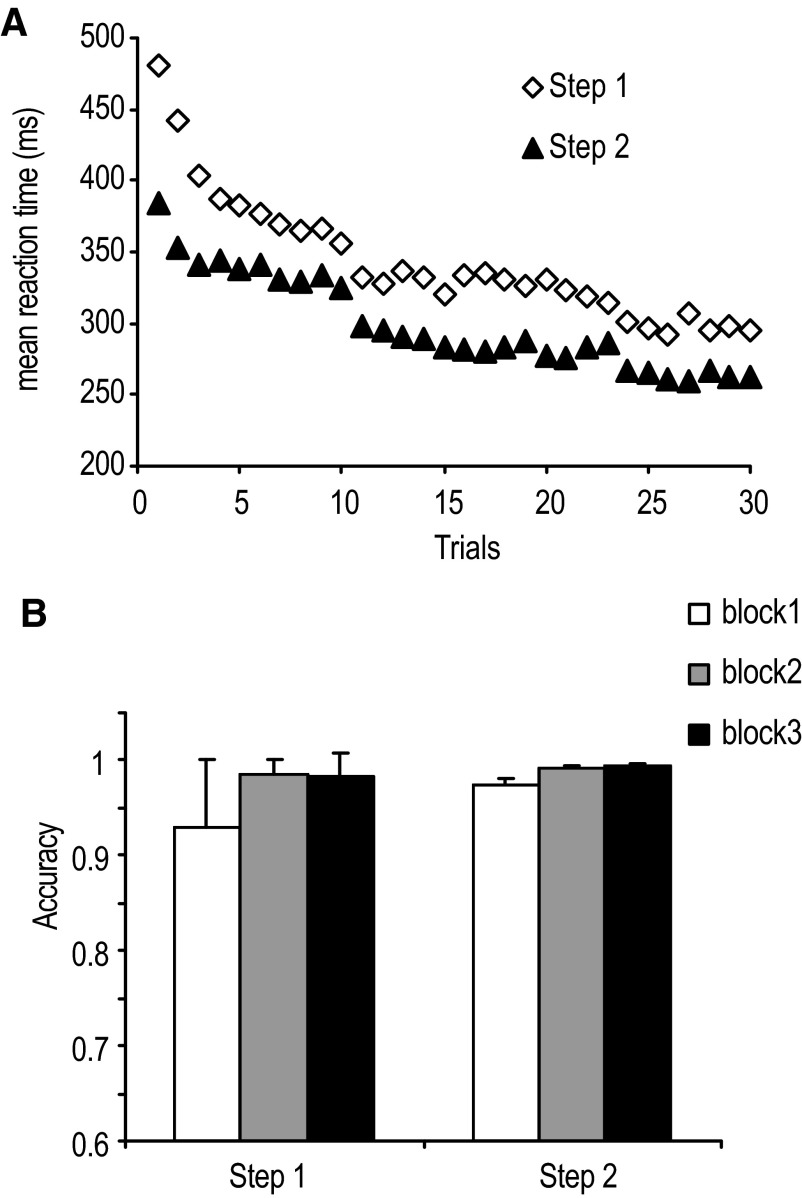
Figure 2, A and B, shows the overall performance for 60 trials from blocks 1–6 in steps 1 and 2. As expected, reaction time got faster as participants practiced. None of the participants had to repeat the steps because all of them were able to reach 90% accuracy in step 2.
FIG. 2.
A: the mean reaction time for each trial in steps 1 and 2. B: the mean accuracy for each block in steps 1 and 2.
LATE LEARNING STEPS: STEPS 3 AND 4 (BLOCKS 7–12).
All participants were able to reach the accuracy criteria after three blocks of practice in steps 3 and 4 (Fig. 3C). They also produced the sequence faster across subsequent practice blocks (F(1,148) = 33.56, P < 0.01; Fig. 3B). Performance for the last three blocks of step 4 from two representative participants is shown in Fig. 2A. Participant A divided the whole sequence into four chunks at the 1st, 4th, 7th, and 10th elements and consistently showed this chunking pattern across three blocks in step 4. However, participant B appeared to have only two chunks for the last two blocks. The additional increase in response time at the ninth element indicates the reorganization of the remaining sequence elements. In addition, this participant's pattern of chunking did not appear until the second block of step 4.
One-tailed paired t-test on the response times from the 3rd to 11th elements of the sequence were used to identify individual chunks. For example, the paired t-test showed that the response time for the fourth element was longer than the response time for both the third and fifth elements for participant A (Fig. 3A). Therefore the fourth element was selected as the beginning of a chunk. We performed the same t-test across all the combinations of pairs. For participant A, additional chunk points were found at the 7th and 10th elements (Fig. 3A).
Reorganization of the chunk points at the group level showed a significant position effect (F(8,171) = 10.66, P < 0.01). A Bonferroni-corrected post hoc test showed that the chunk point RT at position 0 was significantly longer than any of the adjacent response times (all P < 0.01; Fig. 3D), validating the empirically identified chunk locations.
Once the chunk locations were determined, we performed a correlation analysis between the chunk length and the response time ratio between and within chunks for the last block of step 4 to test whether longer chunks required a longer initial reaction time. A significant positive correlation (R = 0.48, P < 0.05; Fig. 3E) provides evidence of “buffer loading” when participants performed longer chunks.
After the sequence elements for every block in steps 3 and 4 were reordered from the longest response time to the shortest response time, we compared whether or not the reordered elements in those blocks matched with the defined chunk points. For example, participant B in Fig. 2A showed that the second block, not the first block, in step 4 matched with the defined chunk points visible in the last block of step 4. Thus we concluded that participant B did not develop his final chunking pattern until the second block in step 4. Table 1 lists the earliest block number for all the participants that showed the defined pattern of chunking. Four of the 25 participants appeared to have a very stable chunking pattern throughout all of the testing blocks. Nine of 25 participants did not show their final chunking patterns until the last block of learning. Three participants developed their stable chunks at the third block of step 3. Six participants showed chunks at the first block of step 4. The remaining three participants had their final chunks at the second block of step 4.
TABLE 1.
Speed at which individual participants developed chunks and whether they showed transfer of the chunking pattern
| Participant | Block at Which the Final Chunk Points Appeared | Transfer Left | Transfer Right |
|---|---|---|---|
| 1 | First block step 3 | Y | Y |
| 2 | First block step 4 | Y | Y |
| 3 | Second block step 4 | N | N |
| 4 | Third block step 4 | N | N |
| 5 | First block step 4 | Y | Y |
| 6 | First block step 3 | Y | Y |
| 7 | Third block step 4 | N | N |
| 8 | Second block step 4 | N | N |
| 9 | Third block step 4 | Y | N |
| 10 | Third block step 4 | N | Y |
| 11 | Third block step 4 | N | Y |
| 12 | Third block step 4 | N | N |
| 13 | First block step 4 | N | N |
| 14 | First block step 3 | Y | Y |
| 15 | First block step 4 | N | N |
| 16 | Third block step 4 | N | N |
| 17 | Third block step 3 | N | N |
| 18 | Third block step 4 | N | N |
| 19 | First block step 3 | Y | N |
| 20 | Third block step 4 | N | N |
| 21 | Second block step 4 | N | N |
| 22 | First block step 4 | Y | Y |
| 23 | Third block step 3 | N | N |
| 24 | First block step 4 | Y | Y |
| 25 | Third block step 3 | Y | N |
Finally, we examined whether there were any correlations between chunk length and learning measures. The measures of goodness-of-fit for all 25 data sets are presented in Table 2; r2 values ranged from 0.37 to 0.93. For 22 of the 25 participants, the fit ranted from medium to very good with the r2 >0.60. We found that the chunk length was significantly correlated with the decay constants within step 1 (R = 0.44, P = 0.03; Fig. 3F). No correlations were found between chunk length and any learning measures for steps 2, 3, and 4.
TABLE 2.
Goodness-of-fit for the power function (step 1) for each participant
| Participant | r2 | Adjusted r2 | RMSE | SSE × 103 |
|---|---|---|---|---|
| 1 | 0.46 | 0.42 | 46.32 | 57.93 |
| 2 | 0.85 | 0.83 | 20.01 | 10.81 |
| 3 | 0.76 | 0.74 | 21.33 | 12.29 |
| 4 | 0.91 | 0.90 | 21.52 | 12.51 |
| 5 | 0.84 | 0.83 | 30.58 | 25.24 |
| 6 | 0.71 | 0.69 | 21.36 | 12.32 |
| 7 | 0.87 | 0.86 | 26.89 | 19.52 |
| 8 | 0.64 | 0.62 | 27.62 | 20.60 |
| 9 | 0.62 | 0.59 | 20.58 | 11.43 |
| 10 | 0.79 | 0.77 | 30.63 | 25.33 |
| 11 | 0.64 | 0.62 | 29.55 | 23.58 |
| 12 | 0.62 | 0.59 | 29.76 | 23.92 |
| 13 | 0.89 | 0.88 | 19.10 | 9.85 |
| 14 | 0.75 | 0.73 | 34.69 | 32.49 |
| 15 | 0.61 | 0.59 | 32.84 | 29.11 |
| 16 | 0.65 | 0.62 | 26.72 | 19.28 |
| 17 | 0.62 | 0.59 | 19.43 | 10.19 |
| 18 | 0.93 | 0.93 | 38.02 | 39.03 |
| 19 | 0.56 | 0.53 | 37.71 | 38.40 |
| 20 | 0.37 | 0.32 | 45.76 | 56.54 |
| 21 | 0.78 | 0.76 | 30.23 | 24.68 |
| 22 | 0.79 | 0.77 | 23.75 | 15.24 |
| 23 | 0.71 | 0.68 | 17.69 | 8.44 |
| 24 | 0.71 | 0.69 | 45.93 | 56.97 |
| 25 | 0.67 | 0.64 | 18.10 | 8.85 |
TRANSFER CONDITIONS.
The same reordering and matching procedures were used to compare the transfer performance with the defined chunks. Table 1 lists the results with “Y” representing transfer of the chunking pattern and “N” representing no transfer. Thirteen of 25 participants did not keep the same pattern of chunking when they changed the response effector. Seven participants appeared to have a consistent chunking pattern regardless of the motor response effector, and the remaining five participants showed partial transfer. We grouped participants into those who showed transfer (including partial transfer) and those that did not and compared mean differences in chunk length and the block at which participants started to show their final chunking pattern (i.e., how efficiently they formed chunks). We found a significant group effect (t(23) = 2.43, P < 0.05) for how fast participants developed chunks (Fig. 3F), supporting that participants who developed their chunking pattern earlier maintained the same pattern when transferring to a new response effector.
Visuospatial working memory
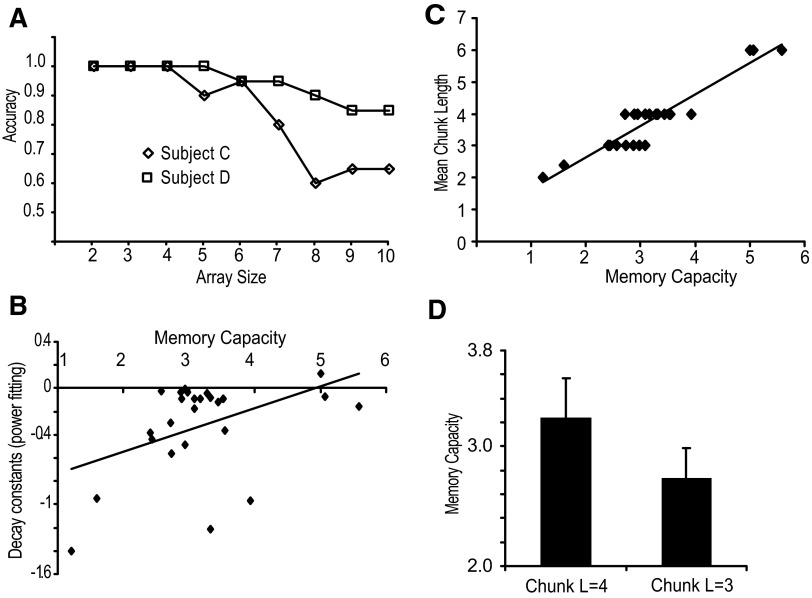
Consistent with previous reports (Luck and Vogel 1997; Vogel and Machizawa 2004), participants showed high accuracy for arrays of two to four items. Figure 4A shows two exemplars: subject C had higher visuospatial working memory capacity and subject D had lower visuospatial working memory capacity. In general, as the array size increased, the accuracy tended to decrease (slope, P < 0.05).
FIG. 4.
A: the accuracy data for each array size of the visuospatial working memory task are plotted for 2 representative participants. B: correlation between the decay constants for step 1 and working memory capacity (K). C: correlation between working memory capacity (K) and mean chunk length. D: the visuospatial working memory capacity in 3- and 4-chunk length groups.
Continuous tapping task
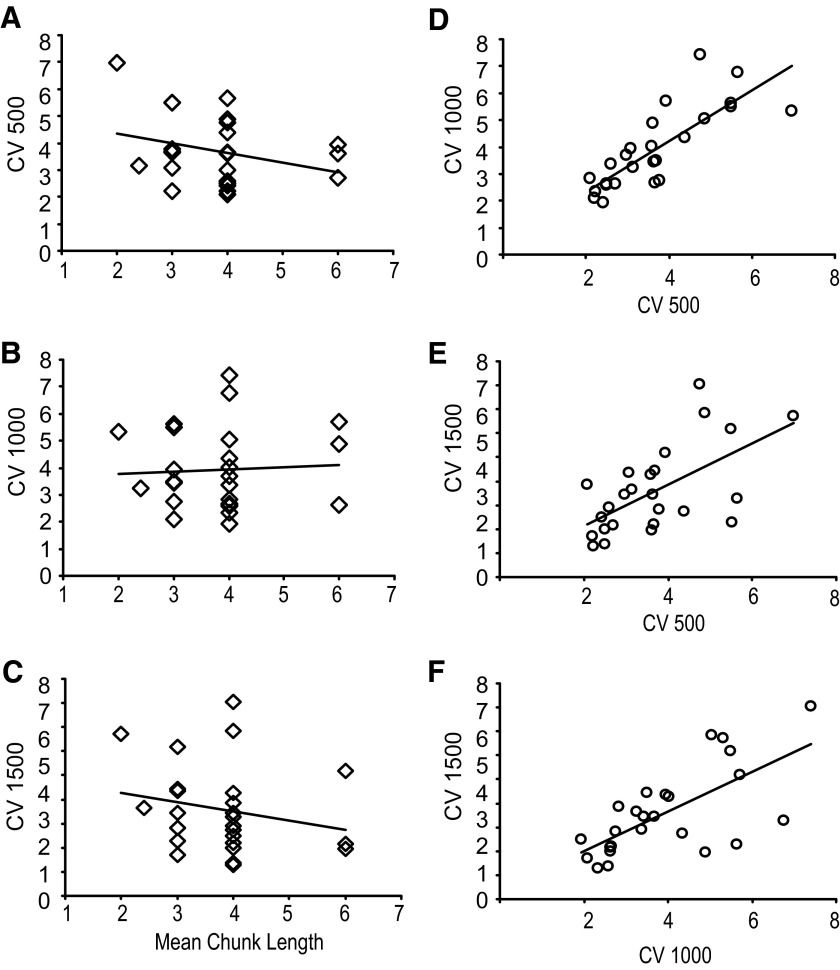
The mean of the CV was 3.7 for the 500-ms interval, 3.9 for the 1,000-ms interval, and 3.6 for the 1500-ms interval condition. The lack of a condition effect (F(2,73) = 1.43, P = 0.25) suggests that participants performed these timing tasks quite consistently across the different conditions. Moreover, high correlations between the CV across the three conditions (R = 0.80, P < 0.01; R = 0.61, P < 0.01; and R = 0.68, P < 0.01 for CV 500 and CV 1,000; CV 500 and CV 1,500; CV 1,000 and CV 1,500 respectively; Fig. 5, D–F) shows that individuals who showed high temporal consistency in one duration also performed well on the other interval conditions.
FIG. 5.
Scatterplots showing the relationship between working memory capacity and CV 500 (A), CV 1,000 (B), and CV 1,500 (C). Scatterplots among the 3 timing intervals: CV 500 vs. CV 1,000 (D), CV 500 vs. CV 1,500 (E), and CV 1,000 vs. CV 1,500 (F).
Relationships among visuospatial working memory, temporal consistency, and sequence learning measures
We first determined whether individual differences in visuospatial working memory capacity and/or temporal control relate to any of the sequence learning rates. Results showed that visuospatial memory capacity was significantly correlated with the decay constants within step 1 (R = 0.46, P < 0.05; Fig. 4B), such that greater memory capacity was associated with faster learning during step 1. No correlations were found between visuospatial working memory capacity and the decay constants for steps 2, 3, and 4. In addition, no correlations were found between any of the timing measures and the decay constants for any sequence learning steps.
In this study, we were particularly interested in examining whether individual differences in visuospatial working memory capacity and/or temporal control explained the chunking patterns that participants formed during motor sequence learning. Figure 4C depicts the scatterplot for memory capacity (K) and mean chunk length across individuals. We found a significant positive correlation (R = 0.78, P < 0.01) between these two, indicating that visuospatial working memory capacity predicts the temporal pattern of the acquired sequence. That is, participants with low working memory capacity formed shorter chunks, whereas high-capacity participants had relatively longer chunks. To further examine whether the correlation between chunk length and working memory was driven by the 20% of the participants with chunk lengths either higher than four or lower than three, we did an independent t-test to compare the working memory differences between the three-chunk and four-chunk groups. We found a significant difference (t = 4.32, P < 0.01; Fig. 4D), further suggesting that visuospatial working memory capacity predicts the temporal pattern of the acquired sequence. No correlations were found in any other combinations between the temporal measures (Fig. 3, A–C), memory capacity, chunk length, and overall response time (all P > 0.05). The three-chunk and four-chunk groups did not differ on any of the timing measures (all P > 0.05). There was a trend for a correlation between memory capacity and overall sequence response time (R = −0.33, P = 0.10).
We performed a multiple linear regression analysis (stepwise procedure) on the temporal measures (CV 500, CV 1,000, CV 1,500) and memory capacity with chunk length as the dependent measure. We found that only the memory capacity was a significant predictor of chunk length (t = 6.02, P < 0.01)). Adding the temporal measures did not significantly change the model fit (all P > 0.05).
To test whether the relationship between chunk length and memory capacity was maintained at transfer, we performed an additional correlation analysis on the transfer data and found a significant positive correlation (R = 0.91, P < 0.01) between chunk length and memory capacity again.
DISCUSSION
Although multiple studies have suggested that cognitive processes such as visuospatial working memory and temporal processing contribute to the organization of motor sequences (cf. Ashe et al. 2006), behavioral evidence has been lacking. Using an individual differences approach, we found a positive correlation between an individual's visuospatial working memory capacity, the rate of early learning, and the length of chunks that participants formed during explicit motor sequence learning. Participants with lower visuospatial working memory capacity had shorter chunk lengths and a slower rate of learning, whereas participants with higher working memory capacity had longer chunk lengths and a faster learning rate. Thus this study documents a link between visuospatial working memory and explicit motor sequence learning, and provides support for previous proposals that the dorsolateral prefrontal cortex is recruited during sequence learning because of the engagement of working memory processes (Pascual-Leone et al. 1996; Robertson et al. 2001).
This finding is consistent with the model of Verwey (1996, 2001), which hypothesizes a close relationship between working memory capacity and sequence learning. This model proposes that participants rely on a cognitive processor, which depends on working memory to allow a certain number of sequence elements (i.e., a chunk) to be programmed in advance of execution. At the same time, a motor processor is running in parallel to execute the actions so that the entire sequence can be performed efficiently.
It is interesting that most of our participants (20/25) chunked together three or four elements for a learned sequence. Such a temporal arrangement indicates that most participants can hold three or four items in short-term working memory when learning a motor sequence. The mean chunk length reported here was shorter than that reported by Kennerley et al. (2004). In their experiment, participants were asked to perform three blocks of 20 memory-guided trials, whereas our participants performed 30 trials of training with visual cues and 30 trials without cues. The longer training period in the study of Kennerley et al. (2004) may have allowed participants to induce a hierarchical “super-chunk” strategy (Ericsson et al. 1980;Rosenbaum et al. 2007), leading to longer chunks.
We were somewhat surprised to find that the pattern of motor chunks was not always transferable; according to Verwey's model, motor chunks should not be context specific. That is, regardless of the response effector, the same chunking pattern should be observed. What we found was that not all of the participants showed transfer of the chunking pattern, and it took participants time to develop a stable chunking pattern. Participants who formed their chunking patterns earlier in the experiment exhibited better transfer of the pattern to new response effectors. This suggests that chunking patterns are initially effector dependent and become more abstractly represented with additional practice. It remains to be seen whether individuals exhibit comparable chunking patterns for multiple sequences, because we only tested participants on one sequence in this study.
We also predicted that temporal processes would contribute to the organization of acquired motor sequences. In contrast to this hypothesis, we did not find a correlation between individual differences in temporal control and the sequence chunking patterns. Similar to other studies of timing, we did find correlations across conditions within an explicit timing task (Robertson et al. 1999). The lack of relationship between the timing conditions and sequence chunk length suggests that the generalized explicit timing mechanism identified by Ivry and Hazeltine (1995) does not play a role in motor sequence learning. It may be that temporal ordering of sequence elements reflects yet another division of temporal processing, in addition to the explicit and emergent timing processes that have already been identified (Merchant et al. 2008; Robertson et al. 1999; Spencer et al. 2003; Zelaznik et al. 2002).
Other potential limitations that might have contributed to a lack of correlation between the temporal measures and the motor sequence chunk length include the sample size and the sensitivity of the temporal measures. The fact that we found strong correlations across participants on the three temporal conditions argues against these criticisms, however. One might also question whether the significant correlation between the motor sequence chunk length and the visuospatial working memory capacity arose simply because of the perceptual similarity between the two tasks; both tasks relied on color and/or location discrimination for their performance. We think it unlikely that this is the major shared component of the two tasks contributing to the correlation, however, particularly because the sequence chunk length was measured when participants produced the sequence from memory as opposed to when it was visually cued. Regardless, our finding that timing did not correlate with the chunking pattern indicates the specificity of the visuospatial working memory correlation; it is not likely that this effect was simply caused by differing motivation levels across participants.
One may also question the limited range of the chunking data because most of our participants (20/25) had chunk lengths of three or four. To ensure that the correlation between chunk length and visuospatial working memory was not driven by 20% of the data set, we performed an additional analysis after removing those participants whose chunk length was longer than four or shorter than three. A significant correlation was still found between chunk length and visuospatial working memory, but not the timing measures, suggesting the robustness of our main findings.
There are limitations of the current approach that should be investigated in future studies. For example, it remains to be seen whether the pattern of chunking, or the correlation between chunks and memory capacity, would vary with sequence structure. Future studies using different structures of sequences would be helpful to answer this question. For the visuospatial working memory test, we do not know whether participants remember the color or the position of stimuli or both. Thus it remains an open question whether different domains of working memory (spatial, visual, verbal, etc.) might make differential contributions to sequence learning. In addition, we studied explicit motor sequence learning in this study. It may be that this relationship is weaker or even nonexistent for implicit sequence learning.
We found that the mean value for chunk length and visuospatial working memory capacity was between three and four. However, Ericsson et al. (1980) reported a case where a participant with average memory abilities increased his memory span from 7 to 79 digits. This individual learned to group chunks of digits together to form “supergroups,” which allowed him to dramatically increase his digit span. Recently, Jaeggi et al. (2008) presented evidence that training on a demanding working memory task can transfer to measures of reasoning and problem solving. Our study suggests that such cognitive training may also benefit the performance of complex motor skills that engage working memory, including learning new sequences of action.
GRANTS
This work was supported by National Institute of Aging Grant R01-AG-024106 to R. D. Seidler.
Acknowledgments
We thank P. Reuter-Lorenz for helpful comments on an earlier draft of this work.
REFERENCES
- Ashe et al. 2006.Ashe J, Lungu OV, Basford AT, Lu X. Cortical control of motor sequences. Curr Opin Neurobiol 16: 213–221, 2006. [DOI] [PubMed] [Google Scholar]
- Baddeley et al. 1986.Baddeley A, Logie R, Bressi S, Dellasala S, Spinnler H. Dementia and working memory. Q J Exp Psychol Hum Exp Psychol 38: 603–618, 1986. [DOI] [PubMed] [Google Scholar]
- Beilock and Carr 2005.Beilock SL, Carr TH. When high-powered people fail - working memory and “choking under pressure” in math. Psychol Sci 16: 101–105, 2005. [DOI] [PubMed] [Google Scholar]
- Boyer et al. 2005.Boyer M, Destrebecqz A, Cleeremans A. Processing abstract sequence structure: learning without knowing, or knowing without learning? Psychol Res 69: 383–398, 2005. [DOI] [PubMed] [Google Scholar]
- Brown and Robertson 2007.Brown RM, Robertson EM. Inducing motor skill improvements with a declarative task. Nat Neurosci 10: 148–149, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen et al. 1990.Cohen A, Ivry RI, Keele SW. Attention and structure in sequence learning. J Exp Psychol Learn Mem Cogn 16: 17–30, 1990. [Google Scholar]
- Cowan 2001.Cowan N The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci 24: 87–138, 2001. [DOI] [PubMed] [Google Scholar]
- Decaro et al. 2008.Decaro MS, Thomas RD, Beilock SL. Individual differences in category learning: sometimes less working memory capacity is better than more. Cognition 107: 284–294, 2008. [DOI] [PubMed] [Google Scholar]
- Doyon et al. 2002.Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA 99: 1017–1022, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson et al. 1980.Ericsson KA, Chase WG, Faloon S. Acquisition of a memory skill. Science 208: 1181–1182, 1980. [DOI] [PubMed] [Google Scholar]
- Henry and Rogers 1960.Henry FM, Rogers DE. Increased response latency for complicated movements and a memory drum theory of neuromotor reaction. Res Q 31: 448–458, 1960. [Google Scholar]
- Howard et al. 2004.Howard DV, Howard JH Jr, Japikse K, DiYanni C, Thompson A, Somberg R. Implicit sequence learning: effects of level of structure, adult age, and extended practice. Psychol Aging 19: 79–92, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry and Hazeltine 1995.Ivry RB, Hazeltine RE. Perception and production of temporal intervals across a range of durations - evidence for a common timing mechanism. J Exp Psychol Hum Percept Perform 21: 3–18, 1995. [DOI] [PubMed] [Google Scholar]
- Jaeggi et al. 2008.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci USA 105: 6829–6833, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides et al. 2008.Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annu Rev Psychol 59: 193–224, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides et al. 1993.Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. Spatial working-memory in humans as revealed by PET. Nature 363: 623–625, 1993. [DOI] [PubMed] [Google Scholar]
- Kennerley et al. 2004.Kennerley SW, Sakai K, Rushworth MF. Organization of action sequences and the role of the pre-SMA. J Neurophysiol 91: 978–993, 2004. [DOI] [PubMed] [Google Scholar]
- Luck and Vogel 1997.Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature 390: 279–281, 1997. [DOI] [PubMed] [Google Scholar]
- Maquet et al. 1996.Maquet P, Lejeune H, Pouthas V, Bonnet M, Casini L, Macar F, TimsitBerthier M, Vidal F, Ferrara A, Degueldre C, Quaglia L, Delfiore G, Luxen A, Woods R, Mazziotta JC, Comar D. Brain activation induced by estimation of duration: a PET study. Neuroimage 3: 119–126, 1996. [DOI] [PubMed] [Google Scholar]
- Mckone 1995.Mckone E Short-term implicit memory for words and nonwords. J Exp Psychol Learn Mem Cogn 21: 1108–1126, 1995. [Google Scholar]
- Merchant et al. 2008.Merchant H, Zarco W, Bartolo R, Prado L. The context of temporal processing is represented in the multidimensional relationships between timing tasks. PLoS ONE 3: e3169, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake and Shah 1999.Miyake A, Shah P. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. New York: Cambridge, 1999.
- Oldfield 1971.Oldfield RC Assessment and analysis of handedness - Edinburgh inventory. Neuropsychologia 9: 97, 1971. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone et al. 1996.Pascual-Leone A, Wassermann EM, Grafman J, Hallett M. The role of the dorsolateral prefrontal cortex in implicit procedural learning. Exp Brain Res 107: 479–485, 1996. [DOI] [PubMed] [Google Scholar]
- Reber 1993.Reber AS Implicit Learning and Tacit Knowledge: An Essay on the Cognitive Unconscious. New York: Oxford, 1993.
- Robertson et al. 2001.Robertson EM, Tormos JM, Maeda F, Pascual-Leone A. The role of the dorsolateral prefrontal cortex during sequence learning is specific for spatial information. Cereb Cortex 11: 628–635, 2001. [DOI] [PubMed] [Google Scholar]
- Robertson et al. 1999.Robertson SD, Zelaznik HN, Lantero DA, Bojczyk KG, Spencer RM, Doffin JG, Schneidt T. Correlations for timing consistency among tapping and drawing tasks: evidence against a single timing process for motor control. J Exp Psychol Hum Percept Perform 25: 1316–1330, 1999. [DOI] [PubMed] [Google Scholar]
- Rosenbaum et al. 2007.Rosenbaum DA, Cohen RG, Jax SA, Weiss DJ, van der Wel R. The problem of serial order in behavior: Lashley's legacy. Hum Mov Sci 26: 525–554, 2007. [DOI] [PubMed] [Google Scholar]
- Shea et al. 2006.Shea CH, Park JH, Braden HW. Age-related effects in sequential motor learning. Phys Ther 86: 478–488, 2006. [PubMed] [Google Scholar]
- Shin and Ivry 2002.Shin JC, Ivry RB. Concurrent learning of temporal and spatial sequences. J Exp Psychol Learn Mem Cogn 28: 445–457, 2002. [DOI] [PubMed] [Google Scholar]
- Shin and Ivry 2003.Shin JC, Ivry RB. Spatial and temporal sequence learning in patients with Parkinson's disease or cerebellar lesions. J Cogn Neurosci 15: 1232–1243, 2003. [DOI] [PubMed] [Google Scholar]
- Smith et al. 2003.Smith A, Taylor E, Lidzba K, Rubia K. A right hemispheric frontocerebellar network for time discrimination of several hundreds of milliseconds. Neuroimage 20: 344–350, 2003. [DOI] [PubMed] [Google Scholar]
- Spencer et al. 2003.Spencer RMC, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science 300: 1437–1439, 2003. [DOI] [PubMed] [Google Scholar]
- Sternberg et al. 1978.Sternberg S, Monsell S, Knoll RL, Wright CE.The latency and duration of rapid movement sequence: comparison of speech and typewriting. In: Information Processing in Motor Control and Learning, edited by Stelmach GE. New York: Academic Press, 1978, p. 117–152.
- Turcotte et al. 2005.Turcotte J, Gagnon S, Poirier M. The effect of old age on the learning of supraspan sequences. Psychol Aging 20: 251–260, 2005. [DOI] [PubMed] [Google Scholar]
- Verwey 1996.Verwey WB Buffer loading and chunking in sequential keypressing. J Exp Psychol Hum Percept Perform 22: 544–562, 1996. [Google Scholar]
- Verwey 2001.Verwey WB Concatenating familiar movement sequences: the versatile cognitive processor. Acta Psychol (Amst) 106: 69–95, 2001. [DOI] [PubMed] [Google Scholar]
- Verwey and Eikelboom 2003.Verwey WB, Eikelboom T. Evidence for lasting sequence segmentation in the discrete sequence-production task. J Motor Behav 35: 171–181, 2003. [DOI] [PubMed] [Google Scholar]
- Vogel and Machizawa 2004.Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature 428: 748–751, 2004. [DOI] [PubMed] [Google Scholar]
- Wing and Kristofferson 1973.Wing A, Kristofferson A. Response delays and the timing of discrete motor response. Percept Psychophysiol 14: 5–12, 1973. [Google Scholar]
- Zelaznik et al. 2002.Zelaznik HN, Spencer RMC, Ivry RB. Dissociation of explicit and implicit timing in repetitive tapping and drawing movements. J Exp Psychol Hum Percept Perform 28: 575–588, 2002. [DOI] [PubMed] [Google Scholar]