Abstract
Recently, adaptively timed, anticipatory changes in hemodynamic responses, independent of neural activity, were described in primate primary visual cortex. Task-related properties of these responses point to a possible link between regional cerebral microcirculation and dopaminergic signaling. In this report, this link is elaborated on the basis of known physiological data and further experiments are proposed to test the possible role of dopamine in task-dependent, “on-demand” allocation of metabolic resources.
Animals are by nature opportunistic. An unexpected encounter with a reward-predicting stimulus leads to a fast redirection of behavior. As with the behavior, at the neural level, it is conceivable that this encounter may also yield an anticipation of an increased demand for neural processing necessary to facilitate the (potential) task in service of collecting the predicted reward. However, in bipeds, cerebral perfusion is maintained constant over a wide range of systemic pressures (termed “cerebral autoregulation”). Autoregulation is critical to neurophysiologic health since too little flow could cause ischemia, whereas too much could raise intracranial pressure. Therefore an “on-demand” allocation of regional blood volume without changing the overall perfusion pressure is likely to be among the key biological abilities. Indeed, in a recent paper, Sirotin and Das (2009) described a new, trial-locked hemodynamic signal in visual cortex in response to anticipation of expected tasks. In their experimental paradigm, two monkeys are trained to perform a “dark-room fixation task,” in which the visual input was minimized compared with standard, periodic, visual tasks while the trial timing was preserved. After training, Sirotin and Das (2009) observed a robust hemodynamic signal (indicative of increased regional blood volume and thus oxygenation) before the onset of the next trial, comparable in magnitude with the stimulus-evoked signal, and tightly linked to trial onset. This hemodynamic signal arose despite the virtual absence of a visual stimulus and was not related to any local neural activity, as confirmed by simultaneous recordings of multiunit spiking activity (although there appeared to be a nonsignificant modulation of the high-frequency local field potential with the task structure). To verify the timing properties of these hemodynamic signals, the authors manipulated the temporal course of the fixation task so that the intertrial interval switched unexpectedly between “short” and “long” trials every 10–20 trials. Interestingly, during such reversals of temporal contingency, anticipatory hemodynamic responses entrained to these slow and fast “rhythms” to conform to the new temporal contingency, although the “switch” in hemodynamic response was slower that the switch in the monkey's behavior. The authors concluded their report with the remark that the mechanism driving this adaptively timed anticipatory signal remains to be elucidated. Both the authors and a recent commentary on the study (Leopold 2009) discussed the significance of this finding mainly from the point of view of imaging methods, in particular, functional magnetic resonance imaging. However, as Sirotin and Das (2009) noted, it is important to explicate the physiologic substrate of this anticipatory behavior.
Another conspicuous population of neurons that exhibit adaptively timed anticipatory responses (among others) is the dopaminergic neurons of the substantia nigra pars compacta and ventral tegmental area. These neurons are part of an adaptive system that uses learned expectations to filter reward-related signals. In particular, their firing patterns are related to learned cues that predict reward (Schultz 2007). The genesis of the dopamine responses has been investigated by numerous researchers and the effects of anticipatory increases in dopamine release on target sites have been discussed only in terms of the effects on learning (and partly on behavioral performance). Thus although a wealth of data is available on the role of dopamine on learning, performance, and cognitive processes in general, its role in regulation of regional blood flow and oxygenation has attracted very little attention from neuroscientists. Historically, it is known that dopamine has both vasodepressor and vasopressor effects on vasculature (Goldberg 1972). More recently, it has been shown that the dynamics of striatal dopamine release, induced by stimulation of median nerve, correlates well with regional cerebral blood volume changes (Chen et al. 2008). Furthermore, this relation appears to be a direct effect of dopamine on the microvasculature, mediated partially through D1/D5 receptors (increasing regional blood volume) and through astroglial D3 receptors (reducing regional blood volume) (Choi et al. 2006). This role of dopamine is also indirectly supported by studies in animal models of Parkinson's disease (marked by a significant degeneration of substantia nigra pars compacta dopaminergic neurons) where a strong relation between the loss of dopamine and changes in regional blood volume has been documented (Brownell et al. 2003). In fact, the effects of dopamine on regulation of cerebral blood flow, perfusion pressure, and oxygenation is well studied in pediatric literature (see, e.g., Tyszczuk et al. 1998). Therefore it should not come as a surprise that anticipatory dopaminergic responses may elicit regional hemodynamic changes in the target structures.
Indeed, although most of the dopaminergic innervation is directed to the frontal and limbic cortical areas, a specific dopaminergic innervation of primary visual cortex that arises from the ventral tegmental area is described in several species, including rats and humans (Phillipson et al. 1987). Furthermore, it appears that the dopamine released in primary visual cortex acts via D1 receptors (Smiley et al. 1994), increasing regional blood volume (Choi et al. 2006). These considerations do not eliminate the possibility that anticipatory hemodynamic responses may be brought about by different neurotransmitter systems that may influence regional blood flow: norepinephrine, acetylcholine, and serotonin (among others; see, e.g., Attwell and Iadecola 2002). Nevertheless, although novel stimuli and reversals in task contingency may induce transient changes in norepinephrine release, these changes disappear once the (new) task contingency is established (Dalley et al. 2001) [in contrast to the observation of Sirotin and Das (2009) that hemodynamic responses entrained to rhythms conform to the new temporal contingency even after reversal]; activation of basal forebrain cholinergic pathway is mostly driven by visual stimuli, rather than being an anticipatory response (Laplante et al. 2005); and serotonin reduces regional cerebral blood flow, at least in rats (Grome and Harper 1983). Furthermore, additional indirect support for involvement of dopamine is provided by the recent report that neural responses in primary visual cortex can accurately predict reward timing (Shuler and Bear 2006) and, by entrainment of hemodynamic responses to reversals in temporal contingency (Sirotin and Das 2009; described earlier), two functions mostly attributed to dopaminergic responses (Schultz 2007). Therefore given the anticipatory responses of ventral tegmental area dopamine cells, the most parsimonious explanation of the findings of Sirotin and Das (2009) appears to be the dopaminergic innervation of primary visual cortex.
Two major implications should be noted. First, it may be necessary to reinterpret the results from functional imaging of the basal ganglia, particularly of the striatum, a major recipient of dopaminergic afferents from substantia nigra pars compacta. In such imaging studies, several research groups attributed task-related activations of the striatum to striatal neurons. If dopaminergic signaling links directly to striatal hemodynamics, the observed activation may be secondary to task-dependent dopamine release, rather than (or perhaps in addition to) striatal cell responses. In fact, a close relation between dopamine release and local blood oxygen level–dependent signal has recently been documented in the ventral striatum (Knutson and Gibbs 2007). Because this point was already discussed by Sirotin and Das (2009) and Leopold (2009), it will not be elaborated here. A second implication of the dopaminergic account of the observation of Sirotin and Das (2009) is that dopaminergic signaling plays a heretofore overlooked role that is nevertheless consistent with the optimization function of its role in reinforcement learning.
As already mentioned, the firing patterns of midbrain dopaminergic neurons are related to learned cues that predict reward. Also important, the magnitude of the dopamine release in response to such cues is shown to be proportional to the expected reward value and its magnitude during the delay period between the predictive cue and predicted (rewarding) event is proportional to the uncertainty of the expected event (Schultz 2007). Furthermore, if the expected (rewarding) event is omitted, dopamine cells respond with a pause in their firing rate (Schultz 2007). Such a multitude of response properties, coupled to different phases of the task, makes these neurons a suitable candidate for task-dependent regulation of regional blood volume and thus oxygenation (Fig. 1). For example, a selective increase in dopamine release in response to an anticipated stimulus would facilitate the blood flow to the relevant region to meet the demands of (expected) activity. Similarly, a gradual increase in the dopamine release during a delay period can ensure sustained regional blood flow during the delay period, when a sustained activation (therefore sustained metabolic demand) of several cortical regions is anticipated. An example is persistent activation of prefrontal cortical neurons during delay or trace conditioning paradigms, commonly associated with working memory processes. Remarkably, when the expected event fails to occur, pauses in dopamine release may ensure an ad hoc reduction in regional blood flow, perhaps in an attempt to facilitate redirection of blood volume to other regions, without the requirement of changing overall cerebral perfusion. Therefore regulation of regional microcirculation by dopaminergic signaling may constitute an important, but overlooked, function of dopaminergic innervation of the brain.
FIG. 1.
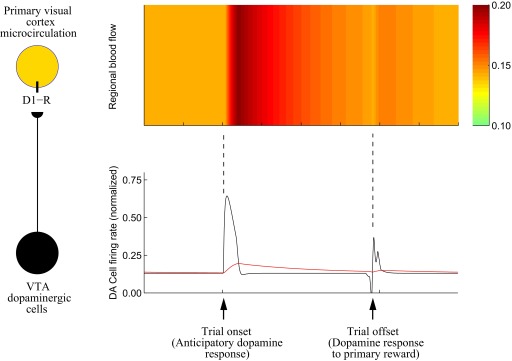
Task-dependent regulation of regional blood volume by dopaminergic neurons. Left panel: a specific dopaminergic projection from ventral tegmental area (VTA) modulates regional blood flow in primary visual cortex via D1 dopamine receptors (D1-R). Right panel: illustration of the hypothesized mechanism. Bottom panel shows the phasic responses of dopaminergic cells during a typical reinforcement learning paradigm (black trace) and corresponding dopamine release (red trace) after learning [simulated using the model of Tan and Bullock (2008)]. Top panel shows hypothesized modulation of regional blood flow by dopamine released.
Nevertheless, although the study by Sirotin and Das (2009) provides a first hint toward this function, further experiments are required to prove or refute this hypothesis. For example, a simple test would be to make use of the fact that the magnitude of dopamine release depends on the expected value of reward. In a typical reinforcement learning paradigm, wherein both the intensity of the predictive cue and the animal's behavior are minimized [similar to the “dark-room fixation task,” but minimizing the feature(s) of the stimulus that are relevant to the task deployed], a relation between the hemodynamic signals and (expected) dopamine release (bursts vs. pauses) would provide an evidence (although indirectly) for the linkage hypothesis. The aforementioned study by Knutson and Gibbs (2007) provides such evidence for ventral striatum, but for other areas a possible link between task-dependent variations in regional cerebral microcirculation and anticipatory dopamine signals awaits verification.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-081693.
Acknowledgments
I thank Prof. Daniel Bullock for a critical reading of the manuscript.
REFERENCES
- Attwell 2002.Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci 25: 621–625, 2002. [DOI] [PubMed] [Google Scholar]
- Brownell 2003.Brownell AL, Canales K, Chen YI, Jenkins BG, Owen C, Livni E, Yu M, Cicchetti F, Sanchez-Pernaute R, Isacson O. Mapping of brain function after MPTP-induced neurotoxicity in a primate Parkinson's disease model. Neuroimage 20: 1064–1075, 2003. [DOI] [PubMed] [Google Scholar]
- Chen 2008.Chen YI, Ren J, Wang FN, Xu H, Mandeville JB, Kim Y, Rosen BR, Jenkins BG, Hui KK, Kwong KK. Inhibition of stimulated dopamine release and hemodynamic response in the brain through electrical stimulation of rat forepaw. Neurosci Lett 431: 231–235, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi 2006.Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage 30: 700–712, 2006. [DOI] [PubMed] [Google Scholar]
- Dalley 2001.Dalley JW, McGaughy J, O'Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J Neurosci 21: 4908–4914, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg 1972.Goldberg LI Cardiovascular and renal actions of dopamine: potential clinical applications. Pharmacol Rev 24: 1–29, 1972. [PubMed] [Google Scholar]
- Grome 1983.Grome JJ, Harper AM. The effects of serotonin on local cerebral blood flow. J Cereb Blood Flow Metab 3: 71–77, 1983. [DOI] [PubMed] [Google Scholar]
- Knutson 2007.Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 191: 813–822, 2007. [DOI] [PubMed] [Google Scholar]
- Laplante 2005.Laplante F, Morin Y, Quirion R, Vaucher E. Acetylcholine release is elicited in the visual cortex, but not in the prefrontal cortex, by patterned visual stimulation: a dual in vivo microdialysis study with functional correlates in the rat brain. Neuroscience 132: 501–510, 2005. [DOI] [PubMed] [Google Scholar]
- Leopold 2009.Leopold DA Neuroscience: pre-emptive blood flow. Nature 457: 387–388, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson 1987.Phillipson OT, Kilpatrick IC, Jones MW. Dopaminergic innervation of the primary visual cortex in the rat, and some correlations with human cortex. Brain Res Bull 18: 621–633, 1987. [DOI] [PubMed] [Google Scholar]
- Schultz 2007.Schultz W Multiple dopamine functions at different time courses. Annu Rev Neurosci 30: 259–288, 2007. [DOI] [PubMed] [Google Scholar]
- Shuler 2006.Shuler MG, Bear MF. Reward timing in the primary visual cortex. Science 311: 1606–1609, 2006. [DOI] [PubMed] [Google Scholar]
- Sirotin 2009.Sirotin YB, Das A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature 457: 475–479, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley 1994.Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA 91: 5720–5724, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan 2008.Tan CO, Bullock D. A local circuit model of learned striatal and dopamine cell responses under probabilistic schedules of reward. J Neurosci 28: 10062–10074, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyszczuk 1998.Tyszczuk L, Meek J, Elwell C, Wyatt JS. Cerebral blood flow is independent of mean arterial blood pressure in preterm infants undergoing intensive care. Pediatrics 102: 337–341, 1998. [DOI] [PubMed] [Google Scholar]


