Abstract
Single muscle fibers with multiple axonal endplates (multiply innervated fibers) are normally present in adult extraocular muscles (EOMs), while most other mammalian skeletal muscles contain fibers with a single myoneural junction. Recent findings by others led us to investigate for the presence of polyneuronal innervation (innervation of a single muscle fiber by >1 motoneuron) in the inferior oblique (IO) muscle of pentobarbital anesthetized cats. The IO muscle nerve branches, as they coursed through the orbit, were further divided for independent or simultaneous electrical stimulation with bipolar electrodes. Four of five established tests for polyneuronal innervation gave positive results. The sum of the twitch (1) and tetanic (2) tensions in response to individual nerve branch stimulation was greater than that for simultaneous (whole) nerve stimulation. The summed electromyographic (EMG) responses (3) gave a similar positive result. The result for crossed tetanic potentiation (4) was negative for polyneuronal innervation while the crossed fatigue (5) test was positive. These results are consistent with recent studies. That the EOMs exhibit polyneuronal innervation further explains the eye-movement system's functional integrity during some neuromuscular disorders as well as its ability to operate with precision after the loss of numerous motoneurons.
INTRODUCTION
In contrast to most other mammalian skeletal muscles in which individual muscle fibers have a single myoneural junction, muscle fibers with multiple endplates (multiply innervated fibers) are normally present in adult extraocular muscles (EOM) (Bach-y-Rita and Ito 1966; Harrison et al. 2007; Hess and Pillar 1963; Lennerstrand 1972; Peachey 1971). It is still not known, however, if these multiple endings on EOM muscle fibers represent axonal inputs from more than one, or only one, motoneuron. If a single muscle fiber is innervated by more than one motoneuron, that would be classified as polyneuronal innervation.
The multiply innervated fibers in the orbital layer of EOM exhibited different electrical properties in the end-plate zone of the muscle as opposed to areas distal to that zone (Jacoby et al. 1989). In addition, two types of nerve endings were identified, morphologically and histochemically, along the length of these fibers in rat and monkey (Jacoby et al. 1989; Pachter 1982 1984). Therefore it would seem likely that the multiply innervated fibers receive polyneuronal (or at least dual) innervation.
Prior to these studies, Bach-y-Rita and Lennerstrand (1975) tested for the possibility of polyneuronal innervation in adult cat lateral rectus muscle. They were not able to demonstrate it physiologically. Their approach used recordings of muscle contraction during stimulation of only two, of several, divisions of the abducens nerve innervating the lateral rectus muscle. They used a ventral approach (through the neck) to expose the VIth nerve at its exit from the brain stem. (While many nerve rootlets are present at this anatomical location, they routinely stimulated only 2 of those several bundles of axons.) The presence of polyneuronal innervation is suggested if the sum of tensions produced by individual stimulation of each nerve root is greater than the tension produced by simultaneous stimulation of both nerve divisions. This approach relies on the muscle fibers' property that any muscle fiber supplied by both nerves (simultaneous stimulation) cannot contract more strongly than when separately stimulated. However, the “whole muscle” twitch tension size in this study was only ∼4 g compared with 10–15 g usually seen in cat lateral rectus muscle (Barmack et al. 1971; Dimitrova et al. 2002; Goldberg and Shall 1997). Judged by the small twitch tension amplitude, a possible source of error that could have lead to negative results would be a reduced stimulation current intensity (to avoid current spread). Using submaximal nerve stimulation would not activate the whole nerve and would greatly reduce the chances of identifying polyneuronal innervation.
The technique itself, applied in earlier studies, has proved to be of accurate value in elucidating the possibility of polyneuronal innervation in other skeletal muscles (Bagust et al. 1973; Brown and Matthews 1960). No consistent evidence for the existence of polyneuronal innervation was obtained by Brown and Matthews (1960) in adult cat hind-limb muscles, but the possibility of its occurrence was not excluded. The same methodological approach gave positive results in kittens ≤2 wk old (Bagust et al. 1973). In addition to developing neuromuscular systems, where polyneuronal innervation is widespread, polyneuronal innervation is also seen during nerve regeneration after injury. This was suggested by Hoffman (1951a,b) and later confirmed by a number of morphological and physiological studies (Gillingwater et al. 2004; Gorio et al. 1983; Guntinas-Lichius et al. 2005; Ito and Kudo 1994; Jansen and Van Essen 1975; Letinsky et al. 1976; McArdle 1975; Rich and Lichtman 1989; Rotshenker and McMahan 1976; Tate and Westerman 1973; Werle and Herrera 1987, 1988, 1991). The nonselectively formed synapses are then, in time, pared back to result in the usual single motor endplates on single muscle fibers (Navarrete and Vrbova 1993).
It is also now known, for EOMs, that there is continuous remodeling of individual myofibers throughout life (McLoon and Wirtschafter 2002; McLoon et al. 2004). Therefore it is reasonable to suggest that other properties (e.g., polyneuronal innervation) seen during development and regeneration would also be retained in normal adult extraocular muscles (McLoon and Wirtschafter 2002; McLoon et al. 2004).
In addition, multiple end-plates exist in some other mammalian muscles and recent electrophysiological evidence strongly suggests polyneuronal innervation of adult human skeletal muscle fibers (Lateva et al. 2002). The approach in the Lateva et al. (2002) study was based on the electromyographic (EMG) potential of individual motor units (found by electrical decomposition) of the EMG activity of brachioradialis muscle. Assuming polyneuronal innervation of a motor unit, the single-unit EMG potential should remain the same regardless of which neuron had discharged to cause the contraction. Also, in the case of simultaneous neuronal discharge close in time, the two components of the motor unit response would collide and one of them then would be blocked in the muscle fiber.
In light of all this recent evidence, the possible existence of polyneuronal innervation in normal adult EOM needed to be revisited. We have employed most of the electrophysiological tests developed by previous experimenters. We searched for a tension and/or EMG excess in the sum of muscle responses to separate versus simultaneous stimulation of the two main nerve branches of cat inferior oblique (IO) muscle. The tension related tests included twitch tension excess, tetanic tension excess, cross-potentiation (posttetanic potentiation of 1 IO nerve branch after tetanic stimulation was delivered to the other branch), and cross-fatigue (decreased tension in 1 branch after fatigue of the other branch). Also, an EMG excess was examined. All but one test (cross-potentiation) gave positive results in relation to polyneuronal innervation.
METHODS
Electrophysiological tests for polyneuronal innervation were performed on eight eye muscle/nerve preparations in adult cats (2.5–3.5 kg) according to procedures and protocols approved by the Animal Care and Use Committee of Virginia Commonwealth University.
Surgical preparation
The cats were initially anesthetized with 45 mg/kg ip sodium pentobarbital. Supplemental doses of sodium pentobarbital were administered intravenously throughout the experiment to maintain deep anesthesia, indicated by the absence of withdrawal reflex to paw pinch. Following topical anesthesia of the larynx with 4% lidocaine, the animals were intubated with a 3- to 5-mm endotracheal tube. A heating pad was used to keep rectal temperature at 38–40°C. Respiratory rate, end-tidal CO2, heart rate, and body temperature were continuously monitored and maintained within normal ranges.
The animal's head was fixed in a Kopf stereotaxic frame and a midline incision from the nose to the posterior neck was made. The superior eyelid and portions of the superior and lateral aspects of the bony orbit were removed to expose the IO muscle and tendon including the insertion on the sclera. The IO muscle/nerve preparation was chosen due to the long, accessible course of the whole nerve from near its origin through the cat orbit. The IO muscle's tendon was looped with 5×0 silk suture to detach the tendon and muscle from the globe. The temporalis muscle was retracted, and both the lateral and superior rectus muscles were detached from the globe to gain access to the optic nerve. The optic nerve and ophthalmic artery were tied off, leaving the blood supply to the IO muscle and nerve intact, and the eye was enucleated to expose the IO nerve as it courses through the orbit. The IO muscle and main IO nerve, including those distal nerve branches entering the middle third of the muscle (endplate zone), were isolated from the surrounding tissues. Similar surgical preparations have been previously used (Nelson et al. 1986; Russell-Mergenthal et al. 1986; Shall and Goldberg 1992; Shall et al. 1995). Additional isolation of the two main IO branches was performed, as described in the following text, to avoid current spread from one IO nerve branch to the other. To keep the muscle/nerve preparation moist and warm during the experiment, it was frequently bathed in warm mineral oil. After all data were collected, each animal was killed with an overdose of sodium pentobarbital delivered intravenously.
Stimulation and recording protocol
A bipolar stainless steel electrode (∼2 mm between poles) was hooked under each IO nerve branch ∼8 mm from their muscle entry. Usually two main natural nerve branches were visible and they are referred to as the lateral and medial branches. IO muscle tension and EMG activity were recorded in response to separate or simultaneous stimulation of these two nerve branches. Simultaneous stimulation of both nerve branches would be equivalent to whole nerve stimulation. Pulses of 0.2-ms duration, 0.5- to 3-mA current intensity and variable frequencies were produced by a programmable pulse generator (AMPI Master-8) and delivered to the stimulating electrodes. The twitch characteristics of the IO muscle and muscle portions innervated by each IO nerve branch were analyzed in response to single pulses delivered at a rate of 1/s (10 stimuli). IO tetanic contractile properties were evaluated in response to constant frequency pulse trains of 200-ms duration using a frequency range from 50 to 220 Hz, delivered at 5-s intervals (3 trials for each frequency). In addition to supramaximal IO nerve stimulation, in several cases submaximal stimulation was tested by an appropriate reduction of the stimulation current intensity until the evoked tension dropped to ∼50% of that evoked by maximal stimulation. In these cases we used submaximal stimulation to determine if the results related to polyneuronal innervation depend on the stimulation current intensity.
The IO muscle suture was attached to a force transducer (MLT050, ADInstruments) and the muscle was set at an optimal length for maximal isometric twitch tension. EMG electrical activity was recorded, simultaneously with muscle tension, using a bipolar electrode consisting of two fine wires (25 μm pole diameter), separated by ∼5 mm, inserted into the belly of the IO muscle (Shall and Goldberg 1995; Shall et al. 1995). One electrode pole was located about 4 mm distal to the central endplate zone and the other pole was ∼9 mm distal to the zone. We wanted to record the electrical response of many IO muscle fibers, but no attempt was made to differentiate responses from the muscle's global and orbital layers as was done in previous studies. The fine wires did not move in the muscle (fixed at isometric tension) and EMG recordings were stable and consistent.
Current spread control
The following measures controlled for the possibility of current spread from the stimulating electrode of one nerve branch to the other nerve branch. 1) Muscle contraction was monitored, and its absolute size was observed on-line. Increasing the intensity of stimulation to one nerve branch resulted in a gradual increase in muscle tension until it reached a plateau. A supramaximal level of stimulation was thus achieved. Further increases of stimulus intensity could lead to current spread to the other (nonstimulated) nerve branch. This current spread was seen as a sudden step increase of evoked tension amplitude. 2) The two “natural” IO nerve branches were further split apart to impose a larger distance between stimulating electrodes and the muscle and thereby avoid direct stimulation of the muscle. One nerve branch (usually lateral) was detached from the whole IO nerve (where it divided from the other branch) and pulled away from the main nerve trunk. Each nerve branch could then be positioned in a separate plastic bath and soaked in mineral oil. This technique for complete physical isolation of the two nerve branches basically eliminated stimulation current spread. 3) Finally, after all other recordings were completed, one nerve branch (usually the lateral) was cut proximal to the muscle and completely removed from the preparation. Muscle responses to stimulation of the remaining branch were compared before and after the cut to assure that none of that response amplitude came from stimulation of the other branch via current spread. Muscle response to stimulation of the remaining IO nerve branch did not differ significantly in comparison with the response recorded just before the cut. Muscle tension in response to 200-Hz supramaximal stimulation of the remaining IO branch was 9.7 ± 1.1 g in comparison with the tension of this branch before the cut (10.4 ± 1.3 g, P > 0.2). The EMG peak-to-peak amplitude was also similar: 1.7 ± 0.5 mV before and 1.6 ± 0.4 mV after the branch was cut (P > 0.2). These insignificant changes represent 4 ± 5% change in tension and 1 ± 9% change in EMG amplitude, showing that eliminating one nerve branch could result in either excess or deficit in muscle tension and EMG activity. This test confirmed that current spread was successfully eliminated during the data collection in this experiment.
Data analysis
The possible existence of polyneuronal innervation in cat IO muscle was explored by five different approaches.
TWITCH AND TETANIC TENSION EXCESS.
The tension elicited by simultaneous stimulation of both IO nerve branches (therefore the whole nerve) was compared with the sum of the tensions evoked by separate stimulation of each nerve branch. For each experimental session, the response of the whole nerve was obtained first, then for each nerve branch, and finally the whole nerve was stimulated again. This was done as a control. No significant differences were found between the first and the control whole nerve tests for both twitch and tetanic stimuli. The whole nerve response was evaluated as a mean of the tension maximal amplitudes of the first and the control tests. The sum of tensions evoked by separate stimulation of the IO branches was calculated by adding the maximal amplitudes of each nerve branch.
CROSS-POTENTIATION.
This test is based on the increase in twitch tension following tetanic stimulation (posttetanic potentiation). In the presence of polyneuronal innervated muscle fibers, an increase in twitch tension of one IO nerve branch could occur after tetanic stimulation is delivered to the other IO nerve branch (cross-potentiation) (Bach-y-Rita and Lennerstrand 1975; Bagust et al. 1973). The twitch tension response (average of 10 trials) to stimulation of one nerve branch was done after the other branch had been subjected to tetanic stimulation consisting of our sequence of 200-ms trains ranging from 50 to 220 Hz (5-s interval between trains), repeated three times. (Fig. 2A illustrates the sequence.)
CROSS-FATIGUE.
This test explored the possibility that fatigue of the muscle fibers in response to stimulation of one nerve branch is present in muscle fibers innervated by the other nerve branch. Fatigue of each IO nerve branch was evaluated by comparing tetanic tensions in response to 200-Hz stimulation frequency of supramaximal current intensity before and after a 2-min fatigue protocol delivered to one of the IO nerve branches. A randomly selected IO nerve branch (data from 4 medial and 3 lateral branches was collected) was presented with 150-Hz frequency, 500-ms train duration, 1 train/s stimulation to fatigue the neuromuscular compartment innervated by this branch.
EMG EXCESS.
The EMG activity of the IO muscle recorded during simultaneous stimulation of both IO nerve branches (whole nerve) was compared with the sum of the EMG activity produced by separate stimulation of each nerve branch (EMG excess similar to the tension excess). The individual EMG signals were zeroed using the mean of 100-ms baseline EMG prior to the stimulus and then aligned by the artifact for summing. In some cases, the EMG signals of the two branches stimulated separately had different latencies. Therefore they were first summed and then the peak-to-peak amplitude of the sum was compared with the whole nerve peak-to-peak amplitude. The first 1 ms of the EMG response was considered as an artifact and was excluded from the analysis.
The results were statistically evaluated using Student's t-test at probability level α = 0.05. The data are presented as means ± SE for the group in both absolute values and percentages. Any excess of the sum of the two IO branches over the whole nerve values was expressed as a positive number.
RESULTS
Tension excess
TWITCH CONTRACTIONS.
The sum of the twitch tensions elicited by each of the two IO nerve branches stimulated separately exceeded the twitch tension elicited by stimulation of the whole IO nerve (simultaneous stimulation of both IO nerve branches). Examples of IO muscle responses to single pulse supramaximal stimulation of each IO nerve branch separately or simultaneously (whole IO nerve stimulation) are given in Fig. 1A in comparison with the algebraic sum of the two IO nerve branches twitch tensions (- - -). Group means ± SE of twitch tensions for each IO nerve branch, the whole IO nerve and the calculated sum of twitch tensions are presented in Fig. 1B (supramaximal stimulation). The sum of IO nerve branches twitch tensions for the two IO nerve branches (3.4 ± 0.2 g) significantly exceeded the whole nerve twitch tension (3.0 ± 0.2 g) by 16 ± 4% on average (paired t-test: P < 0.005, n = 8). In several cases (n = 5), the stimulation intensity for each branch was intentionally reduced (submaximal stimulation). This current intensity resulted in 40–50% decrease of IO muscle tension in comparison with the corresponding tensions of the branches stimulated supra-maximally. Whole nerve submaximal stimulation was achieved with both branches stimulated at equivalent current intensities. In contrast to supramaximal IO stimulation, submaximal twitch stimulation of IO nerve and its branches did not reveal any significant twitch tension excess (Fig. 1B). Additionally, any twitch tension excess found during submaximal stimulation was quite variable, averaging 2 ± 11% (SD 24%) as it ranged from negative to positive values.
FIG. 1.
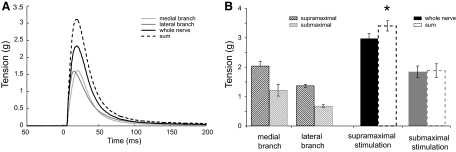
Comparison of twitch tension produced by stimulation of whole inferior oblique (IO) nerve and the sum of twitch tensions of each IO branch stimulated separately. A: example of twitch tensions for the whole IO nerve and each of its branches when stimulated with supramaximal current intensity. B: group means ± SE of twitch tensions of the whole IO nerve and its branches evoked by supra (n = 8)- and submaximal current intensity (n = 5, *P < 0.05).
TETANIC CONTRACTIONS.
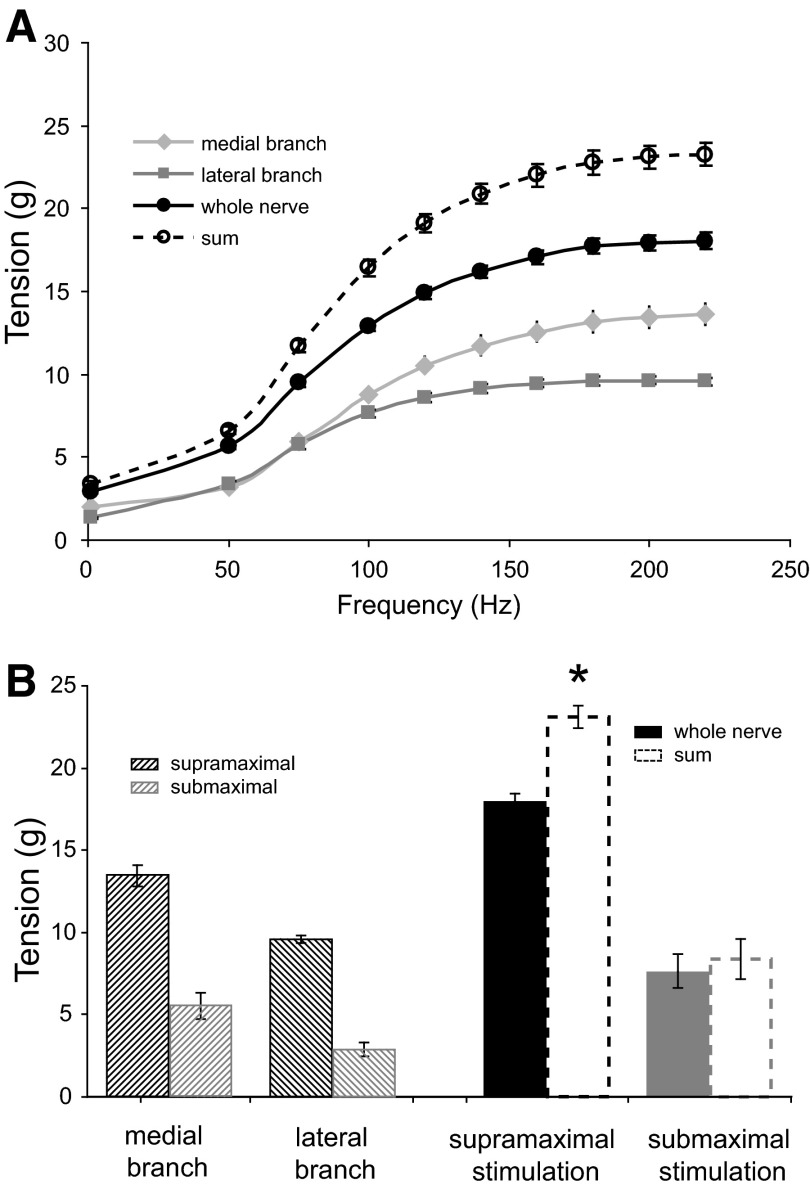
Similar to the twitch tension, the sum of tetanic tensions for the two IO nerve branches exceeded the whole IO nerve tetanic tension (P < 0.05). Tetanic tension excess was consistent with all the frequencies used and gradually increased with the higher stimulation frequencies. Figure 2A illustrates maximal tetanic tensions at different stimulation frequencies for each IO nerve branch and the whole IO nerve. It can be seen that the sum of tetanic tensions of the two IO branches (- - -) always “exceeds” the whole IO nerve maximal tetanic tension. For the lowest stimulation frequency (50 Hz), tetanic tension excess was 17 ± 3%, similar to the twitch tension excess (16 ± 4% at 1 Hz in Fig. 2A). Maximal tetanic tension excess was achieved at 140 Hz (28 ± 1%) and remained at this level regardless of further stimulation frequency increase (Fig. 2A).
FIG. 2.
Comparison of tetanic tension produced by stimulation of whole IO nerve and the sum of tensions of each IO branch stimulated separately. A: group means ± SE for each frequency of stimulation of IO with supramaximal current intensity (twitch responses to stimulation pulses at 1 Hz and to pulse trains of 50- to 220-Hz frequency). All data point differences are significant (P < 0.05). B: group means ± SE of muscle responses to 200-Hz stimulation with supra- and submaximal current intensity. Supramaximal, but not submaximal, current intensity resulted in significant tension excess (*P < 0.05).
Submaximal current intensity at 200-Hz stimulation frequency showed 8.4 ± 1.2 g summed maximal tetanic tension of the two IO branches stimulated separately versus 7.7 ± 1.1 g tetanic tension of the whole IO nerve stimulation (Fig. 2B). Whereas supramaximal IO nerve stimulation at 200 Hz gained 28 ± 1% tension excess (P < 0.05), submaximal stimulation at the same stimulation frequency resulted in statistically insignificant tension excess of 8 ± 2% (P > 0.1).
Posttetanic potentiation
Tetanic stimulation (200-ms trains of 50–220 Hz) of one IO nerve branch caused posttetanic potentiation in the same branch, but not in the other branch (cross-potentiation). The twitch size in the tetanus-stimulated branch increased to 2.6 ± 0.9 g after the tetanic stimulation in comparison with 2.2 ± 0.7 g before the tetanic stimulation (P < 0.05), presenting 19 ± 6% of posttetanic potentiation. The twitch size of the other, not tetanus-stimulated, IO nerve branch did not change significantly (−1 ± 2%). Thus the cross-potentiation test for polyneuronal innervation did not produce a positive result.
Summation of muscle electrical activity
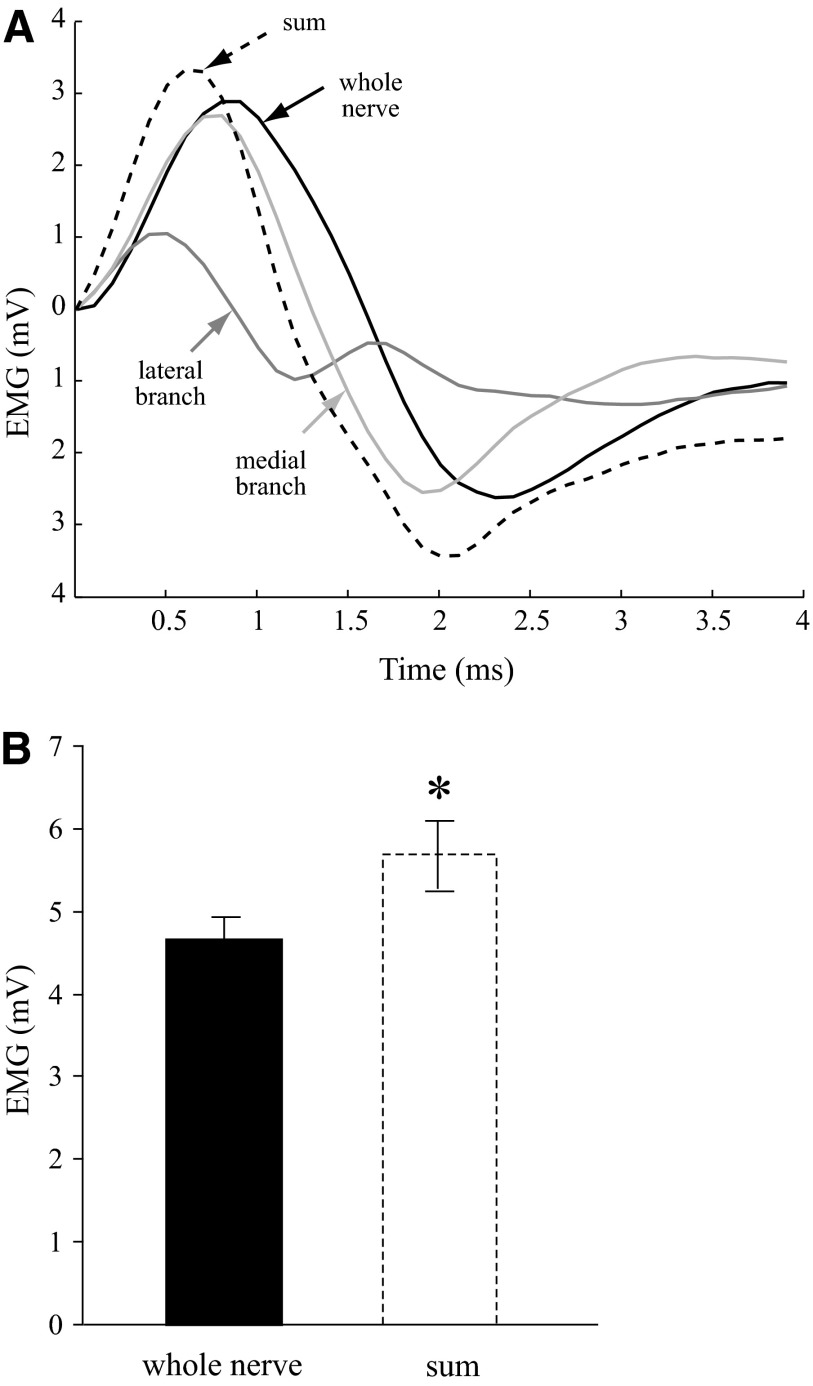
The summed EMG activity of IO muscle when the two IO nerve branches were stimulated separately significantly exceeded the IO EMG activity of the whole IO muscle (simultaneous stimulation of the branches). Examples of EMG signals from each IO nerve branch during separate or simultaneous stimulation with supramaximal current intensity are shown in Fig. 3A. The summed EMG activity following separate branch stimulation (Fig. 3A, - - -) exceeds the EMG activity of the whole IO nerve. The peak-to-peak EMG amplitude for the sum of separately stimulated nerve branches was 5.8 ± 0.4 mV, significantly larger in comparison with the EMG amplitude for simultaneously stimulated IO nerve branches, 4.8 ± 0.3 mV (P < 0.05) as also illustrated in the bar graph of Fig. 3B. The average percentage EMG excess for the group was 19 ± 5%. Interestingly, submaximal IO branch stimulation also showed EMG excess similar to supramaximal stimulation (20 ± 7%). Separate IO branch stimulation summed to 1.6 ± 0.2 mV, whereas simultaneous IO branch stimulation resulted in 1.4 ± 0.2 mV EMG amplitude of IO muscle.
FIG. 3.
Comparison of electromyographic (EMG) signals in response to IO branch or whole nerve stimulation. A: example of IO EMG activity produced by supramaximal stimulation of whole IO nerve, each IO nerve branch stimulated separately, and their sum. Note: the EMG signals are aligned by the onset to facilitate amplitude comparison. B: group means ± SE of EMG peak-to-peak amplitudes of the whole IO nerve and the summed EMG activity of the branches (n = 8, * P < 0.05).
Cross-fatigue
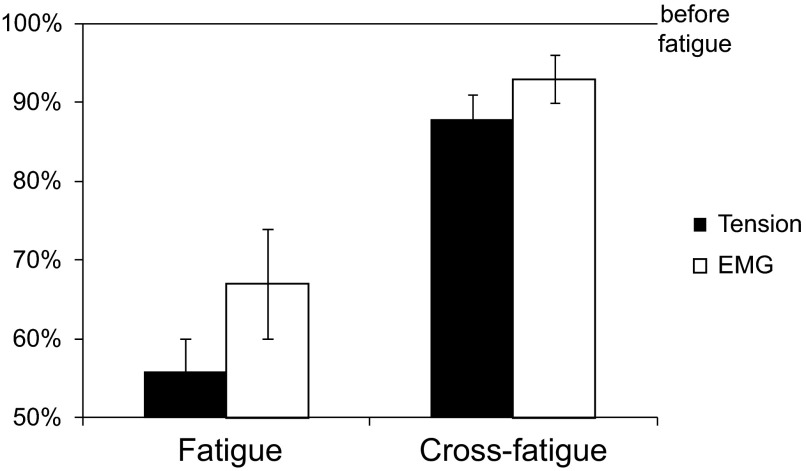
Muscle response to 200-Hz stimulation of the branch that had received fatiguing stimulation decreased to 56 ± 4% of the response prior to fatigue (Fig. 4, left). Muscle response to 200-Hz stimulation frequency of the other (nonfatigued) branch should remain unaltered if no polyneuronal innervation exists (cross-fatigue test). However, the nonfatigued IO nerve branch stimulation with 200-Hz frequency showed smaller but significant reduction in tetanic tension to 88 ± 3% (P < 0.05) of the initial response (Fig. 4, right). The EMG data simultaneously collected with the tension also showed significant reduction in peak-to-peak amplitude to 67 ± 7% for the fatigued IO branch and 93 ± 3% for the nonfatigued IO branch compared with the EMG size before the fatigue protocol was applied (P < 0.05). The results of the cross-fatigue test suggest that some muscle fibers innervated by the nonfatigued branch were already fatigued without being subjected to the fatiguing protocol.
FIG. 4.
Muscle tension and EMG amplitude (group mean ± SE, n = 7) of 1 IO nerve branch after a 2-min fatigue protocol (150-Hz frequency, 500-ms train duration, 1 train/s). Muscle response to 200-Hz stimulation is used for comparison. Fatigue: reduction in tension and EMG amplitude in response to 200-Hz stimulation following fatiguing stimulation of the same branch (P < 0.05). Cross-fatigue: reduction in tension and EMG amplitude although fatiguing stimulation was applied to the other branch (P < 0.05).
DISCUSSION
Four out of five electrophysiological tests developed to indicate polyneuronal innervation gave consistently positive results when applied to cat IO muscle and the two main divisions of its nerve. Excision of one nerve branch did not change, significantly, IO muscle responses to stimulation of the remaining branch, indicating that any changes within a branch or the interaction between the two IO nerve branches were not due to current spread.
The sum of twitch tensions with separate stimulation of the two IO nerve branches exceeded the twitch tension of the whole IO muscle by 16%. While this tension excess could be a sign of polyneuronal innervation, it could be also due to mechanical effects of the series elastic component of the muscle instead. It has been shown by Brown and Matthews (1960) that if a spring is inserted between the isometrically contracting muscle and the force transducer, the tension excess (called in their study “tension deficit” due to a reverse method of percent calculation) rises from 23 to 65%. However, the effect of the series elastic component of the muscle should be minimized during tetanic contractions, and the individual fibers would be able to develop their full tension. Indeed the tension excess during tetanic contractions of IO muscle was still present and even larger (≤28% at the higher frequencies of stimulation) in comparison with the tension excess during twitch muscle contractions. The larger extent of tension excess during tetanic over twitch stimulation could also reflect the presence of nontwitch multiply innervated muscle fibers (Bondi and Chiarandini 1983; Jacoby et al. 1989) and/or nontwitch motor units (Nelson et al. 1986). Obviously, nontwitch units would not be activated using single twitch stimulation paradigms.
Both of the preceding tests for polyneuronal innervation use the arithmetic sum of the tensions developed on stimulation of each IO nerve branch to compare with the muscle tension during whole nerve stimulation. These tests proved to be successful in establishing polyneuronal innervation in skeletal muscle during the early postnatal development period (Bagust et al. 1973). When the major wiring of the neuromuscular system has been accomplished as in normal adults and only minor adjustments may be required, the electrophysiological tests for polyneuronal innervation failed to produce positive results (Brown and Matthews 1960). It may be argued that the tension excess observed here in EOM and in previous studies in other skeletal muscles could be due to the complex muscle architecture (Alvarado-Mallart and Pinçon-Raymond 1976; Goldberg et al. 1997; Mayr et al. 1975; Trotter et al. 1995). The internal properties of the muscle can result in nonlinear summation of motor-unit forces (Goldberg et al. 1997; Monti et al. 2001; Perreault et al. 2003; Shall et al. 2003; Troiani et al. 1999). The problem of nonlinear force summation in the tests for polyneuronal innervation that rely only on tension excess can be solved by the other methods we have used.
The EMG activity of the IO muscle being free of nonlinear tension summation, also showed excess of the sum of the two IO nerve branches separately activated over their simultaneous activation. The EMG excess (∼20%) was smaller than the observed tension excess (∼30% during tetanic stimulation). This could be an indication that a portion of the tension excess might be due to a nonlinear force summation. It is also possible that the peak-to-peak analysis of IO EMG activity might miss some EMG components contributed exclusively by multiply innervated muscle fibers.
Cross-potentiation (potentiation of twitch tension when single pulses are delivered to one IO branch following tetanic stimulation of the other IO branch) was not found in the present study. It is quite possible that the multiply innervated fibers of EOM may not be subjected to posttetanic potentiation. Thus the twitch tension potentiation in the neuromuscular compartment supplied by the IO branch that had received tetanic stimulation may be produced only by the singly innervated muscle fibers. Also in our attempt to avoid any undue fatigue of the muscle, the tetanic stimulation sequence to evoke crossed-potentiation may have been too mild.
In contrast, the cross-fatigue test (decrease of muscle response to 200-Hz stimulation of one IO nerve branch after a fatigue protocol had been applied to the other branch) was always positive. Whereas both the tension and the EMG activity from the neuromuscular compartment of the fatigued IO branch were greatly reduced, a smaller but significant decrease was noted in the other, nonfatigued IO branch. Such a phenomenon could only be observed if axons from the “fresh,” nonfatigued IO branch reach muscle fibers that had already been fatigued. A recent review (Cairns and Lindinger 2008) indicates that ionic interactions may play a role in fatigue spreading to non stimulated muscle fibers. Very intense stimulation or exercise of leg muscles was used to induce the response. How this might translate to EOM using well established motor system fatigue tests is not known.
To check if reduced current intensity was the reason Bach-y-Rita and Lennestrand (1975) did not see tension excess in cat lateral rectus muscle, submaximal stimulation was applied to some of the preparations. Both twitch and tetanic IO muscle responses were very variable and quite similar to the results of Bach-y-Rita and Lennerstrand (1975), especially the twitches. Thus the submaximal IO nerve branch stimulation resulted in small and nonsignificant tension excesses. Therefore the absence of polyneuronal innervation in lateral rectus muscle (Bach-y-Rita and Lennerstrand 1975) seems to be more likely due to insufficient nerve activation. With a partially activated nerve, the effects of the series elastic component of the muscle will be largely involved, including the tetanic stimulation. In contrast to the lack of consistent tension excess with submaximal nerve stimulation, the EMG activity excess in the IO muscle was notable and equal to the EMG excess found during supramaximal nerve stimulation. As with the supramaximal nerve stimulation, the EMG activity proved to be a reliable source as a test for polyneuronal innervation. The consistency of EMG activity excess regardless of stimulation current intensity also indicates that mechanical factors had prevented registration of tension excess during submaximal nerve stimulation.
In conclusion, multiple electrophysiological tests on cat IO muscle and the natural branches of its nerve suggest the presence of polyneuronal innervation of EOM fibers. This result is consonant with many recent findings, both anatomical and physiological, in extraocular as well as other skeletal muscles. And the result also adds to the body of evidence showing how the eye-movement system appears to maintain its functional integrity in the face of insults to the CNS (reviewed in McClung et al. 2004). That is, a loss of motoneurons does not necessarily translate into a loss of muscular control because more than one motoneuron may control the same muscle fiber (McClung et al. 2004).
GRANTS
This work was supported by National Eye Institute Grant EY-11249 and the Jeffress Memorial Trust.
Acknowledgments
The authors greatly appreciate the technical support of M. E. Goldberg.
Present address of D. M. Dimitrova: Dept. of Neurology, Oregon Health and Sciences University, 3181 SW Sam Jackson Park Rd, Portland, OR 97233.
REFERENCES
- Alvarado-Mallart and Pinçon-Raymond 1976.Alvarado-Mallart RM, Pinçon-Raymond M. Nerve endings on the intramuscular tendons of cat extraocular muscles. Neurosci Lett 2: 121–125, 1976. [DOI] [PubMed] [Google Scholar]
- Bach-y-Rita and Ito 1966.Bach-y-Rita P, Ito F. In vivo studies of fast and slow muscle fibers in cat extraocular muscles. J Gen Phsyiol 49: 1177–1198, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach-y-Rita and Lennestrand 1975.Bach-y-Rita P, Lennestrand G. Absence of polyneuronnal innervation in cat extraocular muscle. J Physiol 244: 613–624, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust et al. 1973.Bagust J, Lewis DM, Westerman RA. Polyneuronnal innervation of kitten skeletal muscle. J Physiol 229: 241–255, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmack et al. 1971.Barmack NH, Bell CC, Rence BG. Tension and rate of tension development during isometric responses of extraocular muscle. J Neurophysiol 34: 1072–1079, 1971. [DOI] [PubMed] [Google Scholar]
- Bondi and Chiarandini 1983.Bondi AY, Chiarandini DJ. Morphologic and electrophysiologic identification of multiply innervated fibers in rat extraocular muscles. Invest Ophthalmol 24: 517–519, 1983. [PubMed] [Google Scholar]
- Brown and Matthews 1960.Brown MC, Matthews PBC. An investigation into the possible existence of polyneuronal innervation of individual skeletal muscle fibers in certain hind-limb muscles of the cat. J Physiol 151: 436–457, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns and Lindinger 2008.Cairns SP, Lindinger MI. Do multiple ionic interactions contribute to skeletal muscle fatigue? J Physiol 586: 4039–4054, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova and Shall 2002.Dimitrova DM, Shall MS. Goldberg SJ. Short-term effects of botulinum toxin on lateral rectus muscle of the cat. Exp Brain Res 147: 449–455, 2002. [DOI] [PubMed] [Google Scholar]
- Gillingwater et al. 2004.Gillingwater TH, Thomson D, Ribchester RR. Myo-GDNF increases non-functional polyinnervation of reinnervated mouse muscle. Neuroreport 15: 21–25, 2004. [DOI] [PubMed] [Google Scholar]
- Goldberg and Shall 1997.Goldberg SJ, Shall MS. Lateral rectus whole muscle and motor unit contractile measures with the extraocular muscles intact. J Neurosci Methods 78: 47–50, 1997. [DOI] [PubMed] [Google Scholar]
- Goldberg et al. 1997.Goldberg SJ, Wilson KE, Shall MS. Summation of extraocular motor unit tensions in the lateral rectus muscle of the cat. Muscle Nerve 20: 1229–1235, 1997. [DOI] [PubMed] [Google Scholar]
- Gorio et al. 1983.Gorio A, Carmignoto G, Finesso M, Polato P, Nunzi MG. Muscle reinnervation. II. Sprouting, synapse formation and repression. Neuroscience 8: 403–416, 1983. [DOI] [PubMed] [Google Scholar]
- Guntinas-Lichius et al. 2005.Guntinas-Lichius O, Irintchev A, Streppel M, Lenzen M, Grosheva M, Wewetzer K, Neiss WF, Angelov D. Factors limiting motor recovery after facial nerve transaction in the rat: combined structural and functional analysis. Eur J Neurosci 21: 391–402, 2005. [DOI] [PubMed] [Google Scholar]
- Harrison et al. 2007.Harrison AR, Anderson BC, Thompson LV, McLoon LK. Myofiber length and three-dimensional localization of NMJs in normal and botulinum toxin-treated adult extraocular muscles. Invest Ophthalmol Vis Sci 48: 3594–3601, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess and Pilar 1963.Hess A, Pilar G. Slow fibers in the extraocular muscles of the cat. J Physiol 169: 780–798, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman 1951a.Hoffman H Fate of interrupted nerve-fibers regenerating into partially denervated muscles. Aust J Exp Biol Med Sci 29: 211–219, 1951a. [DOI] [PubMed] [Google Scholar]
- Hoffman 1951b.Hoffman H A study of the factors influencing innervation of muscles by implanted nerves. Aust J Exp Biol Med Sci 29: 289–307, 1951b. [DOI] [PubMed] [Google Scholar]
- Ito and Kudo 1994.Ito M, Kudo M. Reinnervation by axon collaterals from single facial motoneurons to multiple muscle targets following axotomy in the adult guinea pig. Acta Anat 151: 124–130, 1994. [DOI] [PubMed] [Google Scholar]
- Jacoby et al. 1989.Jacoby J, Chiarandini DJ, Stefani E. Electrical properties and innervation of fibers in the orbital layer of rat extraocular muscles. J Neurophysiol 61: 116–125, 1989. [DOI] [PubMed] [Google Scholar]
- Jansen and Van Essen 1975.Jansen JK, Van Essen DC. Re-innervation of rat skeleton muscle in the presence of alpha-bungarotoxin. J Physiol 250: 651–67, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lateva et al. 2002.Lateva ZC, McGill KC, Johanson ME. Electrophysiological evidence of adult human skeletal muscle fibers with multiple endplates and polyneuronnal innervation. J Physiol 544: 549–565, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennerstrand 1972.Lennerstrand G Fast and slow units in extrinsic eye muscles of cat. Acta Physiol Scand 86: 286–288, 1972. [DOI] [PubMed] [Google Scholar]
- Letinsky et al. 1976.Letinsky MS, Fischbeck KH, McMahan UJ. Precision of reinnervation of original postsynaptic sites in frog muscle after a nerve crush. J Neurocytol 5: 691–718, 1976. [DOI] [PubMed] [Google Scholar]
- Mayr et al. 1975.Mayr R, Gottschall J, Gruber H, Neuhuber W. Internal structure of cat extraocular muscle. Anat Embryol 148: 25–34, 1975. [DOI] [PubMed] [Google Scholar]
- McArdle 1975.McArdle JJ Complex end-plate potentials at the regenerating neuromuscular junction of the rat. Exp Neurol 49: 629–638, 1975. [DOI] [PubMed] [Google Scholar]
- McClung et al. 2004.McClung JR, Cullen KE, Shall MS, Dimitrova DM, Goldberg SJ. Effects of electrode penetrations in the abducens nucleus of the money: eye movement recordings and histopathological evaluation of the nuclei and lateral rectus muscles. Exp Brain Res 158: 180–188, 2004. [DOI] [PubMed] [Google Scholar]
- McLoon et al. 2004.McLoon LK, Rowe J, Wirtschafter JD, McCormick KM. Continuous myofiber remodeling in uninjured extraocular myofibers: myonuclear turnover and evidenc for apoptosis. Muscle Nerve 29: 707–715, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoon and Wirtschafter 2002.McLoon LK, Wirtschafter JD. Continuous myonuclear addition to single extraocular myofibers in uninjured adult rabbits. Muscle Nerve 25: 348–358, 2002. [DOI] [PubMed] [Google Scholar]
- Monti et al. 2001.Monti RJ, Roy RR, Edgerton VR. Role of motor unit structure in defining function. Muscle Nerve 24: 848–846, 2001. [DOI] [PubMed] [Google Scholar]
- Navarrete and Vrbova 1993.Navarrete R, Vrbova G. Activity-dependent interactions between motoneurons and muscles: their role in the development of the motor unit. Prog Neurobiol 41: 93–124, 1993. [DOI] [PubMed] [Google Scholar]
- Nelson et al. 1986.Nelson JS, Goldberg SJ, McClung JR. Motoneuron electrophysiological and muscle contractile properties of superior oblique motor units in the cat. J Neurophysiol 55: 715–726, 1986. [DOI] [PubMed] [Google Scholar]
- Pachter 1982.Pachter BR Fiber composition of the superior rectus extraocular muscle of the rhesus macaque. J Morphol 174: 237–250, 1982. [DOI] [PubMed] [Google Scholar]
- Pachter 1984.Pachter BR Rat extraocular muscle. III. Histochemical variability along the length of multiply-innervated fibers of the orbital surface layer. Histochemistry 80: 535–538, 1984. [PubMed] [Google Scholar]
- Peachey 1972.Peachey L The structure of the extraocular muscle fibers of mammals. In: The Control of Eye Movements, edited by. Bach-y-Rita P, Collins CCC. New York: Academic, 1972, p. 47–66.
- Perreault et al. 2003.Perreault EJ, Day SJ, Hulliger M, Heckman CJ, Sandercock TG. Summation of forces from multiple motor units in the cat soleus muscle. J Neurophysiol 89: 738–744, 2003. [DOI] [PubMed] [Google Scholar]
- Rich and Lichtman 1989.Rich MM, Lichtman JW. In vivo visualization of pre- and postsynaptic changes during synapse elimination in reinnervated mouse muscle. J Neurosci 9: 1781–805, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker and McMahan 1976.Rotshenker S, McMahan UJ. Altered patterns of innervation in frog muscle after denervation. J Neurocytol 5: 719–730, 1976. [DOI] [PubMed] [Google Scholar]
- Russell-Mergenthal et al. 1986.Russell-Mergenthal H, McClung JR, Goldberg SJ. The determination of dendrite morphology of lateral rectus motoneurons in cat. J Comp Neurol 245: 116–122, 1986. [DOI] [PubMed] [Google Scholar]
- Shall et al. 2003.Shall MS, Dimitrova DM, Goldberg SJ. Extraocular motor unit and whole muscle contractile properties in the squirrel monkey: summation of forces and fiber morphology. Exp Brain Res 151: 338–345, 2003. [DOI] [PubMed] [Google Scholar]
- Shall and Goldberg 1992.Shall MS, Goldberg SJ. Extraocular motor units: type classification and motoneuron stimulation frequency-muscle unit force relationships. Brain Res 587: 291–300, 1992. [DOI] [PubMed] [Google Scholar]
- Shall and Goldberg 1995.Shall MS, Goldberg SJ. Lateral rectus EMG and contractile reponses elicited by cat abducens motoneurons. Muscle Nerve 18: 948–955, 1995. [DOI] [PubMed] [Google Scholar]
- Shall et al. 1995.Shall MS, Sorg PJ, McClung JR, Gilliam EE, Goldberg SJ. Relationship of the mechanical properties of the cat inferior oblique muscle to the anatomy of its motoneurons and nerve branches. Acta Anat 153: 151–160, 1995. [DOI] [PubMed] [Google Scholar]
- Tate and Westerman 1973.Tate K, Westerman RA. Polyneuronnal self-reinnervation of a slow twitch muscle (soleus) in the cat. Proc Aust Phys Pharm Soc 4: 174–175, 1973. [Google Scholar]
- Troiani et al. 1999.Troiani D, Filippi GM, Bassi FA. Nonlinear tension summation of different combinations of motor units in the anesthetized cat peroneus longus muscle. J Neurophysiol 81: 771–780, 1999. [DOI] [PubMed] [Google Scholar]
- Trotter et al. 1995.Trotter JA, Richmond FJR, Purslow PP. Functional morphology and motor control of series-fibered muscles. Exercise Sports Sci Rev 23: 167–213, 1995. [PubMed] [Google Scholar]
- Werle and Herrera 1987.Werle MJ, Herrera AA. Synaptic competition and the persistence of polyneuronal innervation at frog neuromuscular junctions. J Neurobiol 18: 375–389, 1987. [DOI] [PubMed] [Google Scholar]
- Werle and Herrera 1988.Werle MJ, Herrera AA. Synaptic competition and the elimination of polyneuronal innervation following reinnervation of adult frog sartorius muscles. J Neurobiol 19: 465–481, 1988. [DOI] [PubMed] [Google Scholar]
- Werle and Herrera 1991.Werle MJ, Herrera AA. Elevated levels of polyneuronal innervation persist for as long as two years in reinnervated frog neuromuscular junctions. J Neurobiol 22: 97–103, 1991. [DOI] [PubMed] [Google Scholar]